Abstract
The clinical characteristics, management, and outcome of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) after solid organ transplant (SOT) remain unknown. We report our preliminary experience with 18 SOT (kidney [44.4%], liver [33.3%], and heart [22.2%]) recipients diagnosed with COVID-19 by March 23, 2020 at a tertiary-care center at Madrid. Median age at diagnosis was 71.0 ± 12.8 years, and the median interval since transplantation was 9.3 years. Fever (83.3%) and radiographic abnormalities in form of unilateral or bilateral/multifocal consolidations (72.2%) were the most common presentations. Lopinavir/ritonavir (usually associated with hydroxychloroquine) was used in 50.0% of patients and had to be prematurely discontinued in 2 of them. Other antiviral regimens included hydroxychloroquine monotherapy (27.8%) and interferon-β (16.7%). As of April 4, the case-fatality rate was 27.8% (5/18). After a median follow-up of 18 days from symptom onset, 30.8% (4/13) of survivors developed progressive respiratory failure, 7.7% (1/13) showed stable clinical condition or improvement, and 61.5% (8/13) had been discharged home. C-reactive protein levels at various points were significantly higher among recipients who experienced unfavorable outcome. In conclusion, this frontline report suggests that SARS-CoV-2 infection has a severe course in SOT recipients.
KEYWORDS: coronavirus, COVID-19, outcome, SARS-CoV-2, solid organ transplantation, treatment
Abbreviations: ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease 2019; ED, emergency department; HCQ, hydroxychloroquine; HT, heart transplant; ICU, intensive care unit; IFN, interferon; IL, interleukin; IVIg, intravenous immunoglobulin; KT, kidney transplant; LPV/r, lopinavir/ritonavir; LT, liver transplant; MMF/MPA, mycophenolate mofetil/mycophenolic acid; mTOR, mammalian target of rapamycin; NAT, nucleic acid testing; RT-PCR, reverse transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SOT, solid organ transplant; Spo2/Fio2, pulse oximetry saturation/fraction of inspired oxygen ratio; TDM, therapeutic drug monitoring
1. INTRODUCTION
In December 2019, a cluster of patients with acute respiratory illness of unknown origin was reported from the city of Wuhan, Hubei Province, China.1 The causative agent of the now termed coronavirus disease 2019 (COVID-19) is a novel coronavirus (severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]), which belongs to a unique clade of the sarbecovirus subgenus within the Orthocoronavirinae subfamily.2 Following the rapid worldwide spread, COVID-19 was declared a pandemic by the World Health Organization on March 11, 2020.3 The current COVID-19 pandemic is creating unprecedented challenges to health care systems across the world and particularly in areas with large community transmission such as the region of Madrid (Spain), with 17 166 cases diagnosed by March 26.4
The clinical features, optimal therapeutic approach, and outcomes of COVID-19 among solid organ transplant (SOT) recipients remain largely unknown.5 First clinical descriptions from China and Singapore did not contain data on the specific SOT population.1 , 6, 7, 8 The first case of posttransplant COVID-19 was reported in a 52-year-old Chinese kidney transplant (KT) recipient, with favorable response following discontinuation of maintenance immunosuppression and low-dose methylprednisolone, nebulized interferon (IFN)-α, and polyclonal intravenous immunoglobulin (IVIg) therapy.9 A second report from Spain did not contain information on final patient outcome at day +12 after diagnosis.10 By analogy to other viral respiratory tract infections, namely the 2009 H1N1 influenza pandemic,11 , 12 the risk of pneumonia and progression to septic shock and acute respiratory distress syndrome is expected to be increased among SOT recipients with COVID-19 compared with the nontransplant population. On the other hand, it could be hypothesized that long-term posttransplant immunosuppression would abrogate to some extent the hyperinflammatory syndrome secondary to the cytokine storm that leads to multiorgan failure and is ultimately responsible for most of SARS-CoV-2–attributable deaths.13
Due to the urgent need of clinical experience to guide the management of this new disease in such rapidly evolving scenario, a multicenter cohort study involving many transplant centers across Spain is ongoing. We report herein our preliminary experience with the first 18 SOT recipients diagnosed with COVID-19 by March 23 at a tertiary-care institution at Madrid with a large multiorgan transplant program.
2. MATERIALS AND METHODS
2.1. Study population and design
Adult (≥18 years) SOT recipients routinely followed at the University Hospital “12 de Octubre” (Madrid, Spain) and diagnosed with COVID-19 from March 5 to March 23, 2020, were included. The local Clinical Research Ethics Committee approved the study protocol (ref. no. 20/151) and granted a waiver of informed consent due to its retrospective observational design.
Patients were enrolled at the time of diagnosis of COVID-19 and followed to April 4, 2020. Clinical characteristics, radiological features, laboratory values, antiviral drugs administered, management of baseline immunosuppression, and patient and graft outcomes were extracted from electronic medical records using a standardized case report form. All laboratory and imaging investigations were performed as part of standard of care. Respiratory function was assessed by means of the pulse oximetry saturation/fraction of inspired oxygen (SpO2/FiO2) ratio, which has a good correlation with the partial pressure of arterial oxygen (PaO2)/FiO2 ratio (SpO2/FiO2 = 64 + 0.84 × PaO2/FiO2).14 Acute respiratory distress syndrome (ARDS) was therefore classified as mild, moderate, and severe according to SpO2/FiO2 ratio thresholds of 316, 232, and 148, respectively.15 Unfavorable outcome was defined by the presence of progressive respiratory failure (i.e., sustained worsening of the SpO2/FiO2 ratio and/or development of ARDS), intensive care unit (ICU) admission and/or death.
2.2. Patient management
All SOT recipients presenting at the emergency room or outpatient clinic with suggestive symptoms and signs were tested for SARS-CoV-2 infection. The diagnosis of COVID-19 was made by means of real-time reverse transcription polymerase chain reaction (RT-PCR) in nasopharyngeal swab or sputum samples according to established methods. Such procedures were performed in a dedicated area for immunocompromised patients at the emergency department (ED). All SOT recipients with suspected or confirmed SARS-CoV-2 infection were placed on droplets and contact precautions, and no visits were allowed in patient rooms. Attending staff wore personal protective equipment according to institutional protocols.
2.3. Antiviral and immunomodulatory therapy
In line with clinical practice guidelines proposed by the Spanish Ministry of Health and local protocols,16 coformulated lopinavir/ritonavir (LPV/r) (200/100 mg twice daily orally for up to 14 days) and/or hydroxychloroquine (HCQ) (400 mg twice daily orally for the first 24 hours, followed by 200 mg twice daily for 5-10 days) were prescribed to patients with pneumonia after written or oral informed consent. Subcutaneous (SC) IFN-β (250 μg every 48 hours) could be added according to the criteria of the treating physician, on the basis of the severity of COVID-19 illness and the perceived risk of rejection. Patients with symptoms restricted to the upper respiratory tract, normal oxygen saturation and no radiologic features of pneumonia could be treated with outpatient HCQ monotherapy. Tocilizumab (single 600 mg intravenous dose) was added in selected cases with progressive respiratory failure and increasing inflammatory parameters. The adjuvant use of low- to medium-dose corticosteroids was not explicitly encouraged. Empirical antibiotic therapy was associated if bacterial superinfection was suspected.
2.4. Adjustment of immunosuppressive regimen
The management of immunosuppression was left to the discretion of the attending transplant physician, although tapering of maintenance therapy was usually attempted. Calcineurin and mammalian target of rapamycin (mTOR) inhibitors were temporarily discontinued on initiation of LPV/r and trough serum levels were obtained after 48-72 hours, with close therapeutic drug monitoring (TDM) thereafter. Baseline daily prednisone dose was usually reduced by 50%. Mycophenolate mofetil/mycophenolic acid (MMF/MPA) was also decreased in patients receiving LPV/r The QT interval was regularly assessed in patients treated with HCQ.
2.5. Statistical analysis
Quantitative data are shown as the mean ± SD or the median with IQR. Qualitative variables were expressed as absolute and relative frequencies. Continuous variables were compared using the Mann-Whitney U test. Statistical analysis was performed with SPSS version 20.0 (IBM Corp.).
3. RESULTS
As of March 23, 18 SOT recipients had been diagnosed with COVID-19. The demographics and clinical characteristics are shown in Table 1. Most patients were KT recipients (44.4% [8/18]), followed by liver transplant (LT) and heart transplant (HT) recipients (33.3% [6/18] and 22.2% [4/18], respectively). Median age was 71.0 ± 12.8 years, with predominance of male patients (77.8% [14/18]), and the median interval since transplant was 9.3 years (IQR: 6.3-16.5). Fever was present in most cases (83.3% [15/18]), whereas gastrointestinal symptoms were not uncommon at illness onset (27.8% [5/18]). As detailed in Table 2, respiratory failure (oxygen saturation [at room air] <91%) was observed in 27.8% (5/18) patients at presentation. The initial chest radiograph revealed some type of radiographic abnormalities in 72.2% (13/18) of cases, either as unilateral (33.3% [6/18]) or bilateral/multifocal consolidations (38.9% [7/18]). No thoracic CT scan was performed in any patient. Laboratory parameters (C-reactive protein [CRP], lactate dehydrogenase, and absolute lymphocyte count) according to the time from hospital admission in patients with available data (n = 17) are shown in Figure 1. Interleukin (IL)-6 levels were available for only 2 patients (15 and 7 pg/mL for patients 2 and 4, respectively).
TABLE 1.
Demographics, clinical characteristics, and symptoms at presentation in 18 SOT recipients diagnosed with COVID-19
| Case | Gender/age (y) | Type of SOT/time interval (y)a | Cause of transplantation and major comorbidities | Maintenance immunosuppression regimen | Previous ACEi/ARB therapy | Symptoms at presentation | Duration of symptoms (d)b |
|---|---|---|---|---|---|---|---|
| #1 | M/78 | Kidney/8.3 | PKD, hypertension, prostatic adenocarcinoma | Prednisone, tacrolimus | No | Fever, shortness of breath | 1 |
| #2 | M/73 | Kidney/1.8 | Hypertensive nephropathy, hypertension, diabetes | Prednisone, tacrolimus, MPA | Enalapril | Fever, shortness of breath, cough | 4 |
| #3 | M/80 | Kidney/3.8 | Hypertensive nephropathy, hypertension, diabetes, peripheral artery disease | Prednisone, tacrolimus, MPA | No | Shortness of breath, cough, myalgia, hypoxia | 7 |
| #4 | F/71 | Kidney/6.0 | ESRD of unknown cause, hypertension | Prednisone, tacrolimus, MPA | No | Fever, shortness of breath, cough, sore throat | 7 |
| #5 | M/71 | Kidney/30.1 | PKD, hypertension, diabetes, coronary artery disease | Tacrolimus | No | Fever, epigastric pain | 1 |
| #6 | M/76 | Kidney/14.8 | IgA nephropathy, hypertension, obesity | Prednisone, MMF, rapamycin | Losartan | Fever, rhinorrhea | 3 |
| #7 | M/39 | Kidney/16.8 | PKD, hypertension | Prednisone, tacrolimus, EVE | Enalapril | Fever, myalgia | 1 |
| #8 | M/65 | Kidney/6.5 | Chronic interstitial nephritis, hypertension, diabetes, obstructive sleep apnea-hypopnea syndrome | Prednisone, tacrolimus, MPA | No | Fever, shortness of breath, cough, myalgia | 5 |
| #9 | M/63 | Liver/7.9 | HBV/HCV cirrhosis with HCC, hypertension, diabetes | EVE | No | Fever, shortness of breath, cough, myalgia, malaise, diarrhea | 7 |
| #10 | M/72 | Liver/5.5 | Cryptogenic cirrhosis, hypertension, diabetes, obesity | MMF, EVE | No | Fever, shortness of breath, cough | 1 |
| #11 | F/79 | Liver/15.3 | HCV cirrhosis, HCC, diabetes, chronic renal failure (KDIGO CKD stage 3b) | Prednisone, azathioprine, EVE | Ramipril | Shortness of breath, cough, malaise, diarrhea | 3 |
| #12 | M/73 | Liver/16.4 | HBV cirrhosis, diabetes, asthma, bronchiectasis, splenectomy | MMF | No | Fever, shortness of breath, cough, malaise | 21 |
| #13 | F/76 | Liver/26.5 | HCV cirrhosis, hypertension | Tacrolimus | No | Fever, thoracic pain | 1 |
| #14 | F/46 | Liver/6.4 | Acute liver failure | Tacrolimus | No | Diarrhea | 1 |
| #15 | M/64 | Heart/13.8 | Coronary heart disease, ischemic dilated cardiomyopathy, inflammatory bowel disease, primary sclerosing cholangitis | Prednisone, cyclosporine, MPA | Losartan | Fever, shortness of breath, cough | 7 |
| #16 | M/67 | Heart/10.0 | Coronary heart disease, ischemic dilated cardiomyopathy, hypertension | Prednisone, cyclosporine, MMF | Valsartan | Fever, productive cough | 7 |
| #17 | M/63 | Heart/17.9 | Coronary heart disease, ischemic dilated cardiomyopathy, hypertension, diabetes, lung cancer, peripheral artery disease | Prednisone, cyclosporine, MMF | Enalapril | Fever, shortness of breath, cough, diarrhea | 6 |
| #18 | M/38 | Heart/8.7 | Congenital heart disease, cardiac allograft vasculopathy | Prednisone, tacrolimus, MPA | No | Fever, cough, sore throat, myalgia, malaise | 1 |
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CKD, chronic kidney disease; ESRD, end-stage renal disease; EVE, everolimus; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; KDIGO, Kidney Disease Improving Global Outcomes; MMF, mycophenolate mofetil; MPA, mycophenolic acid; PKD, polycystic kidney disease; SOT, solid organ transplant.
Time interval from transplant to diagnosis of COVID-19.
Time interval from onset of symptoms to admission.
TABLE 2.
Vital signs and radiological characteristics at presentation, therapeutic approaches, management of immunosuppression, and outcomes
| Case | Respiratory rate (bpm) | Pulse oximetry O2 saturation/SpO2/FiO2 | Radiological features (on initial chest radiograph) | Initial antiviral therapya | Respiratory support | Management of immunosuppression | Complications and therapeutic modificationsa | Outcomeb |
|---|---|---|---|---|---|---|---|---|
| #1 | 13 | 89%/424 | Unilateral diffuse consolidation | LPV/r (day +2) | HFOT | Reduction of tacrolimus dose | Paroxysmal atrial fibrillation (day +3), ARDS | Death (day +5)c |
| #2 | 20 | 94%/448 | Unilateral focal consolidation | LPV/r, HCQ (day +5) | NC | Reduction of tacrolimus dose, discontinuation of MPA and prednisone | Discontinuation of LPV/r (day +8), IVIg (day +14), acute myopericarditis (day +22) | Clinical improvement (SpO2/FiO2 400), multifocal consolidations (day +23) |
| #3 | 16 | 90%/429 | No findings | LPV/r, HCQ (day +7) | NC | Reduction of tacrolimus dose, discontinuation of MPA | Delirium (day +10), suspicion of bacterial superinfection (day +20) | Persistent respiratory failure (SpO2/FiO2 303 → 329), multifocal consolidations (day +28) |
| #4 | 23 | 97%/462 | Bilateral interstitial pneumonia, patchy consolidations | LPV/r, HCQ (day +7) | HFOT, CPAP (day +9) | Reduction of tacrolimus dose, discontinuation of MPA and prednisone | Methylprednisolone (day +10), IVIg (day +10) | Death (day +16) |
| #5 | 12 | 100%/471 | No findings | HCQ, IVIg (day +2) | None | Reduction of tacrolimus dose | Concomitant Klebsiella pneumoniae bacteremia | Discharge to home in the absence of abnormalities on follow-up chest radiographs (day +8), readmission due to clinical worsening and bilateral consolidations (day +9) |
| #6 | 12 | 96%/457 | Multifocal consolidations | HCQ (day +4) | NC, CPAP | Discontinuation of MMF | Methylprednisolone (day +6) | Discharge to home (day +13) |
| #7 | 14 | 100%/471 | No findings | HCQ (day +1) (outpatient therapy) | HFOT | Discontinuation of tacrolimus and EVE | Hospital admission due to clinical worsening and bilateral consolidations (day +3), IVIg, methylprednisolone (day +3), tocilizumab (day +8) | Severe ARDS (SpO2/FiO2 116 → 125), mild radiological improvement (day +16) |
| #8 | 16 | 93%/443 | Unilateral focal consolidation | HCQ (+5), LPV/r (day +8) | HFOT, CPAP (day +9) | Reduction of tacrolimus and MPA dose | Early discontinuation of LPV/r (day +10), renal failure due to supratherapeutic tacrolimus levels requiring RRT (day +11) | Mild ARDS (SpO2/FiO2 256 → 269), multifocal consolidations (day +17) |
| #9 | 14 | 96%/457 | Unilateral focal consolidation | HCQ (day +8), LPV/r (day +9) | NC | Transitory conversion from EVE to tacrolimus and MMF | None | Discharge to home on EVE and MMF (day +15), asymptomatic (day +19) |
| #10 | 14 | 95%/452 | Multifocal consolidations | HCQ (day +1), LPV/r, IFN-β (day +2) | HFOT | Transitory conversion from MMF and EVE to tacrolimus | Progressive respiratory failure with ARDS, renal failure, delirium | Death (day +7) |
| #11 | 16 | 96%/457 | Bilateral patchy consolidations | HCQ, IFN-β (day +3) | None | No changes | None | Discharge to home (day +14) |
| #12 | 22 | 76%/361 | Multifocal consolidations | None | HFOT, IMV (day +21) | Discontinuation of MMF | ICU admission, ARDS, refractory shock | Death (day +24) |
| #13 | 14 | 98%/467 | No findings | HCQ (day +1) (outpatient therapy) | None | None | None | Outpatient follow-up, low grade fever (day +15) |
| #14 | 12 | 100%/471 | No findings | None, discharge to home | None | None | None | Outpatient follow-up, asymptomatic (day +18) |
| #15 | 30 | 82%/390 | Bilateral patchy consolidations | HCQ (unknown) | HFOT, IMV (day +8) | Discontinuation of cyclosporine | ICU admission | Severe ARDS, ICU (day +21) |
| #16 | 12 | 96%/457 | Unilateral focal consolidation | HCQ (day +8), LPV/r (day +10) | None | Discontinuation of cyclosporine | None | Discharge to home (day +23) |
| #17 | 32 | 91%/433 | Bilateral diffuse consolidations | LPV/r, HCQ, IFN-β (day +6) | HFOT | Discontinuation of cyclosporine and MMF | Progressive respiratory failure, ARDS, delirium | Death (day +10)c |
| #18 | 14 | 98%/467 | Unilateral focal consolidation | HCQ (day +1) | None | Discontinuation of MPA | Prolongation of QT interval (510 mseg) | Discharge to home (day +21) |
Abbreviations: ARDS, acute respiratory distress syndrome; bpm, breaths per minute; CPAP, continuous positive airway pressure; EVE, everolimus; HCQ, hydroxychloroquine; HFOT, high-flow oxygen therapy (i.e., nonrebreather oxygen mask with reservoir bag); ICU, intensive care unit; IFN, interferon; IMV, invasive mechanical ventilation; IVIg, intravenous immunoglobulin; LPV/r, lopinavir/ritonavir; MMF, mycophenolate mofetil; MPA, mycophenolic acid; NC, nasal cannula; RRT, renal replacement therapy; SpO2/FiO2, pulse oximetry saturation/fraction of inspired oxygen ratio.
Time interval from onset of symptoms to the initiation or modification of treatment.
Time interval from onset of symptoms to the time of outcome assessment.
The patient was not deemed eligible for ICU admission due to high comorbidity burden and short life expectancy.
FIGURE 1.
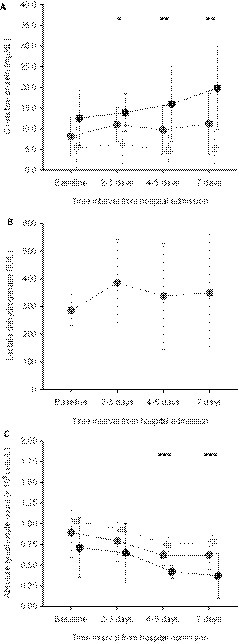
Evolution of laboratory parameters in SOT recipients with available data (n = 17): A, C-reactive protein; B, lactate dehydrogenase; and C, absolute lymphocyte count. Dots represent median values and bars indicate 95% confidence intervals in the overall cohort and patients with favorable and unfavorable outcome (middle gray, clear gray, and black, respectively). Cases 1, 3, 4, 7, 8, 10, 12, 15, and 17 were classified within the unfavorable outcome group. *P < 0.05; **P < 0.01; ***P ≤ 0.001 (for comparisons between recipients with favorable and unfavorable outcomes)
Regarding therapeutic approaches, LPV/r was administered in 50.0% (9/18) of patients, usually in association with HCQ (88.9% [8/9]). LPV/r had to be prematurely discontinued in 2 patients (patient 2 and patient 8) due to the impossibility to achieve target tacrolimus levels and severe gastrointestinal symptoms, respectively. HCQ monotherapy was used in 27.8% (5/18) patients. In addition, SC IFN-β was coadministered with other agents in 16.7% (3/18) patients. IVIg therapy and tocilizumab were added in 2 and 1 patient, respectively. The median interval from symptom onset to the initiation of therapy was 4 days (IQR: 2-7). Two patients did not receive any antiviral agent, either due to the rapid clinical deterioration (patient 12) or the absence of respiratory symptoms (patient 14). Most patients (55.6% [10/18]) underwent transitory discontinuation or dose reduction of the calcineurin inhibitor. Results of TDM in the 5 patients treated with LPV/r that were receiving tacrolimus as maintenance immunosuppression are depicted in Figure 2. Target trough levels (5-10 ng/mL) were achieved at 48-72 hours from the initiation of LPV/r therapy in only 1 patient (20.0%). One of the KT recipients (patient 8) with supratherapeutic levels (49.8 ng/mL) developed tacrolimus-induced nephrotoxicity and required renal replacement therapy (4 hemodialysis sessions). On the other hand, 2 LT recipients (patients 9 and 10) were converted from mTOR inhibitor to tacrolimus in order to facilitate the coadministration of LPV/r 3 recipients (patient 7, patient 13, patient 14) were managed as outpatients. One of them, a 39-year-old KT recipient who had been discharged from the ED on HCQ monotherapy with mild respiratory symptoms and no findings on the chest radiography, was admitted to hospital 48 hours later due to clinical worsening and the development of bilateral consolidations (patient 7). Admission to ICU and invasive mechanical ventilation were required in 11.1% (2/18) of cases. As of April 4, the case-fatality rate was 27.8% (5/18). Patients with a fatal outcome received empirical broad-spectrum antibiotic therapy at some time during their clinical course, although the cause of death was eventually attributed to SARS-CoV-2 in all the cases. Among surviving recipients, and after a median of 18 days (IQR: 14.5-22) from the initiation of symptoms, 4 patients (30.8%) experienced progressive respiratory failure and/or ARDS, 1 patient (7.7%) exhibited stable clinical condition or improvement, and eight (61.5%) had been discharged from hospital. Of note, 1 patient (patient 5) in the latter group had to be readmitted within the first 24 hours due to the reappearance of fever and development of new-onset consolidations on the chest radiograph. No patient developed acute graft rejection. The CRP levels at various times were significantly higher among recipients experiencing unfavorable outcome (n = 9) compared with those with favorable outcome (n = 9) (Figure 1A), whereas absolute lymphocyte counts were lower (Figure 1C). No significant differences were found for other clinical (e.g., age, time interval from transplant, delay in the initiation of therapy) and laboratory variables (data not shown).
FIGURE 2.
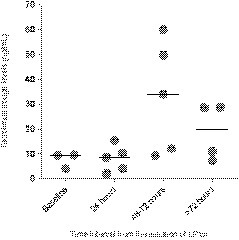
Results of therapeutic drug monitoring of tacrolimus in 5 recipients that received lopinavir/ritonavir (LPV/r). Circles and horizontal bars represent individual and median values, respectively
4. DISCUSSION
There are still many uncertainties associated with the ongoing COVID-19 pandemic in the SOT population, including its short- and medium-term impact in terms of patient and graft outcomes, the effect of baseline immunosuppression, or the role of antiviral agents that are not exempt from adverse events or drug interactions. The present case series from 1 of the regions suffering the highest incidence in Spain (cumulative incidence of 278.84 cases per 100 000 population over the last fortnight) is the largest to date examining the clinical characteristics of posttransplant SARS-CoV-2 infection.4
Despite the notable heterogeneity observed in both the recipient age at diagnosis (ranging from 38 to 80 years) and the time interval from transplant (1.8-30.1 years), the clinical picture was usually severe, in form of fever, respiratory symptoms and pneumonia with unifocal or multifocal/bilateral involvement on the chest radiograph. Moreover, about one-fourth of the cases showed respiratory failure at admission. Due to the emergency caused by the pandemic, a detailed description of the clinical features of patients diagnosed with COVID-19 in Spain is still lacking, although various research initiatives are ongoing. However, basic demographics and selected clinical characteristics extracted from administrative records are provided on a daily basis by the Spanish national epidemiological surveillance network.4 According to this report, SOT recipients in our cohort would have higher rates of pneumonia (72.2% [13/18] vs 31.1%), ARDS (38.9% [7/18] vs 5.6%) and ICU admission (11.1% [2/18] vs 5.1%) compared with the overall Spanish population, although these comparisons must be interpreted cautiously due to differences in the nature and granularity of both datasets.
Interestingly, diarrhea was a common symptom, even as an isolated manifestation (case patient 14). It has been recently reported the case of a 50-year-old KT recipient in which the first manifestations were compatible with viral gastroenteritis, with development of respiratory failure within the next days.10 Whether posttransplant immunosuppression may modify the clinical presentation of COVID-19, making SOT recipients more prone to gastrointestinal complaints compared with nontransplant patients, remains to be confirmed. In the meanwhile, a low suspicion should be maintained for SARS-CoV-2 testing in recipients with atypical, nonrespiratory symptoms. On the other hand, caution should be exercised when ambulatory therapy is planned, as exemplified by the rapid clinical deterioration with development of bilateral consolidations in 1 of our patients (case patient 7). Close follow-up must be ensured even in recipients that are discharged home with mild symptoms and no evidence of pneumonia on the chest radiograph. The sensitivity of the thoracic CT scan to detect pulmonary infiltrates at an early stage is higher compared with conventional radiograph imaging.17 Nevertheless, this increased diagnostic yield should be balanced against the risk of intrahospital transmission during patient transfers and overloading of radiology departments. In addition, the apparent dissociation between the clinical course and the radiological features was not uncommon, with patients experiencing an improvement in respiratory and inflammatory parameters despite radiological progression on serial chest radiographs (patients 2, 3, and 8). In contrast, 1 patient with no radiological findings on follow-up had to be readmitted due to the worsening of symptoms and the onset of bilateral consolidations (patient 5). Therefore, clinical and practical considerations lead us not to systematically recommend ordering CT scans on a routine basis, although we cannot exclude that such an exploration may have a role in detecting incipient infiltrates in selected cases. In contrast to posttransplant influenza,11 , 12 no bacterial superinfection or invasive aspergillosis following COVID-19 was observed, although the follow-up period might have not been long enough to allow these complications to emerge.
Long-standing experience with influenza in SOT recipients has shown that the prompt initiation of neuraminidase inhibitors reduces the risk of ICU admission.11 , 12 It could be assumed that this approach would be also valid for posttransplant COVID-19. Unfortunately, no antiviral drug has been clinically proved to be effective to treat this condition,18 although several agents are being used on the basis of in vitro activity against SARS-CoV-2 (or related viruses)19 or very limited clinical experience.20 A recently published clinical trial failed to demonstrate benefit in the time to clinical improvement with the use of LPV/r for patients with severe COVID-19.21 In accordance with national and local guidelines,16 most of the patients in our series were treated with a combination regimen including LPV/r, although some of them only received HCQ monotherapy. The observational nature of the present study—which makes it susceptible to confusion by indication—does not allow to draw any conclusion regarding the efficacy of such regimens. Nevertheless, the difficulty to manage baseline immunosuppression when LPV/r is coadministered was evident, with tacrolimus trough levels after 72 hours increasing above 30 ng/mL in 3 of 5 recipients despite transitory discontinuation and in-hospital TDM (Figure 2). Previous experiences with SOT recipients infected with human immunodeficiency virus suggest that weekly doses lower than 0.5-1.5 mg may be sufficient to maintain adequate tacrolimus levels.22 Until further evidence supporting the use of LPV/r for treating COVID-19 eventually emerges, any therapeutic decision should take into account both the severity of illness and the feasibility to perform close TDM of calcineurin and mTOR inhibitors.
A notable finding of the present experience is the case-fatality rate found (27.8%), which is much higher than that reported for the overall population.1 , 6, 7, 8 Of note, all the deaths but 1 occurred in male recipients older than 60 years, in keeping with previous studies in nontransplant patients.1 , 6, 7, 8 The limited case reports published at the time of writing 9 , 10 , 23, 24, 25, 26, 27, 28 also suggest that the severity of posttransplant SARS-CoV-2 infection is increased (pooled case-fatality rate of 20.0% [2/10]), not clearly supporting the hypothesis that long-term immunosuppression would exert a protective effect.29 However, these figures should be taken with caution since no reliable population estimates of the attack rate of COVID-19 are currently available. Therefore, it is likely that the mortality rate is overestimated due to the effect of inclusion bias (i.e., the sicker patients were more likely to be tested for SARS-CoV-2 infection). Although SOT recipients are being instructed to contact their transplant center if symptoms suggestive of COVID-19 appear, patients with mild manifestations might have been managed in primary health care facilities without RT-PCR testing or discharged directly home from the ED, remaining unrecognized. In line with previous reports in the nontransplant population,7 recipients experiencing an unfavorable outcome showed increasing CRP levels and decreasing lymphocyte counts, which likely reflected an exacerbated inflammatory response that could benefit from the early use of the anti–IL-6 receptor monoclonal antibody tocilizumab or other immunomodulatory agents.
Although limited by its small sample size, inclusion of a mixed transplant population and short follow-up period, some clinically relevant messages can be derived from our single-center experience at the frontline of the COVID-19 pandemic response. Future larger studies should confirm whether SARS-CoV-2 infection has a more severe course among SOT recipients compared with immunocompetent hosts, as well as the potential impact of early initiation of antiviral and immunomodulatory agents provided that new evidence on the management of COVID-19 become available. A prospective study is being performed at various Spanish transplant centers to try to elucidate some of these questions.
Acknowledgments
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding information This study was supported by the Spanish Ministry of Science and Innovation, Instituto de Salud Carlos III (Proyecto Integrado de Excelencia [PIE] 13/00045). Dr Fernández-Ruiz holds a research contract “Miguel Servet” (CP 18/00073 and COV 20/00181) from the Spanish Ministry of Science and Innovation, Instituto de Salud Carlos III.
REFERENCES
- 1.Zhu NA, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO). Coronavirus disease 2019 (COVID-19). Situation Report 67. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200327-sitrep-67-covid-19.pdf?sfvrsn=b65f68eb_4. Accessed March 28, 2020.
- 4.Instituto de Salud Carlos III, Centro Nacional de Epidemiología. Informe sobre la situación de COVID-19 en España nº 16 (26 de marzo de 2020). https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Documents/INFORMES/Informes%20COVID-19/Informe%20nº%2016.%20Situación%20de%20COVID-19%20en%20España%20a%2026%20marzo%20de%202020.pdf. Accessed March 28, 2020.
- 5.Michaels MG, La Hoz RM, Danziger-Isakov L, et al. Coronavirus disease 2019: implications of emerging infections for transplantation [published online ahead of print 2020]. Am J Transplant. 10.1111/ajt.15832. [DOI] [PMC free article] [PubMed]
- 6.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China[published online ahead of print 2020]. JAMA Intern Med. 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed]
- 8.Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore [published online ahead of print 2020]. JAMA. 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed]
- 9.Zhu L, Xu X, Ma K, et al. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression [published online ahead of print 2020]. Am J Transplant. 10.1111/ajt.15869. [DOI] [PMC free article] [PubMed]
- 10.Guillen E, Pineiro GJ, Revuelta I, et al. Case report of COVID-19 in a kidney transplant recipient: Does immunosuppression alter the clinical presentation? [published online ahead of print 2020]. Am J Transplant. 10.1111/ajt.15874. [DOI] [PMC free article] [PubMed]
- 11.Manuel O, Lopez-Medrano F, Keiser L, et al. Influenza and other respiratory virus infections in solid organ transplant recipients. Clin Microbiol Infect. 2014;20(suppl 7):102–108. doi: 10.1111/1469-0691.12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cordero E, Perez-Romero P, Moreno A, et al. Pandemic influenza A(H1N1) virus infection in solid organ transplant recipients: impact of viral and non-viral co-infection. Clin Microbiol Infect. 2012;18:67–73. doi: 10.1111/j.1469-0691.2011.03537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression [published online ahead of print 2020]. Lancet 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed]
- 14.Rice TW, Wheeler AP, Bernard GR, et al. Comparison of the SpO2/FiO2 ratio and the PaO2/FiO2 ratio in patients with acute lung injury or ARDS. Chest. 2007;132:410–417. doi: 10.1378/chest.07-0617. [DOI] [PubMed] [Google Scholar]
- 15.ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 16.Ministerio de Sanidad. Centro de Coordinación de Alertas y Emergencias Sanitarias. Documento técnico de manejo clínico de pacientes con enfermedad por el nuevo coronavirus (COVID-19) (versión 3 de Marzo de 2020). https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov-China/documentos/Protocolo_manejo_clinico_COVID-19.pdf. Accessed March 21, 2020.
- 17.Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020:1-7. 10.2214/AJR.20.23034 [DOI] [PubMed]
- 18.Martinez MA. Compounds with therapeutic potential against novel respiratory 2019 coronavirus [published online ahead of print 2020]. Antimicrob Agents Chemother. 10.1128/AAC.00399-20. [DOI] [PMC free article] [PubMed]
- 19.Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [published online ahead of print 2020]. Clin Infect Dis. 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed]
- 20.Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial [published online ahead of print 2020]. Int J Antimicrob Agents. 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Retracted]
- 21.Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19 [published online ahead of print 2020]. N Engl J Med. 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed]
- 22.Jain AB, Venkataramanan R, Eghtesad B, et al. Effect of coadministered lopinavir and ritonavir (Kaletra) on tacrolimus blood concentration in liver transplantation patients. Liver Transpl. 2003;9:954–960. doi: 10.1053/jlts.2003.50171. [DOI] [PubMed] [Google Scholar]
- 23.Gandolfini I, Delsante M, Fiaccadori E, et al. COVID-19 in kidney transplant recipients [published online ahead of print 2020]. Am J Transplant. 10.1111/ajt.15891. [DOI] [PMC free article] [PubMed]
- 24.Chen S, Yin Q, Shi H, et al. A familial cluster, including a kidney transplant recipient, of Coronavirus Disease 2019 (COVID-19) in Wuhan, China [published online ahead of print 2020]. Am J Transplant. 10.1111/ajt.15903. [DOI] [PMC free article] [PubMed]
- 25.Huang J, Lin H, Wu Y, et al. COVID-19 in post-transplantation patients - report of two cases [published online ahead of print 2020]. Am J Transplant. 10.1111/ajt.15896. [DOI]
- 26.Bin L, Yangzhong W, Yuanyuan Z, Huibo S, Fanjun Z, Zhishui C. Successful treatment of severe COVID-19 pneumonia in a liver transplant recipient [published online ahead of print 2020]. Am J Transplant. 10.1111/ajt.15901. [DOI]
- 27.Seminari E, Colaneri M, Sambo M, et al. Cov2 infection in a renal transplanted patients. A case report [published online ahead of print 2020]. Am J Transplant. 10.1111/ajt.15902. [DOI]
- 28.Li F, Cai J, Dong N. First cases of COVID-19 in heart transplantation from China [published online ahead of print 2020]. J Heart Lung Transplant. 10.1016/j.healun.2020.03.006. [DOI] [PMC free article] [PubMed]
- 29.Antonio R, Silvia M. Immunosuppression drug-related and clinical manifestation of Coronavirus disease 2019: a therapeutical hypothesis [published online ahead of print 2020]. Am J Transplant. 10.1111/ajt.15905. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


