Abstract
COVID-19 has profoundly affected the American health care system; its effect on the liver transplant (LT) waitlist based on COVID-19 incidence has not been characterized. Using SRTR data, we compared observed LT waitlist registrations, waitlist mortality, deceased donor LTs (DDLT), and living donor LTs (LDLT) 3/15/2020-8/31/2020 to expected values based on historical trends 1/2016-1/2020, stratified by statewide COVID-19 incidence. Overall, from 3/15 to 4/30, new listings were 11% fewer than expected (IRR = 0.84 0.890.93), LDLTs were 49% fewer (IRR = 0.37 0.510.72), and DDLTs were 9% fewer (IRR = 0.85 0.910.97). In May, new listings were 21% fewer (IRR = 0.74 0.790.84), LDLTs were 42% fewer (IRR = 0.39 0.580.85) and DDLTs were 13% more (IRR = 1.07 1.151.23). Centers in states with the highest incidence 3/15-4/30 had 59% more waitlist deaths (IRR = 1.09 1.592.32) and 34% fewer DDLTs (IRR = 0.50 0.660.86). By August, waitlist outcomes were occurring at expected rates, except for DDLT (13% more across all incidences). While the early COVID-affected states endured major transplant practice changes, later in the pandemic the newly COVID-affected areas were not impacted to the same extent. These results speak to the adaptability of the transplant community in addressing the pandemic and applying new knowledge to patient care.
KEYWORDS: clinical research/practice, health services and outcomes research, liver disease, liver transplantation/hepatology, waitlist management
Abbreviations: CDC, Centers for Disease Control and Prevention; DCD, Donation After Circulatory Death; DDLT, Deceased Donor Liver Transplant; IRR, Incidence Rate Ratio; LT, Liver Transplant; LDLT, Living Donor Liver Transplant; COVID-19, Coronavirus Disease 2019; HRSA, Health Resources and Services Administration; MELD, Model of End Stage Liver Disease; OPTN, Organ Procurement and Transplantation Network; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; SRTR, Scientific Registry of Transplant Recipients; US, United States
1. INTRODUCTION
Coronavirus Disease-2019 (COVID-19) has profoundly affected the United States (US) health care system.1, 2, 3, 4 Given the novelty of the disease, there is tremendous uncertainty about the potential impact on transplant candidates and recipients. Early in the pandemic, transplant centers and patients had reservations about the reliable detection of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in candidates, recipients, and donors.5 , 6 Furthermore, due to the rapidly evolving knowledge about management and prognosis for patients with COVID-19,7 there were no evidence-based guidelines for management pre- and posttransplant.5 , 6 , 8, 9, 10, 11 Because of these uncertainties, transplant centers have been cautious to hospitalize, operate, and immunosuppress patients in the setting of COVID-19.12
These uncertainties along with considerable increases in COVID-19–related hospitalizations and deaths1 may have impacted liver transplant (LT) across the US. A national survey between March 24 and 31, 2020, found 67.7% of LT centers had stopped performing live donor LT (LDLT).12 Although 73.3% of centers reported some restrictions for deceased donor LT (DDLT),12 there was substantial heterogeneity in the criteria used to determine transplant restrictions and minimal use of treatment protocols. Furthermore, COVID-19 disease burden varies by state,13 , 14 which may differentially impact transplant center behavior, but data-driven inference about the center-level changes and their correlation of geographic disease burden has not been characterized.
To address this knowledge gap, we used data from Scientific Registry of Transplant Recipients (SRTR) to retrospectively quantify changes to LT waitlist registration, waitlist mortality, and LT rates March-August, 2020. We further investigated these outcomes based on transplant candidate characteristics and local COVID-19 incidence, with the goal of providing insight about early pandemic changes in LT.
2. METHODS
2.1. Data source
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donor, waitlisted candidates, and transplant recipients in the United States, submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration (HRSA), US Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. The SRTR data system has previously been described elsewhere.15
2.2. State-level COVID-19 incidence
We determined the incidence of COVID-19 cases per 100,000 per day for each state (including the District of Columbia and Puerto Rico) over five 1-week periods in 2020. Outcomes from 3/15 to 4/30 were stratified based on state-level per-capita COVID-19 incidence in the last week of March. Outcomes from each month of May through August were stratified based on per-capita COVID-19 incidence during the last week of the prior month. We used publicly available data to evaluate the relationship between COVID-19 disease burden and LT waitlist events.13 , 14 Each state in each time period was stratified based on the incidence into “low” (0-3 cases/100,000/day), “medium” (4-7 cases/100,000/day), “high” (8-11 cases/100,000/day), and “very high” (>11 cases/100,000/day).
2.3. Daily counts of LT waitlist events
To characterize national changes to the liver waitlist over time, we plotted daily counts of waitlist events between February 1 and September 2, 2020 with a LOWESS smooth curve. Waitlist events included new waitlist registrations, newly inactive registrants, and waitlist removals due to DDLT, LDLT, death, or deteriorating condition. For new registrations, changes to inactive status, and LDLT, only weekday counts were included since >97.5% of these events between 2016 and 2019 fell on weekdays. We made similar plots of daily counts for DDLT, donation after circulatory death (DCD), regional import DDLT, and national import DDLT. Due to the sudden decrease in LDLT after March 15, we plotted center-level counts of LDLT between March 15 and September 2,2020 by state-level COVID-19 cases per 100,000 per day.
2.4. Calculating center-level expected numbers of LT waitlist events
To determine the expected number for each waitlist event for comparison to observed counts, we used data from each center by month from February 1, 2016 to January 31, 2020. Using multilevel Poisson regression with a center-level random intercept, we modeled the number of outcomes per center per month. We adjusted at the center-level for distributions of candidate characteristics at the start of each month: age, sex, prior transplant, race/ethnicity, history of diabetes, insurance type, Model of End-stage Liver Disease (MELD), and ABO blood type. We used these models to predict the expected number of monthly waitlist events in 3/15-4/30/2020, May, June, July, August, overall, and for subgroups of patients based on candidate characteristics.
2.5. Comparing observed to expected center-level numbers of LT waitlist events
We used the Pearson’s chi-square test to compare the observed to expected frequencies for each waitlist event. Furthermore, we used Poisson regression to compare the observed versus expected events among centers in states with different levels of COVID-19 burden. We did this by modeling the observed counts, including the log of the expected counts with the coefficient constrained to 1, and adjusted for state-level COVID-19 burden. The incidence rate ratios (IRRs) represent the observed counts as a proportion of expected counts.
2.6. Statistical analysis
All p-values are two-sided with an alpha of 0.05. Confidence intervals are reported as per the method of Louis and Zeger.16 All analyses were performed using Stata 16.1/MP for Linux (College Station, TX).
3. RESULTS
3.1. State-level COVID-19 incidence
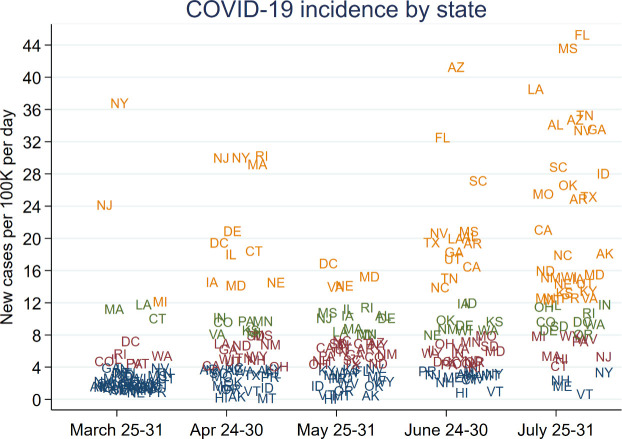
During the last week of March, 2020 (3/25-3/31), there were 39 states with “low” COVID-19 incidence (as described above), 7 states with “medium” incidence, 3 states with “high” incidence, and 3 states with “very high” incidence ( Figure 1). The states with “very high” incidence were New York, New Jersey, and Michigan. During the last week of April (4/24-4/30), the states with “very high” incidence were New York, New Jersey, Rhode Island, Massachusetts, Delaware, Washington, D.C., Connecticut, Illinois, Maryland, Iowa, and Nebraska. During the last week of May (5/25-5/31), the states with “very high” incidence were Washington, D.C., Maryland, Virginia, and Nebraska. During the last week of June (6/24-6/30), states were almost evenly distributed among the four incidence categories. During the last week of July (7/25-7/31), the distribution of states in the “very high” and “very low” incidence groups had reversed with “very low” consisting of only Vermont, Maine, New Hampshire, and New York, and the majority of states in the “very high” category.
FIGURE 1.
COVID-19 incidence by state for a representative week during 5 periods that outcomes were measured. Orange = very high incidence; green = high incidence; red = medium incidence; blue = low incidence [Color figure can be viewed at wileyonlinelibrary.com]
3.2. Daily counts of LT waitlist events
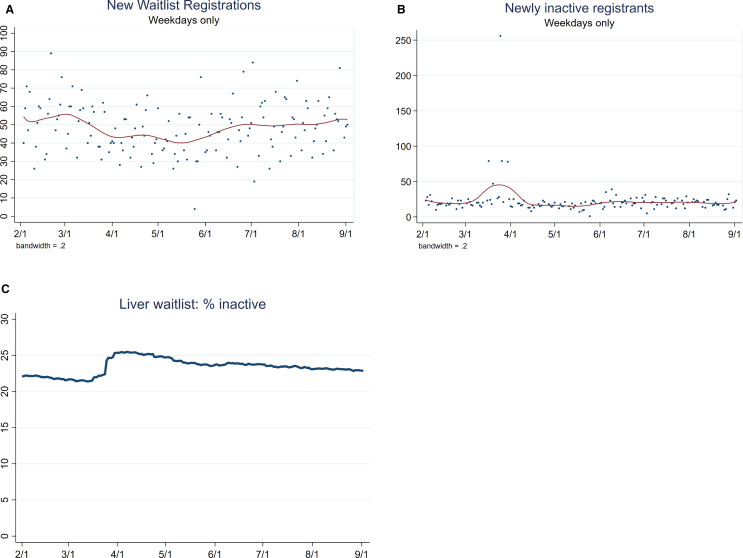
From February 1 to March 15, 2020, the average number of new waitlist registrants was 54.3 per weekday ( Figure 2A). Subsequently, the average number of new waitlist registrants dropped to 44.6 per weekday during March 16-May 3 and then increased. The number of patients newly inactivated remained stable from February 1 to September 2 except for 5 days in the second half of March that were higher than average (Figure 2B). An outlier value on March 25, 2020 was caused by a center in a “very high” COVID-19 burden state inactivating its entire waitlist on that date. The percentage of inactive patients remained stable during April-September (Figure 2C).
FIGURE 2.
Liver transplant waitlist registrations and active status for deceased donor liver transplants from February 1 to September 2, 2020 with LOWESS curve. (A) The number of new waitlist registrations began decreasing by March 15 and then increased starting mid-May. (B) The newly inactive registrants per weekday was stable. Of note, a center in a state with one of the highest COVID-19 levels inactivated its entire waitlist on March 25, 2020. (C) The percentage of the LT waitlist candidates that were inactive per day increased in late March [Color figure can be viewed at wileyonlinelibrary.com]
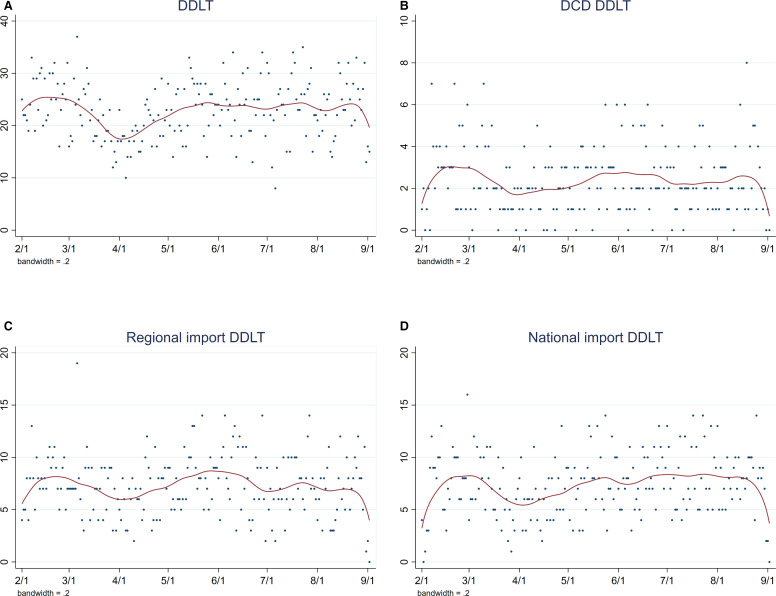
From February 1 to September 2, the number of patients removed from the waitlist due to death averaged 2.8 per day ( Figure 3A). The upward and downward trends at either end of the LOWESS curves are a typical statistical aspect of the smoothing process and may not represent a meaningful change. The number of patients removed from the waitlist due to deteriorating condition averaged 3.8 per day from February 1 to March 15. There was a downward trend mid-March to mid-April and an upward trend to baseline from mid-April to September (Figure 3B). The number of DDLT decreased from an average of 25.1 per day from February 1-March 15 to a nadir on April 5 ( Figure 4A). Afterwards, DDLT increased to baseline and plateaued from April to September. From February 1 to September 2, the average number of DCD DDLTs was 2.4 per day, regional imports were 7.3 per day, and national imports were 7.4 per day (Figure 4B-D).
FIGURE 3.
Counts of deceased donor liver transplant waitlist removals per day, February 1-September 2, 2020, with LOWESS smooth. (A) Waitlist removals due to death remained stable. (B) Waitlist removals due to deteriorating condition trended downward slightly after March 15 to a nadir in mid-April and then increased back to baseline [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 4.
Counts of DDLTs per day, February 1-September 2, 2020, with LOWESS smooth. (A) DDLT decreased after March 15, increased April to mid-May, and plateaued back at baseline mid-May to September. (B) DCD DDLTs, (C) regional imports, and (D) national imports were stable. DCD, donation after circulatory death; DDLT, deceased donor liver transplant [Color figure can be viewed at wileyonlinelibrary.com]
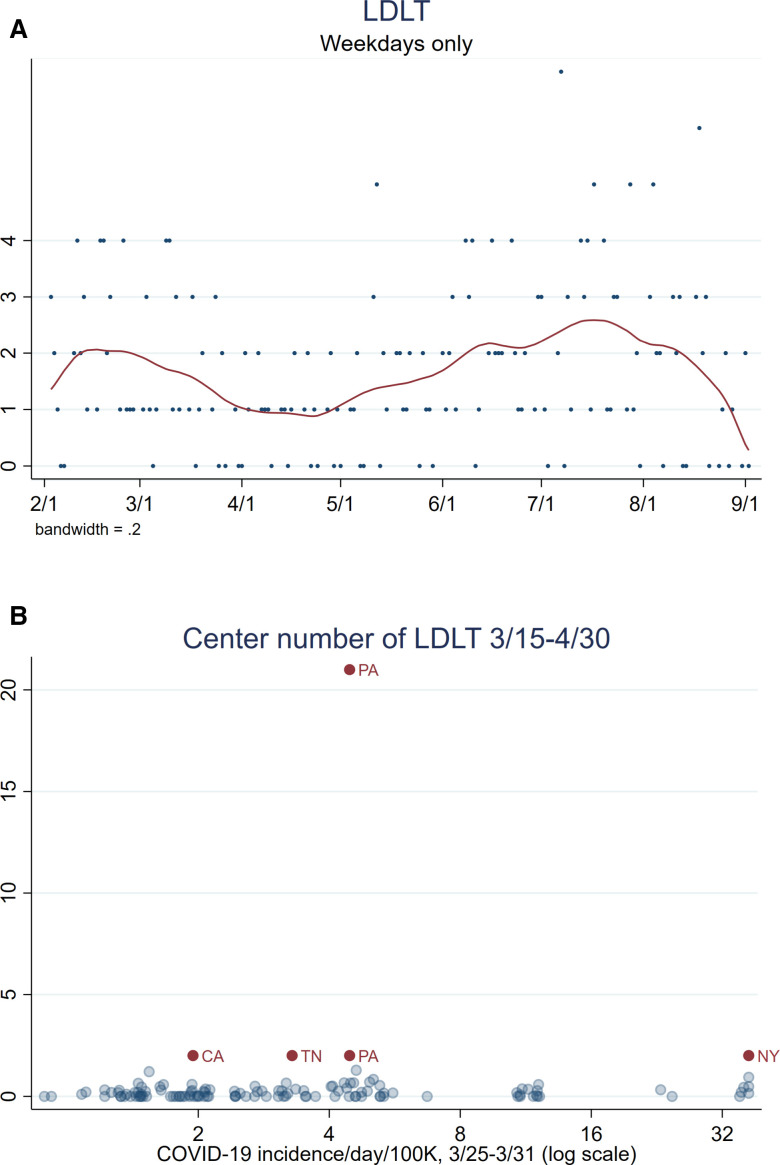
The average number of LDLTs performed per weekday was 2 before March 15 ( Figure 5A). After a rapid decline from March 15 to April 1, the average number of LDLTs plateaued at 1 throughout April. Throughout May to September, the average number of LDLTs increased slowly. After March 15, only five centers performed more than 1 LDLT (Figure 5B). Two of the centers were in states with fewer than four COVID-19 cases per day per 100,000 population. One of the centers was in a state with more than 32 COVID-19 cases per day per 100,000 population.
FIGURE 5.
LDLT events. (A) Counts of LDLT per weekday from February 1 to September 2, 2020, with LOWESS curve show a decrease after mid-March, a plateau through April, and an increase back to baseline from May to September. (B) Center-level number of LDLTs performed between March 15 and April 30, 2020, and COVID-19 cases per day per 100,000 population. Points labeled based on that center’s state and centers from states where more than one LDLT was performed are dark red. LDLT, living donor liver transplant [Color figure can be viewed at wileyonlinelibrary.com]
3.3. Comparing observed to expected center-level numbers of LT waitlist events
Overall, from March 15 to April 30, the number of new listings observed was 11% fewer than expected (IRR = 0.84 0.890.93, Table 1), 21% fewer in May (IRR = 0.75 0.790.84), and as expected during June-August. Overall, the number of waitlist deaths was not different than expected for any period. The overall number of LDLTs observed were 49% lower than expected from March 15 to April 30 (IRR = 0.37 0.510.72) and 42% less than expected in May (IRR = 0.390.580.85). However, in June-August, the overall number of LDLTs observed was similar to expected. Overall, the number of DDLTs observed was 9% lower than expected during March 15-April 30 (IRR = 0.850.910.97), 13% higher in May (IRR = 1.051.131.21), 15% higher in June (IRR = 1.071.151.23), 14% higher in July (IRR = 1.06 1.141.23), and 13% higher in August (IRR = 1.05 1.131.21).
TABLE 1.
Observed center-level events as a proportion of expected events, March 15-August 31, 2020
| COVID–19 incidence | New listings | Waitlist death | LDLT | DDLT | DCD DDLT | Regional import | National import |
|---|---|---|---|---|---|---|---|
| March 15-April 30 | |||||||
| Overall | 0.84 0.890.93 | 0.98 1.151.35 | 0.37 0.510.72 | 0.85 0.910.97 | 0.76 0.941.15 | 0.93 1.041.16 | 3.13 3.523.96 |
| Low | 0.91 0.961.02 | 0.91 1.121.37 | 0.07 0.160.38 | 0.89 0.961.03 | 0.84 1.051.32 | 1.02 1.151.30 | 3.69 4.274.95 |
| Medium | 0.69 0.790.90 | 0.52 0.891.50 | 0.87 1.281.89 | 0.84 1.001.20 | 0.19 0.461.09 | 0.52 0.801.24 | 5.47 7.309.75 |
| High | 0.56 0.690.85 | 0.65 1.101.86 | 0 | 0.44 0.590.79 | 0.15 0.451.41 | 0.35 0.681.30 | 0.68 1.091.75 |
| Very high | 0.55 0.670.80 | 1.09 1.592.32 | 0.13 0.350.94 | 0.50 0.660.86 | 0.43 0.962.15 | 0.13 0.290.65 | 1.71 2.383.31 |
| May 1-May 31 | |||||||
| Overall | 0.74 0.790.84 | 0.80 0.991.22 | 0.39 0.580.85 | 1.05 1.131.21 | 1.01 1.261.57 | 1.15 1.301.47 | 4.11 4.665.29 |
| Low | 0.74 0.830.92 | 0.61 0.941.46 | 0.30 0.922.84 | 1.05 1.181.34 | 1.08 1.522.12 | 1.50 1.802.17 | 4.04 5.316.99 |
| Medium | 0.68 0.770.87 | 0.54 0.811.23 | 0.28 0.581.22 | 0.97 1.101.25 | 0.79 1.191.79 | 0.96 1.181.45 | 2.88 3.734.82 |
| High | 0.63 0.740.87 | 0.38 0.751.50 | 0.43 0.771.40 | 0.96 1.161.40 | 0.48 0.921.76 | 0.76 1.131.67 | 6.44 8.4110.98 |
| Very high | 0.69 0.790.91 | 0.93 1.291.80 | 0.13 0.320.78 | 0.89 1.051.25 | 0.66 1.162.04 | 0.42 0.661.02 | 3.09 3.874.84 |
| June 1-June 30 | |||||||
| Overall | 0.92 0.971.03 | 0.80 1.001.24 | 0.79 1.061.41 | 1.07 1.151.23 | 1.06 1.321.64 | 1.15 1.301.48 | 4.09 4.665.29 |
| Low | 0.89 1.031.19 | 0.69 1.212.13 | 0.41 1.636.53 | 1.05 1.241.48 | 1.29 2.053.25 | 1.40 1.832.40 | 5.15 7.6911.47 |
| Medium | 0.90 0.981.06 | 0.79 1.051.38 | 0.74 1.041.47 | 1.05 1.151.27 | 0.98 1.311.74 | 1.08 1.271.49 | 3.72 4.415.22 |
| High | 0.78 0.901.05 | 0.37 0.671.22 | 0.49 1.032.17 | 0.83 1.001.20 | 0.53 0.961.74 | 0.74 1.101.62 | 2.80 3.724.95 |
| Very high | 0.81 1.001.24 | 0.58 1.071.99 | 0.39 1.042.77 | 0.95 1.241.62 | 0.25 0.782.42 | 0.55 0.911.48 | 5.01 7.3110.65 |
| July 1-July 31 | |||||||
| Overall | 0.98 1.031.10 | 0.78 0.961.20 | 0.90 1.181.54 | 1.06 1.141.23 | 0.85 1.081.37 | 1.02 1.171.33 | 4.31 4.875.52 |
| Low | 0.87 1.001.17 | 0.48 0.801.33 | 0.63 1.121.97 | 0.97 1.171.43 | 0.66 1.322.63 | 0.37 0.671.20 | 3.25 4.135.26 |
| Medium | 0.88 0.981.09 | 0.78 1.121.63 | 0.74 1.101.64 | 1.13 1.281.46 | 0.97 1.392.00 | 0.86 1.111.43 | 5.14 6.247.59 |
| High | 0.79 0.991.23 | 0.08 0.331.34 | 0.20 1.4310.16 | 1.23 1.572.00 | 0.29 0.772.04 | 1.08 1.742.80 | 4.53 7.6512.92 |
| Very high | 1.00 1.091.18 | 0.76 1.041.43 | 0.84 1.362.23 | 0.89 0.991.11 | 0.59 0.871.28 | 1.03 1.221.44 | 3.16 4.005.05 |
| August 1-August 31 | |||||||
| Overall | 0.91 0.961.02 | 0.71 0.881.11 | 0.63 0.871.19 | 1.05 1.131.21 | 0.99 1.241.55 | 0.97 1.111.27 | 4.20 4.765.39 |
| Low | 0.77 0.971.23 | 0.40 0.831.75 | 0.15 0.471.45 | 0.99 1.321.77 | 0.77 2.065.49 | 0.05 0.210.83 | 2.72 3.795.28 |
| Medium | 0.79 0.921.07 | 0.38 0.701.31 | 0.44 0.801.44 | 0.84 1.041.28 | 0.25 0.671.79 | 0.69 1.131.84 | 2.58 3.695.28 |
| High | 0.75 0.881.03 | 0.54 0.981.77 | 0.57 1.142.28 | 1.06 1.261.49 | 0.88 1.342.06 | 0.91 1.271.77 | 3.96 5.146.66 |
| Very high | 0.92 0.991.07 | 0.69 0.921.22 | 0.60 0.961.54 | 1.00 1.101.20 | 0.94 1.251.66 | 0.98 1.141.33 | 4.47 5.346.37 |
Note. COVID-19 incidence stratified based on cases per 100,000 population per day during the last week in March (for March 15-April 30) or during the last week of the prior month (for May and August). Bold denotes statistically significant IRRs; underline denotes IRRs that are significantly different from the IRR in states with the lowest per-capita COVID-19 cases.
Abbreviations: DCD, donation after circulatory death; DDLT, deceased donor liver transplant; LDLT, living donor liver transplant.
From March 15 to April 30, among centers in states with “medium” COVID-19 incidence, there were 21% fewer new waitlist registrants than expected (IRR = 0.69 0.790.90, Table 1). The decrease in new listings for centers in states with “very high” COVID-19 burden was more severe, with 33% fewer new listings than expected (IRR = 0.55 0.670.80) which was also a statistically significantly stronger decrease than for centers in states with “low” COVID-19 burden. There were 59% more waitlist deaths than expected among centers in states with “very high” COVID-19 rates (IRR = 1.091.592.32). Among centers in states with “low” incidence, there were 84% fewer LDLT than expected (IRR = 0.07 0.160.38) and 65% fewer for “very high” incidence states (IRR = 0.13 0.350.94).
From March 15 to April 30, the observed number of DDLTs was also 41% less than expected at centers in states with “high” COVID-19 burden (IRR = 0.44 0.590.79) (Table 1) and 34% at centers in states with “very high” COVID-19 incidence (IRR = 0.50 0.660.86). The IRRs were both statistically significantly less than the IRRs for centers in states with “low” COVID-19 rates. The decline in DDLT was greatest among patients with MELD less than 20 (eg, for MELD 15-19, 92 observed versus 142.4 expected, –35.4%, p < .001) ( Table 2), and patients who received exception points (67 observed versus 154.5 expected, p < .001). Among patients with MELD 25-29, there were 21.1% (144 observed versus 118.9 expected, p < .001), and for MELD 30-34 there were 50.4% (154 observed versus 102.4 expected, p < .001) more DDLT observed than expected.
TABLE 2.
Characteristics of DDLT recipients by expected and observed counts from March 15 to April 30, 2020
| Category | Observed | Expected | % change | p-value |
|---|---|---|---|---|
| Overall | 882 | 971.2 | –9.2 | <.01 |
| Age | ||||
| 0-11 | 33 | 36.8 | –10.3 | |
| 12-17 | 8 | 8.9 | –10.1 | |
| 18-29 | 24 | 29.4 | –18.4 | |
| 30-39 | 78 | 76.2 | 2.4 | .7 |
| 40-49 | 133 | 130.1 | 2.2 | |
| 50-59 | 272 | 283.9 | –4.2 | |
| 60-69 | 296 | 339.5 | –12.8 | |
| 70+ | 38 | 47.8 | –20.5 | |
| Prior transplant | ||||
| No | 839 | 925.7 | –9.4 | .4 |
| Yes | 43 | 38.1 | 12.9 | |
| Race/Ethnicity | ||||
| White | 613 | 668.7 | –8.3 | |
| Black | 62 | 74.8 | –17.1 | |
| Hispanic | 158 | 160.1 | –1.3 | .9 |
| Asian | 34 | 37.6 | –9.6 | |
| Other | 15 | 18.8 | –20.2 | |
| Insurance payor type | ||||
| Private | 453 | 482.4 | –6.1 | |
| Medicaid | 169 | 175.0 | –3.4 | |
| Medicare | 222 | 239.6 | –7.3 | .4 |
| Other public | 13 | 24.1 | –46.1 | |
| Other | 22 | 24.7 | –10.9 | |
| Sex | ||||
| Male | 570 | 618.2 | –7.8 | .9 |
| Female | 312 | 347.5 | –10.2 | |
| Status | ||||
| MELD <15 | 101 | 159.8 | –36.8 | |
| MELD 15-19 | 92 | 142.4 | –35.4 | |
| MELD 20-24 | 126 | 146.4 | –13.9 | <.001 |
| MELD 25-29 | 144 | 118.9 | 21.1 | |
| MELD 30-34 | 154 | 102.4 | 50.4 | |
| MELD 35+ | 162 | 166.2 | –2.5 | |
| Status 1 | 36 | 40.0 | –10.0 | |
| Exception | 67 | 154.5 | –56.6 | |
Note. The overall observed number was 9.2% less than expected.
Abbreviation: MELD, Model for End Stage Liver Disease.
In May, centers in all categories of COVID-19 incidence had 19-25% fewer than expected new listings (IRRlow = 0.75 0.840.94; IRRmed = 0.69 0.780.88; IRRhigh = 0.64 0.750.88; IRRveryhigh = 0.70 0.810.93) (Table 1). Among centers in states with “very high” COVID-19 burden, there were 68% fewer LDLT than expected (IRR = 0.13 0.320.78). The observed number of DDLTs was 18% more than expected at centers in states with “low” COVID-19 burden (IRR = 1.05 1.181.34). Centers in states with “low” COVID-19 incidence were more willing to engage in DCD DDLTs and had 52% more than expected (IRR = 1.081.522.12).
In June, centers across all categories had observed rates similar to expected for new listings, waitlist death, and LDLT (Table 1). The observed number of DDLTs was 24% more than expected at centers in states with “low” COVID-19 burden (IRR = 1.05 1.241.48). Centers in states with “low” COVID-19 incidence continued to be more engaged in DCD DDLT with two times more than expected (IRR = 1.292.053.25). In July and August, the rates of most waitlist events were similar to expected across the incidence categories. Of note, the observed number of DDLT in July in “high” incidence states were 57% more than expected (IRR = 1.23 1.572.00) (Table 1). The observed number of DDLT in August in “high” incidence states were 26% more than expected (IRR = 1.06 1.261.49).
The overall number of observed regional imports were higher than expected in May (IRR = 1.151.301.47) and June (IRR = 1.151.301.48), but as expected by August (Table 1). While there were 15% more observed regional imports to states with “low” COVID-19 burden than expected during March 15-April 30 (IRR = 1.02 1.151.30), there were 71% fewer (IRR = 0.130.290.65) observed regional imports to centers in states with “very high” COVID-19 rates. In June, observed regional imports were still more than expected for centers in “low” COVID-19 burden states (IRR = 1.501.802.17) and similar to that expected (IRR = 0.55 0.891.46) in centers in “very high” burden states. Additionally, the IRRs for medium, high, and very high COVID-19 levels in May and June were each statistically significantly different from the centers in states with low COVID-19 burden. The overall observed number of national imports was 3.5 times higher than expected during March 15-April 30 (IRR = 3.133.523.96), 4.7 times higher in May (IRR = 4.114.665.29), and 4.7 times higher in June (IRR = 4.094.665.29). In July and August, national imports continued to be higher than expected across all incidence levels.
4. DISCUSSION
In this national study of LT waitlist activity compared to pre-COVID-19 trends and stratified by COVID-19 incidence across five periods of the pandemic, we found the overall number of new listings, LDLT, and DDLT were vastly different than expected early in the pandemic. The early decreases in events also occurred differentially across COVID-19 incidence levels. Specifically, from March 15 to April 30, there was no change in new listings or DDLT in states with the lowest COVID-19 burden, but in states with the highest incidence, there were 33% fewer new listings than expected and 34% fewer DDLTs. The changes in DDLT occurred differently across MELD scores; there were 35.4% fewer DDLTs than expected for MELD 15-19 and 50.4% more DDLT than expected for MELD 30-34. Early in the pandemic, LDLTs were 65% fewer than expected in states with the highest burden. Importantly, while the overall number of waitlist deaths across all time periods was as expected, in the states with the highest COVID-19 incidence early in the pandemic, there was a 59% increase in deaths on the waitlist observed compared to expected. By August, waitlist outcomes were occurring at expected rates based on prepandemic averages, except for DDLT which was occurring at 13% more than expected across all incidence levels. While early in the pandemic the COVID-affected areas had major changes to their transplant practice, later in the pandemic the new COVID-affected areas did not seem to be affected to the same extent. Perhaps these results speak to the adaptability of the transplant community in addressing the pandemic and applying new knowledge to patient care, so our colleagues in Florida and Arizona did not require the same extreme reactions as earlier states, such as New York.
Our finding of decreased new listings during March 15-May 31 is likely multifactorial. The Centers for Disease Control and Prevention (CDC) and the AASLD Expert Panel made recommendations that shifted clinical operations, such as safety precautions and telemedicine17 , 18; therefore, it might be that transplant center evaluation processes may not have been functioning at full capacity. Additionally, patients older than 65 years, those with comorbid conditions (eg, diabetes mellitus and obesity), and on immunosuppressive medications have likely been at high risk for severe COVID-19 manifestations,19 so may have been avoiding risky interactions in clinical environments such as transplant centers, laboratories, and imaging centers. These healthcare system avoidant behaviors have been seen in other conditions such as cardiac patients.20
Our findings confirm the anecdotal reports from our recent national survey of transplant centers and are consistent with a prior COVID-19 era study. The survey described restrictions to DDLT reported by 73.3 of centers%.12 The overall decrease in DDLT and differential reduction of DDLT among states of higher incidence rates of COVID-19 is likely reflective of lower hospital capacity and healthcare resources.21 While decrease in LDLT among centers in states with high COVID-19 burden is likely representative of the 67.7% cessation of LDLT among LDLT programs reported in the survey (potentially from the dilemma of increased donor risk), the numbers in the other COVID-19 burden groups were likely too small to reach statistical significance. Additionally, our findings are also consistent with the report from Agopian et al that 39% of centers had a difference of greater than or equal to 6 DDLT during February-March between 2019 and 2020.22 Our study extends the Agopian study by estimating expected rates from 4 years of data instead of 3 months, by accounting for secular trends and center-level changes in waitlist population, and by providing inference through April 2020. National societies have acknowledged the uncertainty among the LT community, and they have provided clinical guidance for LT centers across the world on maintaining care according to pre-COVID-19 clinical guidelines while considering minimizing SARS-COV-2 transmission (eg, personal protective equipment use, social distancing, telemedicine).17 , 23 , 24
Our results show a striking increase in waitlist deaths in the states with the highest COVID-19 incidence in the earliest wave of the pandemic. There are several potential explanations. Our data do not include the cause of death, but some of these deaths may have been directly caused by COVID-19 infection and/or comorbidities. A study from China about patients with metabolic-associated fatty liver disease demonstrated a sixfold increased risk for severe COVID-19 in obese patients.25 However, according to the CDC, chronic liver disease has had few severe outcomes reported, so the risk for severe COVID-19 is not yet known.26 Furthermore, since transplant centers in these states were performing fewer transplants, patients with higher MELD scores may have been dying of their end-stage liver disease before they could be transplanted. Another possibility is that hospitals at full capacity with COVID-19 patients may not have been able to admit the patients whom they would normally manage for decompensated liver disease.
Our model of observed versus expected must be interpreted in the context of the major policy change on February 4, 2020 to organ allocation based on the geographic distance between the recipient and deceased donor (“acuity circles”).27 Under acuity circles, organs are allocated based on distance from recovery hospital, replacing prior donor service areas and regions. The increase in national imports across all levels of statewide COVID-19 incidence, might reflect the impact of acuity circles rather than the impact of COVID-19. However, our finding of a dose-response relationship between state-level COVID-19 burden and observed imports suggests that COVID-19 may have played a role. For example, lower regional imports to centers in states with relatively higher COVID-19 rates may be due to these centers performing fewer transplants, and shifting deceased donor livers to centers in lower COVID-19 burden states that were still actively transplanting. Crucially, studies of changes to liver allocation following implementation of acuity circles will have to properly account for the COVID-19 pandemic. Based on simulated models, the acuity circles allocation is expected to decrease waitlist deaths and geographic variation in MELD at transplant, and increase the overall number of DDLTs.28 , 29 The expected values from February 1, 2016 to January 31, 2020 represent a counterfactual for what would have happened from February 1, 2020 to September 2, 2020 without the effects of either the COVID-19 pandemic or the acuity circles policy change. It is striking to see that despite the policy change, there is a decrease in the number of DDLTs. Compared to expected values, the reduction in transplants to patients with HCC and low MELDs as well as more transplants at high MELDs may reflect the influence of the acuity circles, HCC related policy changes, the pandemic, or a combination of multiple effects. The trends of fewer transplants at low MELDs and exceptions points as well as more transplants at high MELDs may be related to acuity circles, the pandemic, or a combination of both effects. Interestingly, the observed DDLT among MELD >35—a group which experienced higher regional waitlist priority before acuity circles—was comparable to expected rates.
This study must be interpreted in the context of its limitations. The data collected by SRTR is obtained though reporting by individual transplant centers, so there may be discrepancies in the quality of the data that is beyond our control. Furthermore, due the nature of registry data, we are unable to ask pertinent and specific clinical questions related to what factors at the transplant center level were being influenced by COVID-19 burden. For example, there may be city- or hospital-level issues, other than local COVID-19 incidence, that have impacted decision-making. Despite this, the fact that we still observed patterns at the state level is striking. However, the use of a large dataset such as this allows for increased generalizability particularly when connected with the state-level COVID-19 incidence data. Secondly, a potential measurement error in waitlist deaths may be due to a delay in reporting to the transplant center and the OPTN. Since this error would result in an underestimation of the true death rate, and the waitlist mortality has a differential effect among varying COVID-19 burden states, our results may in fact underestimate the true mortality impact of the epidemic in these states. Testing strategies and availability for SARS-CoV-2 have been changing since the beginning of the pandemic,30 , 31 which may have also led to an underestimation of COVID-19 incidence.32 However, misclassification of COVID-19 burden would be expected to bias our results toward the null.
In summary, these findings suggest that COVID-19 has impacted all aspects of LT, and a better understanding of COVID-19 may benefit this patient population. In states with the highest COVID-19 burden early on, there was a dramatic reduction in DDLT and increased mortality for LT candidates. Future research related to post-LT COVID-19 incidence and survival outcomes is needed.
ACKNOWLEDGMENTS
This research was made possible with generous support of the Ben-Dov family. This work was supported by grant number F32DK124941 (Boyarsky), T32AI007291 (Werbel), F32DK113719 (Jackson), F32DK117563 (Kernodle), K01KD101677 (Massie), and K23DK115908 (Garonzik-Wang) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH). This work was supported by grant number K24AI144954 (Segev) from the National Institute of Allergy and Infectious Diseases (NIAID). The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government. The data reported here have been supplied by the Hennepin Healthcare Research Institute (HHRI) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibilities of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the SRTR. Restrictions apply to the availability of these data, which were used under license for this study. Data are available at https://www.srtr.org/ with the permission of SRTR.
Funding information National Institutes of Health (NIH), Grant/Award Number: F32DK124941, T32AI007291, F32DK113719, F32DK117563, K01KD101677 and K23DK115908; National Institute of Allergy and Infectious Diseases (NIAID), Grant/Award Number: K24AI144954
REFERENCES
- 1.Lai C-C, Shih T-P, Ko W-C, Tang H-J, Hsueh P-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and corona virus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.del Rio C, Malani PN. 2019 Novel coronavirus—important information for clinicians. JAMA. 2020;323(11):1039–1040. doi: 10.1001/jama.2020.1490. [DOI] [PubMed] [Google Scholar]
- 3.Truog RD, Mitchell C, Daley GQ. The toughest triage—allocating ventilators in a pandemic. N Engl J Med. 2020;382(21):1973–1975. doi: 10.1056/NEJMp2005689. [DOI] [PubMed] [Google Scholar]
- 4.Cavallo JJ, Donoho DA, Forman HP. Hospital capacity and operations in the coronavirus disease 2019 (COVID-19) pandemic—planning for the nth patient. JAMA Health Forum. 2020;1(3) doi: 10.1001/jamahealthforum.2020.0345. e200345. [DOI] [PubMed] [Google Scholar]
- 5.Qin J, Wang H, Qin X, et al. Perioperative presentation of COVID-19 disease in a liver transplant recipient. Hepatol. 2020. 10.1002/hep.31257 [DOI] [PubMed]
- 6.Michaels MG, La Hoz RM, Danziger-Isakov L, et al. Coronavirus disease 2019: Implications of emerging infections for transplantation. Am J Transplant. 2020. 10.1111/ajt.15832 [DOI] [PMC free article] [PubMed]
- 7.CDC COVID-19 Response Team. MMWR: Severe outcomes among patients with coronavirus disease 2019 (COVID-19) — United States, February 12–March 16, 2020; 2020. http://www.ecie.com.ar/images/paginas/COVID-19/4MMWR-Severe_Outcomes_Among_Patients_with_Coronavirus_Disease_2019_COVID-19-United_States_February_12-March_16_2020.pdf [DOI] [PMC free article] [PubMed]
- 8.Hammami MB, Garibaldi B, Shah P, et al. Clinical course of COVID-19 in a liver transplant recipient on hemodialysis and response to Tocilizumab therapy: A case report. Am J Transplant. 2020. 10.1111/ajt.15985 [DOI] [PMC free article] [PubMed]
- 9.Huang J-F, Zheng KI, George J, et al. Fatal outcome in a liver transplant recipient with COVID-19. Am J Transplant. 2020. 10.1111/ajt.15909 [DOI] [PMC free article] [PubMed]
- 10.Gandolfini I, Delsante M, Fiaccadori E, et al. COVID-19 in kidney transplant recipients. Am J Transplant. 2020. 10.1111/ajt.15891 [DOI] [PMC free article] [PubMed]
- 11.Fishman JA, Grossi PA. Novel Coronavirus-19 (COVID-19) in the immunocompromised transplant recipient: #Flatteningthecurve. Am J Transplant. 2020. 10.1111/ajt.15890 [DOI] [PMC free article] [PubMed]
- 12.Boyarsky BJ, Chiang TP-Y, Werbel WA, et al. Early impact of COVID-19 on transplant center practices and policies in the United States. Am J Transplant. 2020. 10.1111/ajt.15915 [DOI] [PMC free article] [PubMed]
- 13.US Census Bureau. State Population Change: 2010 to 2018. https://www.census.gov/library/visualizations/2018/comm/population-change-2010-2018.html. Accessed March 25, 2020.
- 14.The COVID Tracking Project. The Atlantic. https://covidtracking.com/. Accessed March 25, 2020.
- 15.Massie AB, Kucirka LM, Segev DL. Big data in organ transplantation: registries and administrative claims. Am J Transplant. 2014;14(8):1723–1730. doi: 10.1111/ajt.12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louis TA, Zeger SL. Effective communication of standard errors and confidence intervals. Biostatistics. 2009;10(1):1–2. doi: 10.1093/biostatistics/kxn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fix OK, Hameed B, Fontana RJ, et al. Clinical best practice advice for hepatology and liver transplant providers during the COVID-19 pandemic: AASLD expert panel consensus statement. Hepatol. 2020. 10.1002/hep.31281 [DOI] [PMC free article] [PubMed]
- 18.Communities CDC, Schools Workplaces, Events. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/community/organizations/cleaning-disinfection.html. Accessed June 3, 2020.
- 19.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020. 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed]
- 20.Moroni F, Gramegna M, Ajello S, et al. Collateral damage: Medical care avoidance behavior among patients with myocardial infarction during the COVID-19 pandemic. JACC: Case Reports. April 2020. 10.1016/j.jaccas.2020.04.010 [DOI] [PMC free article] [PubMed]
- 21.Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020. 10.1056/NEJMsb2005114 [DOI] [PubMed]
- 22.Agopian V, Verna E, Goldberg D. Changes in liver transplant center practice in response to COVID-19: Unmasking dramatic center-level variability. Liver Transpl. 2020. 10.1002/lt.25789 [DOI] [PMC free article] [PubMed]
- 23.Boettler T, Newsome PN, Mondelli MU, et al. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. 2020;2(3):100113. doi: 10.1016/j.jhepr.2020.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saigal S, Gupta S, Sudhindran S, et al. Liver transplantation and COVID-19 (Coronavirus) infection: guidelines of the liver transplant Society of India (LTSI). Hepatol Int. 2020. 10.1007/s12072-020-10041-1 [DOI] [PMC free article] [PubMed]
- 25.Zheng KI, Gao F, Wang X-B, et al. Letter to the Editor: Obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020;108:154244. doi: 10.1016/j.metabol.2020.154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.CDC COVID-19 Response Team Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 - United States, february 12-March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):382–386. doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.UNOS. System notice: Liver and intestinal organ distribution based on acuity circles implemented Feb. 4. https://unos.org/news/system-implementation-notice-liver-and-intestinal-organ-distribution-based-on-acuity-circles-implemented-feb-4/. Accessed June 3, 2020.
- 28.Mogul D, Perito ER, Wood N, et al. Impact of acuity circles on outcomes for pediatric liver transplant candidates. Transplantation. 2019. 10.1097/TP.0000000000003079 [DOI] [PMC free article] [PubMed]
- 29.Bertsimas D, Papalexopoulos T, Trichakis N, Wang Y, Hirose R, Vagefi PA. Balancing efficiency and fairness in liver transplant access: tradeoff curves for the assessment of organ distribution policies. Transplantation. 2020;104(5):981–987. doi: 10.1097/TP.0000000000003017. [DOI] [PubMed] [Google Scholar]
- 30.Gronvall G, Connell N, Kobokovich A, et al. Johns Hopkins University; Baltimore, MD: 2020. Developing a National Strategy for Serology (Antibody Testing) in the United States. [Google Scholar]
- 31.Cheng MP, Papenburg J, Desjardins M, et al. Diagnostic testing for severe acute respiratory syndrome–related coronavirus-2: A narrative review. Ann Intern Med. 2020. https://www.acpjournals.org/doi/abs/10.7326/m20-1301 [DOI] [PMC free article] [PubMed]
- 32.Omori R, Mizumoto K, Chowell G. Changes in testing rates could mask the novel coronavirus disease (COVID-19) growth rate. Int J Infect Dis. 2020;94:116–118. doi: 10.1016/j.ijid.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the SRTR. Restrictions apply to the availability of these data, which were used under license for this study. Data are available at https://www.srtr.org/ with the permission of SRTR.