Abstract
Deleting transmembrane α-helix motifs from Plasmodium falciparum sporozoite surface protein (SSP-2) allowed its secretion from Salmonella enterica serovar Typhimurium SL3261 and S. enterica serovar Typhi CVD 908-htrA by the Hly type I secretion system. In mice immunized intranasally, serovar Typhimurium constructs secreting SSP-2 stimulated greater gamma interferon splenocyte responses than did nonsecreting constructs (P = 0.04).
Combining antigens from the sporozoite, intrahepatic, and asexual erythrocytic stages of Plasmodium falciparum into a multivalent vaccine should increase the prospect of achieving protective efficacy (26, 29). Immunologic intervention against the preerythrocytic stages should reduce the number of merozoites emerging from the liver cells, thereby allowing other immune mechanisms to more successfully attack the asexual erythrocytic stages. We have embarked on a program to express in a suitable attenuated Salmonella enterica serovar Typhi strain protective antigens derived from the various stages in the life cycle of P. falciparum and, ultimately, to determine whether the live vector vaccine can stimulate relevant immune responses in humans (14).
SSP-2, also designated thrombospondin-related adhesive protein, which is expressed by the sporozoite once it reaches the mosquito salivary gland, contains a sulfated glycoconjugate-binding peptide sequence needed for parasite invasion (34). Immunization with P. yoelii SSP-2 protects mice against experimental malaria by induction of cytotoxic lymphocytes (CTL) specific to two independent T-cell epitopes (21, 36). Analogously, the specific SSP-2 CTL responses induced following immunization of volunteers with irradiated P. falciparum are thought to contribute to protection (42). Humoral responses may also play a protective role since antibodies to SSP-2 prevent sporozoites from invading human hepatocytes in vitro. Thus, SSP-2 should be included in a multivalent vaccine to prevent P. falciparum malaria (35).
The feasibility of using attenuated serovar Typhi expressing P. falciparum antigens as an oral live vector vaccine was demonstrated in a clinical trial in which attenuated serovar Typhi strain CVD 908 carrying a recombinant P. falciparum circumsporozoite protein gene integrated in the chromosome stimulated serum antibodies and cytotoxic lymphocytes in several vaccinees (14). Serovar Typhi live oral vaccine strain CVD 908-htrA, an improved live vector that harbors attenuating deletion mutations in aroC, aroD, and htrA (3, 23), is well tolerated and elicits antibody and cell-mediated immune responses to serovar Typhi following a single oral dose (40). CVD 908-htrA also functions well in humans as a live vector. One of three seronegative subjects who ingested a single ∼109 CFU dose of CVD 908-htrA expressing fragment C of tetanus toxin mounted a strong serum tetanus antitoxin response (39).
The immune response to foreign antigens expressed by Salmonella live vectors, particularly CTL, is significantly enhanced if the heterologous proteins are secreted externally from the bacteria (11, 18, 20). However, whereas many bacterial proteins are readily expressed in attenuated Salmonella as either cytoplasmic, periplasmic, or secreted moieties (1, 9, 13, 30), expression of eukaryotic P. falciparum proteins, particularly as secreted proteins, has been much more problematic (24). Factors responsible include differences in codon usage between bacteria and Plasmodium, protein sequences (e.g., hydrophobic) deleterious to bacteria, and apparent posttranslational destruction of foreign proteins by bacterial proteases. A tactic to circumvent some problems and achieve adequate expression and secretion of plasmodial proteins in serovar Typhi is the use of a plasmid-based expression-secretion system such as the type I hemolysin (Hly) secretion system of uropathogenic Escherichia coli (12, 18). Although this system increases the number of copies of the foreign gene (thereby potentially elevating expression and increasing deleterious effects on the live vector), the likelihood of toxicity to the bacterial host is decreased since the foreign antigen is secreted. This system requires three membrane proteins, HlyB, HlyD, and TolC, and a signal sequence located at the C terminus of the wild-type HlyA (28). TolC, which is not part of the hly operon, is encoded in the Salmonella chromosome. Herein we describe the expression and secretion of P. falciparum SSP-2 in attenuated serovar Typhi CVD 908-htrA and serovar Typhimurium and demonstrate the immunogenicity of the serovar Typhimurium construct in mice immunized mucosally.
E. coli DH5α and aroA mutant serovar Typhimurium SL3261 were grown in Luria broth (LB) or agar supplemented with 100 μg of ampicillin per ml when required (19). Serovar Typhi CVD 908-htrA was grown in LB supplemented with 0.0001% 2,3-dihydroxybenzoic acid (Sigma, St. Louis, Mo.). The plasmids used in this study are described in Table 1. In adapting pMOhly1, which carries the E. coli hemolysin secretion system, the gene encoding the protein to be exported is inserted into the unique Nsil site located within a truncated hlyA, immediately upstream of the C-terminal secretion signal and downstream of the initiation codon. P. falciparum ssp-2 and truncated ssp-2 derivatives were inserted into pBluescript II SK and pMOhly1, and the plasmid constructions were transferred to E. coli DH5α, serovar Typhimurium SL2361, and Serovar Typhi CVD 908-htrA strains by electroporation. Genomic DNA from the 3D7 clone (41) of P. falciparum strain NF54 was amplified with primers 1 and 2 to obtain full-length ssp-2 (35), which was subsequently cloned into pBluescript II KS. The resulting pKS-SSP-2 plasmid served as the template DNA for all amplification reactions (which utilized Deep Vent DNA polymerase with proofreading enzymatic activity). The primers used for PCR amplification are described in Table 2.
TABLE 1.
Plasmids used in this study
| Plasmid | Insert | Determinantsa | Source or reference |
|---|---|---|---|
| pBluescript II KS | Ampr, β-gal | Stratagene | |
| pKS-SSP-2 | Wild-type ssp-2 | Ampr, β-gal | This study |
| pBluescript II Sk | Ampr | Stratagene | |
| pMOhly1 | Ampr, Hly | 12 | |
| pSK-2023 | Wild-type ssp-2 | Ampr | This study |
| pSK-2123 | ssp-2 with 5′deletion | Ampr | This study |
| pSK-2027 | ssp-2 with 3′deletion | Ampr | This study |
| pSK-3027 | ssp-2 with 5′ and 3′deletions | Ampr | This study |
| pMO-2023 | Wild-type ssp-2 | Hly, Ampr | This study |
| pMO-2123 | ssp-2 with 5′deletion | Hly, Ampr | This study |
| pMO-2027 | ssp-2 with 3′deletion | Hly, Ampr | This study |
| pMO-3027 | ssp-2 with 5′ and 3′ deletions | Hly, Ampr | This study |
| VR2519 | Wild-type ssp-2 preceded by the translation initiation complex and leader peptide sequences of human tissue plasminogen activator. | Kmr | 17 |
β-gal, β-galactosidase.
TABLE 2.
Primers used in this study for polymerase chain reaction amplifications
| Primer | Sequence | ssp-2 position (nt) |
|---|---|---|
| 1 sense | AATAATGAATCATCTTGGG | 1–15 |
| 2 antisense | TATTTAATTCCACTCGTTTTC | 1728–1708 |
| 20 sense | ACTAGTAGCATGCCTGCAGACCCAATAATGAATCATCTTG | 1–13 |
| 21 sense | ACTAGTAGCATGCCTGCAGACGAATGGTCTCCATGTAG | 742–761 |
| 23 antisense | TCTGCAGTGGATCCTCTAGATTTCCACTCGTTTTCTTCAG | 1721–1703 |
| 27 antisense | AACTGCAGCTTCACTATTAGGTACGTGC | 1387–1368 |
| 30 sense | TTCTGCAGATGTGCAAAACAATATAGTGG | 78–100 |
A Pstl site was incorporated at the 5′ end of each oligonucleotide primer to allow cloning into the compatible Nsil site of pMOhly1. The 1.7-kb PCR product obtained with primers 20 and 23 was cloned in pBluescript II SK to get pSK-2023. From this plasmid a Pstl insert carrying the entire wild type ssp-2 was cloned into pMOhly1 to obtain pMO-2023. The same strategy was used to construct pMO-2123, pMO-2027, and pMO-3027 (Table 1; also see Fig. 3).
FIG. 3.
Amplification of full-length and truncated versions of ssp-2. The wild-type ssp-2 was amplified from DNA of P. falciparum clone 3D7 of strain NF54 using primers 1 and 2 and cloned into pBluescript II KS. This cloned plasmid DNA was thereafter used as the template for deriving different versions of ssp-2 that were amplified by PCR and cloned into the Nsil site of pMOhly1. Oligonucleotide primers 20 and 23 amplified the wild-type ssp-2, primers 20 and 27 amplified ssp-2 without the C terminus, primers 21 and 23 amplified ssp-2 without the N terminus, and the primers 30 and 27 amplified SSP-2 without the N and C termini. Summarizing data from Fig. 1 and 4, expression refers to the detection of SSP-2 in whole-cell lysates by immunoblotting with monoclonal antibody SSP-2.1; secretion results in detection of SSP-2 in immunoblots of culture supernatants. LP, leader peptide; TM, transmembrane domain.
Whole-cell lysates prepared from centrifuged pellets of late-logarithmic-phase aerated cultures of E. coli and Salmonella grown in LB at 37°C were boiled and then separated by sodium dodecyl sulfate-polyacylamide gel electrophoresis (SDS-PAGE) on a 12% polyacrylamide gel. Separated proteins were electrotransferred to nitrocellulose membranes, labeled with the SSP-2.1 monoclonal antibody for recognition of SSP-2 protein (2), and detected by chemiluminescence using the ECL kit (Amersham-Pharmacia-Biotech, Piscataway, N.J.).
The whole-cell lysate from E. coli DH5α(pSK-2023) revealed the expression of a 65-kDa protein (Fig. 1A), i.e., a heterologous protein with the expected molecular mass of SSP-2. Other proteins of 90, 75, and 52 kDa were also observed. The 90- and 75-kDa proteins show a molecular mass in SDS-PAGE migration slightly larger than expected, possibly due to the high proline content of SSP-2 (21). The 52-kDa protein apparently represents a degradation product of SSP-2 or a complete product of a truncated SSP-2 transcript. As expected, no SSP-2 was observed in the supernatant (Fig. 1B). DH5α(pMO-2023) expressed a 75-kDa SSP-2 in the cytosol and the smaller band of 52 kDa. The 75-kDa protein correlated very well with the expected molecular mass of the SSP-2–HlyA secretion signal fusion, whereas the 52-kDa protein may correspond to a degradation product of the same protein. Lower expression of SSP-2 was observed with DH5α(pMO-2023) than with DH5α(pSK-2023), suggesting that the lower copy number of pMO-2023 may account for the difference. Protein analysis of bacterial culture supernatants of DH5α(pMO-2023) showed no export of SSP-2 to the extracellular space (Fig. 1B).
FIG. 1.
Expression of the wild type and truncated SSP-2 by the type I secretion system. (A) Immunoblot of whole-cell lysates of E. coli DH5α carrying pSK or pMOhly1 (pMO) vectors (as controls) or pSK-2023, pMOhly1-2023, pMOhly1-2027, pMOhly1-2123, or pMOhly1-3027, encoding either full-length or truncated SSP-2. (B) immunoblot of culture supernatants from the above samples. M, molecular weight markers. The control lane (+) contains the bacterial lysate from E. coli DH5α(pSK-2023) which, expresses full-length SSP-2.
Identical protein analysis results were obtained with whole-cell and supernatants samples from serovar Typhimurium SL3261 and serovar Typhi CVD 908-htrA strains carrying the above plasmids (data not shown), further indicating that the full-length SSP-2 cannot be exported by the hemolysin secretion system and that further engineering of the protein was required.
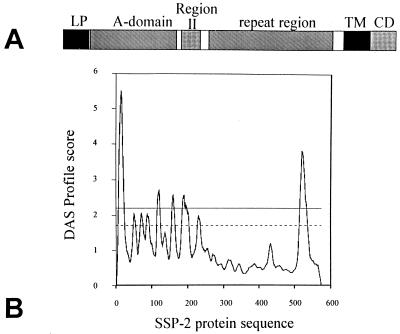
We surmised that the lack of secretion of SSP-2 was most probably the result of interference with the hemolysin secretion machinery (28). Analysis of the SSP-2 sequence using the dense alignment surface method (4) revealed transmembrane α-helices located at the N terminus and at the C terminus which would be expected to inhibit secretion (Fig. 2). We hypothesized that obliteration of these transmembrane α-helices would eliminate interference with the Hly system and allow secretion of SSP-2. Accordingly, truncated versions of ssp-2 were amplified with primers designed to eliminate the regions encoding either the N-terminal signal sequence, the C-terminal transmembrane domain, or both (Fig. 3), and the products were cloned into pBluescript II SK; Pstl cassettes carrying the truncated ssp-2 derivatives were then introduced into pMOhly1 at the Nsil site. Potential pMOhly1 clones were screened by PCR employing the same primers to construct the truncated versions of ssp-2. The constructs were initially recovered in E. coli DH5α and then transferred to serovar Typhimurium SL3261 and serovar Typhi CVD 908-htrA using electroporation.
FIG. 2.
Transmembrane α-helix regions within SSP-2. (A) Diagram of SSP-2 showing the main protein regions including the leader peptide (LP), the A-domain, region II with homology to the circumsporozoite protein, the repeat region, the transmembrane domain (TM), and the cytoplasmic domain (CD). (B) Dense alignment surface (DAS) analysis of the SSP-2 protein sequence. Sequences with scores above 2.2 are predicted to form transmembrane α-helices with a high degree of statistical significance.
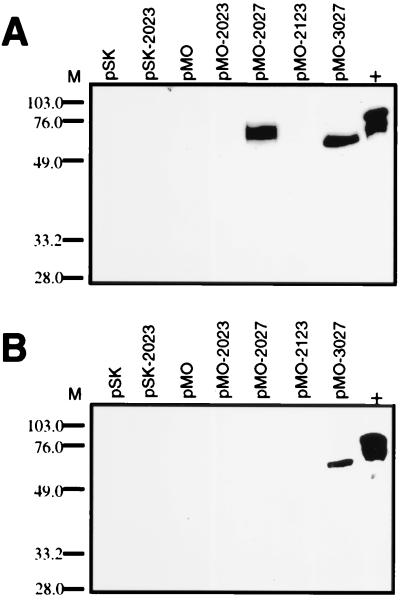
Supernatant and whole-cell lysates of E. coli and Salmonella carrying pMOhly1 constructs were evaluated by immunoblotting using the SSP-2.1 monoclonal antibody. E. coli DH5α carrying pMOhly1 encoding wild-type or truncated SSP-2 showed expression in all whole-cell lysates (Fig. 1A). However, only two of the plasmid constructs, pMO-2027 and pMO-3027, were able to secrete the protein, as indicated by the detection of truncated SSP-2 in supernatants (Fig. 1B). E. coli DH5α(pMO-2027) expressed a modified SSP-2 that lacks the C-terminal transmembrane domain, whereas DH5α(pMO-3027) secreted the engineered SSP-2 lacking both the N-terminal signal sequence and the C-terminal transmembrane domain. Identical results were obtained with whole-cell lysates (data not shown) and supernatants from serovar Typhimurium SL3261 (Fig 4A), indicating that the SSP-2 C-terminal transmembrane region also interferes with protein secretion in serovar Typhimurium. Most importantly, in the serovar Typhi CVD 908-htrA background, only serovar Typhi CVD 908-htrA(pMO-3027), which encodes the SSP-2 truncated at both the N and C termini, achieved secretion of the malarial protein (Fig. 4B).
FIG. 4.
Expression and secretion of SSP-2 by the hemolysin secretion system detected by immunoblot analysis of culture supernatants using monoclonal antibody SSP-2.1. (A) Culture supernatants of serovar Typhimurium SL3261 carrying pSK and pMOhly1 (pMO) constructs encoding full-length SSP-2 and truncated versions without the transmembrane α-helix domains. (B) Culture supernatants of CVD 908-htrA carrying pSK and pMOhly1 encoding full-length SSP-2 and truncated versions without transmembrane α-helix domains. M, molecular weight markers. Control lane (+) contains the bacterial lysate from E. coli DH5α(pSK-2023), which expresses full length SSP-2.
The effectiveness of the HlyA secretion system was highlighted by comparison of results with those obtained with pBluescript II SK, an otherwise excellent expression system that does not encode a secretion apparatus. Whereas whole-cell lysates from E. coli DH5α carrying pBluescript II SK encoding wild-type and truncated SSP-2 demonstrated expression of these proteins, in no supernatant from any construct was there evidence of secretion of SSP-2 (data not shown).
Immunity to preerythrocytic-stage antigens of the malaria parasites, including SSP-2, is largely mediated by CD8+ T cells and involves gamma interferon (IFN-γ) (shown to be involved in the killing of developing liver-stage parasites in infected hepatocytes), nitric oxide, and interleukin-12 (IL-12) production (6, 8, 16, 25, 43). To assess the immunogenicity of serovar Typhimurium strains carrying plasmids encoding SSP2, we measured IFN-γ production by effector cells from immunized mice in response to target cells infected with a vaccinia virus expressing PfSSP-2, using an enzyme-linked immunospot (ELISPOT) technique (15, 27). Groups of 8 to 10 female C57BL-6 (H-2b) mice (Charles River Breeding Laboratories, Wilmington, Mass.) aged 6 to 8 weeks, were immunized intranasally (i.n.) with 10 μl containing 1 × 109 to 2 × 109 CFU of SL3261(pMO2023), SL3261(pMO3027), SL3261(pMO), or SL3261(VR2519). Mice serving as the positive control were inoculated intramuscularly with an SSP-2 DNA vaccine consisting of eukaryotic expression plasmid VR2519 encoding full-length SSP-2 under the control of a cytomegalovirus promoter (17); a total of 100 μg of DNA was injected into the tibialis anterioris, 50 μg in each leg. The mice were given a total of three doses, at 3-week intervals. The negative control mice received phosphate-buffered saline i.n.
Ninety-six-well nitrocellulose plates (Multiscreen-HA; Millipore, Bedford, Mass.) were coated with 5 μg of anti-mouse IFN-γ monoclonal antibody (Pharmingen, San Diego, Calif.) per ml overnight at 4°C. After incubation, the plates were washed four times with RPMI and blocked with RPMI containing 2 mM l-glutamine, 10 mM HEPES, 50 μg of gentamicin per ml, and 10% heat-inactivated fetal calf serum (HyClone, Logan, Utah). Serial dilutions of effector splenocytes (1 × 106 to 1.25 × 105 cells/well) from immunized and control mice in culture medium supplemented with 20 U of recombinant murine IL-2 (R&D Systems Inc., Minneapolis, Minn.) per ml were incubated for 36 h at 37°C in 5% CO2 in the presence of irradiated major histocompatibility complex-matched (H-2b) EL4 cells (5 × 104 to 1 × 105 cells/well) that were previously infected with recombinant vaccinia virus carrying the PfSSP-2 gene (vP1254; WR-PfSSP-2) or parental vaccinia virus (WR) (Virogenetics, Troy, N.Y.). Vaccinia virus infection was performed the day before the assay. Briefly, EL-4 cells were centrifuged, resuspended in 0.5 to 1 ml of RPMI, and incubated for 2 h with vaccinia virus at 5 PFU per cell. The efficiency of vaccinia virus infection was assessed by flow cytometry using a fluorescein isothiocyanate-labeled rabbit anti-vaccinia virus Lister strain antiserum (Virostat, Portland, Maine). Following undisturbed incubation, the plates were washed with PBS-Tween 20 (0.05%) and incubated with biotin-labeled anti-IFN-γ monoclonal antibody (Pharmingen) for 2 h at 37°C. The wells were washed, incubated with 100 μl of streptavidin-peroxidase (diluted 1:500 in PBS-Tween) for 1 h at 37°C, and then washed again, whereupon 50 μl of TrueBlue Peroxidase substrate (Kirkergaard & Perry Laboratories Inc., Gaithersburg, Md.) was added per well. The number of spots corresponding to IFN-γ-secreting cells in different spleen cell dilutions was counted using a stereomicroscope. The results were recorded as mean counts per 106 cells from quadruplicate wells per sample. The number of cells secreting IFN-γ per spleen was calculated based on the number of splenocytes obtained per mouse in each group. The results are shown in Fig. 5 as spot counts/spleen after subtraction of the number of spots in cultures containing EL-4 cells infected with vaccinia alone.
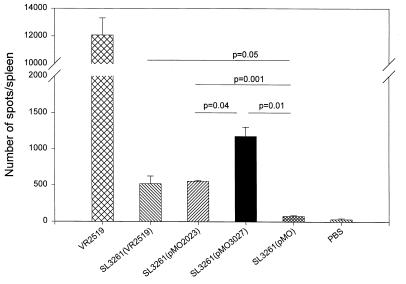
FIG. 5.
Frequency of P. falciparum SSP-2-specific IFN-γ-secreting cells in mice immunized with serovar Typhimurium strain SL3261 carrying SSP-2 constructs, measured by ELISPOT. C57BL/6 mice were immunized i.n. with SL3261(pMO2023) (prokaryotic expression, nonsecreted full-length SSP-2), SL3261(pMO3027) (prokaryotic expression, secreted truncated SSP-2), SL3261(pMO) (prokaryotic expression negative control, carrying the Hly secretion system without an insert), SL3261(VR2519) (eukaryotic expression, full-length SSP-2) (negative control) and PBS (negative control). Positive control mice were immunized intramuscularly with DNA vaccine plasmid VR2519 encoding full-length SSP-2. Splenocytes were stimulated in vitro with irradiated major histocompatibility complex-matched (H-2b) EL-4 cells infected with SSP-2 expressing recombinant vaccinia virus or native vaccinia virus for detection of IFN-γ secretion. Each bar represents the mean number of specific spots per spleen, and the error bars show standard deviation. Significant differences among the number of secreting cells measured in the different effector populations were determined by Student's t test. Data are representative of two independent experiments.
Mice immunized parenterally with the positive control preparation, the SSP-2 DNA vaccine plasmid VR2519, mounted the strongest ELISPOT response in terms of IFN-γ-secreting cells/per spleen (Fig. 5). Mice immunized mucosally with serovar Typhimurium SL3261(VR2519) carrying the identical SSP-2 DNA vaccine plasmid also exhibited a significant IFN-γELISPOT response, corroborating previous reports that Salmonella live vectors can successfully deliver eukaryotic expression plasmids and elicit immune responses (5, 32). However, the magnitude of the IFN-γ ELISPOT response in mice that received serovar Typhimurium SL3261(VR2519) i.n. was markedly lower than that in mice that received parenteral inoculations with VR2519 DNA. Mice immunized mucosally with the two Serovar Typhimurium constructs carrying the SSP-2 gene under a prokaryotic expression system also exhibited significant increases in the number of cells producing IFN-γ (Fig. 5). The magnitude of the response elicited by serovar Typhimurium construct SL3261(pMO2023), which encodes full-length SSP-2 that cannot be secreted, was very similar to that observed in mice immunized with serovar Typhimurium SL3261(VR2519) (Fig. 5). In contrast, in mice immunized i.n. with Serovar Typhimurium construct SL3261(pM3027), which encodes a truncated SSP-2 that is secreted, the IFN-γ cell ELISPOT response was significantly stronger than that elicited by the other serovar Typhimurium constructs (P = 0.04) (Fig. 5). These data support previous observations that with Salmonella live vectors, foreign antigens are more immunogenic if they are secreted, including the stimulation of cell-mediated immune responses (18).
Development of an effective malaria vaccine would provide a new tool to help control malaria. Because 90% of P. falciparum deaths and severe disease occur in sub-Sahara Africa among populations inhabiting some of the world's poorest countries, the characteristics of a malaria vaccine will affect its suitability in such venues. The strategy that we are pursuing, i.e., expressing protective antigens of P. falciparum in an attenuated serovar Typhi live vector, has several theoretical advantages, including oral administration and likely economy of manufacture. However, a drawback that has so far slowed the pace of vaccine development is the difficulty in expressing many eukaryotic proteins in bacterial live vectors.
The high AT/GC ratio of ssp-2 and the difference in codon usage with respect to E. coli and Salmonella strains did not prevent the expression of ssp-2 in E. coli DH5α, serovar Typhimurium SL3261, and serovar Typhi CVD 908-htrA by two different expression systems. The Hly system cloned into pMOhly1 allowed secretion of a modified SSP-2 by serovar Typhi CVD 908-htrA once the transmembrane α-helices at the N terminus and C terminus were removed. Despite the high copy number of each plasmid, the level of SSP-2 protein expressed was not toxic in vitro based on a comparison of bacterial growth and colony morphology of Salmonella carrying or lacking these plasmids. The visualization of smaller moieties of SSP-2 in the protein gels suggests that differences in codon usage of ssp-2 with respect to E. coli or Salmonella codon usage may stall or slow transcription.
The Hly secretion system incorporates a complex regulatory system that is dependent on a positive regulator that allows transcription of the hly operon. However, little is known about the in vivo induction of the promoter that drives expression of the hly operon. Therefore, one likely approach to improve the HlyA secretion system would be to substitute a promoter for which the in vivo inducing conditions are known.
So far, we have succeeded in expressing three preerythrocytic-stage antigens of P. falciparum in attenuated serovar Typhi, including circumsporozoite protein (14), SSP-2 (this work), and liver-stage antigen 1 (LSA-1) (unpublished data). We can now undertake practical steps to improve the expression of malarial antigens by serovar Typhi. These include (i) optimizing the codon usage of malarial genes to match Salmonella expression; (ii) utilizing regulated promoters that are activated by in vivo conditions, such as PompC or PdmsA, to drive expression of the malaria gene as well as of the entire hly secretion system (31, 33); and (iii) utilizing expression plasmids that encode stabilization and plasmid maintenance functions (10).
Antigen-specific CD8+ T lymphocytes and IFN-γ production are essential effector mechanisms that contribute to the protective responses against malaria infection in the murine model (7). CD8+ CTL against preerythrocytic stages of the malaria parasites, including PySSP2, protect against sporozoite challenge (6, 22). It is believed that IFN-γ and CD8+ T cells together contribute to the killing of developing liver-stage parasites either by regulating the production of nitric oxide in the liver or by stimulating mononuclear cells to produce IL-12, which in turn activates other lymphocytes and NK cells to further increase IFN-γ levels (6, 8, 16, 25, 38). Moreover, protection induced by previous vaccine strategies was associated with high levels of CD8+ IFN-γ-secreting splenocytes (37). Thus, we assessed the immunogenicity of our vaccine constructs by measuring the frequency of IFN-γ secreting cells in short-term cultures of effector splenocytes incubated in the presence of MHC-matched P. falciparum SSP-2-expressing cells by ELISPOT. This technique has proven suitable to monitor antigen-specific CD8+ T lymphocyte responses to malaria antigens. Furthermore, quantification of IFN-γ-secreting cells in short-term cultures at the single-cell level has been proposed as a reliable tool to assess the efficacy of malaria vaccines (15). The serovar Typhimurium strains encoding P. falciparum SSP-2 successfully delivered the foreign antigen and induced specific immune responses. Of particular note, the highest responses were observed in mice immunized with a live vector vaccine engineered to secrete P. falciparum SSP-2 extracellularly. The demonstration of the ability of the serovar Typhimurium constructs to elicit a relevant cell-mediated immune response (IFN-γ secreting cells) in a pre clinical mouse model provides a rationale for undertaking phase I clinical trials with the analogous serovar Typhi construct, bringing our ambitious quest to develop a mucosally administered, multivalent, live vector-based malaria vaccine one step closer.
Acknowledgments
This research was supported by RO1AI40297 and RO1AI29471 and research contract NO1-AI-45251 from the National Institute of Allergy and Immunology (M.M.L. Principal Investigator) and by Naval Medical Research and Development Command Work Unit AE284 STO F 6.2 622787A 0101.870.EFX.1432.
We thank Werner Goebel, University of Würzberg, Würzberg, Germany, for generously providing pMOhly1 carrying the hemolysin secretion system of uropathogenic E. coli.
REFERENCES
- 1.Barry E M, Gomez-Duarte O, Chatfield S, Pizza M, Rappuoli R, Losonsky G A, Galen J E, Levine M M. Expression and immunogenicity of pertussis toxin S1 subunit-tetanus toxin fragment C fusions in Salmonella typhi vaccine strain CVD 908. Infect Immun. 1996;64:4172–4181. doi: 10.1128/iai.64.10.4172-4181.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charoenvit Y, Fallarme V, Rogers W O, Sacci J B J, Kaur M, Aguiar J C, Yuan L F, Corradin G, Andersen E, Wizel B, Houghten R A, Oloo A, De la Vega P, Hoffman S L. Development of two monoclonal antibodies against Plasmodium falciparum sporozoite surface protein 2 and mapping of B-cell epitopes. Infect Immun. 1997;65:3430–3437. doi: 10.1128/iai.65.8.3430-3437.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chatfield S N, Strahan K, Pickard D, Charles I G, Hormaeche C E, Dougan G. Evaluation of Salmonella typhimurium strains harbouring defined mutations in htrA and aroA in the murine salmonellosis model. Microb Pathog. 1992;12:145–151. doi: 10.1016/0882-4010(92)90117-7. [DOI] [PubMed] [Google Scholar]
- 4.Cserzo M, Wallin E, Simon I, von Heijne G, Elofsson A. Prediction of transmembrane alpha-helices in prokaryotic membrane proteins: the dense alignment surface method. Protein Eng. 1997;10:673–676. doi: 10.1093/protein/10.6.673. [DOI] [PubMed] [Google Scholar]
- 5.Darji A, Guzman C A, Gerstel B, Wachholz P, Timmis K N, Wehland J, Chakraborty T, Weiss S. Oral somatic transgene vaccination using attenuated S. typhimurium. Cell. 1997;91:765–775. doi: 10.1016/s0092-8674(00)80465-1. [DOI] [PubMed] [Google Scholar]
- 6.Doolan D L, Hoffman S L. IL-12 and NK cells are required for antigen-specific adaptive immunity against malaria initiated by CD8+ T cells in the Plasmodium yoelii model. J Immunol. 1999;163:884–892. [PubMed] [Google Scholar]
- 7.Doolan D L, Hoffman S L. The complexity of protective immunity against liver-stage malaria. J Immunol. 2000;165:1453–1462. doi: 10.4049/jimmunol.165.3.1453. [DOI] [PubMed] [Google Scholar]
- 8.Doolan D L, Sedegah M, Hedstrom R C, Hobart P, Charoenvit Y, Hoffman S L. Circumventing genetic restriction of protection against malaria with multigene DNA immunization: CD8+ cell-, interferon gamma-, and nitric oxide-dependent immunity. J ExpMed. 1996;183:1739–1746. doi: 10.1084/jem.183.4.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galen J E, Gomez-Duarte O G, Losonsky G A, Halpern J L, Lauderbaugh C S, Kaintuck S, Reymann M K, Levine M M. A murine model of intranasal immunization to assess the immunogenicity of attenuated Salmonella typhi live vector vaccines in stimulating serum antibody responses to expressed foreign antigens. Vaccine. 1997;15:700–708. doi: 10.1016/s0264-410x(96)00227-7. [DOI] [PubMed] [Google Scholar]
- 10.Galen J E, Nair J, Wang J Y, Wasserman S S, Tanner M K, Sztein M B, Levine M M. Optimization of plasmid maintenance in the attenuated live vector vaccine strain Salmonella typhi CVD 908-htrA. Infect Immun. 1999;67:6424–6433. doi: 10.1128/iai.67.12.6424-6433.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gentschev I, Glaser I, Goebel W, McKeever D J, Musoke A, Heussler V T. Delivery of the p67 sporozoite antigen of Theileria parva by using recombinant Salmonella dublin: secretion of the product enhances specific antibody responses in cattle. Infect Immun. 1998;66:2060–2064. doi: 10.1128/iai.66.5.2060-2064.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gentschev I, Mollenkopf H, Sokolovic Z, Hess J, Kaufmann S H, Goebel W. Development of antigen-delivery systems, based on the Escherichia coli hemolysin secretion pathway. Gene. 1996;179:133–140. doi: 10.1016/s0378-1119(96)00424-6. [DOI] [PubMed] [Google Scholar]
- 13.Gomez-Duarte O G, Galen J, Chatfield S N, Rappuoli R, Eidels L, Levine M M. Expression of fragment C of tetanus toxin fused to a carboxyl-terminal fragment of diphtheria toxin in Salmonella typhi CVD 908 vaccine strain. Vaccine. 1995;13:1596–1602. doi: 10.1016/0264-410x(95)00094-h. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez C, Hone D, Noriega F, Tacket C O, Davis J R, Losonsky G, Nataro J P, Hoffman S, Malik A, Nardin E, Sztein M B, Heppner D G, Fouts T R, Isibasi A, Levine M M. Salmonella typhi vaccine strain CVD 908 expressing the circumsporozoite protein of Plasmodium falciparum: strain construction and safety and immunogenicity in humans. J Infect Dis. 1994;169:927–931. doi: 10.1093/infdis/169.4.927. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez J M, Peter K, Esposito F, Nebie I, Tiercy J M, Bonelo A, Arevalo-Herrera M, Valmori D, Romero P, Herrera S, Corradin G, Lopez J A. HLA-A*0201 restricted CD8+ T-lymphocyte responses to malaria: identification of new Plasmodium falciparum epitopes by IFN-gamma ELISPOT. Parasite Immunol. 2000;22:501–514. doi: 10.1046/j.1365-3024.2000.00331.x. [DOI] [PubMed] [Google Scholar]
- 16.Good M F, Doolan D L. Immune effector mechanisms in malaria. Curr Opin Immunol. 1999;11:412–419. doi: 10.1016/S0952-7915(99)80069-7. [DOI] [PubMed] [Google Scholar]
- 17.Hedstrom R C, Doolan D L, Wang R, Kumar A, Sacci J B, Jr, Gardner M J, Aguiar J C, Charoenvit Y, Sedegah M, Tine J A, Margalith M, Hobart P, Hoffman S L. In vitro expression and in vivo immunogenicity of Plasmodium falciparum pre-erythrocytic stage DNA vaccines. Int J Mol Med. 1998;2:29–38. doi: 10.3892/ijmm.2.1.29. [DOI] [PubMed] [Google Scholar]
- 18.Hess J, Gentschev I, Miko D, Welzel M, Ladel C, Goebel W, Kaufmann S H E. Superior efficacy of secreted over somatic antigen display in recombinant Salmonella vaccine induced protection against listeriosis. Proc Natl Acad Sci USA. 1996;93:1458–1463. doi: 10.1073/pnas.93.4.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoiseth S, Stocker B A D. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;292:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 20.Kaufmann S H, Hess J. Impact of intracellular location of and antigen display by intracellular bacteria: implications for vaccine development. Immunol Lett. 1999;65:81–84. doi: 10.1016/s0165-2478(98)00128-x. [DOI] [PubMed] [Google Scholar]
- 21.Khusmith S, Charonevit Y, Kumar S, Sedegah M, Beaudoin R L, Hoffman S L. Protection against malaria by vaccination with sporozoite surface protein 2 plus CS protein. Science. 1991;252:715–717. doi: 10.1126/science.1827210. [DOI] [PubMed] [Google Scholar]
- 22.Khusmith S, Sedegah M, Hoffman S L. Complete protection against Plasmodium yoelii by adoptive transfer of a CD8+ cytotoxic T-cell clone recognizing sporozoite surface protein 2. Infect Immun. 1994;62:2979–2983. doi: 10.1128/iai.62.7.2979-2983.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levine M M, Galen J, Barry E, Noriega F, Chatfield S, Sztein M, Dougan G, Tacket C. Attenuated Salmonella as live oral vaccines against typhoid fever and as live vectors. J Biotechnol. 1995;44:193–196. doi: 10.1016/0168-1656(95)00094-1. [DOI] [PubMed] [Google Scholar]
- 24.Levine M M, Galen J E, Sztein M B, Beier M, Noreiga F. Salmonella expressing protozoal antigens. In: Levine M M, Woodrow G C, Kaper J B, Cobon G S, editors. New generation vaccines. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 351–361. [Google Scholar]
- 25.Mellouk, S., S. L. Hoffman, Z. Z. Liu, I. De, V, T. R. Billiar, and A. K. Nussler. 1994. Nitric oxide-mediated antiplasmodial activity in human and murine hepatocytes induced by gamma interferon and the parasite itself: enhancement by exogenous tetrahydrobiopterin. Infect. Immun. 62:4043–4046. [DOI] [PMC free article] [PubMed]
- 26.Miller L H, Hoffman S L. Research toward vaccines against malaria. NatMed. 1998;4:520–524. doi: 10.1038/nm0598supp-520. [DOI] [PubMed] [Google Scholar]
- 27.Miyahira Y, Murata K, Rodriguez D, Rodriguez J R, Esteban M, Rodrigues M M, Zavala F. Quantification of antigen specific CD8+ T cells using an ELISPOT assay. J Immunol Methods. 1995;181:45–54. doi: 10.1016/0022-1759(94)00327-s. [DOI] [PubMed] [Google Scholar]
- 28.Mollenkopf H J, Gentschev I, Goebel W. Conversion of bacterial gene products to secretion-competent fusion proteins. BioTechniques. 1996;21:854. doi: 10.2144/96215st03. , 856–860. [DOI] [PubMed] [Google Scholar]
- 29.Nussenzweig R S, Long C A. Malaria vaccines: multiple targets. Science. 1994;265:1381–1383. doi: 10.1126/science.8073276. [DOI] [PubMed] [Google Scholar]
- 30.Orr N, Galen J E, Levine M M. Expression and immunogenicity of a mutant diphtheria toxin molecule, CRM(197), and its fragments in Salmonella typhi vaccine strain CVD 908-htrA. Infect Immun. 1999;67:4290–4294. doi: 10.1128/iai.67.8.4290-4294.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orr, N., J. E. Galen, and M. M. Levine. Novel use of anaerobically induced promoter, dmsA, for controlled expression of fragment C of tetanus toxin in live attenuated Salmonella enterica serovar Typhi strain CVD 908-htrA. Vaccine, in press. [DOI] [PubMed]
- 32.Pasetti M F, Anderson R J, Noriega F R, Levine M M, Sztein M B. Attenuated guaBA Salmonella typhi vaccine strain CVD 915 as a live vector utilizing prokaryotic or eukaryotic expression systems to deliver foreign antigens and elicit immune responses. Clin Immunol. 1999;92:76–89. doi: 10.1006/clim.1999.4733. [DOI] [PubMed] [Google Scholar]
- 33.Pickett T E, Pasetti M F, Galen J E, Sztein M B, Levine M M. In vivo characterization of the murine intranasal model for assessing the immunogenicity of attenuated Salmonella enterica serovar Typhi strains as live mucosal vaccines and as live vectors. Infect Immun. 2000;68:205–213. doi: 10.1128/iai.68.1.205-213.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robson K J, Frevert U, Reckmann I, Cowan G, Beier J, Scragg I G, Takehara K, Bishop D H, Pradel G, Sinden R. Thrombospondin-related adhesive protein (TRAP) of Plasmodium falciparum: expression during sporozoite ontogeny and binding to human hepatocytes. EMBO J. 1995;14:3883–3894. doi: 10.1002/j.1460-2075.1995.tb00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rogers W O, Malik A, Mellouk S, Nakamura K, Rogers M D, Szarfman A, Gordon D M, Nussler A K, Aikawa M, Hoffman S L. Characterization of Plasmodium falciparum sporozoite surface protein 2. Proc Natl Acad Sci USA. 1992;89:9176–9180. doi: 10.1073/pnas.89.19.9176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogers W O, Rogers M D, Hedstrom R C, Hoffman S L. Characterization of the gene encoding sporozoite surface protein 2, a protective Plasmodium yoelii sporozoite antigen. Mol Biochem Parasitol. 1992;53:45–51. doi: 10.1016/0166-6851(92)90005-5. [DOI] [PubMed] [Google Scholar]
- 37.Schneider J, Gilbert S C, Blanchard T J, Hanke T, Robson K J, Hannan C M, Becker M, Sinden R, Smith G L, Hill A V. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat Med. 1998;4:397–402. doi: 10.1038/nm0498-397. [DOI] [PubMed] [Google Scholar]
- 38.Seguin M C, Klotz F W, Schneider I, Weir J P, Goodbary M, Slayter M, Raney J J, Aniagolu J U, Green S J. Induction of nitric oxide synthase protects against malaria in mice exposed to irradiated Plasmodium berghei infected mosquitoes: involvement of interferon gamma and CD8+ T cells. J Exp Med. 1994;180:353–358. doi: 10.1084/jem.180.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tacket C O, Galen J, Sztein M B, Losonsky G, Wyant T L, Nataro J, Wasserman S S, Edelman R, Chatfield S, Dougan G, Levine M M. Safety and immune responses to attenuated Salmonella enterica serovar Typhi oral live vector vaccines expressing tetanus toxin fragment C. Clin Immunol. 2000;97:146–153. doi: 10.1006/clim.2000.4924. [DOI] [PubMed] [Google Scholar]
- 40.Tacket C O, Sztein M B, Losonsky G A, Wasserman S S, Nataro J P, Edelman R, Pickard D, Dougan G, Chatfield S N, Levine M M. Safety of live oral Salmonella typhi vaccine strains with deletions in htrA and aroC aroD and immune response in humans. Infect Immun. 1997;65:452–456. doi: 10.1128/iai.65.2.452-456.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walliker D, Quakyi I A, Wellems T E, McCutchan T F, Szarfman A, London W T, Corcoran L M, Burkot T R, Carter R. Genetic analysis of the human malaria parasite Plasmodium falciparum. Science. 1987;236:1661–1666. doi: 10.1126/science.3299700. [DOI] [PubMed] [Google Scholar]
- 42.Wizel B, Houghten R, Church P, Tine J A, Lanar D E, Gordon D M, Ballou W R, Sette A, Hoffman S L. HLA-A2-restricted cytotoxic T lymphocyte responses to multiple Plasmodium falciparum sporozoite surface protein 2 epitopes in sporozoite-immunized volunteers. J Immunol. 1995;155:766–775. [PubMed] [Google Scholar]
- 43.Wizel B, Rogers W O, Houghten R A, Lanar D E, Tine J A, Hoffman S L. Induction of murine cytotoxic T lymphocytes against Plasmodium falciparum sporozoite surface protein 2. Eur J Immunol. 1994;24:1487–1495. doi: 10.1002/eji.1830240705. [DOI] [PubMed] [Google Scholar]