Abstract
Introduction: Polymyxin B is a last-line therapy for carbapenem-resistant microorganisms. However, a lack of clinical pharmacokinetic/pharmacodynamic (PK/PD) data has substantially hindered dose optimization and breakpoint setting.
Methods: A prospective, multi-center clinical trial was undertaken with polymyxin B [2.5 mg/kg loading dose (3-h infusion), 1.25 mg/kg/12 h maintenance dose (2-h infusion)] for treatment of carbapenem-resistant K. pneumoniae (CRKP) bloodstream infections (BSI). Safety, clinical and microbiological efficacy were evaluated. A validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) method was applied to determine the concentrations of polymyxin B in blood samples. Population pharmacokinetic (PK) modeling and Monte Carlo simulations were conducted to examine the susceptibility breakpoint for polymyxin B against BSI caused by CRKP.
Results: Nine patients were enrolled and evaluated for safety. Neurotoxicity (5/9), nephrotoxicity (5/9), and hyperpigmentation (1/9) were recorded. Blood cultures were negative within 3 days of commencing therapy in all 8 patients evaluated for microbiological efficacy, and clinical cure or improvement occurred in 6 of 8 patients. Cmax and Cmin following the loading dose were 5.53 ± 1.80 and 1.62 ± 0.41 mg/L, respectively. With maintenance dosing, AUCss,24 h was 79.6 ± 25.0 mg h/L and Css,avg 3.35 ± 1.06 mg/L. Monte Carlo simulations indicated that a 1 mg/kg/12-hourly maintenance dose could achieve >90% probability of target attainment (PTA) for isolates with minimum inhibitory concentration (MIC) ≤1 mg/L. PTA dropped substantially for MICs ≥2 mg/L, even with a maximally recommended daily dose of 1.5 mg/kg/12-hourly.
Conclusion: This is the first clinical PK/PD study evaluating polymyxin B for BSI. These results will assist to optimize polymyxin B therapy and establish its breakpoints for CRKP BSI.
Keywords: polymyxins, pharmacokinetics/pharmacodynamics, CRKP, bloodstream infection, neurotoxicity, nephrotoxicity, breakpoint
Introduction
The increasing global prevalence of carbapenem-resistant Gram-negative organisms (CRO) represents a major threat to health (WHO, 2021). In China alone, ∼20%–30% of Pseudomonas aeruginosa and Klebsiella spp. and >70% of Acinetobacter spp. are carbapenem resistant (http://www.chinets.com/), and such infections are associated with high morbidity and mortality (Gu et al., 2018; Wang et al., 2018). Carbapenem-resistant K. pneumoniae (CRKP) is a particular problem, with pooled mortality among patients infected with CRKP reported to be approximately 42% and mortality among bloodstream infection (BSI) patients estimated to exceed 50% (Xu et al., 2017). Although new drug combinations such as ceftazidime-avibactam have shown good clinical efficacy against KPC-producing K. pneumoniae, treatment options remain limited (Wright et al., 2017). Polymyxin B may be the only accessible or affordable therapeutic option for CRKP in many countries (Satlin et al., 2020).
While use of polymyxin B for the treatment of CRKP is increasing, a lack of pharmacokinetic/pharmacodynamic (PK/PD) evidence in support of appropriate susceptibility breakpoints has hindered its clinical use (Medeiros et al., 2019). Susceptibility breakpoints for polymyxin B are difficult to determine from existing clinical studies given the large interpatient variability observed with polymyxin B exposures, varying sites of infection examined (e.g., lung and bloodstream), and a wide range of MIC values of the infecting pathogens (Tsuji et al., 2019). While organizations such as the European Committee on Antimicrobial Susceptibility Testing (EUCAST) provide a susceptible breakpoint category for colistin (polymyxin B breakpoints are not reported) (Version 12.0, 2022), in 2020 the Clinical and Laboratory Standards Institute (CLSI) removed the susceptible interpretive category for the polymyxins (previously ≤2 mg/L in all cases) (Satlin et al., 2020). The decision by the CLSI is not rational as it was primarily based on the data that showed polymyxins are not efficacious for the treatment of lung infections in mice and patients following intravenous administration (Rigatto et al., 2013; Cheah et al., 2015; Landersdorfer et al., 2018; Satlin et al., 2020; Nang et al., 2021). Both EUCAST and CLSI agree that polymyxins should have clinical breakpoints for Enterobacterales, P. aeruginosa and Acinetobacter baumannii; and have highlighted the urgent need for clinical PK/PD data to support the establishment of breakpoints and optimize the dosage regiments of polymyxins (Satlin et al., 2020).
Ideally, susceptibility breakpoints of antibiotics should be determined based on their PK/PD at the infection site and administration route (Bian et al., 2022); however, clincial investigations on the efficacy of intravenous polymyxins in the literature are from mixed types of infections predominantly with pneumonia (Paul et al., 2018; Almangour et al., 2021). Notably, favorable PK/PD relationships of colistin and polymyxin B have been reported in neutropenic mouse thigh infection models (Cheah et al., 2015; Landersdorfer et al., 2018). Here, we investigated the clinical PK/PD of intravenous polymyxin B (2.5 mg/kg loading dose and maintenance dose of 1.25 mg/kg 12-hourly) in patients with CRKP bloodstream infection using an intensive sampling strategy. Both microbiological and clinical efficacy were evaluated to determine the dose-response relationship of polymyxin B. Our study aims to provide clinical evidence for the rational determination of PK/PD driven susceptibility breakpoints of polymyxins in patients with bloodstream infection.
Materials and methods
Study design and ethics approval
This prospective, multi-centered, single-armed and open-label clinical trial was conducted in accordance with Good Clinical Practice, International Conference on Harmonization guidelines, and in compliance with the World Medical Association Declaration of Helsinki and was approved by the Ethics Committee of Sir Run Run Shaw Hospital, College of Medicine, Zhejiang University, Hangzhou, China, and other participating centers (Williams, 2008). Written informed consent was obtained from each patient or his/her representative before enrollment. Patients with CRKP-positive blood cultures and with an estimated glomerular filtration rate (eGFR) of 60–120 ml/min were included. The inclusion and exclusion criteria are shown in Supplementary Table S1. This clinical trial was registered at http://www.chictr.org.cn (registration number: ChiCTR 1900021137) and was conducted across 2019 and 2020.
Drug administration
Polymyxin B Sulphate for Injection (Lot No. A-1411137) containing 72.0% polymyxin B1 and B1-Ile and 12.1% polymyxin B2 was provided by SPH No. 1 Biochemical & Pharmaceutical Co., Ltd. (Shanghai, China). Prior to administration, polymyxin B was dissolved in 50–100 ml normal saline as per the manufacturer’s instructions then administered intravenously at a dose of 1.25 mg/kg every 12 h (2-h infusion) with an initial loading dose of 2.5 mg/kg (3-h infusion). Treatment lasted for 7–14 days as per treatment guidelines (Rhodes et al., 2017). Adverse events were closely monitored and recorded for each patient.
Susceptibility testing
The minimum inhibitory concentrations (MICs) to polymyxin B and meropenem of the collected CRKP strains were determined by broth microdilution according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines and interpreted according to EUCAST breakpoints of colistin (Breakpoint tables for interpretation of MICs and zone diameters, Version 11.0); Escherichia coli ATCC 25922 was used as the control strain. Polymerase chain reaction (PCR) was employed to determine the presence of resistance genes (KPC, NDM, IMP, OXA, VIM). The primers were the same as in a published protocol and the sample process procedures were adapted a little bit (Poirel et al., 2011). To be brief, a single colony of the bacteria were picked and resuspended in ultra-pure water, and total DNA was extracted by heating bacterial suspension at 100°C for 10 min to lysis and release DNA. The suspension was centrifuged for total DNA which was dissolved in supernatant. Then 2 µl total DNA, 1 µl forward and 1 µl reverse primers (10 µM) for each targeted gene, 12.5 µl 2 × Hieff® Robust PCR Master Mix (Yeasen, Shanghai, China) and ultra-pure water were mixed into a 25 µl reaction mixture. The mixture was subjected to amplification process, which was 5 min at 94°C, and 30 cycles of amplification consisting of 30 s at 94°C, 30 s at 55°C, and 50 s at 72°C, with 5 min at 72°C for the final extension. Finally amplified DNA fragments were analyzed by electrophoresis in a 1% agarose gel. Multilocus sequence typing (MLST) analysis of the isolates was performed following Seemann T, mlst (https://github.com/tseemann/mlst), according to PubMLST website (Jolley et al., 2018).
Safety and clinical efficacy
The definition of different population groups for safety and efficacy analysis is shown in Supplementary Table S2. Briefly, safety was evaluated based on adverse events (AEs) in patients who received at least one polymyxin B dose. Adverse events were recorded based on patient self-reporting as well as abnormal laboratory test results including serum chemistry and routine blood and urinary testing. Acute kidney injury (AKI) was graded according to the RIFLE criteria (Bellomo et al., 2004). The primary endpoint was culture-confirmed bacterial eradication of CRKP from the blood evaluated by blood cultures taken every 3 days following the first dose of polymyxin B administration. Secondary endpoints were clinical outcome (clinical cure or improvement, Supplementary Table S3) evaluated in patients who received at least 3 days of polymyxin B treatment, and 28-day all-cause mortality evaluated in patients who completed 14 days of polymyxin B treatment.
Determination of polymyxin B concentrations in blood samples
Blood samples (4 mL) for pharmacokinetic analysis were collected in tubes with EDTA-K2 as the anticoagulant immediately before and after administration of the polymyxin B loading dose (the first dose) and immediately before administration of the second dose. Subsequent blood samples were collected during the second visit (5th, 6th or 7th dosing) consisting of a sample immediately prior to the infusion and at .5, 1, 2, 3, 4, 5, 6, 7, 8, 10, and 12 h following the commencement of the infusion. If possible, a final blood sample was collected 24 h after the commencement of the last infusion. All blood samples were centrifuged (4°C, 2,000 g) and plasma collected and stored at −80°C until analysis.
The concentrations of polymyxin B1 (including B1-Ile) and polymyxin B2 were determined using a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) method (Liu et al., 2020). Calibration and quality control (QC) samples were prepared using USP standard polymyxin B. This method showed excellent linearity (average R 2 = 0.9931), precision (3.2%–10.0%), and accuracy (91.1%–105.1%). The calibration range was .050–5.00 mg/L for polymyxin B1 and .011–.549 mg/L for polymyxin B2. The concentration of polymyxin B was calculated by the sum of concentrations of polymyxin B1 and B2 using molar terms and their molecular weights (polymyxin B1 is 1203.48 g/mol, polymyxin B2 is 1189.45 g/mol).
PK analyses and population pharmacokinetic (PPK) modeling
The PK parameters of polymyxin B were calculated using Phoenix WinNonlin 8.0 (CertaraTM, USA) with non-compartmental and compartmental methods. PPK analysis was conducted using NONMEM 7.4 (Icon Development Solutions, Ellicott City, MD) with G77 FORTRAN complier and FOCEI algorithm. Results from BSI patients in the present study and healthy subjects in a previous study were combined (Liu et al., 2021). One- and two-compartment models were tested during development of the base model. Models were developed and evaluated based on an objective function value and goodness-of-fit plots. The covariates were selected using a forward inclusion and backward elimination strategy. The screened covariates were sex, age, body weight, height, body mass index (BMI), creatinine clearance (CLcr), albumin, hemoglobin, total protein, total bilirubin, serum alanine aminotransferase (ALT), serum aspartate aminotransferase (AST), serum creatinine, Sequential Organ Failure Assessment (SOFA) score, and disease state (BSI patients were defined as 1 and healthy subjects were defined as 2). Covariates were included in the model based on the criteria of OFV requiring a decrease of 3.84 (p < .05) in the forward inclusion and an increase of greater of 10.83 (p < .001) in the backward elimination. Bootstrap sampling and visual predictive checks (VPCs) were utilized to validate the robustness of the final model.
PK/PD analysis and simulations
Monte Carlo simulations were conducted for the probability of target attainment (PTA) analysis to guide the selection of optimal dosage regimens. Different dosage regimens were simulated by the PPK model and AUCss,24h were calculated. An unbound fraction (f) of polymyxin B in human plasma of 42% (Sandri et al., 2013) was used to calculate the fAUCss,24h/MIC (the PK/PD index that best correlates with optimal microbiological outcomes) at steady state (Dudhani et al., 2010b; 2010a; Bergen et al., 2010; Cheah et al., 2015; Landersdorfer et al., 2018). The maximal reductions in colony forming units (CFU)/thigh derived from murine thigh infection models using polymyxin B or colistin were set as PK/PD targets. Specifically, fAUC24h/MIC targets of 17.4 for polymyxin B for a 1-log10 reduction against K. pneumoniae (Landersdorfer et al., 2018), and 13.5 and 17.6 for colistin for a 2-log10 reduction against P. aeruginosa and A. baumannii, respectively, were used in the Monte Carlo simulations (Cheah et al., 2015). The colistin targets were adopted for polymyxin B given that both polymyxins have essentially identical in vitro potencies (as measured by MICs), spectra of antibacterial activity and efficacy against thigh infection in mice (Landersdorfer et al., 2018; Satlin et al., 2020).
Results
Patient enrollment and adverse events
Nine Chinese patients (seven males and two females) were enrolled in the present study, with four patients receiving the full 14 days of polymyxin B treatment (Table 1). Baseline demographic data including SOFA score and original infection sites are shown in Table 2, and the co-administered drugs are shown in Supplementary Table S4. All nine patients were administered at least one dose of polymyxin B and all were included in the safety analysis. Adverse events are summarized in Table 3. At least one of neurotoxicity (5/9), nephrotoxicity (5/9), and hyperpigmentation (1/9) were recorded in 88.9% (8/9) of patients. Five of seven conscious patients (two patients being sedated during the treatment period) reported neurotoxicity. Of these patients, four reported pruritus and paresthesia on the face and head after the polymyxin B loading dose while three were evaluated as ataxic with the symptoms resolving by days 3–5 without any additional treatment. Five patients (four males and one female) experienced an increase in serum creatinine within 7 days, with all graded “I” according to the RIFLE criteria. Of these five patients, one (patient 7) continued in the study and completed 14 days of treatment, three (patients 1, 2 and 8) withdrew from the study and continued on a reduced polymyxin B dose (50 mg, 12-hourly), and one (patient 5) withdrew from the study and stopped polymyxin B treatment on day 6. The changing trends in eGFR and serum creatinine for all patients across the study period are shown in Supplementary Table S5. Hyperpigmentation on the head and neck was observed in patient 9 on day 4 which did not resolve until after the study was completed on day 28. Unlike in healthy subjects, no abdominal pain was reported by any patient during polymyxin B treatment (Liu et al., 2021).
TABLE 1.
Polymyxin B MICs, pharmacokinetic/pharmacodynamic indices, and clinical outcome.
| Patient no. | K. pneumoniae strain no. | Polymyxin B MIC (mg/L) | Treatment duration (day) | Study completion per protocol (Withdrawal reasons) | Continued polymyxin B treatment after withdrawal | AUC24 h,ss/MIC | Microbiological efficacy | Clinical efficacy** | 28-day survival |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | .5 | 6 | Withdraw (AKI) | Yes | 110.4 | Eradication | Not improved | - |
| 2 | 2–1, 2–2* | .5, .5 | 6 | Withdraw (AKI) | Yes | 266.8 | Eradication | Improved | - |
| 3 | 3 | 1 | 14 | Yes | - | 68.8 | Eradication | Cure | Yes |
| Withdraw (pruritus and numbness on face and head, ataxia) | |||||||||
| 4# | 4 | .5 | 1 | No | - | - | - | - | |
| Withdraw (AKI, pruritus and numbness on face and head, ataxia) | |||||||||
| 5 | 5 | .5 | 5 | No | 172 | Eradication | Improved | - | |
| 6 | 6 | .5 | 14 | Yes | - | 149.6 | Eradication | Cure | Yes |
| 7 | 7 | 1 | 14 | Yes | - | 87.8 | Eradication | Cure | Yes |
| Withdraw (AKI, pruritus and numbness on face and head, ataxia) | |||||||||
| 8 | 8 | .5 | 4 | Yes | 154.4 | Eradication | Improved | - | |
| 9 | 9 | 1 | 14 | Yes | - | 54.4 | Eradication | Failure | Yes |
MIC, minimum inhibitory concentration; AUC24 h,ss, area under the plasma concentration-time curve across 24 h at steady state; *, two CRKP strains were isolated; -, not applicable or not evaluated; **, evaluated when the subjects withdrew or completed the study; #, the PK/PD and efficacy could not be evaluated as the patient withdrew after receiving only the loading dose. All strains had a meropenem MIC of >64 mg/L except for strain No. 2–2 (MIC of 4 mg/L).
TABLE 2.
Baseline characteristics of enrolled patients.
| Characteristics |
n (%) or median (IQR) (N = 9) |
|---|---|
| Age (year) | 68 (63–73) |
| Sex | |
| Male | 7 (78) |
| Female | 2 (22) |
| Weight (kg) | 60 (55–65) |
| Body mass index (kg/m2) | 22.2 (17.6–24.4) |
| Serum creatinine (μmol/L) | 61 (52–73) |
| Creatinine clearance (ml/min) | 89 (68–106) |
| Serum albumin concentration (g/L) | 28.8 (26.9–30.3) |
| SOFA score | 4 (2–5) |
| Original infection site | |
| Intra-abdominal infection | 6 (67) |
| Testicular infection | 1 (11) |
| Urinary tract infection | 1 (11) |
| Cather related blood infection | 1 (11) |
IQR, interquartile range; SOFA, sequential organ failure assessment.
TABLE 3.
Adverse events following intravenous administration of polymyxin B in patients with CRKP bloodstream infections.
| Adverse effect | Event |
|---|---|
| Total | 16 |
| Neurotoxicity | |
| Pruritus | 4 |
| Ataxia | 2 |
| Dizziness | 2 |
| Weakness | 1 |
| Numbness of extremities | 1 |
| Nephrotoxicity | |
| Acute kidney injury (Grade I) | 5 |
| Other | |
| Skin hyperpigmentation | 1 |
| Back pain | 1 |
Pharmacokinetics of polymyxin B in BSI patients
The average concentration–time profile of polymyxin B at steady state across all patients is shown in Figure 1. The mean (±SD) Cmax and Cmin (12 h) following the loading dose was 5.53 ± 1.80 mg/L and 1.62 ± 0.41 mg/L, respectively. The corresponding PK parameters of polymyxin B derived from non-compartmental analyses at steady state are shown in Table 4. The pharmacokinetics of polymyxin B was best described by a two-compartmental model with the parameters shown in Supplementary Table S6.
FIGURE 1.
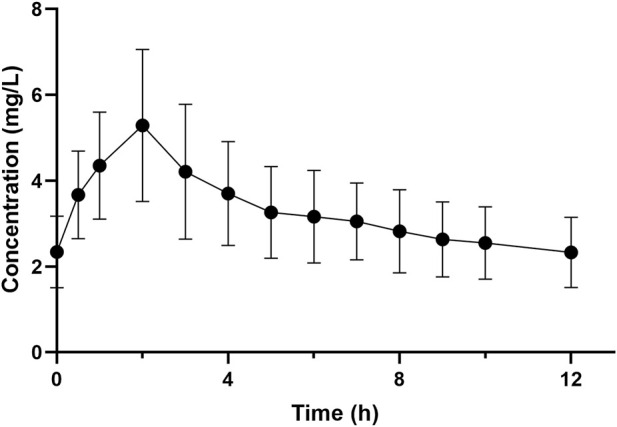
Mean (±SD) plasma concentration-time profile of polymyxin B at steady state in patients with carbapenem-resistant K. pneumoniae bloodstream infections. The X-axis is normalized to the dosing time at steady state. Time “0 h” is equivalent to immediately before polymyxin B dosing with the remaining times calculated from the commencement of dosing at steady state.
TABLE 4.
Pharmacokinetic parameters by non-compartmental analysis.
| Parameter | Unit | Mean value ± SD |
|---|---|---|
| Cmax | mg/L | 5.42 ± 1.69 |
| AUC12h * | mg·h/L | 39.8 ± 12.5 |
| Cavg | mg/L | 3.35 ± 1.06 |
| T1/2 | h | 12.5 ± 3.11 |
| CL | L/kg/h | 0.028 ± 0.007 |
| Vd | L/kg | 0.490 ± 0.142 |
SD, standard deviation; AUC12h, area under the concentration-time curve across 12 h; Cavg, average concentration; T1/2, half-life; CL, clearance; Vd, volume of distribution. All values are calculated at steady state.
The corresponding AUC24h (area under the concentration-time curve across 24 h) is 79.6±25.0 mg h/L.
Microbiological and clinical efficacy
The polymyxin B MICs of ten K. pneumoniae strains isolated from the nine patients (two strains isolated from one patient) are shown in Table 1. All strains were susceptible to polymyxin B (MIC ≤1 mg/L) and were resistant to meropenem (MICs >64 mg/L for 9 strains and 4 mg/L for 1 strain). PCR results showed that all strains harbored bla-KPC2 and that New Delhi metallo-beta-lactamase (NDM), imipenem-resistant carbapenemase (IMP), Verona integron metallo-β-lactamase (VIM) or oxacillinase (OXA) genes were absent (Supplementary Figure S1). Except isolates from patient No. 8 belonged to ST14, all the rest isolates were belonged to ST11, which is the predominant clone type in ICU in China (Qin et al., 2020).
One patient withdrew from the study following the loading dose due to intolerable pruritus on the face and head and was excluded from the microbiological and clinical efficacy analysis. Blood cultures showed eradication of all isolated CRKP from the remaining 8 patients by the third day of treatment and remained negative until the patients completed the study or withdrew. Clinical cure or improvement occurred in 75% (6/8) of these patients, with all 4 patients who completed the 14-day polymyxin B treatment protocol alive 28 days after the commencement of therapy.
Population PK and PK/PD analysis
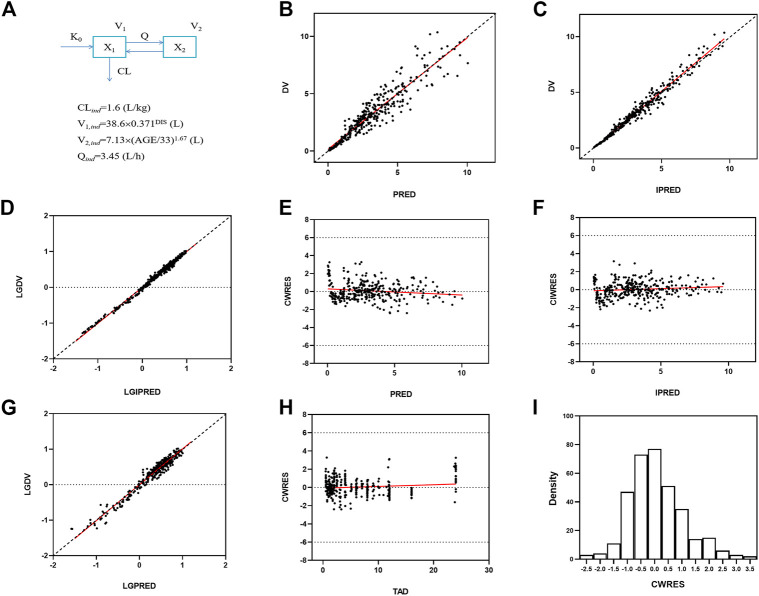
The polymyxin B concentration data were fitted into a two-compartmental model with the OFV value of −988 during the base model development. An exponential model and a proportional model were used to describe the interindividual variability and residual variability, respectively. Two covariates [BSI on the volume of central compartment (V1) and age on the volume of peripheral compartment (V2)] were included in the final population PK model where the OFV value decreased from −988 to −1087. The final PPK model and parameters are shown in Figure 2, and the progression of model building was shown in Supplementary Table S7. The final model was evaluated by goodness-of-fit plots and showed no apparent visual bias for the predictions (Figure 2; Supplementary Figures S2, S3). The parameter estimates and between-subject variability from the final model and 1,000 bootstrap runs are presented in Table 5. Both bootstrap and VPC plots (Figure 3) indicated the robustness of the final model.
FIGURE 2.
Goodness-of-fit plots for the final population PK model. Predicted concentrations are in micrograms per liter and time is in hours. (A) PPK model schematic figure and final model, DIS = 1 for patients and DIS = 2 for healthy subjects; (B) Observed polymyxin B concentrations (DV) versus population predictions (PRED). (C) DV versus individual predictions (IPRED). (D) Log transformed DV versus Log transformed IPRED. (E) Conditional weighted residuals (CWRES) versus PRED. (F) Conditional individual weighted residuals (CIWRES) versus IPRED. (G) Log transformed DV versus Log transformed PRED. (H) CWRES versus time after dose (TAD). (I) distribution of CWRES.
TABLE 5.
Population pharmacokinetic parameter estimates and between-subject variability.
| Parameter (Unit) | Estimate | Between-subject variability | ||
|---|---|---|---|---|
| Original dataset (Typical value) | Bootstrap dataset (Median and 95% interval confidence) | Original dataset (%) | Bootstrap dataset (%) | |
| CL (L/h) | 1.60 | 1.60 (1.5, 1.7) | 18.2 | 17.7 |
| V1 (L) | 38.6 | 38.6 (27.7, 54.4) | 20.0 | 18.7 |
| V2 (L) | 7.13 | 7.04 (5.93, 7.98) | 27.2 | 25.6 |
| Q (L/h) | 3.45 | 3.39 (2.31, 4.48) | 0 (FIX) | — |
| θDIS on V1 | 0.371 | 0.372 (0.31, 0.45) | NA | NA |
| θAGE on V2 | 1.67 | 1.68 (1.34, 1.95) | NA | NA |
| Proportional error (%) | 11.8 | 11.6 (10.4, 12.9) | NA | NA |
CL, clearance from the central compartment; V1, central volume of distribution; V2, peripheral volume of distribution; Q, intercompartmental clearance.
FIGURE 3.
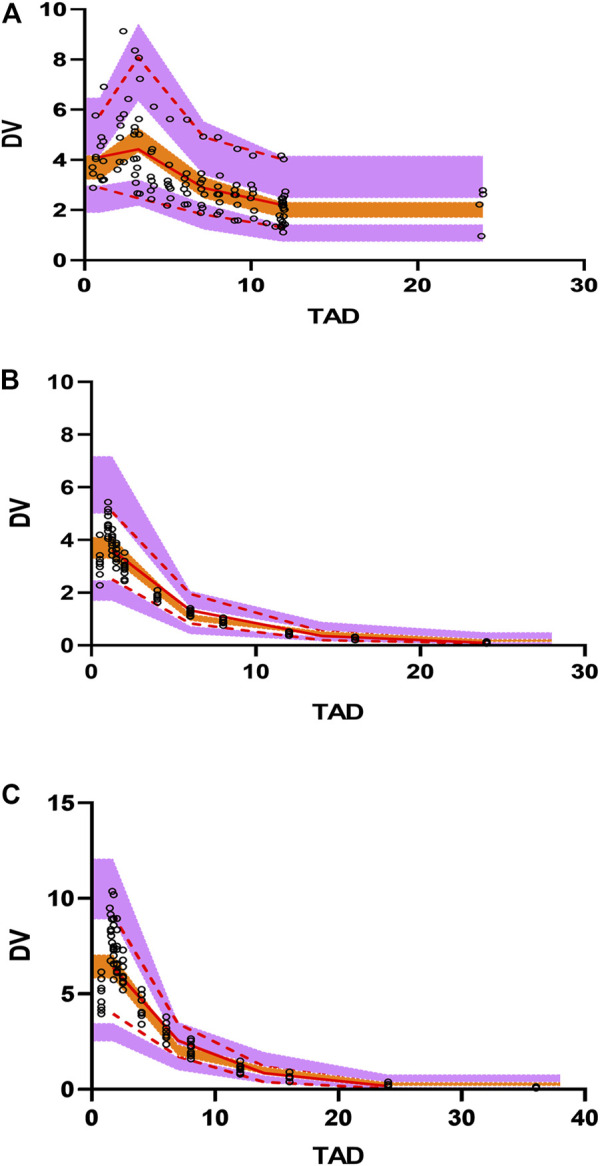
Visual predictive check (VPC) plots for the polymyxin B concentration in plasma. (A) VPC for BSI patients. (B) VPC for healthy subjects with .75 mg/kg dosing administration. (C) VPC for healthy subjects with 1.5 mg/kg dosing administration. Open circles represent observed concentrations. The solid red line represents the median of the observations. The red dashed lines represent the 5th and 95th percentiles of the observations. The purple shaded areas represent the 95% confidence intervals for the 5th and 95th percentiles, and the orange shaded areas represents the median of the predicted data.
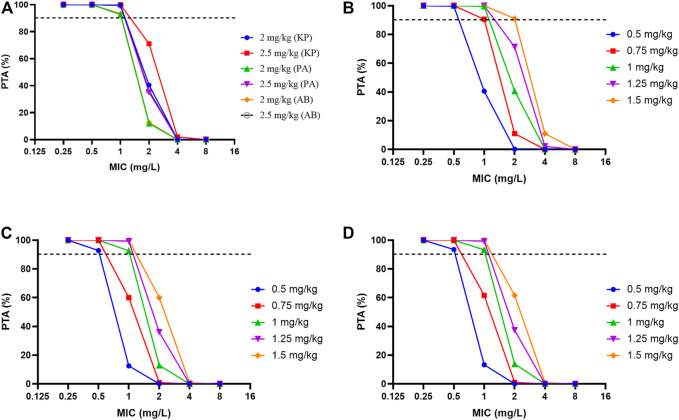
Probability of target attainment for different dosage regimens of polymyxin B against common carbapenem-resistant organisms according to PK/PD simulations was shown in Figure 4 and Supplementary Table S8. For K. pneumoniae, P. aeruginosa and A. baumannii with MICs ≤1 mg/L, Monte Carlo simulations of polymyxin B revealed >90% PTA for fAUCss,24h/MIC targets with the 1 mg/kg dosing regimen (Figure 4). However, with an MIC of 2 mg/L the PTA with this regimen dropped below 50% for each organism and remained below 90% even with the maximum dose of 3 mg/kg/d (corresponding to the 1.5 mg/kg 12-hourly regimen).
FIGURE 4.
Probability of target attainment for different dosage regimens of polymyxin B against common carbapenem-resistant organisms according to PK/PD simulations. (A) Loading dose for three bacterial species. (B) K. pneumoniae (KP), (C) P. aeruginosa (PA), (D) A. baumannii (AB); The fAUCss,24h/MIC targets were 13.5 for a 1-log10 reduction against K. pneumoniae and 17.6 and 17.4 for a 2-log10 reduction against P. aeruginosa and A. baumannii, respectively. The unbound fraction in plasma was .42.
Discussion
In our prospective clinical PK/PD study, CRKP with a polymyxin B MIC ≤1 mg/L was eradicated from the bloodstream of Chinese patients with an achieved polymyxin B AUCss,24h/MIC of ≥54.4 (equivalent to an fAUCss,24h/MIC of ≥22.8). Neurotoxicity and nephrotoxicity were the major dose-limiting factors associated with polymyxin B administration. Intensive PK sampling and PPK modeling showed that disease status and age were covariates of V1 and V2, respectively. Monte Carlo simulations suggested that a polymyxin B dose of 1 mg/kg 12-hourly was effective for pathogens with an MIC ≤1 mg/L.
Nephrotoxicity following intravenous administration of polymyxin B may occur in up to ∼30% of patients and constitutes the major dose-limiting factor with polymyxin therapy (Ouderkirk et al., 2003; Sobieszczyk et al., 2004; Phe et al., 2014; Oliota et al., 2019). The inability to substantially increase the daily dose of polymyxins may lead to suboptimal dosing which compromises efficacy and increases the likelihood of resistance emerging (Zavascki et al., 2007; Rigatto et al., 2014). In the present study, no pathological factors affecting renal function were present in any of the participants prior to enrollment, and no concomitant nephrotoxic drugs were administered during the study period. Polymyxin B-induced AKI as defined by the RIFLE criteria occurred with an incidence of 55.6% (5/9) 4–7 days after commencing therapy; this incidence is approximately twice that reported previously (29.8%) (Oliota et al., 2019). This discrepancy might be at least partially explained by the different definitions of nephrotoxicity (e.g., RIFLE, AKIN, KDIGO) applied in earlier studies which also included a wide range of polymyxin B doses (12–225 mg/day) patient variability (baseline characteristics and clinical conditions), and variations in interventions being assessed. However, the study by Phe et al. used the same RIFLE criteria to define nephrotoxicity and reported a considerably lower incidence of polymyxin B-associated nephrotoxicity than we observed (23.1% vs. 55.6%) (Phe et al., 2014). The high dose of polymyxin B administered (2.5 mg/kg/day) in the present study could be a risk factor. Since polymyxin dose is known to be the most important variable associated with the development of nephrotoxicity, with total drug exposure and longer duration of treatment also associated with its development (Nation et al., 2019).
Neurotoxicity is another major dose-limiting factor for the polymyxins, although it often goes unreported given many patients receiving polymyxins are mechanically ventilated which makes assessment difficult. Polymyxin-induced neurotoxicity is very likely dose-dependent and is characterized by symptoms such as dizziness, vertigo, visual disturbances, confusion, hallucinations, seizures, ataxia, facial and peripheral parenthesis, and pruritus (Falagas and Kasiakou, 2006; Weinstein et al., 2009; Wahby et al., 2010; Honore et al., 2013). While neurologic side effects such as parenthesis have been reported to be as high as 27% with intravenous polymyxin therapy (Wallace et al., 2008), our recently published study examining polymyxin B toxicity in healthy Chinese patients reported perioral paraesthesia, dizziness, and numbness of extremities occurred in 7 of 10 (70%) subjects who received a single dose of 0.75 mg/kg via a 1-h infusion, and all subjects who received a single dose of 1.5 mg/kg via a 1.5-h infusion (Liu et al., 2021). The high incidence of neurotoxic adverse events (5 of 7 conscious patients) in the present study is thus similar to our earlier report. Unfortunately, it appears that there is considerable overlap in plasma concentrations required to achieve the desired antibacterial effect and those causing the major adverse effects of nephrotoxicity and neurotoxicity (Nation et al., 2019). While we had initially planned to include a higher-dose polymyxin B regimen (loading dose of 2.5 mg/kg with a maintenance dose of 3 mg/kg/d) in the research protocol for the present study, consideration of the potential for nephrotoxicity and neurotoxicity meant that the higher dose regimen was terminated.
Polymyxin B exhibits rapid, concentration-dependent bacterial killing in vitro against a range of Gram-negative organisms including K. pneumoniae (Poudyal et al., 2008); and several reports suggest polymyxin B had a good clinical potential in bloodstream infection (Kvitko et al., 2011; Medeiros et al., 2019; Falcone et al., 2021). In the present study, microbiological eradication was achieved by the third day of treatment in all eight patients assessed and blood culture remained negative until they had completed the study or withdrew. Clinical improvement occurred in 6 of 8 (75%) of these patients, with all 4 who completed the full 14 days of treatment alive 28 days after therapy commenced. Although direct comparison with alternative therapies are lacking, clinical efficacy of this study was satisfied. Pooled mortality among patients with CRKP BSI receiving various treatment was 54.3% according to a meta-analysis (Xu et al., 2017). A retrospective study analyzing clinical efficacy of amikacin and its combinations for CRKP infection found that 30-day mortality was 34.5% (Rodrigues et al., 2021). Recent retrospective study about efficacy of ceftazidime-avibactam for CRKP BSI showed that 30-day mortality was around 25%, whether alone or combined with other antibiotics (Tumbarello et al., 2021). While acknowledging the small number of patients in our cohort, two factors may have contributed to this result. First, the SOFA score of our patient cohort, which is a risk factor for mortality generally and has been shown to be independently associated with mortality in patients with CRE BSI, was generally low (range, 1–10; median, 4; only one patient was receiving vascular-active drugs) (Falcone et al., 2021). Second, the rapid eradication of CRKP within 3 days of commencing therapy likely benefited the disease recovery process.
Our study is the first to specifically examine patients with BSI using the recommended dosage regimen of polymyxin B (2.5–3.0 mg/kg/d) (Tsuji et al., 2019). Previous studies utilizing PPK models to examine polymyxin B PK have included primarily critically ill patients with various medical conditions or patients with cystic fibrosis, and have employed a wide range of doses (0.45–3.38 mg/kg/day) (Sandri et al., 2013; Avedissian et al., 2018; Kubin et al., 2018; Manchandani et al., 2018; Miglis et al., 2018; Yu et al., 2021). Interestingly, it is noteworthy that the PK of polymyxin B in patients with BSI was very different from in healthy subjects (Liu et al., 2021). Although the clearance was similar in both groups (0.028 ± 0.007 L/kg/h in BSI patients and 0.026 ± 0.004 L/kg/h in healthy subjects), the volume of distribution and half-life in BSI patients were over twice that of healthy subjects (0.490 ± 0.142 L/kg vs. 0.204 ± 0.026 L/kg and 12.5 ± 3.11 vs. 5.55 ± 0.942 h, respectively). Increases in volume of distribution and half-life in critically ill patients are not surprising and was reported in other antibiotics previously (Bergen et al., 2011; Pea, 2013; Blot et al., 2014). However, when built PPK model from BSI patients, a full PPK model with covariates could not be built possibly due to a small number of patients. Considering plenty of studies built PPK models by combining data from all available trials (Lu et al., 2019; Gaudy et al., 2020; Naik et al., 2021), and population pharmacokinetic guidance by FDA also encourages combining data from early- and late-stages of trials to build models using the non-linear mixed-effects modeling approach (https://www.fda.gov/media/128793/download).We therefore included all plasma concentrations acquired from healthy subjects and BSI patients in the PPK model. Not surprisingly, BSI was included as a category covariate on the volume of the central compartment (V1), whereas CLcr before and during treatment was not included as a covariate in the model. On this latter point, polymyxin B is known to be predominantly non-renally cleared with ≤4% of the dose excreted unchanged in the urine (Zavascki et al., 2008). Although body weight has been included in several reported PPK models (Sandri et al., 2013; Xie et al., 2020), it was not incorporated as a covariate in our model most likely due to the relatively narrow range of body weight of enrolled subjects (range, 55–65 kg; median 60 kg). Although age is commonly considered as an underlying covariate of weight or progressive decline in the functional reserve of multiple organs and systems (ElDesoky, 2007; Klotz, 2009; Shi and Klotz, 2011), it is incorporated as a covariate that influences polymyxin B distribution (included on V2).
A good understanding of the PK of polymyxin B is essential for optimizing its clinical use. A previous population PK study examining the use of polymyxin B (0.45–3.38 mg/kg/day) in 24 critically ill patients reported an AUCss,24h of 66.9 ± 21.6 mg h/L (range, 16.4–117 mg h/L) and Css,avg of 2.79 ± 0.90 mg/L; no loading dose was administered (Sandri et al., 2013). In our present study utilizing a 2.5 mg/kg loading dose and 1.25 mg/kg maintenance dose of polymyxin B for BSI patients, non-compartmental analysis showed that the AUCss,24h (calculated as 2× AUCss,12h) and Css,avg were 79.6 ± 25.0 mg h/L and 3.35 ± 1.06 mg/L, respectively, somewhat higher than the values reported by Sandri et al. and well within the recommended targets for AUCss,24h and Css,avg of 50–100 mgh/L and 2–4 mg/L, respectively (Tsuji et al., 2019).
Similar to colistin, fAUC/MIC has been shown to be the most predictive PK/PD index for polymyxin B (Lin et al., 2017; Landersdorfer et al., 2018). In preclinical studies, the fAUC/MIC required for various magnitudes of bacterial killing varied among bacterial strains and infection sites. In a mouse thigh infection model undertaken with three strains of K. pneumoniae, target values of fAUC/MIC for polymyxin B of 3.7–28.0 were required for 1 log10 kill, while 2 log10 kill could not be achieved with even the highest tolerated dose (Landersdorfer et al., 2018). Against P. aeruginosa and A. baumannii, an fAUC/MIC target for colistin of ∼17 has been established for 2 log10 kill in a mouse thigh infection model (Dudhani et al., 2010a; 2010b). A strength of our study is that our PK/PD results were obtained from the infection site. In our patient cohort with CRKP BSI, the AUCss,24h/MIC was ≥54.4 (fAUCss,24h/MIC of 22.8) in the eight patients in whom clinical efficacy was assessed, with microbiological eradication from the blood achieved in all patients within 3 days of commencing therapy.
Monte Carlo simulations showed that fAUCss,24h/MIC targets of 13.5 for K. pneumoniae and 17.6 and 17.4 for P. aeruginosa and A. baumannii, respectively, were achieved in >90% of patients for pathogens with MIC ≤1 mg/L with our maintenance dosing regimen of 2.5 mg/kg/day (Figure 4). However, the likelihood of achieving these targets with this dosage regimen for organisms with an MIC of 2 mg/L was poor and remained <90% even with the maximum recommended daily dose of 3 mg/kg (except for K. pneumoniae). These results are very similar to those reported by Sandri et al. (2013) for critically ill patients generally. But these results should be interpreted with caution because the PK/PD target is based on small numbers of animal studies. For pathogens with MIC ≥2 mg/L it would seem prudent to use polymyxin B in combination with other antibiotics such as a carbapenem, tigecycline and fosfomycin to maintain efficacy and reduce toxicity (Zhang et al., 2017). Importantly, despite achieving microbiological eradication from the bloodstream in the eight assessed patients, intra-abdominal infection in two patients with low fAUCss,24h/MICs (46.4 and 28.9) did not resolve and they subsequently died on days 11 and 45. Therefore, it is critical to determine the specific fAUC/MIC targets of polymyxins for different infection sites after intravenous administration.
There are some limitations in the present study. The relatively small number of BSI patients is limited for the clinical and microbiological efficacy evaluation. More patients are needed to enrolled in the study to validate the efficacy of the dosing regimens, as well as to collect more PK samples for the PPK model and PK/PD analysis.
Conclusion
This is the first study to examine the clinical PK/PD of polymyxin B in BSI patients infected with CRKP. Polymyxin B administered at a recommended dose of 1.25 mg/kg 12-hourly achieved an AUCss,24h/MIC of ≥54.4 and was sufficient to achieve microbiological eradication from the blood of all patients. Monte Carlo simulations indicated that the recommended dose of polymyxin B would be suitable for bloodstream infections caused by pathogens with an MIC ≤1 mg/L. These results will assist to establish polymyxin B breakpoints and to optimize its therapy in difficult-to-treat patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Sir Run Run Shaw Hospital, College of Medicine, Zhejiang University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
YY and JZ conceived of and designed the study; XD, ZZ, LF, YZ, and RS recruited patients and collected clinical data; ZY and HC supervised drug administration and sample collection; ZY, XL, HW, YW, YF, and BG performed drug concentration test; FZ, WL, and XL performed microbiology test; XL and ZY performed PK and PK/PD analysis; ZY, XL, XD, PB, and JL drafted the manuscript; all authors read and approved the final version.
Funding
This research was supported by National natural science foundation of China (Grant Number. 81903667), the Shanghai Municipal Science and Technology Commission (Grant Number. 19411964900) and Zhejiang Provincial Natural Science Foundation of China (Grant Number. LYY21H310006).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The authors are grateful to SPH No. 1 Biochemical & Pharmaceutical Co., Ltd. (Shanghai, China) for providing the product of Polymyxin B Sulphate for Injection and the standard of polymyxin E2.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.975066/full#supplementary-material
References
- Almangour T. A., Garcia E., Zhou Q., Forrest A., Kaye K. S., Li J., et al. (2021). Polymyxins for the treatment of lower respiratory tract infections: Lessons learned from the integration of clinical pharmacokinetic studies and clinical outcomes. Int. J. Antimicrob. Agents 57, 106328. 10.1016/j.ijantimicag.2021.106328 [DOI] [PubMed] [Google Scholar]
- Avedissian S. N., Miglis C., Kubin C. J., Rhodes N. J., Yin M. T., Cremers S., et al. (2018). Polymyxin B pharmacokinetics in adult cystic fibrosis patients. Pharmacotherapy 38, 730–738. 10.1002/phar.2129 [DOI] [PubMed] [Google Scholar]
- Bellomo R., Ronco C., Kellum J. A., Mehta R. L., Palevsky P. (2004). Acute renal failure – definition , outcome measures , animal models , fluid therapy and information technology needs : The second international consensus conference of the acute dialysis quality initiative ( ADQI ) group. Crit. Care 8, R204–R212. 10.1186/cc2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen P. J., Bulitta J. B., Forrest A., Tsuji B. T., Li J., Nation R. L. (2010). Pharmacokinetic/pharmacodynamic investigation of colistin against Pseudomonas aeruginosa using an in vitro model. Antimicrob. Agents Chemother. 54, 3783–3789. 10.1128/AAC.00903-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen P., Li J., Nation R. (2011). Dosing of colistin-back to basic PK/PD. Curr. Opin. Pharmacol. 11, 464–469. 10.1016/j.coph.2011.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian X., Liu X., Hu F., Feng M., Chen Y., Bergen P. J., et al. (2022). Pharmacokinetic/pharmacodynamic based breakpoints of polymyxin B for bloodstream infections caused by multidrug-resistant gram-negative pathogens. Front. Pharmacol. 12, 785893. 10.3389/fphar.2021.785893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blot S. I., Pea F., Lipman J. (2014). The effect of pathophysiology on pharmacokinetics in the critically ill patient - concepts appraised by the example of antimicrobial agents. Adv. Drug Deliv. Rev. 77, 3–11. 10.1016/j.addr.2014.07.006 [DOI] [PubMed] [Google Scholar]
- Cheah S. E., Wang J., Nguyen V. T. T., Turnidge J. D., Li J., Nation R. L. (2015). New pharmacokinetic/pharmacodynamic studies of systemically administered colistin against Pseudomonas aeruginosa and acinetobacter baumannii in mouse thigh and lung infection models: Smaller response in lung infection. J. Antimicrob. Chemother. 70, 3291–3297. 10.1093/jac/dkv267 [DOI] [PubMed] [Google Scholar]
- Dudhani R. V., Turnidge J. D., Coulthard K., Milne R. W., Rayner C. R., Li J., et al. (2010a). Elucidation of the pharmacokinetic/pharmacodynamic determinant of colistin activity against Pseudomonas aeruginosa in murine thigh and lung infection models. Antimicrob. Agents Chemother. 54, 1117–1124. 10.1128/AAC.01114-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudhani R. V., Turnidge J. D., Nation R. L., Li J. (2010b). fAUC/MIC is the most predictive pharmacokinetic/pharmacodynamic index of colistin against Acinetobacter baumannii in murine thigh and lung infection models. J. Antimicrob. Chemother. 65, 1984–1990. 10.1093/jac/dkq226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElDesoky E. S. (2007). Pharmacokinetic-pharmacodynamic crisis in the elderly. Am. J. Ther. 14 (5), 488–498. 10.1097/01.mjt.0000183719.84390.4d [DOI] [PubMed] [Google Scholar]
- Falagas M. E., Kasiakou S. K. (2006). Toxicity of polymyxins: A systematic review of the evidence from old and recent studies. Crit. Care 10, R27. 10.1186/cc3995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone M., Daikos G. L., Tiseo G., Bassoulis D., Giordano C., Galfo V., et al. (2021). Efficacy of ceftazidime-avibactam plus aztreonam in patients with bloodstream infections caused by metallo-β-lactamase-producing Enterobacterales. Clin. Infect. Dis. 72, 1871–1878. 10.1093/cid/ciaa586 [DOI] [PubMed] [Google Scholar]
- Gaudy A., Hwang R., Palmisano M., Chen N. (2020). Population pharmacokinetic model to assess the impact of disease state on thalidomide pharmacokinetics. J. Clin. Pharmacol. 60, 67–74. 10.1002/jcph.1506 [DOI] [PubMed] [Google Scholar]
- Gu D., Dong N., Zheng Z., Lin D., Huang M., Wang L., et al. (2018). A fatal outbreak of ST11 carbapenem-resistant hypervirulent Klebsiella pneumoniae in a Chinese hospital: A molecular epidemiological study. Lancet. Infect. Dis. 18, 37–46. 10.1016/S1473-3099(17)30489-9 [DOI] [PubMed] [Google Scholar]
- Honore P., Jacobs R., Lochy S., De Waele E., De Regt J., Van Gorp V., et al. (2013). Acute respiratory muscle weakness and apnea in a critically ill patient induced by colistin neurotoxicity: Key potential role of hemoadsorption elimination during continuous venovenous hemofiltration. Int. J. Nephrol. Renov. Dis. 6, 107–111. 10.2147/ijnrd.s42791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolley K. A., Bray J. E., Maiden M. (2018). Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 3, 124. 10.12688/wellcomeopenres.14826.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz U. (2009). Pharmacokinetics and drug metabolism in the elderly. Drug Metab. Rev. 41, 67–76. 10.1080/03602530902722679 [DOI] [PubMed] [Google Scholar]
- Kubin C. J., Nelson B. C., Miglis C., Scheetz M. H., Rhodes N. J., Avedissian S. N., et al. (2018). Population pharmacokinetics of intravenous Polymyxin B from clinical samples. Antimicrob. Agents Chemother. 62, e01493. 10.1128/AAC.01493-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvitko C. H., Rigatto M. H., Moro A. L., Zavascki A. P. (2011). Polymyxin B versus other antimicrobials for the treatment of Pseudomonas aeruginosa bacteraemia. J. Antimicrob. Chemother. 66, 175–179. 10.1093/jac/dkq390 [DOI] [PubMed] [Google Scholar]
- Landersdorfer C. B., Wang J., Wirth V., Chen K., Kaye K. S., Tsuji B. T., et al. (2018). Pharmacokinetics/pharmacodynamics of systemically administered polymyxin B against Klebsiella pneumoniae in mouse thigh and lung infection models. J. Antimicrob. Chemother. 73, 462–468. 10.1093/jac/dkx409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. W., Zhou Q., Onufrak N. J., Wirth V., Chen K., Wang J., et al. (2017). Aerosolized polymyxin B for treatment of respiratory tract infections: Determination of pharmacokinetic-pharmacodynamic indices for aerosolized polymyxin B against Pseudomonas aeruginosa in a mouse lung infection model. Antimicrob. Agents Chemother. 61, e00211–e00217. 10.1128/AAC.00211-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Chen Y., Yang H., Li J., Yu J., Yu Z., et al. (2021). Acute toxicity is a dose-limiting factor for intravenous polymyxin B: A safety and pharmacokinetic study in healthy Chinese subjects. J. Infect. 82, 207–215. 10.1016/j.jinf.2021.01.006 [DOI] [PubMed] [Google Scholar]
- Liu X., Yu Z., Wang Y., Wu H., Bian X., Li X., et al. (2020). Therapeutic drug monitoring of polymyxin B by LC-MS/MS in plasma and urine. Bioanalysis 12, 845–855. 10.4155/bio-2020-0051 [DOI] [PubMed] [Google Scholar]
- Lu Z., Bonate P., Keirns J. (2019). Population pharmacokinetics of immediate- and prolonged-release tacrolimus formulations in liver, kidney and heart transplant recipients. Br. J. Clin. Pharmacol. 85, 1692–1703. 10.1111/bcp.13952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchandani P., Thamlikitkul V., Dubrovskaya Y., Babic J. T., Lye D. C., Lee L. S., et al. (2018). Population pharmacokinetics of polymyxin B. Clin. Pharmacol. Ther. 104, 534–538. 10.1002/cpt.981 [DOI] [PubMed] [Google Scholar]
- Medeiros G. S., Rigatto M. H., Falci D. R., Zavascki A. P. (2019). Combination therapy with polymyxin B for carbapenemase-producing Klebsiella pneumoniae bloodstream infection. Int. J. Antimicrob. Agents 53, 152–157. 10.1016/j.ijantimicag.2018.10.010 [DOI] [PubMed] [Google Scholar]
- Miglis C., Rhodes N. J., Avedissian S. N., Kubin C. J., Yin M. T., Nelson B. C., et al. (2018). Population pharmacokinetics of polymyxin B in acutely ill adult patients. Antimicrob. Agents Chemother. 104, e01475. 10.1128/AAC.01475-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik H., Zhao Y., Forrestal F., Cleall S., Bockbrader H., Chapel S. (2021). Population pharmacokinetics of vixotrigine in healthy volunteers and subjects with trigeminal neuralgia, painful lumbosacral radiculopathy and erythromelalgia. Eur. J. Drug Metab. Pharmacokinet. 46, 395–404. 10.1007/s13318-021-00678-0 [DOI] [PubMed] [Google Scholar]
- Nang S. C., Azad M. A. K., Velkov T., Tony Zhou Q., Li J. (2021). Rescuing the last-line polymyxins: Achievements and challenges. Pharmacol. Rev. 73, 679–728. 10.1124/pharmrev.120.000020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nation R. L., Maria M. H., Falci D. R., Zavascki A. P. (2019). Polymyxin acute kidney injury: Dosing and other strategies to reduce toxicity. Antibiotics 8, 24. 10.3390/antibiotics8010024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliota A. F., Penteado S. T., Tonin F. S., Fernandez-Llimos F., Sanches A. C. (2019). Nephrotoxicity prevalence in patients treated with polymyxins: A systematic review with meta-analysis of observational studies. Diagn. Microbiol. Infect. Dis. 94, 41–49. 10.1016/j.diagmicrobio.2018.11.008 [DOI] [PubMed] [Google Scholar]
- Ouderkirk J. P., Nord J. A., Turett G. S., Kislak J. W. (2003). Polymyxin B nephrotoxicity and efficacy against nosocomial infections caused by multiresistant gram-negative bacteria. Antimicrob. Agents Chemother. 47, 2659–2662. 10.1128/AAC.47.8.2659-2662.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul M., Daikos G. L., Durante-mangoni E., Yahav D., Carmeli Y., Benattar Y. D., et al. (2018). Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant gram-negative bacteria : An open-label , randomised controlled trial. Lancet. Infect. Dis. 18, 391–400. 10.1016/S1473-3099(18)30099-9 [DOI] [PubMed] [Google Scholar]
- Pea F. (2013). Plasma pharmacokinetics of antimicrobial agents in critically ill patients. Curr. Clin. Pharmacol. 8, 5–12. 10.2174/157488413804810585 [DOI] [PubMed] [Google Scholar]
- Phe K., Lee Y., McDaneld P. M., Prasad N., Yin T., Figueroa D. A., et al. (2014). In vitro assessment and multicenter cohort study of comparative nephrotoxicity rates associated with colistimethate versus polymyxin b therapy. Antimicrob. Agents Chemother. 58, 2740–2746. 10.1128/AAC.02476-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirel L., Walsh T. R., Cuvillier V., Nordmann P. (2011). Multiplex PCR for detection of acquired carbapenemase genes. Diagn. Microbiol. Infect. Dis. 70, 119–123. 10.1016/j.diagmicrobio.2010.12.002 [DOI] [PubMed] [Google Scholar]
- Poudyal A., Howden B. P., Bell J. M., Gao W., Owen R. J., Turnidge J. D., et al. (2008). In vitro pharmacodynamics of colistin against multidrug-resistant Klebsiella pneumoniae . J. Antimicrob. Chemother. 62, 1311–1318. 10.1093/jac/dkn425 [DOI] [PubMed] [Google Scholar]
- Qin X., Wu S., Hao M., Zhu J., Ding B., Yang Y., et al. (2020). The colonization of carbapenem-resistant Klebsiella pneumoniae: Epidemiology, resistance mechanisms, and risk factors in patients admitted to intensive care units in China. J. Infect. Dis. 221 (S2), S206–S214. 10.1093/infdis/jiz622 [DOI] [PubMed] [Google Scholar]
- Rhodes A., Evans L. E., Alhazzani W., Levy M. M., Antonelli M., Ferrer R., et al. (2017). Surviving sepsis campaign: International guidelines for management of sepsis and septic shock. Intensive Care Med. 43, 304–377. 10.1007/s00134-017-4683-6 [DOI] [PubMed] [Google Scholar]
- Rigatto M. H., Behle T. F., Falci D. R., Freitas T., Lopes N. T., Nunes M., et al. (2014). Risk factors for acute kidney injury (AKI) in patients treated with polymyxin B and influence of AKI on mortality: A multicentre prospective cohort study. J. Antimicrob. Chemother. 70, 1552–1557. 10.1093/jac/dku561 [DOI] [PubMed] [Google Scholar]
- Rigatto M. H., Ribeiro V. B., Konzen D., Zavascki A. P. (2013). Comparison of polymyxin B with other antimicrobials in the treatment of ventilator-associated pneumonia and tracheobronchitis caused by Pseudomonas aeruginosa or Acinetobacter baumannii. Infection 41, 321–328. 10.1007/s15010-012-0349-z [DOI] [PubMed] [Google Scholar]
- Rodrigues D., Baldissera G. S., Mathos D., Sartori A., Zavascki A. P., Rigatto M. H. (2021). Amikacin for the treatment of carbapenem-resistant Klebsiella pneumoniae infections: Clinical efficacy and toxicity. Braz. J. Microbiol. 52 (4), 1913–1919. 10.1007/s42770-021-00551-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri A. M., Landersdorfer C. B., Jacob J., Boniatti M. M., Dalarosa M. G., Falci D. R., et al. (2013). Population pharmacokinetics of intravenous polymyxin B in critically Ill patients: Implications for selection of dosage regimens. Clin. Infect. Dis. 57, 524–531. 10.1093/cid/cit334 [DOI] [PubMed] [Google Scholar]
- Satlin M. J., Lewis J. S., Weinstein M. P., Patel J., Humphries R. M., Kahlmeter G., et al. (2020). Clinical and laboratory standards Institute and European committee on antimicrobial susceptibility testing position statements on polymyxin B and colistin clinical breakpoints. Clin. Infect. Dis. 71, E523–E529. 10.1093/cid/ciaa121 [DOI] [PubMed] [Google Scholar]
- Shi S., Klotz U. (2011). Age-related changes in pharmacokinetics. Curr. Drug Metab. 12, 601–610. 10.2174/138920011796504527 [DOI] [PubMed] [Google Scholar]
- Sobieszczyk M. E., Furuya E. Y., Hay C. M., Pancholi P., Della-Latta P., Hammer S. M., et al. (2004). Combination therapy with polymyxin B for the treatment of multidrug-resistant Gram-negative respiratory tract infections. J. Antimicrob. Chemother. 54, 566–569. 10.1093/jac/dkh369 [DOI] [PubMed] [Google Scholar]
- Tsuji B. T., Pogue J. M., Zavascki A. P., Paul M., Daikos G. L., Forrest A., et al. (2019). International consensus guidelines for the optimal use of the polymyxins: Endorsed by the American College of clinical pharmacy (ACCP), European society of clinical microbiology and infectious diseases (ESCMID), infectious diseases society of America (IDSA), international society for anti-infective Pharmacology (ISAP), society of critical care medicine (SCCM), and society of infectious diseases pharmacists (SIDP). Pharmacotherapy 39, 10–39. 10.1002/phar.2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbarello M., Raffaelli F., Giannella M., Mantengoli E., Mularoni A., Venditti M., et al. (2021). Ceftazidime-avibactam use for Klebsiella pneumoniae carbapenemase-producing K. pneumoniae infections: A retrospective observational multicenter study. Clin. Infect. Dis. 73 (9), 1664–1676. 10.1093/cid/ciab176 [DOI] [PubMed] [Google Scholar]
- Wahby K., Chopra T., Chandrasekar P. (2010). Intravenous and inhalational colistin-induced respiratory failure. Clin. Infect. Dis. 50, e38–e40. 10.1086/650582 [DOI] [PubMed] [Google Scholar]
- Wallace S. J., Li J., Nation R. L., Rayner C. R., Taylor D., Middleton D., et al. (2008). Subacute toxicity of colistin methanesulfonate in rats: Comparison of various intravenous dosage regimens. Antimicrob. Agents Chemother. 52, 1159–1161. 10.1128/AAC.01101-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Wang X., Wang J., Ouyang P., Jin C., Wang R., et al. (2018). Phenotypic and genotypic characterization of carbapenem-resistant enterobacteriaceae: Data from a longitudinal large-scale CRE study in China (2012-2016). Clin. Infect. Dis. 67, S196–S205. 10.1093/cid/ciy660 [DOI] [PubMed] [Google Scholar]
- Weinstein L., Doan T. L., Smith M. A. (2009). Neurotoxicity in patients treated with intravenous polymyxin B: Two case reports. Am. J. Health. Syst. Pharm. 66, 345–347. 10.2146/ajhp080065 [DOI] [PubMed] [Google Scholar]
- WHO (2021). Global antimicrobial resistance and use surveillance system (GLASS) report 2021. Geneva: World Health Organization. [Google Scholar]
- Williams J. R. (2008). The Declaration of Helsinki and public health. Bull. World Health Organ. 86, 650–652. 10.2471/BLT.08.050955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright H., Bonomo R. A., Paterson D. L. (2017). New agents for the treatment of infections with gram-negative bacteria: Restoring the miracle or false dawn? Clin. Microbiol. Infect. 23, 704–712. 10.1016/j.cmi.2017.09.001 [DOI] [PubMed] [Google Scholar]
- Xie J., Roberts J. A., Lipman J., Cai Y., Wang H., Zhao N., et al. (2020). Pharmacokinetic/pharmacodynamic adequacy of polymyxin B against extensively drug-resistant Gram-negative bacteria in critically ill, general ward and cystic fibrosis patient populations. Int. J. Antimicrob. Agents 55, 105943. 10.1016/j.ijantimicag.2020.105943 [DOI] [PubMed] [Google Scholar]
- Xu L., Sun X., Ma X. (2017). Systematic review and meta-analysis of mortality of patients infected with carbapenem-resistant Klebsiella pneumoniae . Ann. Clin. Microbiol. Antimicrob. 16, 18. 10.1186/s12941-017-0191-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Jiao Z., Zhang C., Dai Y., Zhou Z., Han L., et al. (2021). Population pharmacokinetic and optimization of polymyxin B dosing in adult patients with various renal functions. Br. J. Clin. Pharmacol. 87, 1869–1877. 10.1111/bcp.14576 [DOI] [PubMed] [Google Scholar]
- Zavascki A. P., Goldani L. Z., Li J., Nation R. L. (2007). Polymyxin B for the treatment of multidrug-resistant pathogens: A critical review. J. Antimicrob. Chemother. 60, 1206–1215. 10.1093/jac/dkm357 [DOI] [PubMed] [Google Scholar]
- Zavascki A. P., Goldani L. Z., Cao G., Superti S. V., Lutz L., Barth A. L., et al. (2008). Pharmacokinetics of intravenous polymyxin B in critically ill patients. Clin. Infect. Dis. 47, 1298–1304. 10.1086/592577 [DOI] [PubMed] [Google Scholar]
- Zhang X., Guo F., Shao H., Zheng X. (2017). Clinical translation of polymyxin-based combination therapy : Facts , challenges and future opportunities. J. Infect. 74, 118–130. 10.1016/j.jinf.2016.11.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.