Abstract
Monoclonal antibodies to the capsular polysaccharide of Cryptococcus neoformans produce distinct capsular reactions and have biological activities that are determined by serotype specificity. In the present study, polyclonal rabbit anticapsular antibodies were cross-absorbed to produce serotype specificities similar to those of monoclonal antibodies. The results showed that polyclonal and monoclonal antibodies with similar serotype specificities have similar capsular reactions and biological activities.
Cryptococcus neoformans is an encapsulated yeast that can produce a life-threatening meningitis. Attention has focused on the immunogenicities of protein conjugates of glucuronoxylomannan (GXM), the major component of the capsular polysaccharide, and the ability of a protein conjugate vaccine to produce protective immunity (1, 2).
We recently described two distinct capsular quellung reactions following incubation of encapsulated cryptococci with monoclonal anticapsular antibodies (MAbs) having different epitope specificities (9). An annular pattern, termed rim, is produced by MAbs having specificity for an epitope shared by cryptococcal serotypes A, B, C, and D. In contrast, a MAb reactive only with serotypes A and D produces a diffuse pattern termed puffy. MAbs producing the rim and puffy patterns have several biological activities. Immunoglobulin G1 (IgG1) antibodies that produce the rim pattern support early deposition of C3 on the yeast via the classical pathway but suppress the overall rate and amount of C3 binding by either the classical or alternative pathway. IgG1 antibodies producing the puffy pattern have no effect on C3 accumulation by either the classical or the alternative pathway. IgM MAbs that are protective in a murine model of cryptococcosis produce the rim pattern and suppress C3 accumulation via the alternative pathway; nonprotective IgM antibodies produce the puffy pattern and have no effect on C3 binding via the alternative pathway.
Given the association between the capsular reaction and biological activities that might be important in host resistance and the potential for GXM as a vaccine candidate to induce protective immunity, we examined the capsular reactions produced by polyclonal rabbit anti-GXM antibodies and the effects of these antibodies on accumulation of C3 on the yeast via the alternative pathway. Our objective was to determine whether results observed with MAbs would also occur with polyclonal antibodies and oligospecific polyclonal antibodies produced by cross-absorption.
Rabbits were immunized with a complex of serotype A GXM and methylated bovine serum albumin (7). Antibodies to GXM were isolated by affinity purification using immobilized GXM (8). Analysis of the affinity-purified antibodies by enzyme-linked immunosorbent assay (4) showed the presence of antibodies that were reactive with GXM of C. neoformans serotypes A, B, C, and D (not shown). Previous studies of antibodies produced in response to immunization with whole cells of serotype A C. neoformans found that the antibodies fell into four categories: antibodies reactive with an epitope (i) shared by serotypes A, B, C, and D (factor 1); (ii) shared only by serotypes A, B, and D (factor 2); (iii) found only on serotypes A and D (factor 3); or (iv) unique to serotype A (factor 7) (5). The affinity-purified antibodies were absorbed with whole formalin-killed cells of serotype B to remove antibodies having the specificities of factors 1 and 2 but to retain reactivity comparable to that of factor 3 antisera (serotypes A and D). A preliminary experiment established that the antiserum used in this study lacked measurable amounts of factor 7 antibodies (serotype A alone), perhaps due to our use of a complex of GXM with methylated bovine serum albumin as the antigen (7). Analysis of the serotype B-absorbed antibodies by enzyme-linked immunosorbent assay using purified GXM in the solid phase showed the expected reactivity with serotypes A and D but no reactivity with serotype B or C (not shown). In this way, two pools of polyclonal antibodies were prepared. The first pool was unabsorbed, affinity-purified antibody reactive with GXM of serotypes A, B, C, and D. The second pool was reactive only with GXM of serotypes A and D. All subsequent experiments were done using the unabsorbed, affinity-purified antibodies and the serotype B-absorbed antibodies at final concentrations of 200 μg/ml (determined by absorbance at 280 nm).
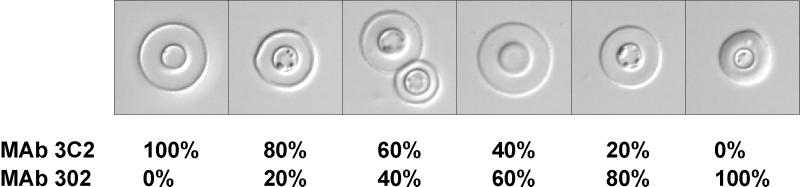
Since polyclonal antibodies likely contained a mixture of antibodies of different epitope specificities, an initial experiment evaluated the capsular reactions produced by various ratios of a MAb reactive with the epitope shared by all four serotypes (MAb 3C2) and a MAb reactive only with serotypes A and D (MAb 302). The production, purification, and characteristics of these MAbs have been described previously (6, 9, 10). The capsular reaction was determined by use of serotype A C. neoformans strain CN6 and was observed by differential interference contrast microscopy (9). The results (Fig. 1) showed the expected result that MAb 3C2 alone produced the rim pattern and MAb 302 alone produced the puffy pattern. When the antibodies were mixed, the rim pattern was observed through most ratios of antibodies until the mixture was 60 to 80% MAb 302, when a hybrid type of reaction that had features of both the rim and puffy patterns occurred.
FIG. 1.
Capsular reactions produced by various ratios of a MAb producing the rim pattern (MAb 3C2) and a MAb producing a puffy pattern (MAb 302). Cells of serotype A strain CN6 were incubated with the antibodies (200 μg of total antibody per ml), and the capsular reaction was observed by differential interference contrast microscopy as described previously (9).
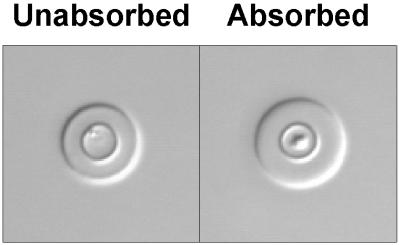
Results from mixtures of MAbs suggested that polyclonal antibodies containing a mixture of factor-like antibodies that include antibodies comparable to factors 1 and 2 will most likely produce the rim pattern as the dominant phenotype. In a second experiment, we examined the capsular reactions produced by affinity-purified polyclonal antibodies that are reactive with all four serotypes and polyclonal antibodies that have been absorbed to limit reactivity to an epitope shared by serotypes A and D (comparable to MAb 302). The results (Fig. 2) showed that the polyclonal antibodies reactive with all four serotypes produced a pattern that contained features of both the rim and puffy reactions but was predominantly rim in appearance. In contrast, the absorbed antibodies produced a puffy pattern similar to results observed with MAb 302.
FIG. 2.
Capsular reactions produced by affinity-purified polyclonal anti-GXM antibodies reactive with GXM serotypes A, B, C, and D (left panel) and affinity-purified polyclonal anti-GXM antibodies that have been absorbed with serotype B cells to yield a polyclonal antibody preparation that is reactive only with serotypes A and D (right panel).
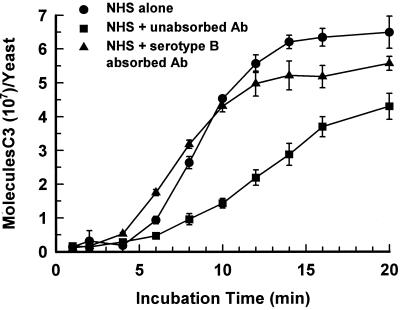
As noted above, a second biological activity of anti-GXM MAbs that is determined by epitope specificity is the effect of the antibody on activation and binding of C3 to the yeast via the alternative pathway. A final experiment examined the effect of polyclonal antibodies on the accumulation of C3 in the presence of EGTA. EGTA chelates the Ca2+ needed for activation of the classical pathway (3). As a consequence, C3 binding is due solely to action of the alternative pathway. C3 binding to serotype A strain CN6 was assessed by use of normal human serum (NHS) containing 125I-labeled C3 in the presence or absence of antibody as described previously (6). The results (Fig. 3) showed that affinity-purified antibodies that were reactive with serotypes A, B, C, and D markedly suppressed the rate of C3 accumulation and the amount of bound C3. In contrast, the absorbed antibodies that were reactive only with serotypes A and D had no effect on the rate of C3 accumulation and showed only a slightly diminished effect on the final amount of bound C3.
FIG. 3.
Kinetics for binding of C3 via the alternative pathway to cells of serotype A strain CN6 that were incubated with (i) 40% NHS alone, (ii) 40% NHS plus a 200-μg/ml concentration of affinity-purified polyclonal anti-GXM antibodies (Ab) reactive with GXM serotypes A, B, C, and D; or (iii) 40% NHS plus a 200-μg/ml concentration of affinity-purified polyclonal anti-GXM antibodies that have been absorbed with serotype B cells to yield polyclonal antibodies reactive only with serotypes A and D. Data are expressed as the mean number of molecules of C3 bound per yeast cell ± standard deviation for three replicate experiments.
Our results demonstrate that the epitope-specific effects of anti-GXM antibodies on the capsular reaction and the accumulation of C3 via the alternative pathway previously observed with anti-GXM MAbs also occur with polyclonal antibodies having similar serotype specificities. This observation is important because the capsular reaction and the effect of antibody on C3 accumulation correlate with the protective efficacy of anti-GXM IgM MAbs. The fact that the rim pattern and suppression of C3 deposition was produced by unabsorbed, affinity-purified antibodies containing a mixture of factor antibodies indicates that the dominant phenotype found in mixtures of antibodies is that associated with protection. This result supports arguments that active immunization with protein conjugates of GXM will be protective.
Acknowledgments
This work was supported by Public Health Service grant AI14209 from the National Institutes of Health.
REFERENCES
- 1.Devi S J. Preclinical efficacy of a glucuronoxylomannan-tetanus toxoid conjugate vaccine of Cryptococcus neoformans in a murine model. Vaccine. 1996;14:841–844. doi: 10.1016/0264-410x(95)00256-z. [DOI] [PubMed] [Google Scholar]
- 2.Devi S J N, Schneerson R, Egan W, Ulrich T J, Bryla D, Robbins J B, Bennett J E. Cryptococcus neoformans serotype A glucuronoxylomannan-protein conjugate vaccines: synthesis, characterization, and immunogenicity. Infect Immun. 1991;59:3700–3707. doi: 10.1128/iai.59.10.3700-3707.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fine D P, Marney S R, Jr, Colley D G, Sergent J S, Des Prez R M. C3 shunt activation in human serum chelated with EGTA. J Immunol. 1972;109:807–809. [PubMed] [Google Scholar]
- 4.Houpt D, Pfrommer G S T, Young B, Larson T, Kozel T R. Characteristics of antibodies in normal human serum that are reactive with the Cryptococcus neoformans glucuronoxylomannan. Infect Immun. 1994;62:2857–2864. doi: 10.1128/iai.62.7.2857-2864.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikeda R, Shinoda T, Fukazawa Y, Kaufman L. Antigenic characterization of Cryptococcus neoformans serotypes and its application to serotyping of clinical isolates. J Clin Microbiol. 1982;16:22–29. doi: 10.1128/jcm.16.1.22-29.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kozel T R, deJong B C H, Grinsell M M, MacGill R S, Wall K K. Characterization of anti-capsular monoclonal antibodies that regulate activation of the complement system by the Cryptococcus neoformans capsule. Infect Immun. 1998;66:1538–1546. doi: 10.1128/iai.66.4.1538-1546.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kozel T R, Gotschlich E C. The capsule of Cryptococcus neoformans passively inhibits phagocytosis of the yeast by macrophages. J Immunol. 1982;129:1675–1680. [PubMed] [Google Scholar]
- 8.Kozel T R, Hermerath C A. Benzoquinone activation of Cryptococcus neoformans capsular polysaccharide for construction of an immunoaffinity column. J Immunol Methods. 1988;107:53–58. doi: 10.1016/0022-1759(88)90008-7. [DOI] [PubMed] [Google Scholar]
- 9.MacGill T C, MacGill R S, Casadevall A, Kozel T R. Biological correlates of capsular (quellung) reactions of Cryptococcus neoformans. J Immunol. 2000;164:4835–4842. doi: 10.4049/jimmunol.164.9.4835. [DOI] [PubMed] [Google Scholar]
- 10.Savoy A C, Lupan D M, Manalo P B, Roberts J S, Schlageter A M, Weinhold L C, Kozel T R. Acute lethal toxicity following passive immunization for treatment of murine cryptococcosis. Infect Immun. 1997;65:1800–1807. doi: 10.1128/iai.65.5.1800-1807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]