Abstract
Coronavirus disease 2019 (COVID-19) pandemic spreads rapidly and may be an increasing challenge for transplant community. Clinical data on COVID-19 infection in transplant population is very limited. Herein we presented the clinical course and outcome of a 50-year-old male post liver transplantation who contracted COVID-19, with subsequent infection of his wife. The process of illness was representative. A therapeutic regime with temporary immunosuppression withdrawal and systemic low-dose corticosteroid as principle was involved in the management of the patient which made him recover from severe COVID-19 pneumonia.
KEYWORDS: clinical research/practice, liver transplantation/hepatology, infectious disease, infection and infectious agents - viral, immunosuppressant
Abbreviations: ARDS, Acute Respiratory Distress Syndrome; CMV, cytomegalovirus; CNI, calcineurin inhibitor; COVID-19, coronavirus disease 2019; CT, computed tomographic; GGO, ground-glass opacities; hsCRP, high sensitivity C-reactive protein; IVIg, intravenous immunoglobulin; LT, liver transplantation; MERS, Middle East Respiratory Syndrome; MMF, mycophenolate mofetil; MP, methylprednisolone; PCT, procalcitonin; RT-PCR, real-time reverse transcriptase polymerase chain reaction; SARS, Severe Acute Respiratory Syndrome; TAC, tacrolimus
1. INTRODUCTION
In December, 2019, an outbreak of pneumonia caused by a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged in Wuhan, Hubei province, and spread to other regions in China.1 , 2 As of March 15 2020, more than 81 000 confirmed cases infected with COVID-19 have been identified in China, of them 3204 patients died. Given that the current COVID-19 outbreak is moving rapidly, there have been 72 469 accumulated confirmed cases reported in over 140 countries/areas around the world (http://www.who.int).3
COVID-19 pandemic may be an increasing challenge for transplant community. Temporary closure of donation and cessation of transplantation would be in consideration in the epidemic area.4 Transplant patients are susceptible population to SARS-CoV-2, with majorities usually require life-long immunosuppressive therapy. Immunosuppression that reduce cell-mediated immunity may prolong shedding of coronavirus and increase the risk of infectious complications.5 However, it is recently reported that immunosuppressed patients are not at increased risk of severe pulmonary disease compared to the general population and there are no reasons to postpone transplantation during COVID-19 outbreaks.6 Indeed, there is not yet enough data to describe the epidemic situation of COVID-19 in transplant population since it is ongoing.
Though the information about COVID-19 is evolving rapidly, whether COVID-19 has donor-to-recipient transmission and its short term or long-term impact on infected immunocompromised patients is still unclear. So far, literature reports on COVID-19 infection in solid organ transplantation are limited. Herein, we report a liver transplant recipient who has recovered from severe COVID-19 pneumonia.
2. MATERIALS AND METHODS
We retrospectively collected and analyzed data on a liver transplant recipient with laboratory-confirmed COVID-19 infection. Data were obtained from electronic medical records. We also directly communicated with the recipient and his family to ascertain epidemiological and symptom data. The patient met the diagnosis criteria of “New Coronavirus Pneumonia Diagnosis and Treatment Program (Trial Version 4)” on the basis of epidemiological history of exposure, clinical manifestation, chest imaging and laboratory test of SARS-CoV-2 from the respiratory specimens by real-time reverse transcriptase polymerase chain reaction (RT-PCR).7
3. CASE REPORT
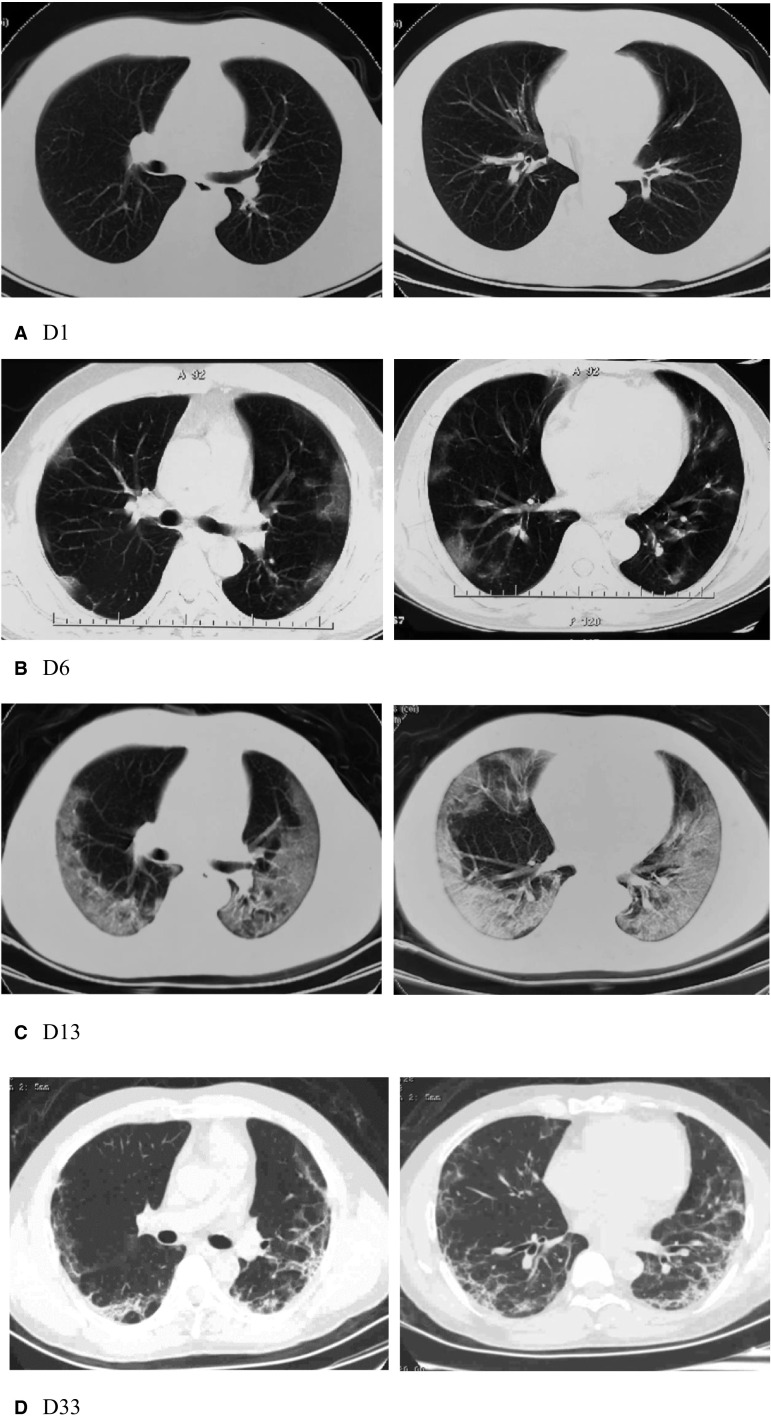
A 50-year-old man received cadaveric liver transplantation (LT) for hepatitis B cirrhosis on July, 2017. The donor was a brain-death 58-year-old male. His current immunosuppression was tacrolimus (TAC) (at a mean dosage of 0.03 mg/kg/d) monotherapy. On the night of January 23 (hereafter assumed as reference day, D0), the patient reported low fever (temperature, 37.7°C). Next day (D1) he went to a fever clinic. There were no abnormal findings on computed tomography (CT) pulmonary imaging ( Figure 1A) and he was approached as influenza by being administered oseltamivir (75 mg twice a day for 5 days). On January 29 (D6), the patient developed high fever (temperature, 39.6°C), and presented again to the fever clinic. CT scan revealed multiple peripheral patchy ground-glass shadows in both lungs (Figure 1B). He was diagnosed as a suspected case of COVID-19 and admitted to a designated hospital at Yunmeng County, Hubei province.
FIGURE 1.
Representative chest CT images of COVID-19 pneumonia. A, Negative on CT imaging (symptom onset). B, Multiple irregular piece of ground-glass shadows under the pleura in both lungs (early phase). C, Bilateral confluent GGO with multifocal patchy consolidations and bronchiectasis (advanced phase). D, Peripheral small patchy consolidations and reticular fibrotic streaks (absorptive phase)
Upon admission, he was taken off to an isolation ward. His laboratory investigations were as follows: total white blood cell count was 5.9 × 109/L, with 7.2% lymphocytes, and elevated high sensitivity C-reactive protein (hsCRP) of 32.1 mg/L. Liver transaminases and procalcitonin (PCT) were normal reference values. Because of persistent fever and lymphocytopenia, he was advised to discontinue tacrolimus, and received systemic methylprednisolone (MP) along with prophylactic antibiotic treatment. On February 2, the patient presented progressive dyspnea with oxygen saturation (SaO2) 89%. Oxygen therapy was given through a nasal cannula to maintain SaO2 at 95%-99%. On February 5 (D13), chest CT showed mixed diffuse ground-glass opacities (GGO) with multifocal patchy consolidations involving both lungs and bronchiectasis in left lower lobe (Figure 1C). The oropharyngeal swab tested positive for SARS-CoV-2. The patient fitted the severe case definition for laboratory-confirmed COVID-19.7
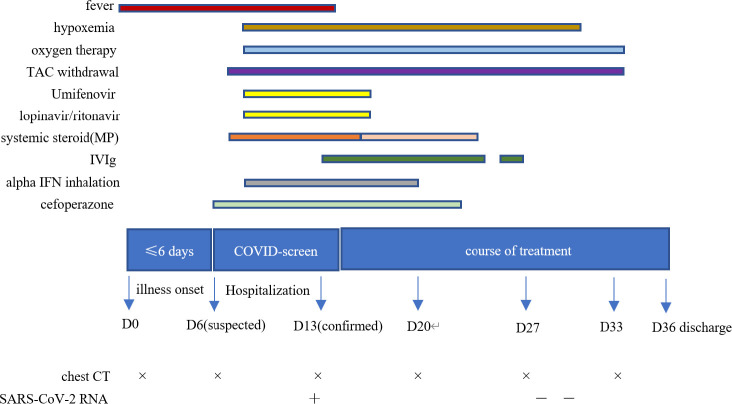
The patient was provided the following therapeutic regime ( Figure 2):
-
•
Immunosuppression withdrawal
-
•
Oxygen therapy
-
•
Umifenovir combined with lopinavir/ritonavir antiviral treatment for 1 week
FIGURE 2.
Clinical course and therapeutic regime [Color figure can be viewed at wileyonlinelibrary.com]
Umifenovir (0.2 g) three times a day.
Lopinavir/ritonavir (400 mg and 100 mg, respectively) twice a day.
-
•
Systemic methylprednisolone (MP, initially 40 mg/d for a week, then tapered to 20 mg/d for a week)
-
•
Intravenous immunoglobulin (IVIg, 10 g/d for 12 days)
-
•
Prophylactic antibiotic (cefoperazone, 2.0 g/d twice a day)
-
•
Alpha interferon (4 million units daily for 10 days, atomization inhalation)
-
•
Nutritional supportive treatment
On February 21, all clinical symptoms had resolved and lymphocyte count markedly increased (0.92 × 109/L). Two consecutive negative nucleic acid tests for respiratory specimens (sampling interval being at least 24 hours) were displayed. On February 25 (D33), chest CT demonstrated bilateral peripheral distribution of small patchy consolidations and reticular fibrosis and exudative lesions had been remarkably absorbed (Figure 1D). After 4 weeks of withdrawal of immunosuppression, TAC restated at half dosage (0.5 mg, twice a day) initially and gradually increased to full dosage. His liver function test was normal. On February 28, the patient was discharged and sent to a rehabilitation station for 14-day quarantine. At present, he has discontinued quarantine and gets home.
4. EXPOSURE HISTORY
The male patient was a resident of Wuhan, Hubei province. The epidemiological history of exposure to confirmed or suspected cases of COVID-19 prior to the onset of illness was uncertain. During the onset course of his illness, his family members were exposed. These included his wife, his son, grandson, and daughter-in-law. His wife presented fever (temperature 38°C) on January 30 and was confirmed with COVID-19 pneumonia and admitted to the hospital on February 5, 2020. The family of his son was quarantined for 14 days as the 95% confidence incubation period fell within the range of 2-14 days,8 and no one was infected.
5. DISCUSSION
In the past 2 decades, the emergence of 3 major deadly coronaviruses resulted in global outbreak of SARS, MERS, and COVID-19. There is limited knowledge regarding the transmission of COVID-19. Infection via respiratory droplets or secretions of infected individuals are thought to be the predominant mode of transmission from person to person. Due to asymptomatic transmission during incubation period, SARS-CoV-2 is more infectious than SARS-CoV and MERS-CoV.9 , 10 The case reports on transplant patients infected with COVID-19 are very limited by far, which may probably owe to low-level exposure of epidemic and strict adherence in immunosuppressed individuals.
In the present case, the clinical course of disease advancing was representative and the diagnosis of COVID-19 was similar to that in the general population, which was conducted by assessing epidemiology, clinical features, radiological findings, and SARS-CoV-2 nucleic acid test. However, we specifically stress the differential diagnosis in this case. Because of overlapping outbreak of COVID-19 and seasonal influenza, the patient was initially approached as influenza due to similar symptoms and negative radiological findings at illness onset. SARS-CoV-2 nucleic acid test should be performed early to facilitate the screening of infected individuals and close contacts since family cluster infection was presented in this case as well.
The management of COVID-19 pneumonia in immunosuppressed patients may be more sophisticated than that in nonimmunocompromised patients. Successful strategies in treatment of opportunistic infections such as CMV pneumonia post-transplantation are significantly used for reference and inspiration. Besides supportive treatment, reduction or temporary removal of immunosuppressive agents may probably be beneficial for rehabilitation of immunity in this case since the outcome of COVID-19 is largely determined by virus-host interaction. The problems are concerned on the risk of acute allograft rejection after weakening immunosuppression. The majority of patients post LT are treated with reduced-dose calcineurin inhibitors (CNIs) in combination with mycophenolate mofetil (MMF). However, this patient was on low dose TAC monotherapy due to intolerance of MMF, and he had normal liver function tests post-transplantation without episodes of rejection. We speculated that he might be low-risk for rejection. While the patient presented viral pneumonia with persistent fever and significant lymphocytopenia on admission, we made decision to withdraw TAC immunosuppression. Thereafter, with reference to clinical symptoms, lymphocyte counting, liver function test and chest CT imaging, we explored the status of pneumonia development and evaluated whether it could restore immunosuppression. A stepwise approach was involved in the resumption of immunosuppressive agents. The next, corticosteroid was administered in low dose not only to eliminate the symptoms of fever and dyspnea but to attenuate inflammatory exudation associated with cytokine storm, which has been concerned with the pathogenesis of ARDS.11 Meanwhile, systemic use of corticosteroid possibly protected him from allograft rejection owe to immunosuppressive activity. The former experience on SARS with high-dose corticosteroids had little benefit. Because of inhibiting clearance of coronavirus, it increased the risk of virus dissemination, opportunistic infection and osteopathy.12 In this case, the duration and dosage of corticosteroid for COVID-19 treatment were confined, so that severe side effects and delayed clearance of coronavirus would be averted. Methylprednisolone was provided 40 mg daily for a week and subsequently tapered to 20 mg daily for a week, which covered the advanced phase of inflammation. Although no evidence endorsed the immunomodulatory effect of IVIg due to lack of specific antibodies against SARS-CoV-2, IVIg was provided in this patient with severe lymphocytopenia, and might be helpful to reduce the incidence of potential coinfections.
In conclusion, clinical data on COVID-19 infection in transplant population is still very limited. In present case, recovery after having severe COVID-19 pneumonia may depend on normalization of immunity. Temporary withdrawal of immunosuppression and administration of corticosteroid in low-dose might be principle components of therapeutic regime. Nevertheless, success of a single positive case indeed does not represent the rationality and necessity of complex strategy. Additional data from immunosuppressed cases need to be collected to further recognize the clinical features of COVID-19 in transplant recipients.
Acknowledgments
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation. Study procedures were approved by the institutional review board (IRB) at Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (IRB approval number: TJ-C20200120). The clinical activities being reported are consistent with the principles of the declaration of Istanbul as outlined in the “Declaration of Istanbul on Organ Trafficking and Transplant Tourism”.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data was created or analyzed in this study.
REFERENCES
- 1.Guan W, Ni Z, Hu YU, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020. 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed]
- 2.Zhou F, Yu T, Du H, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective Cohn study [published online ahead of print 2020]. Lancet. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed]
- 3.World Health Organization. Coronavirus disease 2019 (COVID-19). https://www.who.int/docs/default-ource/coronaviruse/situation-reports. Accessed March 15, 2020.
- 4.Michaels MG, La Hoz RM, Danziger-Isakov L, et al. Coronavirus disease 2019: implications of emerging infections for transplantation[published online ahead of print 2020]. Am J Transplant. 10.1111/ajt.15832 [DOI] [PMC free article] [PubMed]
- 5.Shahani L, Ariza-Heredia E, Chemaly R. Antiviral therapy for respiratory viral infections in immunocompromised patients. Expert Rev Anti Infect Ther. 2017;15(4):401–415. doi: 10.1080/14787210.2017.1279970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Antiga L. Coronaviruses and immunosuppressed patients. The facts during the third epidemic [published online ahead of print 2020]. Liver Transpl. 10.1002/lt.25756 [DOI] [PubMed]
- 7.National Health Commission of the People’s Republic of China. http://www.nhc.gov.cn. Accessed March 15, 2020. [DOI] [PMC free article] [PubMed]
- 8.Linton NM, Kobayashi T, Yang Y, et al. Incubation period and other epidemiological characteristics of 2019 novel coronavirus infections with right truncation: a statistical analysis of publicly available case data [published online ahead of print 2020]. J Clin Med. 10.3390/jcm9020538 [DOI] [PMC free article] [PubMed]
- 9.Peeri NC, Shrestha N, Rahman MS, et al. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: what lessons have we learned? Int J Epidemiol. 2020:1-10. 10.1093/ije/dyaa033. [DOI] [PMC free article] [PubMed]
- 10.Yu P, Zhu J, Zhang Z, et al. A familial cluster of infection associated with the 2019 novel coronavirus indicating possible person-to-person transmission during the incubation period [published online ahead of print 2020]. J Infect Dis. 10.1093/infdis/jiaa077 [DOI] [PMC free article] [PubMed]
- 11.Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39(5):529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Auyeung T, Lee J, Lai W, et al. The use of corticosteroid as treatment in SARS was associated with adverse outcomes: a retrospective cohort study. J Infect. 2005;51(2):98–102. doi: 10.1016/j.jinf.2004.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data was created or analyzed in this study.