Abstract
The spread of Coronavirus Disease 2019 (COVID-19) has already reached a pandemic dimension within a few weeks. Italy has been one of the first countries dealing with the outbreak of COVID-19, and severe measures have been adopted to limit viral transmission. The spread of COVID-19 may have several implications in organ transplant activity that physicians should be aware of. The initial experience gained during the COVID-19 outbreak shows that around 10% of infected patients in Italy need intensive care management to overcome the acute respiratory distress syndrome. Due to the exponential rise of infected patients we are now facing an actual risk of saturation of intensive care unit (ICU) beds. A restriction in the number of ICU beds available for both donors and transplant recipients may unfavorably influence the overall donation activity, and eventually lead to a reduced number of transplants. Preliminary Italian data show that a 25% reduction of procured organs has already occurred during the first 4 weeks of COVID-19 outbreak. This underlines the need to closely monitor what will be further happening in ICUs due to the COVID-19 spread in the attempt to preserve transplant activity, especially in Western countries where deceased donors represent the major organ resource.
KEYWORDS: critical care/intensive care management, donors and donation: donor-derived infections, editorial/personal viewpoint, epidemiology, health services and outcomes research, infection and infectious agents – viral, infectious disease, organ procurement and allocation, organ procurement organization, organ transplantation in general
Abbreviations: ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease 2019; DD, deceased donor; ICU, intensive care unit; MERS-CoV, middle east respiratory syndrome coronavirus; SARS-CoV, acute respiratory syndrome coronavirus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WHO, World Health Organization
1. INTRODUCTION
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a new, highly contagious pathogen that spreads quickly via human-to-human transmission.1 The outbreak of Coronavirus Disease 2019 (COVID-19), initially started in Wuhan city, Hubei, China,2 was declared a public health emergency of international concern with pandemic spread by the World Health Organization (WHO),3 having infected more than 332 935 people in 189 countries.4
As of March 22, 2020, Italy developed the highest incidence of confirmed COVID-19 cases outside China.4 The first Italian case was detected in an Italian citizen on February 18, 2020. Thereafter, infected patient numbers have grown at an exponential rate.5 The infection, first limited to Lombardy and Veneto in Northern Italy, eventually spread to all other Italian regions.5 According to the Italian National Institute of Health, on March 22, 2020, the number of infected patients across Italy was 46 638, of whom 19 846 were hospitalized for symptomatic disease, 3009 patients required intensive care unit (ICU) admission for ventilator support, and 5476 deaths.6
To limit viral transmission, the Italian government introduced drastic emergency restrictions, including quarantine and strict self-isolation measures. Initially, these were limited to restricted areas of Northern Italy, and on March 10, 2020, restrictions were extended across the whole country.7
1.1. COVID-19 spread and measures adopted for organ transplantation in Italy
As the first cases of COVID-19 were detected in Italy, the Italian National Institute of Health and the National Transplant Centre (CNT) defined regulatory measures for organ transplantation to maintain transplantation activity, from both deceased and living-related donors.8 Ordinary allocation policies were maintained to ensure treatments for patients who were candidates for liver, kidney, heart, lung, or pancreas transplantation, accounting for a total of 8615 patients listed by December 31, 2019.8
From March 3, 2020, a systematic COVID-19 surveillance was imposed for deceased and living donors. Since then, all potential donors are screened for SARS-CoV-2 using real-time reverse transcription PCR (RT-PCR), on samples from nasopharyngeal swabs or bronchoalveolar-lavage fluids, in accordance with WHO recommendations.9 Only negative COVID-19 donors are considered for organ donation. Organ transplant recipients must undergo COVID-19 screening in presence of symptoms or when there is a suspicion of infection due to close contact with someone known, or suspected, of being infected with SARS-CoV-2.8
1.2. Potential implications of COVID-19 outbreak for transplantation activities
A variety of clinical, organizational, and logistical aspects, as well as several professional health care specialists, are required for organ transplantation activities. Every step of the organ transplantation process, from donor evaluation to recipient surgery, is already highly complex in “ordinary” times, yet it is expected to be dramatically exacerbated in a pandemic era.
In 2019, Italy accounted for 1743 donors for organ transplantation, of which 79% were deceased.8 Therefore, in Italy, as in most Western countries, the management of deceased donors by intensivists is essential in allowing adequate organ availability to transplant candidates, for many of whom transplantation represents a life-saving treatment. During the ongoing COVID-19 outbreak, close to 10% of infected patients require intensive care management to overcome acute respiratory distress syndrome (ARDS).6 , 10 Hence, the limited number of ICU beds (approximately 5200 across the country) may unfavorably influence donation activities, due to possible ICU bed restrictions for both donor and transplantation recipients.
As of March 22, 2020, 3009 ICU beds in Italy are already utilized for the management of critical patients with COVID-19.6 This number is estimated to increase approximately to 4000 beds in the next few weeks.11 Therefore, great efforts of the government and national health system, supported by the scientific community, have been made to strengthen the overall ICU capacity at national level.12 Indeed, in Lombardy, which is currently the most affected region by COVID-19, the health care system and ICU network had already made 482 new ICU beds within the first 18 days of the COVID-19 outbreak.13
Meanwhile, logistics for organ procurement, as well as transplantation surgery, are becoming every day more challenging due to travel and working restrictions, and due to the fact that healthcare transplant professionals are progressively employed to fight against COVID-19.
Strict adherence to the COVID-19 precautions recommended by the WHO3 is absolutely mandatory to minimize the risk of nosocomial transmission to recipients, procurement teams and healthcare transplant workers. As suggested by Michaels et al,14 since SARS-CoV-2 is rather an unknown new pathogen, we should rely on the lessons learned from previous experiences of other coronavirus outbreaks, namely acute respiratory syndrome coronavirus (SARS-CoV)15 and Middle East respiratory syndrome coronavirus (MERS-CoV).16
The actual risk of donor-derived transmission of SARS-CoV-2 in transplant recipients is yet unclear. In most countries national guidelines recommend the routine testing of donors for SARS-CoV-2.17
The Transplantation Society (TTS) has put forward interim recommendations18 to consider SARS-CoV-2 testing of upper and lower airway specimens using PCR in both deceased and living donors. According to TTS directives, to minimize the risk of false positive testing and organ wastage, the routine screening for SARS-CoV-2 infection in donors should be performed only in areas with significant ongoing community transmission, or when there is a clinical suspicion of infection. In addition, TTS recommends to consider the temporary suspension of both deceased and living-related transplant programs in countries with widespread community viral transmission. For any case, when transplantation is required for a life-saving procedure, appropriate exclusion of SARS-CoV-2 infection, both in the donors and recipients, should be mandatory.
1.3. Organ donation and transplantation in the initial spread of COVID-19 in Italy
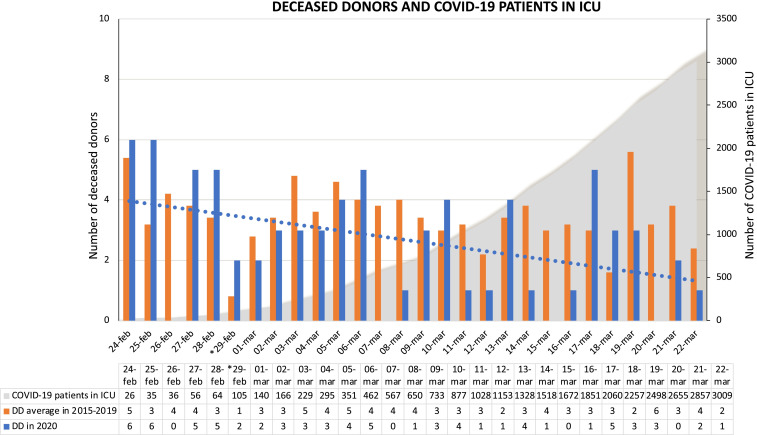
We compared the numbers of deceased donors used for organ transplantation from February 24, 2020 to March 22, 2020—the period of the initial COVID-19 outbreak in Italy—with the number of procurements performed during the same timeframe from 2015 to 2019. A total of 73 deceased donors were used in the current year compared to an average of 97 in the 2015-2019 timeframe ( Figure 1).
FIGURE 1.
Deceased donors used for organ transplantation during the initial Coronavirus Disease 2019 (COVID-19) outbreak, according to patients with COVID-19 admitted in intensive care unit in Italy. The figure describes the daily number of deceased donors used for organ transplantation from February 24, 2020 to March 22, 2020 (the first 4 wk of COVID-19 outbreak in Italy) compared with the daily average of deceased donors used during the same time frame of the last 5 y (2015-2019). The dotted line corresponds to the trend line of deceased donors since the COVID-19 spread in Italy. Data of patient with COVID-19 admitted in ICU were retrieved by the Italian National Institute of Health.5 *February 29, 2020 was a leap day, present only in 2016 and 2020. DD, deceased donor; ICU, intensive care unit; COVID-19, Coronavirus Disease 2019
In 2020, 214 solid organ transplantations were performed (including 114 kidney, 74 liver, 16 heart, 7 lung and 3 pancreas transplantations) compared to an average of 249 in the years 2015-2019 ( Table 1). According to the type of transplantation, during the COVID-19 outbreak the transplantation activity remained stable for each type of solid organ, compared to the control period. Also, the number of liver, heart and lung urgent transplantations were similar, accounting overall 17 (7.9%) in 2020 and 18 (7.2%) in 2015-2019 (Table 1).
TABLE 1.
Number of organ transplantation during the first 4 wk of Coronavirus Disease 2019 outbreak in Italy, compared to the average number performed in the same time fame of the last 5 y (2015-2019)
| Type of organ transplantation | 2015-2019 (February 24-March 22) | 2020 (February 24-March 22) | ||
|---|---|---|---|---|
| Total number (%) | Urgency (%)a | Total number (%) | Urgency (%)a | |
| Kidney transplantation | 128 (51.5%) | — | 114 (53.3%) | — |
| Liver transplantation | 90 (36.1%) | 8 (8.8%) | 74 (34.6%) | 9 (12.2%) |
| Heart transplantation | 18 (7.2%) | 8 (44.4%) | 16 (7.5%) | 6 (37.5%) |
| Lung transplantation | 10 (4%) | 2 (20%) | 7 (3.3%) | 2 (28.6%) |
| Pancreas transplantation | 3 (1.2%) | — | 3 (1.4%) | — |
| Total | 249 (100%) | 18 (7.2%) | 214 (100%) | 17 (7.9%) |
Percentage are calculated on the total number in each category of organ transplantation.
As a whole, during the first 4 weeks of the COVID-19 outbreak, there was a 25% reduction in overall deceased donors procured at the national level. This reduction could be due to multiple factors. Among these it is conceivable that it might be a consequence of the dramatic higher spread of COVID-19 in Northern Italy, especially in Lombardy, where there is a trend towards saturation of ICU beds availability, due to their extensive recruitment for COVID-19 patients with severe ARDS. As March 22, 2020, of 3009 patients infected with SARS-CoV-2 in ICU, 2406 (80%) are in the regions of the Northern-macroarea of the transplant network (1142 in Lombardy), while 603 (20%) in the Southern-macroarea.5 So far, in the regions of the Northern-macroarea, where 70% of all Italian deceased donors are procured, we observed a 30% of reduction of deceased donation rate (52 donors in 2020 vs 74 donors in 2015-2019), while 9% of decrease of donation rate was observed in the Southern-macroarea (21 donors in 2020 vs 23 donors in 2015-2019). However, due to the rapid evolution of the COVID-19 outbreak, the situation of the Southern regions might change in the next few weeks.
Transplant programs in an epidemic area not only need to face the scarce medical resources of ICU beds, ventilators and health care specialists,19, 20, 21 but also have to define an adequate pathway to avoid post-transplant recipient’s infection. In epidemic regions, transplant centers need to carefully balance the cost and benefits in performing a transplant during the COVID-19 outbreak.22 , 23 On this regard, the transplant program of the Ospedale Maggiore Policlinico in Milan, which is in an epidemic red-zone, defined a local policy for liver transplant candidates. In this center, liver transplantation is currently limited to the most urgent cases for candidates living in the epidemic area, while regular allocation policy is used for patients coming from outside the epidemic regions.22
Since the number of COVID-19 patients is dramatically increasing, we believe that all transplant programs should constantly adapt their protocols in relation to the risk of viral transmission in each region and need to be prepared how to face the expanding pandemia.
Yet, the rapidly changing epidemic scenario may eventually lead to further reduced activities in organ donation and transplantation across the whole country over the next few weeks, unless the imposed quarantine conditions to the population will effectively control viral spread.
After 4 weeks from the COVID-19 spread in Italy, the strict collaboration between national and local health organizations and the strong economic support by the government, mainly finalized to increase the number of ICU facilities and healthcare providers, have allowed the continuation of all transplant-related activities for all type of solid organs, without facing unfavorable results. Yet, continued efforts will be required to overcome further expected difficulties in the next few months.
2. CONCLUSIONS
Italy has been one of the first countries dealing with the spread of COVID-19 outside of China and has imposed severe mobility restriction to limit the outbreak. So far, this unique situation has caused only limited harm to the overall transplant activity across the country, though the data available represent only an initial picture of what could eventually be the effects SARS-CoV-2 pandemia in a longer term. On the other hand, the initial experience gained in Italy already provides a strong warning for the transplant community and the healthcare systems for all countries facing the COVID-19 outbreak. Indeed, deceased donors represent the major organ resources in Western countries. Hence, in order to maintain the number of transplants and preserve their quality, maximal attention to what will be happening in ICU due to the COVID-19 spread is mandatory. Since the current evolving pandemia may pose severe restrictions in organ availability, transplant physicians will need to use even more stringent prioritization criteria to select transplant candidates. Meanwhile, any effort should be undertaken to ensure that all transplant candidates may safely access organ resources in the current pandemic scenario.
Acknowledgments
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
REFERENCES
- 1.Zhun N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Coronavirus disease (COVID-19) outbreak. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed March 22, 2020.
- 4.World Health Organization. Novel Coronavirus (COVID-19) situation. https://experience.arcgis.com/experience/685d0ace521648f8a5beeeee1b9125cd. Accessed March 22, 2020.
- 5.COVID-19 spread in the Italian regions. http://opendatadpc.maps.arcgis.com/apps/opsdashboard/index.html#/b0c68bce2cce478eaac82fe38d4138b1. Accessed March 22, 2020.
- 6.Italian National Institute of Health. Report of COVID-19 patients. http://www.salute.gov.it/portale/nuovocoronavirus/dettaglioContenutiNuovoCoronavirus.jsp?lingua=italiano&id=5351&area=nuovoCoronavirus&menu=vuoto. Accessed March 22, 2020.
- 7.Italian Government measures for COVID-19 outbreak. http://www.salute.gov.it/portale/nuovocoronavirus/archivioNormativaNuovoCoronavirus.jsp. Accessed March 22, 2020.
- 8.Italian National Transplant Centre. Information for transplant programs regarding novel Coronavirus 2019. http://www.trapianti.salute.gov.it/trapianti/homeCnt.jsp. Accessed March 22, 2020.
- 9.World Health Organization. Coronavirus disease (COVID-19) technical guidance: laboratory testing for 2019-nCoV in humans. https://www.who.int/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117. Accessed March 22, 2020.
- 10.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? [published online ahead of print 2020]. Lancet. 10.1016/S0140-6736(20)30627-9 [DOI]
- 12.Italian Government Measures. Legislative decree for COVID-19 emergency. http://www.governo.it/it/articolo/decreto-legge-17-marzo-2020/14333. Accessed March 22, 2020.
- 13.Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response [published online ahead of print 2020]. JAMA. 10.1001/jama.2020.4031 [DOI] [PubMed]
- 14.Michaels MG, La Hoz RM, Danziger Isakov L, et al. Coronavirus disease 2019: implications of emerging infections for transplantation [published online ahead of print 2020]. Am J Transplant. 10.1111/ajt.15832 [DOI] [PMC free article] [PubMed]
- 15.Kumar D, Tellier R, Draker R, et al. Severe acute respiratory syndrome (SARS) in the liver transplant recipient and guidelines for donor SARS screening. Am J Transpl. 2003;3:977–981. doi: 10.1034/j.1600-6143.2003.00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.AlGhamdi M, Mushtaq F, Awn N, et al. MERS CoV infection in two renal transplant recipients: case report. Am J Transpl. 2015;15:1101–1104. doi: 10.1111/ajt.13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Recommendations for epidemic disease occurrence (SARS-CoV-2). https://www.notifylibrary.org/background-documents#SARS-CoV-2. Accessed March 22, 2020.
- 18.An update and guidance on 2019 novel coronavirus (2019-nCov) pretransplant ID clinicians. https://tts.org/23-tid/tid-news/657-tid-update-and-guidance-on-2019-novel-coronavirus-2019-ncov-for-transplant-id-clinicians. Accessed March 22, 2020.
- 19.Emanuel EJ, Persad G, Fair UR, et al. Allocation of scarce medical resources in the time of Covid-19 [published online ahead of print 2020]. N Engl J Med. 10.1056/NEJMsb2005114 [DOI] [PubMed]
- 20.Truog RD, Mitchell C, Daley QG. The toughest triage — allocating ventilators in a pandemic [published online ahead of print 2020]. N Engl J Med. 10.1056/NEJMp2005689 [DOI] [PubMed]
- 21.Italian Society of Anesthesiology and Intensive Care. Ethical recommendation for the use of hospital resources in emergency. http://www.siaarti.it/SiteAssets/News/COVID19%20%20documenti%20SIAARTI/SIAARTI%20-%20Covid19%20-%20Raccomandazioni%20di%20etica%20clinica.pdf. Accessed March 22, 2020.
- 22.Gori A, Dondossola D, Antonelli B, et al. Coronavirus Disease 2019 and transplantation: a view from the inside [published online ahead of print 2020]. Am J Transplant. [DOI] [PMC free article] [PubMed]
- 23.D’Antiga L. Coronaviruses and immunosuppressed patients. The facts during the third epidemic [published online ahead of print 2020]. Liver Transpl. 10.1002/lt.25756 [DOI] [PubMed]