Abstract
In December 2019, an outbreak of COVID-19 occurred in Wuhan, China, and spread to the whole of China and to multiple countries worldwide. Unlike SARS and MERS, where secondary transmission mostly occurred in hospital settings, COVID-19 transmission occurs in large numbers within families. Herein we report three cases of a familial cluster with one family member being a kidney transplant recipient. The initial clinical symptoms of COVID-19 in these three patients were the same, but their progression was different. Based on the severity of clinical symptoms, chest computer tomography findings and SARS-Cov-2 RNA test results, we admitted the husband to the respiratory intensive care unit (RICU) and used a treatment consisting of immunosuppressant reduction/cessation and low dose methylprednisolone-based therapy, and his wife to the respiratory isolation ward. In contrast, the son received in-home isolation and home-based care. All three family members made a full recovery.
KEYWORDS: COVID-19, kidney, novel coronavirus, pneumonia, transplantation, treatment
Abbreviations: COVID-19, coronavirus disease 2019; CT, computed tomography; IVIG, intravenous immunoglobulin; MMF, mycophenolate mofetil; MP, methylprednisolone; PCR, polymerase chain reaction; PED, prednisone; RICU, respiratory intensive care unit; SARS-Cov-2, severe acute respiratory syndrome coronavirus 2; TAC, tacrolimus
1. INTRODUCTION
In December 2019, a cluster of patients with pneumonia of unknown origin was reported in Wuhan, China. Biological testing indicated that this pneumonia was caused by a novel coronavirus, 2019-nCoV,1 which was officially labeled severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2).2 An outbreak of this novel coronavirus disease (COVID-19) then occurred and spread to the whole of China as well as multiple countries worldwide.3 As of 24:00 on Mar 20, 2020, the number of patients in China diagnosed with COVID-19 reached 81 008 and there were 3255 deaths (4.0% mortality rate).4 By Mar 20, in Wuhan, where the outbreak first began, there were 50 005 confirmed cases and 2504 deaths (5.0% mortality rate).4 To date, there have been several public health and intervention-based recommendations from different organizations, and a lot of debate concerning optimal COVID-19 treatment strategies. The present report describes a familial cluster consisting of three cases: the husband (49 years old), the wife (49 years old) and the son (24 years old). The husband is a kidney transplant recipient who has experienced long-term immunosuppression. The treatment strategies differed for each family member but they all made a full recovery.
2. CASE REPORT
All three patients in this family presented with loss of appetite beginning on Jan 25, 2020, which was the second day of lockdown in Wuhan city.
2.1. Case 1, a severe confirmed case who is a kidney transplant recipient, was treated in the respiratory intensive care unit (RICU)
Case 1, a 49-year-old male who is a kidney transplant recipient, has been taking immunosuppressive agents (Tacrolimus, Mycophenolate mofetil and Prednisone) for over 6 years. The patient received a kidney transplant from a brain-dead deceased donor on June 21, 2013, for end-stage renal disease due to chronic glomerulonephritis. Following the transplant, the patient has regular follow-up at our outpatient clinic with serum creatinine (Cr) of 110-120 μmol/L, estimated glomerular filtration rate (eGFR) of 62-68 mL/min/1.73 m2 and Tacrolimus trough level of 5-7 ng/mL. In addition, the patient also has a history of hypertension, which is well controlled with Valsartan (80 mg, once per day). There are no other medical conditions.
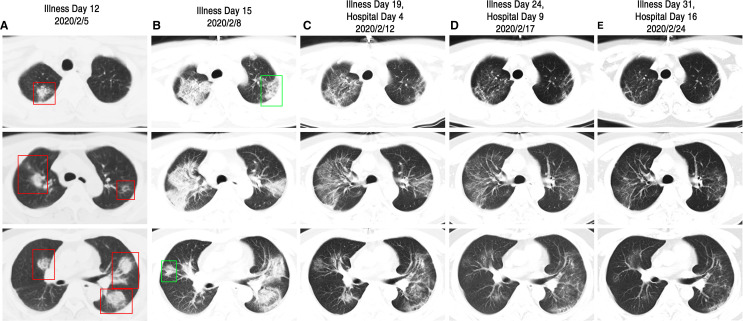
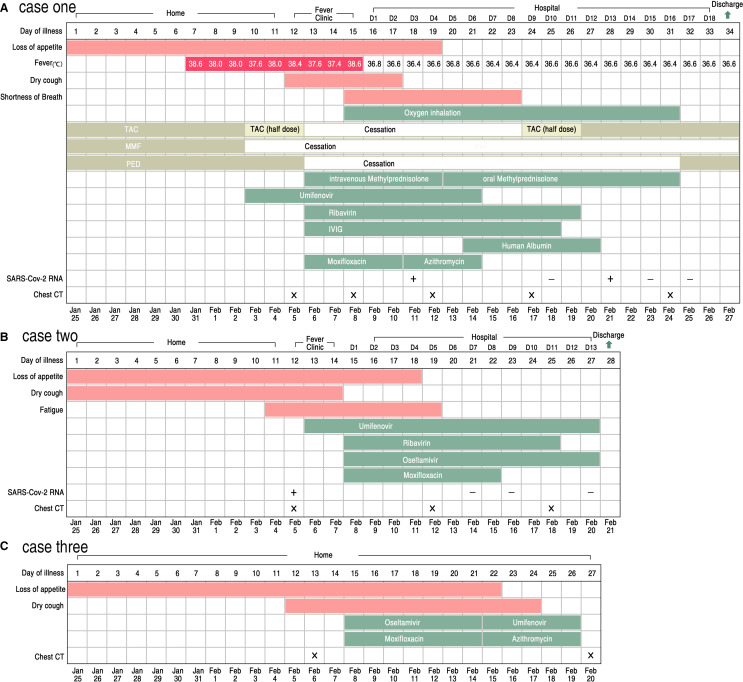
On Jan 25, 2020, this patient presented with poor appetite. Six days later, on Jan 31, he had a fever with a body temperature of 38.6°C. He took Tylenol Cold Relief Tablets for 3 days but still experienced a persistent fever with a peak daily body temperature of 38.0°C. On Feb 3, after a telephone consultation with his transplant specialist, the patient stopped taking Mycophenolate mofetil (MMF). In addition, the dose of Tacrolimus (TAC) was halved and he began receiving oral Umifenovir (200 mg, three times per day). On Feb 5, the patient reported a serious dry cough and his chest computer tomography (CT) showed multiple patchy ground glass opacity and consolidation shadow in the bilateral lung view ( Figure 1A). It was strongly suspected that he had COVID-19 associated pneumonia and thus he was moved to an isolated area of the fever clinic. TAC and Prednisone (PED) treatment were discontinued, and, instead, he was treated with intravenously injected Methylprednisolone (MP) (20 mg, once per day), Ribavirin (500 mg, twice per day), intravenously injected Immunoglobin (IVIG, 5 g, once per day), and oral Moxifloxacin (400 mg, once per day). This new treatment regimen reduced the patient’s fever to around 37.4°C. However, on Feb 8, the patient’s fever increased with a body temperature of 38.6°C, he experienced shortness of breath, and his blood oxygen saturation fluctuated from 90% to 95%. A second chest CT scan indicated that his pneumonia had been aggravated during the past 3 days and several new shadows were apparent (Figure 1B). The patient then received oxygen inhalation, increased intravenous MP (20 mg, twice per day), and he was admitted to the respiratory intensive care unit (RICU). On Feb 9, the patient was no longer febrile. The symptoms of dry cough and poor appetite disappeared soon thereafter. On Feb 11, an oropharyngeal swab specimen was obtained and subsequent analysis was positive for the presence of SARS-Cov-2 RNA ( Figure 2). On Feb 12, after administering 40 mg intravenous MP once per day for 3 days and then 20 mg intravenous MP once per day for 2 days, orally administered MP (16 mg per day initially, with a daily reduction of 4 mg per day for 4 days down to a final dose of 4 mg per day) was given instead. This adjusted regimen was associated with improved clinical symptoms and significant absorption of chest CT infections (Figure 1C). After Feb 16, all of his symptoms were eliminated and the fourth chest CT scan (Figure 1D) showed a remarkable absorption of the shadow compared to previous scans. TAC was gradually increased to the original dosage level. After two negative SARS-Cov-2 RNA tests (Figure 2) and a fifth (clean) chest CT scan (Figure 1E), the patient was discharged from hospital on Feb 27, 2020. The association of his symptoms with treatment is presented in Figure 2A and his clinical laboratory results are contained in Table 1.
FIGURE 1.
Chest CT images of case 1. A, CT images taken on Feb 5, 2020, showed multiple ground glass opacity and consolidation shadow in the bilateral lung view (as shown in red box) on Illness Day 12. B, Images taken on Feb 8, 2020, showed aggravation of pneumonia and appearance of several new shadows (as shown in green box) on Illness Day 15. C, Images taken on Feb 12, 2020, showed significant absorption of bilateral ground glass opacity on Illness Day 15 and Hospital Day 4. D, Images taken on Feb 17, 2020, showed the remarkable absorption of the shadow compared to previous scans on Illness Day 24 and Hospital Day 9. E, Images taken on Feb 24, 2020, showed almost a clean chest CT scan on Illness Day 31 and Hospital Day 16
FIGURE 2.
Symptoms, maximum body temperatures, treatment timeline and results of testing for the SARS-Cov-2 RNA according to day of illness and day of hospitalization of cases. A, Chronology of case 1. B, Chronology of case 2. C, Chronology of case 3 [Color figure can be viewed at wileyonlinelibrary.com]
TABLE 1.
Clinical laboratory results of case 1
| Reference | Fever clinic | D1 | D2 | D7 | D11 | D17 | |
|---|---|---|---|---|---|---|---|
| Measure | Range | 2020/2/8 | 2020/2/9 | 2020/2/10 | 2020/2/15 | 2020/2/19 | 2020/2/25 |
| Alanine aminotransferase (U/L) | 9-50 | 57↑ | – | 34 | 44 | 104↑↑ | 70↑ |
| Aspartate aminotransferase (U/L) | 15-40 | 50↑ | 24 | 16 | 23 | 61↑ | 44↑ |
| Albumin (g/L) | 40-55 | 42.3 | – | 35.2↓ | 37.8↓ | 41.3 | 39.3↓ |
| Total bilirubin (μmol/L) | 5.13-22.2 | 9.7 | – | 11.5 | 14.9 | 16.4 | 10.0 |
| Blood urea nitrogen (mmol/L) | 2.3-8.2 | 7.8 | – | 10.04↑ | 9.05↑ | 9.97↑ | 7.50↑ |
| Creatinine (μmol/L) | 53-132 | 167.3↑ | – | 122.9 | 119.8 | 121.7 | 119.2 |
| Sodium (mmol/L) | 137-147 | 136.0↓ | 140 | 140 | 139.9 | 137.3 | – |
| Potassium (mmol/L) | 3.5-5.3 | 4.10 | 4.00 | 4.21 | 4.10 | 4.26 | – |
| Calcium (mmol/L) | 1.97-2.85 | 2.61 | 1.90↓ | 2.45 | 2.53 | 2.44 | – |
| Carbon dioxide (mmol/L) | 20-34 | 19.8↓ | 21.2 | 20.5 | 29.4 | 30 | 28.5 |
| Glucose (mmol/L) | 4.4-8.0 | – | 6.30 | – | – | – | – |
| Venous lactate (mmol/L) | 1.25-1.78 | – | 2.0 | – | – | – | – |
| Creatine kinase (U/L) | 15-130 | – | 80 | – | – | – | – |
| C-reactive protein (mg/L) | <10 | 74.34↑↑ | 39.81↑ | 37.70↑ | 21.99↑ | 4.46 | 2.66 |
| White-cell count (×109 cells per L) | 3.30-9.60 | 3.44 | – | 4.29 | 5.14 | 6.38 | 4.25 |
| Red-cell count (×1012 cells per L) | 4-5.5 | 4.77 | – | 3.93↓ | 3.59↓ | 3.39↓ | 3.20↓ |
| Neutrophil count (×109 cells per L) | 2.0-7.0 | 2.59 | – | 3.36 | 3.86 | 4.95 | 2.74 |
| Lymphocyte count (×109 cells per L) | 0.80-4.00 | 0.43↓ | – | 0.42↓ | 0.59↓ | 0.82 | 1.03 |
| Platelet count (×109 cells per L) | 97-350 | 207 | – | 213 | 378↑ | 340 | 218 |
| Hemoglobin (g/L) | 120-160 | 145 | – | 119↓ | 108↓ | 100↓ | 96↓ |
| Urine protein | Neg | – | – | – | – | – | Neg |
| Urine gravity | 1.01-1.03 | – | – | – | – | – | 1.01 |
| Prothrombin time (s) | 11-14 | 12.8 | – | – | 12.8 | 11.0 | 11.3 |
| Fibrinogen (g/L) | 2-4 | 5.22↑ | – | – | 4.33↑ | 2.12 | 1.70↓ |
| International normalized ratio | 0.88-1.26 | 1.07 | – | – | 1.07 | 0.91 | 0.94 |
↓=The value in the patient was below normal.
↑=The value in the patient was above normal. [Color table can be viewed at wileyonlinelibrary.com]
2.2. Case 2, a moderately ill confirmed case, was treated in the respiratory isolation ward
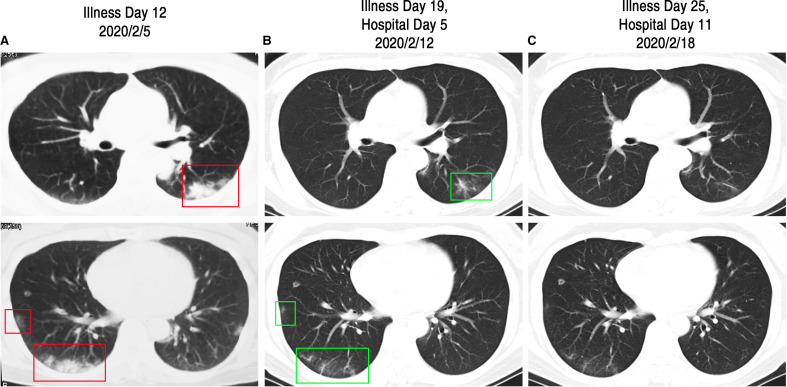
Case 2, a 49-year-old female and the wife of case 1, is a healthy nonsmoker. On Jan 25, 2020, she presented with mild poor appetite and a dry cough without fever. She stayed at home but was kept under medical observation by her physician. On Feb 4, the patient reported feeling un-well and she experienced fatigue. The next day, on Feb 5, her chest CT scan showed multiple patchy ground glass opacity and consolidation shadow in the bilateral lung and subpleural views ( Figure 3A). On the same day, an oropharyngeal swab specimen was obtained and analysis was positive for the presence of SARS-Cov-2 RNA. She was moved to an isolated area of the fever clinic and began taking oral Umifenovir (200 mg, three times per day). Three days later, on Feb 8, the patient was admitted to the respiratory isolation ward and began receiving intravenous Ribavirin (500 mg, twice per day), oral Moxifloxacin (400 mg, once per day), and Oseltamivir (75 mg, twice per day). On Feb 12, her second chest CT scan showed significant absorption of the bilateral lung shadow (Figure 3B). The patient was discharged from hospital on Feb 21, when all her symptoms disappeared and she received two negative SARS-Cov-2 RNA tests ( Figure 4). The association of her symptoms with treatment is presented in Figure 2B.
FIGURE 3.
Chest CT images of case 2. A, Chest CT images obtained on Feb 5, 2020, showed multiple patchy ground glass opacity and consolidation shadow in the bilateral lung and subpleural views (as shown in red box) on Illness Day 12. B, Images taken on Feb 12, 2020, showed significant absorption of the bilateral lung shadow (as shown in green box) on Illness Day 19 and Hospital Day 5. C, Images taken on Feb 18, 2020, show a near normal CT finding before discharge on Illness Day 25 and Hospital Day 11
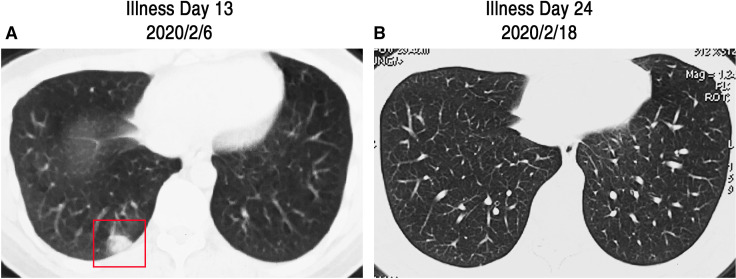
FIGURE 4.
Chest CT images of case 3. A, CT images obtained on Feb 6, 2020, showed a single consolidation shadow in right lower lung subpleural (as shown in red box) on Illness Day 13. B, Images taken on Feb 28, 2020, show the disappearance of the previous single lesion on Illness Day 24
2.3. Case 3, a mild suspected case, received in-home isolation and home care
Case 3, a 24-year-old male and the son of cases 1 and 2, presented with mild poor appetite beginning on Jan 25, 2020, and remained at home under medical observation. On Feb 5, the patient reported a mild dry cough. The next day, on Feb 6, his chest CT scan showed a single consolidation shadow in the right lower lung subpleural (Figure 4A). Considering his close contact history with his parents and his mild clinical symptoms, he received in-home isolation and home care. Oral Moxifloxacin (400 mg, once per day) and Oseltamivir (75 mg, twice per day) were administered for 7 days, and then switched to oral Umifenovir (200 mg, three times per day) and Azithromycin (500 mg, once per day) for 5 days. As of Feb 18, all of his symptoms had resolved. On Feb 20, his second chest CT scan showed that the previous single consolidation shadow had disappeared. The association of his symptoms with treatment is presented in Figure 2C.
3. DISCUSSION
There are three members in this close family: the husband, the wife, and the son. They live together in one apartment. The family stayed at home beginning on Jan 23, 2020, when the Chinese government locked down all of Wuhan city. However, only 2 days later, all members of this family began to lose their appetites. We carefully traced each family member’s contacts but still don’t know when or where they got infected. This familial pattern suggests a rapid and strong person-to-person transmission of COVID-19. In contrast to SARS and MERS, COVID-19 appears to transmit rapidly within families. By Feb 11, 20 provinces outside of Hubei, China have reported 1183 case clusters of COVID-19, 64% of which have been within family households.5
By Feb 2020, it was clear that with limited medical resources in Wuhan, we had to quickly stratify patients to ensure that everyone received proper care. Briefly, patients with mild symptoms and suspected infection were considered for in-home isolation and home care.6 Confirmed infected patients (positive for SARS-Cov-2 RNA test) with mild symptoms stayed in the hospital for further treatment. Patients with severe symptoms and a typical chest CT scan result, regardless of whether the SARS-Cov-2 RNA test was positive, were admitted to the isolation ward of the hospital or in some cases to the Intensive Care Unit (ICU). These three different treatment strategies for COVID-19 were all used in the familial cluster that we described. As a renal recipient with long-term immunosuppression, we kept very close attention on case 1. Confirming our concerns, his illness progressed rapidly. The patient suffered a dry cough and shortness of breath within a few days, and subsequently was admitted to the RICU for further treatment. The wife had the same illness onset time and initial symptoms as her husband. However, she never had a fever and shortness of breath, and was kept in the respiratory isolation ward. Their son had the mildest symptoms in the family. He only experienced a mild poor appetite and cough, without fever. His chest CT images showed only a single shadow. He never received a SARS-Cov-2 RNA test because of the severe shortage of test kits at the time. Based on his young age, exposure history, mild symptom presentation, and CT image results, he received in-home isolation and home care.
As Wuhan is one of the cities with the largest number of renal transplants in China, the presence of COVID-19 infection in renal transplant recipients deserves attention. As a special population with immunosuppression, the clinical manifestations, treatment recommendations and outcomes, and long-term prognosis of COVID-19 infection for renal transplant recipients may be different from those of the general population. For the three familial cases that we presented, the initial clinical symptoms and illness onset time were almost identical. However, for the renal transplant recipient case, COVID-19 associated pneumonia was severe and progressed rapidly. In the absence of a proven treatment strategy for COVID-19, we treated our renal transplant recipient case by implementing the following: immunosuppressant reduction/cessation; early oxygen therapy; planned and regulated application of methylprednisolone (MP); prevention of secondary infection; IVIG; timely support treatment; and antiviral treatment. Of note, use of steroids is controversial and the efficacy is not proven in clinical trials generally or transplant patients specifically. In the absence of these data, based on our experience, our local policy in transplant patients is to use MP in the early stages of typical CT imaging manifestations caused by significant lung interstitial exudation, especially when accompanied by persistent fever symptoms. The principle behind MP administration is to use the minimum dose needed to control body temperature below 37.3°C. The total daily dose of MP should not exceed 80 mg. Reducing the dose level over time and switching to oral MP is also important. In case 1, 20 mg of intravenous MP per day was unable to maintain the body temperature in the normal range. Consequently, we doubled the daily dose to 40 mg per day. Once the fever was relieved for at least 3 days, the use of MP was gradually reduced. During the same period of time, appropriate antibiotic treatment (not Carbapenems) and IVIG were administered to prevent potential secondary infections. Other adjunctive treatments included diuretic administration to prevent water-sodium retention and nutritional support therapy. At present there is no specific treatment for COVID-19, and the efficacy of Umifenovir, Ribavirin and Oseltamivir, as used for the cases discussed herein, is not clear.
In conclusion, the present report described three familial cases having the same illness onset time and clinical manifestations, but different progressions associated with COVID-19 infection and different optimal treatment strategies. The renal transplant recipient case reminds us that immunocompromised patients often have more severe disease progressions. Finally, we note that our renal transplant recipient case made a full recovery from a treatment consisting of immunosuppressant reduction/cessation and low dose methylprednisolone-based therapy, thereby providing a reference for the informed treatment of such patients.
ACKNOWLEDGMENTS
This work was funded by a Key Project of Health and Family Planning Commission of Hubei Province of China (grant number WJ2019Z006).
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation. Study procedures were approved by the institutional review boards (IRBs) at Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (IRB approval number: TJ-C20200120). The clinical activities reported in this article are consistent with the principles of the declaration of Istanbul as outlined in the “Declaration of Istanbul on Organ Trafficking and Transplant Tourism.”
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Funding information Health and Family Planning Commission of Hubei Province, Grant/Award Number: WJ2019Z006
REFERENCES
- 1.Zhu NA, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu X-W, Wu X-X, Jiang X-G, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Health Commission of the People’s Republic of China. March 21: daily briefing on novel coronavirus cases in China. http://en.nhc.gov.cn/index.html. Accessed March 21, 2020.
- 5.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the chinese center for disease control and prevention [published online ahead of print 2020]. JAMA 10.1001/jama.2020.2648 [DOI] [PubMed]
- 6.Jin Y-H, Cai L, Cheng Z-S, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil Med Res. 2020;7(1):4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.