Abstract
Background
Treatment with fingolimod for multiple sclerosis (MS) reduces the efficacy of COVID-19 vaccination. The aim of this exploratory study was to evaluate whether main lymphocyte subsets and demographic features correlated to the subsequent increase in anti-SARS-CoV2 antibodies following the third dose of COVID-19 vaccination in fingolimod-treated MS patients.
Methods
This was a prospective single-center observational exploratory study including a subgroup of adult patients with MS (pwMS) in treatment with fingolimod who underwent COVID-19 vaccination. The association of anti-SARS-CoV2 antibody levels (reported as the Log10 of the difference between the post and pre third dose levels) with the total number and percentage of CD3+ T and CD19+ B was assessed by a linear regression model adjusted for age and sex.
Results
We found that peripheral blood CD19+ B lymphocytes before the third dose of vaccination in pwMS treated with fingolimod predict the subsequent increase of anti-SARS-CoV2 antibodies.
Conclusion
This work suggests that evaluating the percentage of CD19+ B cells may be important to identify patients at risk of not producing SARS-CoV-2 antibodies, with possible reduced protection from COVID-19.
Keywords: Multiple sclerosis, Fingolimod, COVID-19 vaccination, B lymphocytes, anti-SARS-CoV2 antibodies
1. Introduction
Coronavirus disease 2019 (COVID-19) vaccination is less effective in patients with multiple sclerosis (MS) treated with fingolimod, as evaluated through antibody response, T-cell specific responses and risk of developing breakthrough COVID-19. Even after the third dose of COVID-19 vaccine, only a fraction of patients treated with fingolimod develop an antibody response, whose mean levels are lower compared to untreated subjects (Achiron et al., 2022). The reason of the lower response to COVID-19 vaccine is only partially explained by the mechanism of action of fingolimod, a functional antagonist of the sphingosine-1-phospgate receptors, which causes a decrease of circulating T- and B- lymphocytes due to impaired recirculation. Total lymphopenia is the only factor which has been associated to impaired humoral response to COVID-19 vaccination in patients treated with fingolimod (Achiron et al., 2022). The objective of the present study was to ascertain whether the numbers and proportion of circulating main lymphocyte subsets predict the antibody response to COVID-19 vaccination in MS patients treated with fingolimod.
2. Methods
2.1. Patients
This is a prospective single-center observational exploratory study including a subgroup of adult patients with MS (pwMS) in treatment with fingolimod just enrolled for COVID-19 vaccine in MS (CovaXiMS) study. All of them underwent COVID-19 vaccination and were willing to provide blood samples for assessment of antibody levels and for the evaluation of immunological status before and after the three vaccination doses. Additional details about inclusion criteria are available elsewhere (Sormani et al., 2021). All participants signed written informed consent before starting any study procedures. The study was conducted in accordance with all national regulations and with principles of the Declaration of Helsinki and the protocol was approved by the regional ethic committee (CER Liguria: 5/2021 - DB id 11,169- 21/01/2021) and at national level by AIFA/Spallanzani (Parere n 351, 2020/21).
2.2. SARS CoV2 antibodies
Levels of anti-SARS-CoV-2 antibodies were measured within one month before the first dose of vaccine, one month after the second dose and within one month before and one month after the third dose, by a centralized laboratory with a double-antigen sandwich-based electrochemiluminescence immunoassay (ECLIA), using a commercial kit (Elecsys, Anti-SARS-CoV-2 S, Roche Diagnostics).
2.3. Lymphocyte subsets
Total number and percentage of CD3+ T lymphocytes and CD19+ B lymphocytes were measured through clinical immune profiling at the IRCCS Ospedale Policlinico San Martino before the third dose of COVID-19 vaccination.
2.4. Statistical analysis
According to previous literature, the distribution of antibody levels was normalized by a Log10 transformation.
A linear regression model was used to identify the association between antibody levels and lymphocytes (total number and percentage of CD3+ T and CD19+ B), after having adjusted for age and sex. The effect on the antibody levels and of all the relevant covariates was reported as a geometric mean, that represents the multiplicative factor for the reference level of the considered covariate.
Differences in antibody levels between two group was assessed by Mann-Whitney U Test.
3. Results
From January 2021 until April 2021, 20 adult pwMS on fingolimod (15 females/5 males) with a mean age of 49.3 ± 10.41 years were enrolled for the study. Fourteen (70.0%) had relapsing-remitting MS, median EDSS was 3.5 (IQR: 1.8 – 4.8), mean disease duration was 15.2 ± 11.38 years, and 75.0% had already been treated with different DMTs before fingolimod.
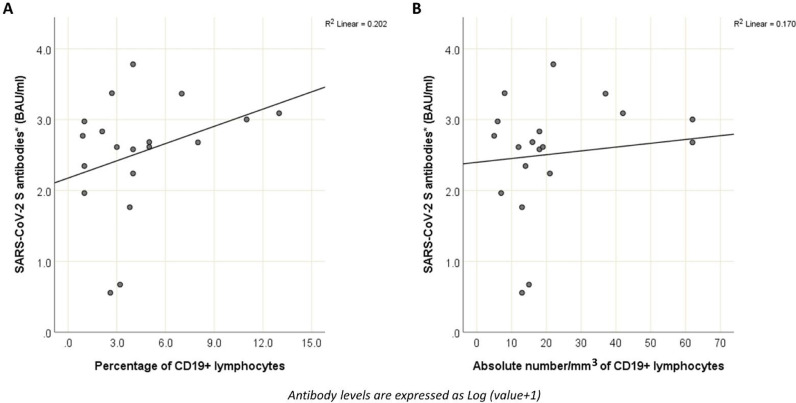
The factors associated to antibody levels after the third vaccination dose were the age (with a 2.46-fold decrease every 10 years of age, p = 0.003) and CD19+ lymphocytes (with a 1.21-fold increase every percentage point increase, p = 0.010) (Table 1 , Fig. 1 ).
Table 1.
Factors associated to antibody levels, after the third vaccination dose.
| Parameter | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| Beta coefficients (SE); | p | Beta coefficients (SE) | Geometric mean * (95%CI) | p | |
| Age (10 years) | −0.359 (0.156) | 0.021 | −0.392 (0.133) | 0.406 (0.223 - 0.738) | 0.003 |
| Sex, females vs males | 0.214 (0.432) | 0.62 | 0.043 (0.358) | 1.104 (0.219 - 5.559) | 0.91 |
| CD19+ lymphocytes (absolute number/mm3) | 0.005 (0.003) | 0.043 | |||
| CD19+ lymphocytes (percentage) | 0.081 (0.036) | 0.024 | 0.084 (0.033) | 1.213 (1.047 - 1.406) | 0.010 |
| CD3+ T lymphocytes (absolute number/mm3) | 0.001 (0.001) | 0.38 | |||
| CD3+ T lymphocytes (percentage) | −0.003 (0.010) | 0.74 | −0.004 (0.008) | 0.991 (0.957 - 1.028) | 0.63 |
Due to collinearity between absolute and percentage values for each class of lymphocytes, only percentage values (that, in case of CD19+ B lymphocytes performs better in univariate, R2=0.20) were included in the multivariate model.
Fig. 1.
Correlation between level of CD19+ lymphocytes (1A: percentage; 1B: absolute number) and antibody level after the third vaccination dose. Antibody levels are expressed as Log (value + 1).
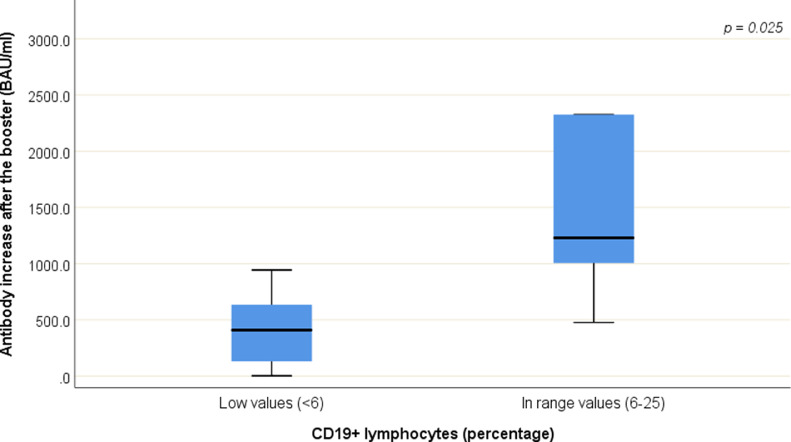
Patients with normal values for percentage of CD19+ lymphocytes (laboratory normal range: 6–25) showed higher gain in antibody levels after the third vaccine dose (median change: 1229.6 BAU/ml; IQR: 1005.4 – 2325.8) compared to patients with lower values (< 6) of CD19+ lymphocytes (median change: 409.0 BAU/ml; IQR: 91.0 – 680.2) (p = 0.025) (Fig. 2 ). The median normal value for the percentage of CD19+ lymphocytes was 11.0% (ranging from 7% to 20%), whereas the median lower value was 3.0% (ranging from 0.9% to 5.0%).
Fig. 2.
Differences in antibody levels after the booster between patients with normal values of percentage of CD19+ lymphocytes and patients with lower values of percentage of CD19+ lymphocytes.
4. Discussion
The results of this exploratory study suggest that the number and proportion of peripheral blood CD19+ B lymphocytes before the third dose of COVID-19 vaccination, in patients treated with fingolimod, predict the subsequent increase of anti-SARS-CoV2 antibodies.
Low or undetectable levels of specific antibodies after COVID-19 vaccination are a concern, since, as we have previously reported, every 10x increase in the antibody concentration reduces by 49% the risk of breakthrough COVID-19 (Sormani et al., 2022). Achiron and colleagues observed a lower rate of seroconversion following the third dose of COVID-19 vaccination in patients who continued fingolimod compared to those who suspended and received the third dose after the total number of lymphocytes was 1000/mm3 (Achiron et al., 2022). A previous study by others did not find correlations between lymphocyte subsets and seroconversion after vaccination in patients treated with modulators of the sphingosine-1-receptor, likely due to the low number of enrolled patients (N = 7) (Sabatino et al., 2022).
In this cohort, total number of lymphocytes, or T lymphocytes did not correlate with changes in the antibody levels. This may be at least in part surprising, since previous work in the animal model has suggested that fingolimod affects production of antibodies in a T-cell dependent fashion (Han et al., 2004). On the contrary, we found that the number and proportion of CD19+ B cells were associated to the increase of specific antibodies following the third dose of vaccine. Besides the number of CD19+ B cells, higher age was associated to lower development of antibodies, confirming that senescence of the immune system is linked to impaired response to vaccination. Similarly, CD19+ levels and age, together with IgM levels, were recently reported to predict the humoral response to anti-SARS-CoV2 vaccination in patients with hematological malignancies (Okamoto et al., 2022).
This study has some limitations: first of all, the low number of enrolled subjects, secondly the fact that immune cell subsets before the first dose of vaccine were not available. For all investigated correlations, nominal p-values were reported, being this is an exploratory analysis not powered for a specific hypothesis (Althouse, 2016; Perneger, 1998; Rothman, 1990). Therefore, future studies (preferably involving larger samples) are necessary to corroborate our results. If confirmed, these results suggest that assessing the percentage of CD19+ B cells in fingolimod-treated patients may be useful for identifying patients at higher risk of responding poorly to COVID-19 vaccination.
Funding
This work was supported by grants from Italian Ministry of Health (Ricerca Corrente). This work was developed within the framework of the DINOGMI Department of Excellence of MIUR 2018–2022 (legge 232 del 2016). The CovaXiMS study was supported by FISM - Fondazione Italiana Sclerosi Multipla - cod. 2021/Special-Multi/001 and co-financed with the '5 per mille' public funding.
Declaration of Competing Interest
I. Schiavetti has acted as a paid consultant to Associazione Commissione Difesa Vista, Eye Pharma, Hippocrates Research, and D.M.G Italia. L. Barcellini: no disclosures. C. Lapucci has received Travel grant from Novartis, Roche and Merck. F. Tazza: no disclosures. M. Cellerino reports no disclosures. E. Capello reports no disclosures. D. Franciotta received personal honoraria from Merck, Biogen, Sanofi-Genzyme, Roche. M. Inglese received grants NIH, NMSS, FISM; received fees for consultation from Roche, Genzyme, Merck, Biogen and Novartis. M. P. Sormani, received consulting fees from Merck, Biogen, Novartis, Sanofi, Roche, Geneuro, GSK, Medday, and Immunic. A. Uccelli, received grants (to his Institution) from FISM, Biogen, Roche, Alexion, Merck Serono; participated on a Data Safety Monitoring Board or Advisory Board (to his Institution) for BD, Biogen, Iqvia, Sanofi, Roche, Alexion, Bristol Myers Squibb. A. Laroni received fees for consultation from Roche, Genzyme, Merck, Biogen, Novartis, Bristol-Myers Squibb.
References
- Achiron A., Mandel M., Gurevich M., Dreyer-Alster S., Magalashvili D., Sonis P., Dolev M., Menascu S., Harari G., Flechter S., Falb R. Immune response to the third COVID-19 vaccine dose is related to lymphocyte count in multiple sclerosis patients treated with fingolimod. J. Neurol. 2022;269(5):2286–2292. doi: 10.1007/s00415-022-11030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althouse A.D. Adjust for multiple comparisons? It's not that simple. Ann. Thorac. Surg. 2016;101(5):1644–1645. doi: 10.1016/j.athoracsur.2015.11.024. [DOI] [PubMed] [Google Scholar]
- Han S., Zhang X., Wang G., Guan H., Garcia G., Li P., Feng L., Zheng B. FTY720 suppresses humoral immunity by inhibiting germinal center reaction. Blood. 2004;104(13):4129–4133. doi: 10.1182/blood-2004-06-2075. [DOI] [PubMed] [Google Scholar]
- Okamoto A., Fujigaki H., Iriyama C., Goto N., Yamamoto H., Mihara K., Inaguma Y., Miura Y., Furukawa K., Yamamoto Y., Akatsuka Y., Kasahara S., Miyao K., Tokuda M., Sato S., Mizutani Y., Osawa M., Hattori K., Iba S., Kajiya R., Okamoto M., Saito K., Tomita A. CD19-positive lymphocyte count is critical for acquisition of anti-SARS-CoV-2 IgG after vaccination in B-cell lymphoma. Blood Adv. 2022;6(11):3230–3233. doi: 10.1182/bloodadvances.2021006302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perneger T.V. What's wrong with Bonferroni adjustments. BMJ. 1998;316(7139):1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman K.J. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–46. [PubMed] [Google Scholar]
- Sabatino J.J., Jr., Mittl K., Rowles W.M., McPolin K., Rajan J.V., Laurie M.T., Zamecnik C.R., Dandekar R., Alvarenga B.D., Loudermilk R.P., Gerungan C., Spencer C.M., Sagan S.A., Augusto D.G., Alexander J.R., DeRisi J.L., Hollenbach J.A., Wilson M.R., Zamvil S.S., Bove R. Multiple sclerosis therapies differentially affect SARS-CoV-2 vaccine-induced antibody and T cell immunity and function. JCI Insight. 2022;7(4) doi: 10.1172/jci.insight.156978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., Inglese M., Schiavetti I., Carmisciano L., Laroni A., Lapucci C., Da Rin G., Serrati C., Gandoglia I., Tassinari T., Perego G., Brichetto G., Gazzola P., Mannironi A., Stromillo M.L., Cordioli C., Landi D., Clerico M., Signoriello E., Frau J., Ferrò M.T., Di Sapio A., Pasquali L., Ulivelli M., Marinelli F., Callari G., Iodice R., Liberatore G., Caleri F., Repice A.M., Cordera S., Battaglia M.A., Salvetti M., Franciotta D., Uccelli A. Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies. EBioMedicine. 2021;72 doi: 10.1016/j.ebiom.2021.103581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., Schiavetti I., Inglese M., Carmisciano L., Laroni A., Lapucci C., Visconti V., Serrati C., Gandoglia I., Tassinari T., Perego G., Brichetto G., Gazzola P., Mannironi A., Stromillo M.L., Cordioli C., Landi D., Clerico M., Signoriello E., Cocco E., Frau J., Ferrò M.T., Di Sapio A., Pasquali L., Ulivelli M., Marinelli F., Pizzorno M., Callari G., Iodice R., Liberatore G., Caleri F., Repice A.M., Cordera S., Battaglia M.A., Salvetti M., Franciotta D., Uccelli A. Breakthrough SARS-CoV-2 infections after COVID-19 mRNA vaccination in MS patients on disease modifying therapies during the Delta and the Omicron waves in Italy. EBioMedicine. 2022;80 doi: 10.1016/j.ebiom.2022.104042. [DOI] [PMC free article] [PubMed] [Google Scholar]