Abstract
The artichoke tuber is full of nutrients, inulin, and phytochemicals. It has been used to treat illnesses including diabetes and colon cancer, as well as in food product formulation, but limited information on the Jerusalem artichoke tuber (JAT) powder characterization exists in the literature, hence in this paper, JAT was freeze and oven-dried. It was powdered into JAT-freeze-dried-(FD)-powder and oven-dried (OD)-powder. This enabled the JAT powder's functional and physical properties to be studied. As a result, JAT powder's morphology, microstructure, and functional groups, as well as the powder foaming, swelling, solubility, antioxidant, color pasting, bulk, packed, and particle distribution properties were studied. Results indicated that the average particle distribution size at Dx90 and Dx80 displayed a distinct difference at p ≤ 0.05, while the bulk (0.39 g/cm−3) and packed (0.48 g/cm−3) densities recorded a lower value for FD powder. The FD powder's foaming capacity (24.0%) was significantly distinct (p ≤ 0.05) from the OD powder. Also, the solubility of FD powder was 6.2 g/g at 50 °C, and that of OD powder was recorded as 2.3 g/g. Again, the FD powder had a higher ABTS+ (34.3 mM (TE)/g dw) and CUPRAC (94.61 mM (TE)/g dw) capacity. Besides, a significant (p ≤ 0.05) dissimilarity among the powder color parameters (L∗, a∗, b∗, C∗, and whiteness) was observed. More so, the XRD and FT-IR characterization established a semi-crystalline or amorphous nature of the powder containing polysaccharides, and a broad halo pattern at an angle 19.3° and 20 ° for FD powder and OD powder respectively. The FD powder particles were more agglomerated than those of OD powder. This was seen as a microscopic image, again FD powder revealed a higher pasting temperature and a drop in peak viscosity. Based on the results obtained, JAT (FD and OD) powder has all the quality attributes required of a powder for culinary product formulation.
Keywords: Jerusalem artichoke, Powder, Functional attributes, Morphology, Microstructure
Jerusalem artichoke; Powder; Functional attributes; Morphology; Microstructure.
1. Introduction
Root vegetables are essential components of human food because they reduce the risk of several diet-related disorders (Shao et al., 2021), and also enhance the nutritional value of meals. New ingredients are always needed for food processing due to the growing and quick food businesses (Dalmış, 2022). The JAT comes to mind when researchers look for new, underused fruit, vegetable, and root tuber needed for optimum health, nutrition, and processing. If food industries and consumers are made aware of inulin and other functional constituents in JAT its economic usability and production JAT may be enhanced. As established, JAT has been a beneficial vegetable for controlling several illnesses, including diabetes and colon cancer (Ahmed et al., 2005; Fontaine et al., 1996; Nizioł-Łukaszewska et al., 2018; Zhang and Kim, 2015; Afoakwah and Mahunu, 2022), and contain a rich source of phytochemicals like coumarin, sesquiterpenes chlorogenic acid, caffeic acid, as well as protein, minerals, and carbohydrates (Cabello-Hurtado et al., 1998; Baba et al., 2005; Kays and Nottingham, 2007; Afoakwah et al., 2015; Afoakwah and Mahunu, 2022).
The main dietary fiber of JAT is inulin (Ozgoren et al., 2019; Dalmış, 2022), which is present in the tuber cell as stored glucose (Alexsandra et al., 2014: Nizioł-Łukaszewska et al., 2018; Zhang and Kim, 2015; Afoakwah and Mahunu, 2022). In terms of health, inulin is a prebiotic and soluble dietary fiber (Afoakwah et al., 2015), and it enables calcium, magnesium, and potassium absorption in the alimentary canal, once again, it has a hypoglycemic effect, liver lipid profile, glucose tolerance, and an anti-diabetic effect (Coudray et al., 2003; Shao et al., 2021; Afoakwah and Mahunu, 2022). Additionally, JAT phytochemicals have anti-inflammatory, anti-bacterial, anti-cancer, and antioxidant properties (Nizioł-Łukaszewska et al., 2018; Zhang and Kim, 2015).
JAT is now an ingredient added to many processed meals due to its health-benefiting effect (Afoakwah et al., 2015; Rodriguez-Furlán et al., 2014), and its inulin is widely employed in a variety of foods, including bakery, confectionery, and pharmaceutical formulations. It acts as a fat replacement and thickening agent in culinary products (Rodriguez-Furlán et al., 2014). High concentrations of fructooligosaccharides, oligosaccharides, phytochemicals, and inulin can be found in the powder made from whole JAT (Afoakwah and Maunu, 2022; Afoakwah et al., 2015), while its sweet taste, does not require extraction to make tasty functional food ingredients available, and can be utilized in place of sugar, fat, and cereal flour in food products (Paznik et al., 2002; Afoakwah et al., 2015).
However, the characterization of JAT (FD and OD) powder is inadequate in literature, hence, in this work, JAT powder was developed using freeze and oven-drying procedure, whereby structural analysis, pasting profile evaluation, functional grouping attributes, color properties, and particle size distribution of the JAT (FD and OD) powder was studied. Converting JAT into powdery form is necessary, and paramount, because it may help to extend JAT’s shelf life, and improve its uses (Paznik et al., 2002; Niemirich et al., 2017). It is believed that results from this research will provide the characteristic properties of JAT (FD and OD) powder, and how it can be applied to food and other products.
2. Materials and methods
2.1. Preparation of Jerusalem artichoke powder
JAT was carried to the Food Science Laboratory in a jute bag after being purchased from a market in Zhenjiang, China PR during winter. The tubers were then washed and cut into slices using a slicing tool. The slices were then dried in an oven at 80 °C until they reached a constant weight, while other slices of JAT were freeze-dried. To produce Jerusalem artichoke [freeze-dried (FD) powder and oven-dried (OD) powder], the samples were processed after being ground to a fine powder using a lab grinder. Before analysis, the powder was kept in a sealed glass bottle at room temperature.
2.2. Physical properties of JAT powder
2.2.1. FD and OD powder particle size evaluation
As suggested by Sonaye and Baxi (2012), the JAT (FD and OD) powder particle size distribution was evaluated by sieving 250.0 g samples for 5 min through seven sieves with openings of 425.0, 300.0, 180.0, 150.0, 106.0, 90.0, and 38.0 μm. The receiver pan was placed at the bottom of a serially stacked, decreasing sieves. The largest sieve was placed on top and loaded with the sample. The column was set on the mechanical shaker that vibrates. The sample on each sieve was weighted when the shaking process was complete. The quantity of the material on every sieve was calculated using Eq. (1).
| (1) |
2.2.2. Bulk density
A 50.0 g sample of powder weighed using ES-A electronic scale (ES200), was put into a 100.0 mL measuring cylinder. The cylinder was repeatedly tapped to produce a constant volume. By dividing the volume of powder (cm3) by the weight of flour (g), the bulk density (g/cm3) was calculated as seen in Eq. (2) (Adegunwa et al., 2017).
| (2) |
2.2.3. Fourier transform infrared spectroscopy (FTIR)
Making use of FTIR Nicolet Nexus 470, Nicolet, USA, the powder samples were characterized after grinding it with potassium bromide (KBr) powder, which was hard-pressed to form pellets.
2.2.4. Scanning electron microscope (SEM)
Using 15.0 kV as an acceleration voltage, JAT powder (FD and OD) SEM image was done with TXL30ESEM, PHILIPS, scanning electron microscope. The FD powder and OD powder were covered with gold before the SEM analysis.
2.2.5. XRD analysis
D8 advance, Bruker X-ray diffractometer was used for the assessment of the diffraction arrangements in 2θ range of 4.0–90.0◦ in a time-step of 1 s 60 min. Sieved powdered samples (less than 50.0 μm) were used for this investigation. Shujun et al. (2005); Niemirich et al. (2017), established methods for XRD studies were used for the evaluation of JAT powder diffractogram analysis.
2.2.6. Thermal analysis of FD and OD
Using Thermogravimetric (TG) and Differential Scanning calorimetry (DSC) (NETZSCH STA 449C Thermos-Analyzer) under 25–600 °C nitrogen atmosphere. The losses in weight at unlike periods of the FD powder and OD powder were studied and have been reported (Afoakwah et al., 2015).
2.3. Measurement of functional properties of JAT powder
2.3.1. Alkaline water retention capacity
Adebowale et al. (2009) procedure was used to calculate the capacity of alkaline water retention. In brief, 1.0 g of FD or OD was weighed into a test tube (P1) and 5.0 mL of 0.1 M NaHCO3 was added to the samples, afterward a 30-second vortex followed. At room temperature, the samples were allowed to equilibrate for 20 min. The supernatant was carefully removed after 15 min of centrifugation at 2000 rpm. To calculate alkaline water retention, the contents of the test tubes were weighed (P2), and using Eq. (3) the alkaline water retention capacity (g/g) was evaluated as:
| (3) |
2.3.2. Foaming capacity (FC)
To determine the foaming capacity of FD and OD, the method of Makri et al. (2005) was modified slightly. In a graded cycler set to 30 ± 2 °C, one gram of the powder samples (FD and OD) was added separately to 50 mL of distilled water. The suspension was mixed and shaken to foam for 30 min. Using formula (4), the volume of foam after 30 s of whipping was calculated as foam capacity:
| (4) |
2.3.3. Swelling power and solubility
The modified method of Adebowale et al. (2009) was used to study how temperature affected the swelling and solubility of FD and OD. Briefly, 1 g of FD and OD were precisely measured separately, placed in a centrifuge tube, and then reweighed (X1). The powder was then mixed with 20 mL of water and heated using a thermostat water bath for 30 min at temperatures between 50 °C and 90 °C. After allowing the mixture to cool to ambient temperature, it was centrifuged at a speed of 25000 g for 15 min. Carefully separating the supernatant, the pellet's swelling power capability was assessed. The centrifuge tube's weight, along with whatever residue and water is absorbed, was calculated as X2. Finally, the power of swelling was determined using Eq. (5).
| (5) |
1 mL of the upper liquid was dried in an oven at 110 °C to a stable mass. The residue left behind after drying the top liquid revealed how much FD or OD powder was soluble in water. On a dry weight basis, solubilization was calculated as g per 100 g of FD or OD powder using Eq. (6).
| (6) |
2.3.4. Pasting characteristics
Utilizing the Rapid Visco Analyzer (RVA), New point Scientific). A total of 25 mL of distilled water was placed into the text canister after 3.5 g of the powder samples were weighed into it. After fully blending the solution, the canister was attached to the RVA. The slurry was heated for 2 min at a temperature of 50 °C. The pasting profiles were read from the thermocline for Windows software that was connected to a computer. The heating rate and cooling at a constant rate were 11.2, 550 °C/min.
2.3.5. Gelation capacity
The ability of FD and OD powder to undergo gelation has been established in previous literature by Afoakwah et al. (2015).
2.3.6. Water holding capacity and oil binding
This has been done and can be found in the literature (Afoakwah et al., 2015). The rationale was to evaluate the water-holding capacity and the oil binding of FD powder and OD powder.
2.3.7. Emulsion activity and stability
The data on emulsion activity and stability of the JAT (FD and OD) powders was reported in a previous paper by Afoakwah et al. (2015).
2.3.8. Extraction procedure, total phenolic, proximate, and inulin contents of FD and OD powder
The extraction procedure, total phenolic, proximate, and inulin values for FD and OD powders have been reported in the literature (Afoakwah et al., 2015).
2.3.9. ABTS+ radical scavenging measurement of JAT powder
Using the method proposed by Petkova et al. (2014), the ABTS radical was produced by combining 3 mL of 2.45 mM potassium persulfate (Merck) and 3 mL of 7.0 mM 2,2′azinobis (3) ethylbenzthiazoline-6-sulfonic acid (ABTS, Sigma) in distilled water. The reaction was carried out at room temperature for 16 h in complete darkness. To get the final absorbance of the working solution, which was measured at 734 nm, 2.0 mL of the produced ABTS+ solution was diluted with methanol at a ratio of 1:30 (v/v). For analysis, 2.85 mL of ABTS+ solution and 0.15 mL of FD and OD extracts were mixed, while the absorbance was measured at 734 nm after 15 min at 37 °C in complete darkness. The antioxidant activity was expressed as mM (TE)/g dw.
2.3.10. CUPRAC measurement of JAT
Applying Petkova et al. (2014) methodology, 1 mL of CuCl2 with 2H2O was combined with 1 mL of Neocuproine in 7.5 mL methanol, 1 mL of 0.1 M ammonium acetate buffer, 0.1 mL of the extracted samples, and 1 mL of distilled H2O. The reaction was allowed to stand for 20 min, in the dark at 50 °C. The absorbance was measured at 450 nm after cooling. Triplicate analyses were used to determine the antioxidant activity, and the results were represented as mM Trolox equivalents (mM TE) by dw.
2.3.11. JAT powder color determination
To measure the color of FD powder and OD powder, A Color Spectrophotometer (Hunter lab, Gretag-Macthbeth, I-5, USA) was used. Color values (L∗, a∗, and b∗) of the powder samples were evaluated. The color was measured in triplicates. From the values obtained the evaluation of whiteness index (WI) and Chroma (C∗) were determined using Eqs. (7) and (8) respectively as described by Afoakwah et al. (2021).
| (7) |
| (8) |
2.4. Statistical analysis
The analyses were carried out three times. Significant differences in treatment means were determined using analysis of variance, and Tukey (p ≤ 0.05) was employed to distinguish the means.
3. Results and discussion
3.1. Functional and physical properties of JAT freeze and oven-dried powder
3.1.1. Particle size distribution
Results for the distribution of particle sizes at a given percentage aggregate of particles passing are in Tables 1A and 1B. The results indicate that FD powder and OD powder have distinct particle sizes (p ≤ 0.05). The particle distribution curve was used to determine the powder particles between 90 and 10 % cumulative particles passing through a sieve (Figure 1). From the curve, FD powder and OD powder had average JAT powder particle sizes at Dx90, Dx80, Dx60, Dx30, and Dx10 recorded (Table 1B). It is important to note that Dx10, Dx30, Dx60, Dx80, and Dx90 correspond to the size of particles at 10%, 30%, and 80% of 90% passing through a sieve at a selected sieve size respectively. These are finer particles smaller than the sieve aperture. As presented in Figure 1, the average particle size at Dx90 and Dx80 displayed a discernible variance at p ≤ 0.05. Comparing OD powder at Dx90, to FD powder at Dx90, a smaller-particle size distribution was recorded for FD powder. At the aperture sieve (38.0, to 106.0 μm) size, and Dx10 to Dx60, the FD powder and the OD powder showed a similar particle size passage (Table 1B and Figure 1).
Table 1A.
Aggregate of particles finer than sieve size (μm).
| Sieve size (μm) | Freeze-dried powder | Oven-dried powder |
|---|---|---|
| 38 | 10.0 ± 0.02a | 8.4 ± 0.03b |
| 90 | 37.0 ± 0.31c | 37.2 ± 0.11d |
| 106 | 62.2 ± 0.03e | 60.6 ± 0.07f |
| 150 | 86.0 ± 0.06g | 77.8 ± 0.02h |
| 180 | 94.8 ± 0.031 | 93.0 ± 0.02j |
| 300 | 99.8 ± 0.00k | 96.8 ± 0.05l |
| 425 | 99.8 ± 0.00m | 98.9 ± 0.02n |
Means within rows with different letters are significantly different (P ≤ 0.05).
Table 1B.
% cumulative particle size finer than sieve-size (μm).
| % cumulative particle size (μm) | Freeze-dried powder | Oven-dried powder |
|---|---|---|
| Dx10 | 37.7 ± 0.02a | 37.9 ± 0.02a |
| Dx30 | 73.2 ± 0.03b | 73.4 ± 0.02b |
| Dx60 | 104.8 ± 0.04c | 104.7 ± 0.02c |
| Dx80 | 140.5 ± 0.02d | 158.2 ± 0.02e |
| Dx90 | 180.6 ± 0.00f | 189.4 ± 0.00g |
Means within rows with different letters are significantly different (P ≤ 0.05); Dx10, Dx30, Dx60, Dx80 and Dx90 correspond to finer particles size at 10%, 30%, 80% of 90% passing through a sieve respectively.
Figure 1.
Particle size distribution curves of freeze dried powder (FD) and oven dried powder (OD)of JAT.
Smaller powder particles may have a lower gelatinization temperature, this can have a substantial impact on how FD and OD powder solutions may behave when heated (Ahmed et al., 2018). Because fine particles have a greater capacity to absorb water quickly, the particle size of JAT (FD and OD) powder may also have an impact on how quickly it may absorb water (Oladunmoye et al., 2014). Grainy flour doughs demonstrated high toughness and resistance to stretching and flow (Lazaridou et al., 2019). According to Farooq et al. (2018), coarse particles were less soluble than finer particles. Additionally, the higher particle size of a powder may have an impact on digestion.
3.2. Bulk and packed density
The bulk density for FD powder and OD powder was 0.39 and 0.34 g/cm3 respectively and differed significantly at p ≤ 0.05. According to Eleazu et al. (2014), cassava flour has a bulk density between 0.59 and 0.68 g/cm3, which is lower than the 0.77 g/cm3 of wheat flour. The bulk density values can be used for processing, packing, and handling needs. Reduced bulk densities are required for instant foods (Sharma et al., 2012). The bulk density, which is crucial for defining packaging needs, and material handling applications are influenced by the flour's density and particle size (Adegunwa et al., 2017). Bulk density affects how well flour flows, as well as how packages are designed, and how much packaging material is needed (Abdullah and Geldart, 1999). Low bulk density, however, would be advantageous when making complementing foods (Adegunwa et al., 2017). The greater the bulk density, the denser package materials must be used. Both the maximum bulk and packed densities were seen in OD powder. The increase in bulk density is preferred because it provides a better packaging benefit by allowing for the packing of more in a given amount of space (Van, 2018).
The packed density recorded for FD powder and OD powder were 0.48 and 0.56 g/cm3 and were varied at p ≤ 0.05. The term “packed” refers to the highest packing density that a powder can achieve when subjected to specific external forces. Bulk densities were lower than packed densities. This variance may be caused by the morphology, shape, solidity, density, and surface characteristics of the flour materials, and it may be reduced by appropriately tapping or agitating the particles, and using the right packaging (Iwe et al., 2016).
3.3. Functional properties
3.3.1. Foaming capacity
Functional characteristics of powders are necessary because they indicate how proteins, fats, fibers, and carbohydrates function in particular food–systems (Adegunwa et al., 2017). Table 2 contains the results for FD powder and OD powder foaming capacity. It was realized that the FD powder had a foaming capacity, which was significantly different (p ≤ 0.05) from the OD powder. This perhaps may be due to the protein concentration in the different powders (Afoakwah et al., 2015). Also, protein-to-protein interaction at the air and water crossing point of the FD powder might cause a difference in the foaming capacities of the powders. Additionally, protein in the dispersion may cause a drop in surface tension at the water-air interface, which is always a result of protein that forms a cohesive film surrounding the foam's air bubbles (Adegunwa et al., 2017). This finding suggests that FD powder may be a potential aerating agent, and can be used in frozen desserts and sponge cake formulation.
Table 2.
Functional properties of Jerusalem artichoke tuber powder.
| Parameter | Freeze-dried powder | Oven-dried powder |
|---|---|---|
| Foaming capacity (%) | 24.00 ± 1.00a | 17.00 ± 1.02b |
| Alkaline water retention capacity (g/g) | 4. 78 ± 0.31c | 5.87 ± 0.09d |
| ABTS (mM (TE)/g dw) | 34.31 ± 0.03e | 29.56 ± 0.00f |
| CUPRAC (mM TE)/g dw) | 94.61 ± 0.01g | 68.42 ± 0.01h |
| Lightness (L∗) | 98.2 ± 0.001 | 89.41 ± 0.01j |
| Redness (a∗) | 0.22 ± 0.01k | 0.43 ± 0.03l |
| Yellowness (b∗) | 5.60 ± 0.00m | 7.51 ± 0.02n |
| Chroma (C∗) | 5.60 ± 0.01° | 7.51 ± 0.00p |
| Whiteness index (WI) | 5.80 ± 0.02q | 8.19 ± 0.00r |
Means within rows with different letters are significantly different (P ≤ 0.05).
dw: (Dry weight).
3.3.2. Alkaline water capacity
Alkaline water capacity (Table 2) is known as an indicator for the cookie's diameter parameter (Yamazaki et al., 1977; Adebowale et al., 2009). For the powders studied, FD powder had an increased value of this potential. The increase in water holding capacity could be ascribed to the exposure of the surface area of the inulin water chains, as well as an increase in the amount of inulin in the FD powder, which probably had a higher potential to associate with a greater percentage of water than OD powder. The result suggests that FD powder can be utilized in flour blends to increase alkaline-water retention abilities.
3.3.3. Swelling and solubility of the powder
The effect of swelling and solubility of JAT (FD and OD) powder are shown in Table 3. The solubility and swelling of JAT (FD and OD) powder increased significantly (p ≤ 0.05) when temperature increased. However, the changes in swelling may be attributed to the dissimilarities in the molecular organization of the JAT powder particle. Other researchers had similar results for cereal and legumes and other tuber starches (Hoover and Manuel, 1996). Changes in the structure within the inulin molecules of the powder after oven drying treatment might be accountable for the drop in the ability of OD powder to swell and solubilize when compare to FD powder. Lower flour solubility values signify the presence of strong bonding forces inside the flour granules, and this might prevent the inulin from leaking from the granules. The size of the flour, density, pH, powder processing, and storage conditions might affect the solubility of flour (Esumaba et al., 2018).
Table 3.
Effect of temperature on swelling power and solubility of freeze dry powder and oven dry powder from Jerusalem artichoke tuber.
| Temperature °C | FD (Swelling power [g/g]) | OD (Swelling power [g/g ]) |
|---|---|---|
| 50 | 6.16 ± 0.00a | 2.17 ± 0.01b |
| 60 | 6.33 ± 0.32c | 3.16 ± 0.02d |
| 70 | 7.85 ± 0.03e | 4.18 ± 0.00f |
| 80 | 8.76 ± 0.14g | 4.56 ± 0.05h |
| 90 | 9.83 ± 0.19i | 4. 71 ± 0.06j |
| Temperature °C | FD (Solubility [g/g]) | OD (Solubility [g/g]) |
| 50 | 0.42 ± 0.02a | 0.22 ± 0.01b |
| 60 | 0.47 ± 0.01c | 0.28 ± 0.01d |
| 70 | 0.75 ± 0.03e | 0.52 ± 0.01f |
| 80 | 1.13 ± 0.06g | 0.72 ± 0.06h |
| 90 | 1.37 ± 0.01i | 1.06 ± 0.03j |
All the values and mean ± SD of three replicate analyses. Means within columns with different letters are significantly different (P ≤ 0.05). FD = Freeze-dried powder; OD = Oven-dried powder.
3.4. Antioxidant capability of freeze and oven-dried powder
Dietary antioxidants are "substances, which scavenge reactive-oxygen/nitrogen to interrupt radical chain reactions, or can block the reactive-oxidants from being generated (Hoover and Manuel, 1996)”. Hence, in this study, the ABTS and cupric-reducing antioxidant potential of FD powder and OD powder were evaluated (Table 2). The results showed that FD powder ABTS scavenging ability and its power to reduce cupric ions were stronger than OD's powder ability to scavenge ABTS and cupric-reducing ions. The low values recorded for OD powder in terms of its antioxidant value (ABTS and CUPRAC) were expected and may be attributed to the procedure employed during the powder-making process, which might cause the phytochemicals to degrade Afoakwah et al., 2015). In a related study, Petkova et al. (2014) demonstrated that JAT powder has antioxidant potential. Also, it has been confirmed that JAT has bioactive ingredients, which have antioxidant activity (Tchone et al., 2006; Yuana et al., 2013; Nizioł-Łukaszewska et al., 2018; Zhang and Kim, 2015; Afoakwah and Mahunu, 2022).
3.5. Freeze and oven-dried powder color attributes
Consumers use color as a sign of product quality. To examine the color characteristics of the JAT powder, L ∗, a ∗, and b∗ color indicators were measured. The lightness (L∗) reported for FD (98.23) was higher than OD (89.41) (Table 2) Omolola et al. (2017) reported L∗ values of cassava-flour-dried at temperatures of 60.0–72.0 °C for 15.0–20.0 h as 88.30–93.57. The differences in L∗ for the JAT powders could be attributed to drying temperatures, and the procedure employed during drying. Drying JAT in hot ovens, can cause scorching and become discolored, which could make the lightness of color reduced. Higher temperatures during drying may cause gelatinization and a loss of the JAT's powder birefringence, which may impair the powder color quality depending on the moisture content of the JAT. In terms of redness (a∗), FD measured 0.22 and OD 0.43. The b∗ was 5.60 for FD and 7.51 for OD. The JAT powder processing method may be the reason for the variations in the a ∗ and b∗ results. In comparison to the FD, the OD had a higher whiteness value (8.19). For the examined JAT (OD and FD) powders, the color considerations (L∗, a∗, b∗, and WI) were statistically distinct (p ≤ 0.05).
The C∗ values significantly varied (p ≤ 0.05) for both JAT (OD and FD) powder indicating a considerably larger intensity of yellow tint in OD powder. Higher L∗ and low C∗ values are used as the desired color quality criteria when choosing flours or powders for industrial applications (Sankhon et al., 2014; Vasconcelos et al., 2017). According to Martinek et al. (2014), Afoakwah et al. (2015), and Rani et al. (2021), variations in the moisture content, ash content, inherent phenolic constituents, and particle size (Martinek et al., 2014; Rani et al., 2021) of the FD and OD powder may also be responsible for variations in the powder color. A prior study on flour suggested that wheat cultivars had L∗ values of 90.8–92.9, a∗: 0.2–0.6, b∗: 7.7–10.8, and C∗:7.7–10.8 (Siddiqi et al., 2020; Rani et al., 2021). Generally speaking, factors influencing the overall color of flours or powders include variety, maturation stage, and powder processing technologies (McClements et al., 2017; Rodriguez-Sandoval et al., 2017; Rani et al., 2021). Processes requiring careful sifting, flaking, and cleaning with fresh water may be required to produce flour or powder with higher lightness (L∗) and whiteness.
3.6. Infrared spectra analysis
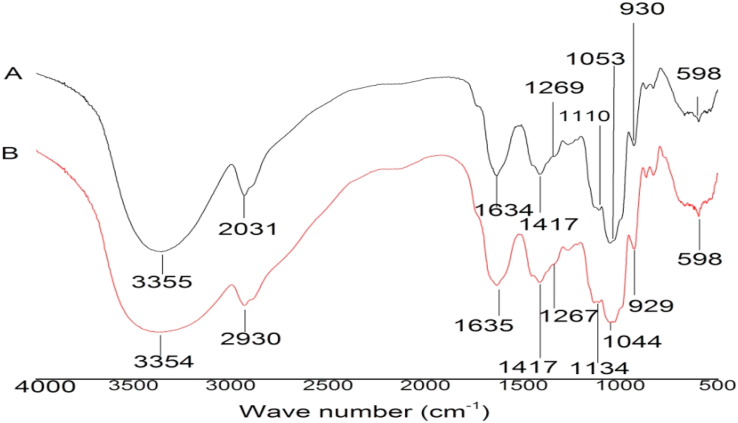
The FT-IR spectra of the dried powders are shown in Figure 2. A sturdy-broad absorption peak at 3355 cm−1 and 3354 cm−1 for O–H stretching vibrations is seen The absorption peak at 2931 cm−1 for C–H stretching vibrations, and a wide region of 900–1200 cm−1 to be joining C–O, and C–C stretching and C–OH bending vibrations were seen in the polysaccharide powder. Signifying a characteristic feature of a polysaccharide (Liu et al., 2008). Additionally, bands at 1634, 1635 cm−1, and 1417 cm−1 spectra (Figure 2) are associated with a deprotonated carboxylic group (COO−). An indication that FD and OD might be acidic polysaccharides (Zou et al., 2010). The peaks observed at 1267 cm−1, 1269 cm−1 1110 cm−1and 1134 cm−1 of the powders (Figure 2) are the asymmetric and symmetric stretching vibrations of S O. A signal of sulfate ester, reveals that powders might be sulfated polysaccharides (Shao et al., 2021). Moreover, the absorption peak at 1634 cm−1 and 1635 cm−1 for N–H bending vibration may be linked to the protein in the powders (Qiao et al., 2009).
Figure 2.
FT-IR Spectrum of freeze-dried powder (A) and oven-dried powder (B) of JAT.
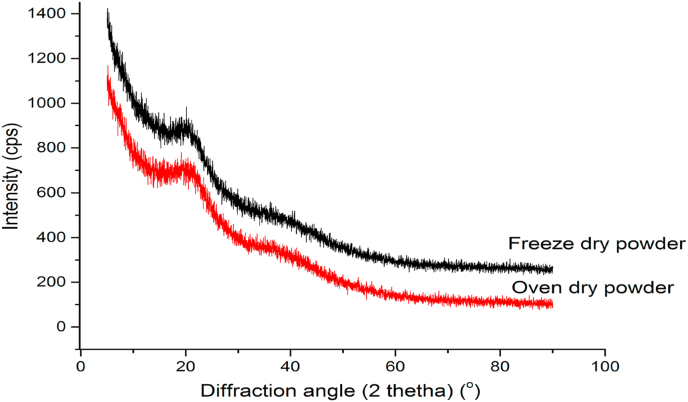
3.7. Xray diffraction of freeze and oven-dried powder
The dried powders’ X-ray diffraction patterns are depicted in Figure 3, it can be deduced from the diffractogram that, a broad halo pattern at an angle of 19.3° (freeze-dried powder) and 20.0° (oven-dried powder) indicates a characteristic feature of a typical semi-crystalline or amorphous natural-polysaccharide powders exhibiting a partially organized structure, whose macromolecules may be capable of rotating the polarization plane of light rays and creating a double helix (Kawai et al., 2011; Niemirich et al., 2017; Cvetkov et al., 1964; Shujun et al., 2005; Cui, 2005; Belitz et al., 2009; Niemirich et al., 2017). In a study, Niemirich et al. (2017) discovered that JAT powder dried utilizing a mixed-heat-supply drying approach had a diffractive reflex at an angle of = 20.5 ° as opposed to a wide fuzzy reflex from the convection drying process. Therefore, it may be inferred that the JAT drying processes could result in distinct angles. The amorphous state of the JAT powder might be responsible for its high hygroscopicity, and the caking of the powder might be due to this phenomenon (Meste et al., 2002).
Figure 3.
X-ray diffractograms of freeze-dry powder and oven-dry powder of JAT.
3.8. Microscopic studies of freeze and oven-dried powder
The microstructure of FD powder and OD powder samples was evaluated by using scanning electron microscopy of different magnification echelons. The scanning electron microscopy revealed the different amorphous features of a freeze-dried (FD) powder and oven-dried (OD) powder. As illustrated in Figure 4A-1 and A-2, a revelation of a slab-like or a cake matrix was seen. Also, In Figure 4(B-1, B-2, and C-1, C-2) images of granules of different sizes and surfaces with some being smooth and some rough are observed. These might be the inulin (polysaccharides) molecules in a mixture of proteins, carbohydrates, and minerals. High surface roughness is necessary to increase the matrix's contact surface area (Dalmış, 2022). Increased surface roughness is beneficial because it improves the adhesion of the fiber to the matrix, and promotes interfacial adhesion between the fiber and the matrix by mechanical interlocking (Dalmış, 2022).
Figure 4.
Scanning electron microscope pictures of oven dried-powder (A-1, B-1 and C-1) and freezedried powder (A-2, B-2 and C-2) processed from JAT.
The powder has a high hygroscopicity leading to agglomeration, which is easily seen as microscopic imagery, showing agglomerated particles pasted on each other (A-1 A-2, B-1, B-2 C-1, C-2). The FD powder particles were more agglomerated than those of OD, probably the FD powder might have its long inulin water chains intact, and may have adsorbed water. The attachment of smaller particles to larger ones and the presence of few cracks and surface pores show the amorphous nature of the powder (Cano-Chauca et al., 2005). Truong et al. (2005) described that sugar-rich powders act as hygroscopic thermoplastic, which causes the sticking of powder particles. The handling, storing, and processing of JAT (OD and FD) powder products may also be responsible for the powder's stickiness, agglomeration, and caking (Leyva-Porras et al., 2014). This could cause the microstructure to collapse and alter the macroscopic structure, which probably caused the JAT (OD and FD) powder particles to stuck (Leyva-Porras et al., 2014). Once more, at the microscopic level, the cohesive and attractive forces operating on the sticky substance and the solid surface may compete. The attractive interactions between particles or molecules in the same matrix are referred to as cohesion; while the interaction between the surfaces of various powder particles is known as adhesion (Leyva-Porras et al., 2014). Cohesion is the force that causes powders to clump together and cake, whereas adhesion is the force that causes stickiness (Leyva-Porras et al., 2014).
According to Foster et al. (2006) and Leyva-Porras et al. (2014), the glass transition temperature and the amount of adsorbed water have a direct impact on the cohesiveness of the powders, while inter-molecular forces, electrostatic forces, liquid-bridges, and solid bridges are all potential causes of the interaction mechanisms between the JAT (OD and FD) powder surfaces, they are highly reliant on particle size (Leyva-Porras et al., 2014). The surface area increases with decreasing particle size. The effects of agglomeration and caking in the powder product may then be more pronounced because stronger attraction forces between the particles are present (Leyva-Porras et al., 2014). If the particle size is smaller, the surface area is increased. Then, bigger attractive forces between the particle occur, thus, larger effects of agglomeration and caking in the powder product may be observed (Leyva-Porras et al., 2014).
3.9. Pasting properties
When determining whether to employ flour or powder as a useful constituent in food or other goods, the pasting properties are taken into consideration (Adegunwa et al., 2017). The pasting attributes of FD powder and OD powder are shown in (Table 4 and Figure 5). Figure 5 displays the pasting characteristics of FD powder and OD powder. The lack of starch in the samples may have caused the Jerusalem artichoke powder pasting curves to deviate from the expected starch pasting properties. The temperature at which FD and OD began to gel is measured as the pasting temperature, which provides information about the processing's gelatinization time. It is an indicator of the initial shift brought on by the swelling since it is the temperature at which the first discernible rise in viscosity is detected (Adegunwa et al., 2017). For FD powder, the pasting temperature was higher than OD powder, while the peak viscosity was found to be lower in FD powder. Peak viscosity is linked to the finished product quality, and it is an indicator of viscous load likely to be met during mixing (Adegunwa et al., 2017). Following oven drying of the sample, the hot paste viscosity of the OD powder marginally decreased. After the sample was freeze-dried, the setback, breakdown rates, and final viscosity significantly decreased. The final viscosity demonstrated that JAT (FD and OD) powders can transform into a gel or viscous paste following heating and cooling, as well as the paste's resistance to shear stress during stirring (Adegunwa et al., 2017).
Table 4.
Pasting attributes of Jerusalem artichoke powder.
| Pasting properties |
Samples |
|
|---|---|---|
| Oven dried powder | Freeze dried powder | |
| Peak viscosity | 2315 | 1527 |
| Hot pasting viscosity | 952 | 977 |
| Breakdown | 1333 | 550 |
| Final viscosity | 3942 | 1930 |
| Setback | 2959 | 953 |
| Pasting temperature | 50.25 | 87.1 |
Figure 5.
Pasting characteristics of freeze-dried powder (a) and oven-dried powder (b) produced from JAT.
The reorientation of the inulin chain molecules in the powder granules may be the cause of an increase in pasting temperature for FD powder. Before structural disintegration, a higher temperature may be necessary due to the strengthening of intra-granular binding pressures to enable paste configuration (Eliasson, 1980). Instead of the usual inverse relationship between thickness (viscosity) and hotness, the viscosity during the cooling phase of FD powder and OD powder did not follow that pattern. Inulin chains may accidently expand and reorganize in the OD powder and FD powder to form low-solubility aggregates, which can lead to gel building. When compared to those of OD powder, the setback value for FD powder was significantly smaller. Also, larger setback values could mean lower dough digestibility (Adegunwa et al., 2017). The FD's powder breakdown figures also significantly dropped.
4. Conclusions
Various functional and physical uniqueness of the JAT powder was determined. The temperature had an obvious effect on the solubility and swelling capacity of the powder and had a link with the powder particle size. The finer particle size recorded for JAT (FD and OD) powder may influence powder water absorption strength, because fine-particle may possess quicker water absorption capability. Besides, the JAT (OD and FD) powder bulk density was affected by the powder particle size. The pasting temperature indicates that viscous load may occur during mixing FD powder, also a higher swelling index, which showed a lower peak viscosity in the FD powder may be associated with higher inulin concentration in the JAT (FD) powder. Probably, it may be due to JAT powder lacking starch. The crystalline structure and the amorphous solid mixture may develop dissimilar properties for the JAT (FD and OD) powder. Also, the amorphous solids of the powder may cause a drop in molecular mobility with limited diffusion phenomena, all the same, the JAT (FD and OD) powder has the potential to be utilized for making food products and other products. Additional studies should be conducted on the packaging, storage, and shelf life stability of the JAT powder (Sathe and Salunkhe, 1981).
Declarations
Author contribution statement
Newlove A. Afoakwah: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interest's statement
The author declared no competing interests.
Additional information
No additional information is available for this paper.
References
- Abdullah E.C., Geldart D. The use of bulk density measurements as flowability indicators. Powder Technol. 1999;102:151–165. [Google Scholar]
- Adebowale K.O., Thomas H., Uwe S., Doert T. Modification and properties of African yam bean (Sphenostylis stenocarpa Hochst. Ex A. Rich.) Harms starch I: heat moisture treatments and annealing. Food Hydrocolloids. 2009;23(7):1947–1957. [Google Scholar]
- Adegunwa M.O., Adelekan E.O., Adebowale A.A., Bakare H.A., Alamu E.O. Evaluation of nutritional and functional properties of plantain (Musaparadisiaca L.) and tiger nut (Cyperus esculentus L.) flour blends for food formulations. Cogent Chem. 2017;3(1) [Google Scholar]
- Afoakwah N.A., Mahunu G.K. Springer; 2022. African Fermented Food Products-New Trends; pp. 525–536. [Google Scholar]
- Afoakwah N.A., Dong Y., Zhao Y., Xiong Z., Owusu J., Wang Y., Zhang J. Characterization of Jerusalem artichoke (Helianthus tuberosus L.) powder and its application in emulsion-type sausage. LWT--Food Sci. Technol. 2015;64(1):74–81. [Google Scholar]
- Afoakwah A.N., Mohammed A.R., Mahunu G.K. Quality evaluation of chinchin produced from wheat flour and orange-fleshed sweet potato puree. Ghana J. Horticult. 2021;15(1):1–15. [Google Scholar]
- Ahmed M.S., El-Sakhawy F.S., Soliman S.N., Abou H.D.M.R. A phytochemical and biological study of Helianthus tuberosus L. Egypt. J. f Biomed. Sci. 2005;18:134–147. [Google Scholar]
- Ahmed J., Thomas L., Arfat Y.A. Functional, rheological, microstructural, and antioxidant properties of quinoa flour in dispersions as influenced by particle size. Food Res. Int. 2018;116:302–311. doi: 10.1016/j.foodres.2018.08.039. [DOI] [PubMed] [Google Scholar]
- Alexsandra C.A., Bolívar P. G-de, Napoleão E-de M.B., Adalbert P., Attilio C., José Alexsandro-da S. Inulin-type fructans: a review on different aspects of biochemical and pharmaceutical technology. Carbohydr. Polym. 2014;101:368–378. doi: 10.1016/j.carbpol.2013.09.081. [DOI] [PubMed] [Google Scholar]
- Baba H., Yaoita Y., Kikuchi M. Sesquiterpenoids from the Leaves of Helianthus Tuberosus L. J. Tohoku Pharmaceut. Univ. 2005;52:21–25. [Google Scholar]
- Belitz H.D., Grosch W., Schieberle Р. Springer; 2009. Food Chemistry; p. 1070. [Google Scholar]
- Cabello-Hurtado F., Durst F., Jorrin J.V., Werck-Reichhart D. Coumarins in Helianthus tuberosus: characterization, induced accumulation and biosynthesis. Phytochemistry. 1998;49:1029–1036. [Google Scholar]
- Cano-Chauca M., Stringheta P.C., Ramos A.M., Cal-Vidal J. Effect of the carriers on the microstructure of mango powder obtained by spray drying and its functional characterization. Innovat. Food Sci. Emerg. Technol. 2005;6(4):420–428. [Google Scholar]
- Coudray C., Demigne C., Rayssiguier Y. Effects of dietary fibers on magnesium absorption in animals and humans. J. Nutr. 2003;33:1–4. doi: 10.1093/jn/133.1.1. [DOI] [PubMed] [Google Scholar]
- Cui S., editor. Food Carbohydrates: Chemistry, Physical Properties, and Applications. CRC Press; 2005. p. 580. [Google Scholar]
- Cvetkov V.N., Jeskin V.E., Frenkel’ S.J. Nauka; Moscow: 1964. Struktura Makromolekul V Rastvorah; p. 720. [Google Scholar]
- Dalmış R. Description of a new natural fiber extracted from Helianthus tuberosus L. as a composite reinforcement material. Res. Square. 2022:1–28. doi: 10.1111/ppl.13960. [DOI] [PubMed] [Google Scholar]
- Eleazu O.C., Eleazu K.C., Kolawole S. Use of indigenous technology for the production of high quality cassava flour with similar food qualities as wheat flour. Sci. Polonorum Technol. Alimentar. 2014;13:249–256. doi: 10.17306/j.afs.2014.3.3. [DOI] [PubMed] [Google Scholar]
- Eliasson A.C. Effect of water content on the gelatinisation of wheat. Starch. 1980;32:270–275. [Google Scholar]
- Esumaba S.A., Adubofuor J., Amoah I., Apeku O.-J.D. Functional and pasting properties of yellow maize–soya bean–pumpkin composite flours and acceptability study on their breakfast cereals. Cogent Food Agric. 2018;4(1) [Google Scholar]
- Farooq A.M., Li C., Chen S. Particle size affects structural and in vitro digestion properties of cooked rice flours. Int. J. Biol. Macromol. 2018;118:160–167. doi: 10.1016/j.ijbiomac.2018.06.071. [DOI] [PubMed] [Google Scholar]
- Fontaine N., Meslin J.C., Lory S., Andrieux C. Intestinal mucin distribution in the germ free rat and in the heteroxenic rat harbouring a human bacterial flora: effect of inulin in the diet. Br. J. Nutr. 1996;75:881–892. doi: 10.1079/bjn19960194. [DOI] [PubMed] [Google Scholar]
- Foster K.D., Bronlund J.E., Paterson A.H.J. Glass transition related cohesion of amorphous sugars powders. J. Food Eng. 2006;77:997–1006. [Google Scholar]
- Hoover R., Manuel H. Effect of heat–moisture treatment on the structure and physicochemical properties of legume starches. Food Res. Int. 1996;29(8):731–750. [Google Scholar]
- Iwe M., Onyeukwu U., Agiriga A. Proximate, functional and pasting properties of FARO 44 rice, African yam bean and brown cowpea seeds composite flour. Cogent Food Agric. 2016;2 [Google Scholar]
- Kawai K., Fukami K., Thanatuksorn P., Viriyarattanasak C., Kajiwara K. Effects of moisture content, molecular weight, and crystallinity on the glass transition temperature of inulin. Carbohydr. Polym. 2011;83(2):934–939. [Google Scholar]
- Kays S.J., Nottingham S.F. CRC Press; Boca Raton, Florida: 2007. Biology and Chemistry of Jerusalem Artichoke: Helianthus tuberosus L; p. 496. [Google Scholar]
- Lazaridou A., Marinopoulou A., Biliaderis C.G. Impact of flour particle size and hydrothermal treatment on dough rheology and quality of barley rusks. Food Hydrocolloids. 2019;87:561–569. [Google Scholar]
- Leyva-Porras C., López-Pablos A.L., Alvarez-Salas C., Pérez-Urizar J., Saavedra-Leos Z. Physical properties of inulin and technological applications. Polysaccharides. 2014:1–22. [Google Scholar]
- Liu C., Li X., Li Y., Feng Y., Zhou S., Wang F. Structural characterization and antimutagenic activity of a novel polysaccharide isolated from Sepiella maindroni ink. Food Chem. 2008;110(4):807–813. doi: 10.1016/j.foodchem.2008.02.026. [DOI] [PubMed] [Google Scholar]
- Makri E., Papalamprou E., Doxastakis G. Study of functional properties of seed storage proteins from indigenous European legume crops (lupin, pea, broad bean) in admixture with polysaccharides. Food Hydrocolloids. 2005;19:583–594. [Google Scholar]
- Martinek P., Jirsa O., Vaculová K., Chrpová J., Watanabe N., Burešová V. 2014. Use of Wheat Gene Resources with Different Grain Color in Breeding (Raumberg Gumpenstein) pp. 75–78. [Google Scholar]
- McClements D.J., Chung C., Wu B. Structural design approaches for creating fat droplet and starch granule mimetics. Food Funct. 2017;8:498–510. doi: 10.1039/c6fo00764c. [DOI] [PubMed] [Google Scholar]
- Meste M.L., Champion D., Roudaut G., Blond G., Simatos D. Glass transition and food technology: a critical appraisal. J. Food Sci. 2002;67(7):2444–2458. [Google Scholar]
- Niemirich A., Melnyk O., Petrusha O., Havrysh A., Koval O. Technological properties of the powder made from jerusalem artichoke obtained by the method of drying with mixed heat supply. Eureka: Life Sci. 2017;2:43–50. [Google Scholar]
- Nizioł-Łukaszewska Z., Furman-Toczek D., Zagórska-Dziok M. Vol. 17. 2018. Antioxidant Activity and Cytotoxicity of Jerusalem Artichoke Tubers and Leaves Extract on HaCaT and BJ Fibroblast Cells Lipids in Health and Disease; p. 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oladunmoye O.O., Aworh O.C., Maziya-Dixon B. Chemical and functional properties of cassava starch, durum wheat semolina flour, and their blends. J. Food Sci. Nutr. 2014;2:132–138. doi: 10.1002/fsn3.83. 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omolola A.O., Kapila P.F., Anyasi T.A. Optimization of colour and thermal properties of sweet cassava (Manihot esculenta Crantz Var. UVLNR 0005) flour using response surface methodology. Asian Res. J. Agric. 2017;11:57–65. [Google Scholar]
- Ozgoren E., Isik F., Yapar A. Effect of Jerusalem artichoke (Helianthus tuberosus L.) supplementation on chemical and nutritional properties of crackers. J. Food Meas. Char. 2019;13(4):2812–2821. [Google Scholar]
- Petkova N., Ivanov I., Denev P., Pavlov A. Bioactive substance and free radical scavenging activities of flour from Jerusalem artichoke (helianthus tuberosus L.) tubers – a comparative study. Turk. J. Agric. Nat. Sci. 2014;2:1774–1778. [Google Scholar]
- Praznik W., CiesÂlik E., Filipiak-Florkiewicz A. Soluble dietary fibers in Jerusalem artichoke powders: composition and application in bread. Nahrung-Food. 2002;46(3):151–157. doi: 10.1002/1521-3803(20020501)46:3<151::AID-FOOD151>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Qiao D., Hu B., Gan D., Sun Y., Ye H., Zeng X. Extraction is optimized by using response surface methodology, purification, and preliminary characterization of polysaccharides from Hyriopsis cumingii. Carbohydr. Polym. 2009;76(3):422–429. [Google Scholar]
- Rani M., Singh G., Siddiqi R.A., Gill B.S., Sogi D.S., Bhat M.A. Comparative quality evaluation of physiochemical, technological, and protein profiling of wheat, rye and barley cereals. Front. Nutr. 2021;8 doi: 10.3389/fnut.2021.694679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Furlán L.T., Pérez P., Padilla A., Campderrós M.E. Development of reduced fat minced meats using inulin and bovine plasma proteins as fat replacers. Meat Sci. 2014;96:762–768. doi: 10.1016/j.meatsci.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Sandoval E., Prasca-Sierra I., Hernandez V. Effect of modified cassava starch as a fat replacer on the texture and quality characteristics of muffins. J. Food Meas. Char. 2017;11:1630–1639. [Google Scholar]
- Sankhon A., Amadou I., Yao W.R. Comparison of physicochemical and functional properties of flour and starch extract in different methods from africa locust bean (Parkia Biglobosa) Seeds. Afr. J. Tradit., Complementary Altern. Med. 2014;11:264–272. doi: 10.4314/ajtcam.v11i2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathe S.K., Salunkhe D.K. Isolation, partial characterization, and modification of the Great Northern Bean (Phaseolus vulgaris L.) starch. J. Food Sci. 1981;46(2):617–621. [Google Scholar]
- Shao T., Yuana P., Zhang W., Dou D., Feng W., Hao C., Li C., Han J., Chen K., Wang G. Preparation and characterization of sulfated inulin-type fructans from Jerusalem artichoke tubers and their antitumor activity. Carbohydr. Res. 2021;509 doi: 10.1016/j.carres.2021.108422. [DOI] [PubMed] [Google Scholar]
- Sharma A., Jana A.H., Chavan R.S. Functionality of milk powders and milk-based powders for end use applications—a review. Compr. Rev. Food Sci. Food Saf. 2012;11:518–528. [Google Scholar]
- Shujun W., Jinglin Y., Wenyuan G. Use of X-ray Diffractometry (XRD) for identification of Fritillaria according to geographical origin. Am. J. Biochem. Biotechnol. 2005;1(4):207–211. [Google Scholar]
- Siddiqi R.A., Singh T.P., Rani M., Sogi D.S., Bhat M.A. Diversity in grain, flour, amino acid composition, protein profiling, and proportion of total flour proteins of different wheat cultivars of North India. Front. Nutr. 2020;7:141. doi: 10.3389/fnut.2020.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonaye S., Baxi R. Particle size measurement and analysis of flour. Int. J. Eng. Res. Afr. 2012;2:1839–1842. [Google Scholar]
- Tchone M., Barwald G., Annemuller G., Fleischer L. Separation and identification of phenolic compounds in Jerusalem artichoke (Helianthus tuberosus L.) Sci. Aliments, Libr. Lavoisier. 2006;26(5):394. [Google Scholar]
- Truong V., Bhandari B.R., Howes T. Optimization of co-current spray drying process of sugar-rich foods. Part I- Moisture and glass transition temperature profile during drying. J. Food Eng. 2005;71(1):55–65. [Google Scholar]
- Van T.N. Preparation and improved quality production of flour and the made biscuits from purple sweet potato. J. Food Nutr. 2018;4:1–14. [Google Scholar]
- Vasconcelos L., Brito A., Carmo C. Phenotypic diversity of starch granules in cassava germplasm. Genet. Mol. Res. 2017;16:1–10. doi: 10.4238/gmr16029276. [DOI] [PubMed] [Google Scholar]
- Yamazaki W.T., Donelson J.R., Kwolek W.F. Effects of flour fraction on cookie diameter. Cereal Chem. 1977;54:352–361. [Google Scholar]
- Yuana X., Chenga M., Gaoa M., Zhuoa R., Zhanga L., Xiaoa H. Cytotoxic constituents from the leaves of Jerusalem artichoke (Helianthus tuberosus L.) and their structure–activity relationships. Phytochem. Lett. 2013;6(1):21–25. [Google Scholar]
- Zhang Q., Kim H.Y. Antioxidant, anti-inflammatory and cytotoxicity on human lung epithelial A549 cells of Jerusalem artichoke (Helianthus tuberosus L.) tuber) Korean J. Polar Res. 2015;28:305–311. [Google Scholar]
- Zou S., Zhang X., Yao W., Niu Y., Gao X. Structure characterization and hypoglycemic activity of a polysaccharide isolated from the fruit of lyceum barbarum l. Carbohydr. Polym. 2010;80(2):1161–1167. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.