Abstract
There have been conflicting data regarding liver transplantation (LT) outcomes for hereditary hemochromatosis (HH), with no recent data on LT outcomes in patients with HH in the past decade. Using the United Network for Organ Sharing registry, we evaluated waitlist and post-LT survival in all adult patients listed for HH without concomitant liver disease from 2003 to 2019. Post-LT survival for HH was compared with a propensity-matched (recipient and donor factors) cohort of recipients with chronic liver disease (CLD). From 2003 to 2019, 862 patients with HH were listed for LT, of which 55.6% (n = 479) patients underwent LT. The 1- and 5-year post-LT survival rates in patients with HH were 88.7% (95% confidence interval [CI], 85.4%–91.4%) and 77.5% (95% CI, 72.8%–81.4%), respectively, and were comparable with those in the propensity-matched CLD cohort (p value = 0.96). Post-LT survival for HH was lower than for Wilson’s disease, another hereditary metabolic liver disease with similar LT volume (n = 365). Predictors for long-term (5-year) post-LT mortality included presence of portal vein thrombosis (hazard ratio [HR], 1.96; 95% CI, 1.07–3.58), obesity measurements greater than Class II (HR, 1.98; 95% CI, 1.16–3.39), and Karnofsky performance status (HR, 0.98; 95% CI, 0.97–0.99) at the time of LT. The leading cause of post-LT death (n = 145) was malignancy (25.5%), whereas cardiac disease was the cause in less than 10% of recipients. In conclusion, short- and long-term survival rates for HH are excellent and comparable with those of other LT recipients. Improving extrahepatic metabolic factors and functional status in patients with HH prior to LT may improve outcomes.
INTRODUCTION
Hereditary hemochromatosis (HH) is an autosomal recessive disorder characterized by systemic iron overload through excessive intestinal iron absorption.[1] It represents one of the most prevalent genetic disorders among individuals of Northern European descent.[2] Over time, increased iron uptake results in progressive iron deposition and dysfunction in the liver, pancreas, heart, joints, and pituitary gland.[3] The lifetime incidence of cirrhosis among untreated men with HH approaches 10%.[4] Periodic phlebotomy remains the first-line treatment for HH[5]; however, the incidence of advanced liver disease requiring liver transplantation (LT) despite undergoing treatment is unknown. LT is the only therapeutic option for severe disease progression in patients with disease refractory to medical treatment.
Although HH accounts for approximately 1% of LTs performed in the United States, there is conflicting evidence regarding post-LT outcomes for HH.[6–13] Prior to 1996, a multicenter study suggested significantly decreased survival in HFE-associated HH when compared with all other LT recipients, with 1- and 5-year survival rates of 64% and 34%, respectively.[10] In contrast, Yu and Ioannou showed that national outcomes prior to 2006 were improved and comparable with LT recipients with viral hepatitis and cholestatic liver disease.[12] However, there are no recent data from the past decade that have explored population-level LT outcomes for HH and in relation to other hereditary metabolic liver diseases. Therefore, our aim was to evaluate temporal trends in waitlist and post-LT outcomes and identify clinical and donor risk factors associated with post-LT mortality in patients with HH.
PATIENTS AND METHODS
Using the United Network for Organ Sharing (UNOS) and Organ Procurement and Transplantation Network (OPTN) registry, we identified all adult patients listed for LT in the United States between January 1, 2003, and December 31, 2019, with follow-up through June 30, 2020. Our primary objective was to assess recent trends in waitlist and post-LT outcomes in adult patients with HH. Our secondary objective included comparing HH post-LT outcomes to a propensity-score matched cohort of patients with chronic liver disease (CLD). In addition, post-LT outcomes for HH were also compared with those of Wilson’s disease (WD), another hereditary metabolic liver disease with a similar number of performed LTs (n = 365). Furthermore, we explored the post-LT cause of death in these patients.
Data collection
The UNOS/OPTN registry compiles information from transplant centers, histocompatibility laboratories, and organ procurement organizations.[14] Standardized data collection forms must be submitted to UNOS for each transplantation. The “Transplant Candidate Registration” form includes pertinent patient information at the time of listing for transplantation, which was used in our analysis of waitlist survival. The “Transplant Recipient Registration” and “Transplant Recipient Follow-up” forms contain patient information at the time of transplant and subsequent follow-ups, respectively, which we used in our analysis of post-LT survival. In addition, donor characteristics from the “Deceased Donor Registration” and “Living Donor Registration” forms were used in our analysis of post-LT survival. We also focused our analysis on the donor characteristic components of the liver donor risk index (LDRI).[15]
Identifying patients with HH
Patients with HH were included only if they had a primary listing diagnosis for HH or a primary listing diagnosis for hepatocellular carcinoma (HCC) and a secondary diagnosis of HH using the UNOS diagnostic codes. We excluded patients with other concomitant etiologies of liver disease unless listed with a primary diagnosis of HCC and secondary diagnosis of HH. Patients listed for or who underwent LT for acute liver failure (Status 1A), simultaneous organ transplantation, or history of any previous transplantation were also excluded.
Outcomes of interest
We evaluated waitlist mortality defined as removal because of death or clinical deterioration from time of waitlist registration. Post-LT survival was determined by patient alive/dead status, and patients were censored if lost to follow-up or after death. Causes of post-LT mortality among recipients with HH were categorized into malignancy, sepsis, cardiovascular disease, graft failure, renal failure, respiratory failure, cerebrovascular accident (CVA), hemorrhage, nonimmunosuppressive drug related, and unknown.
Statistical analysis
Demographic characteristics of the study cohort were presented with frequencies and proportions for categorical variables and mean and standard deviation (SD) or median and interquartile range (IQR) for continuous variables.
Waitlist mortality and post-LT survival rates over time were reported using the Kaplan–Meier curve method and log-rank testing for equality of survivor functions.[16] In addition, we compared post-LT survival in patients with HH with a comparable cohort of patients with CLD.
For the purposes of this study, the CLD cohort was composed of adult recipients from the same study period with liver disease attributed to any etiology other than HH. Similar to the HH cohort, patients listed for or who underwent LT for acute liver failure (Status 1A), simultaneous organ transplantation, or history of any previous transplantation were excluded. LT recipients with HH were propensity matched to recipients with CLD with respect to recipient and donor demographic information (age, sex, race/ethnicity), Model for End-Stage Liver Disease (MELD) score at LT, transplant year, LDRI parameters (donor cause of death, donation after circulatory death, partial/split-liver graft, organ location, cold ischemia time), HCC, and living donation. Separately, we compared survival to an unmatched cohort of recipients with WD. Characteristics of propensity matching for the CLD cohort and differences between recipients with WD are shown in Tables S1 and S2, respectively.
Multivariable Cox proportional hazards models were developed to assess for clinical risk factors predictive of waitlist mortality and post-LT survival.[17] General stepwise regression was performed with a significance threshold for inclusion of clinical characteristics in the multivariable models with a univariable p value <0.05. Patient age, sex, and race/ethnicity were included in the multivariable Cox proportional hazards models; a priori covariates of interest included age, sex, and race/ethnicity in the waitlist and post-LT models. Donor age, sex, and race/ethnicity were included in the post-LT model as well. All multivariable models were run with and without Karnofsky performance status (KPS) given the limitations in its reliability in the UNOS registry.[18] Harrell’s C indexes were calculated to determine the goodness of fit measures for these risk models.[19] Statistical analysis was performed using Stata/IC Version 16.1 (StataCorp LLC, College Station, TX).
RESULTS
Clinical characteristics
A total of 862 patients with HH were listed for LT from 2003 to 2019, and 55.6% (n = 479) of these patients underwent LT during the study period. Table 1 shows the characteristics of patients with HH who were listed for and underwent LT. The mean age at listing was 57.5 years (SD, 8.9), and most waitlisted patients were men (81.9%) and non-Hispanic White (91.1%). The following indicators of hepatic decompensation characterized the waitlisted HH population: 69.3% with ascites, 57.1% with any encephalopathy, and 7.0% with a history of spontaneous bacterial peritonitis (SBP). Of the patients, 10.7% (n = 92) had a concomitant diagnosis of HCC attributable to HH. Severity of hepatic decompensation, as expected, increased in patients who underwent LT. UNOS Regions 3 and 4, which correspond to the South and Southeast, had the largest proportion of listings and LT for HH.
Table 1.
Clinical characteristics of patients with HH who were listed for and underwent LT from 2003 to 2019 with recipient and donor demographics
| Demographics | Patients who were on the waiting list | Patients who received an LT |
|---|---|---|
| Patients, n | 862 | 479 |
| Recipient characteristics | ||
| Age, mean (SD), y | 57.5 (8.9) | 58.2 (8.2) |
| Female, No. (%) | 156 (18.1) | 67 (14.0) |
| Race/ethnicity, No. (%) | ||
| Non-Hispanic white | 785 (91.1) | 433 (90.4) |
| Black | 16 (1.9) | 8 (1.7) |
| Hispanic | 47 (5.5) | 29 (6.1) |
| Obtained associate or bachelor degree, No. (%) | 263 (30.5) | 156 (32.6) |
| Employed, No. (%) | 236 (27.4) | 94 (19.6) |
| Karnofsky performance status, mean (SD), % rating | 65.2 (21.9) | 56.0 (23.6) |
| Albumin, mean (SD), g/dL | 3.2 (0.7) | 3.2 (0.8) |
| Previous abdominal surgery, No. (%) | 310 (36.0) | 195 (40.7) |
| BMI, mean (SD), kg/m2 | 29.2 (5.6) | 28.8 (5.4) |
| Diabetes mellitus, No. (%) | 289 (33.5) | 161 (33.6) |
| Ascites, No. (%) | 597 (69.3) | 348 (72.7) |
| Any encephalopathy, No. (%) | 492 (57.1) | 302 (63.0) |
| Spontaneous bacterial peritonitis, No. (%) | 60 (7.0) | 40 (8.4) |
| Portal vein thrombosis, No. (%) | 33 (3.8) | 52 (10.9) |
| MELD score, mean (SD) | 17.5 (9.0) | 21.2 (10.2) |
| HCC, No. (%) | 92 (10.7) | 59 (12.3) |
| TIPS, No. (%) | 44 (5.1) | 36 (7.5) |
| On life support, No. (%) | 23 (2.7) | 23 (4.8) |
| Creatinine, mean (SD), mg/dL | 1.2 (0.9) | 1.3 (0.9) |
| Dialysis, No. (%) | 35 (4.1) | 34 (7.1) |
| Wait time, median (IQR), days | ||
| Overall on the waitlist | 137 (391) | - |
| To transplant | 61 (196) | - |
| To death on the waitlist | 141 (389) | - |
| UNOS region, No. (%) | ||
| 1: CT, ME, MA, NH, RI, E. VT | 55 (6.4) | 22 (4.6) |
| 2: DE, DC, MD, NJ, PA, WV, N. VA | 112 (13.0) | 52 (10.9) |
| 3: AL, AR, FL, GA, LA, MS, PR | 113 (13.1) | 84 (17.5) |
| 4: OK, TX | 140 (16.2) | 64 (13.4) |
| 5: AZ, CA, NV, NM, UT | 78 (9.1) | 35 (7.3) |
| 6: AK, HI, ID, MT, OR, WA | 14 (1.6) | 9 (1.9) |
| 7: IL, MN, ND, SD, WI | 85 (9.9) | 48 (10.0) |
| 8: CO, IA, KS, MO, NE, WY | 45 (5.2) | 30 (6.3) |
| 9: NY, W. VT | 58 (6.7) | 30 (6.3) |
| 10: IN, MI, OH | 72 (8.4) | 48 (10.0) |
| 11: KY, NC, SC, TN, VA | 90 (10.4) | 57 (11.9) |
| Year of listing/transplant, No. (%) | ||
| 2003 – 2009 | 344 (39.9) | 190 (39.7) |
| 2010 – 2014 | 230 (26.7) | 122 (25.5) |
| 2015 – 2019 | 288 (33.4) | 167 (34.9) |
| Retransplant, No. (%) | - | 24 (5.0) |
| Donor characteristics | - | n = 479 |
| Age, mean (SD), y | - | 44.6 (16.6) |
| Female, No. (%) | - | 165 (34.4) |
| Race/ethnicity, No. (%) | ||
| Non-Hispanic white | - | 327 (68.3) |
| Black | - | 89 (18.6) |
| Hispanic | - | 50 (10.4) |
| BMI, mean (SD), kg/m2 | - | 28.3 (6.7) |
| Cold ischemia time, mean (SD), h | - | 6.2 (2.8) |
| Cause of death, No. (%) | ||
| Anoxia | - | 124 (25.9) |
| CVA | - | 186 (38.8) |
| Trauma | - | 134 (28.0) |
| Organ location, No. (%) | ||
| Local | - | 351 (73.3) |
| Regional | - | 107 (22.3) |
| National | - | 21 (4.4) |
| Donation after cardiac death, No. (%) | - | 29 (6.1) |
| Macrovesicular steatosis > 5%, No. (%) | - | 65 (13.6) |
| History of hypertension, No. (%) | - | 195 (40.7) |
| History of diabetes, No. (%) | - | 65 (13.6) |
| High risk for blood-borne disease transmission, No. (%) | - | 63 (13.2) |
| Positive for anti-HBc, No. (%) | - | 27 (5.6) |
All waitlist characteristics were ascertained at the time of listing. All transplant characteristics were ascertained at the time of transplant, except the presence of diabetes, SBP, and HCC, which were only specified at the time of listing.
Abbreviations: BMI = body mass index, MELD = model for end-stage liver disease, HCC = hepatocellular carcinoma, TIPS = transjugular intrahepatic portosystemic shunt, CVA= cerebrovascular accident, anti-HBc = hepatitis B core antibody
After censoring post-LT follow-up survival time in those who underwent LT, the 1-year waitlist survival rate was 80.1% (95% confidence interval [CI], 75.7%–83.8%). The median time to death while on the waiting list was 141 days (IQR, 389 days). In the multivariable model shown in Table 2, waitlisted patients with increasing age (hazard ratio [HR], 1.03; 95% CI, 1.01–1.05) and MELD score (HR, 1.12; 95% CI, 1.09–1.16) and those requiring life support measures in the intensive care unit (HR, 7.18; 95% CI, 2.73–18.91) were at higher risk for waitlist mortality. Of note, patients with increasing KPS (HR, 0.98; 95% CI, 0.97–0.99) and those who were employed at the time of listing (HR, 0.40; 95% CI, 0.21–0.77) had decreased waitlist mortality. In addition to KPS, hypoalbuminemia, another factor associated with sarcopenia and functional status, was associated with waitlist mortality in the univariable analysis, but did not reach statistical significance in the multivariable analysis. Harrell’s C index for the multivariable model was 0.85/0.82 (with and without KPS).
Table 2:
Univariable and multivariable models assessing clinical risk factors for waitlist mortality in patients with HH.
| Waitlist Mortality Cox Proportional Hazard Ratios | ||||
|---|---|---|---|---|
| Waitlist mortality Univariable model Cox proportional HR (95% CI) | Multivariable model Cox proportional HR (95% CI) | |||
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age at listing | 1.01 (0.99 – 1.04) | 0.22 | 1.03 (1.01 – 1.06)/1.03 (1.01 – 1.05) a | < 0.01/0.02 a |
| Female | 1.81 (1.19 – 2.75) | < 0.01 | 1.41 (0.89 – 2.24)/1.29 (0.83 – 2.00)a | 0.15/0.25a |
| Race/ethnicity | ||||
| Non-Hispanic white | [Reference] | [Reference] | ||
| Black | 7.59 (2.74 – 21.00) | < 0.01 | 2.42 (0.78 – 7.48)/2.14 (0.70 – 6.51)a | 0.13/0.18a |
| Hispanic | 0.99 (0.40 – 2.44) | 0.98 | 0.87 (0.34 – 2.22)/0.95 (0.38 – 2.35)a | 0.77/0.91a |
| Obtained associate or bachelor degree | 0.62 (0.39 – 0.99) | 0.04 | 0.70 (0.41 – 1.19)/0.63 (0.38 – 1.04)a | 0.19/0.07a |
| Employed at listing | 0.27 (0.15 – 0.51) | < 0.01 | 0.51 (0.25 – 1.02)/0.40 (0.21 – 0.77)a | 0.06/< 0.01a |
| Karnofsky performance status at listing | 0.96 (0.95 – 0.97) | < 0.01 | 0.98 (0.97 – 0.99) | 0.04 |
| Albumin at listing | 0.52 (0.39 – 0.69) | < 0.01 | 0.91 (0.65 – 1.28)/0.80 (0.59 – 1.10)a | 0.59/0.17a |
| Previous abdominal surgery at listing | 1.32 (0.89 – 1.95) | 0.18 | ||
| BMI at listing, kg/m2 | ||||
| < 18.5 | 1.18 (0.16 – 8.65) | 0.87 | ||
| 18.5 – 24.9 | [Reference] | |||
| 25 – 29.9 | 0.70 (0.42 – 1.16) | 0.17 | ||
| 30 – 34.9 | 0.74 (0.43 – 1.27) | 0.27 | ||
| ≥ 35 | 0.91 (0.51 – 1.61) | 0.74 | ||
| Diabetes mellitus at listing | 1.13 (0.76 – 1.68) | 0.55 | ||
| Ascites at listing | 2.06 (1.26 – 3.36) | < 0.01 | 1.34 (0.76 – 2.37)/1.32 (0.78 – 2.23)a | 0.32/0.30a |
| Encephalopathy at listing | 1.68 (1.20 – 2.35) | < 0.01 | 0.93 (0.64 – 1.36)/0.99 (0.69 – 1.42)a | 0.72/0.95a |
| Spontaneous bacterial peritonitis at listing | 2.23 (1.12 – 4.43) | 0.02 | 0.93 (0.42 – 2.06)/0.72 (0.33 – 1.56)a | 0.86/0.40a |
| History of portal vein thrombosis at listing | 0.66 (0.16 – 2.69) | 0.56 | ||
| MELD score at listing | 1.14 (1.11 – 1.17) | < 0.01 | 1.11 (1.07 – 1.15)/1.12 (1.09 – 1.16) a | < 0.01/< 0.01 a |
| HCC at listing | 0.28 (0.09 – 0.90) | 0.03 | 0.40 (0.09 – 1.67)/0.58 (0.18 – 1.89)a | 0.21/0.37a |
| TIPS at listing | 0.62 (0.23 – 1.68) | 0.35 | ||
| On life support at listing | 19.68 (9.25 – 41.85) | < 0.01 | 4.13 (1.46 – 11.67)/7.18 (2.73 – 18.91) a | < 0.01/< 0.01 a |
| Dialysis at listing | 5.81 (3.00 – 11.24) | < 0.01 | 0.74 (0.33 – 1.65)/0.78 (0.34 – 1.76)a | 0.46/0.54a |
| Year of listing | ||||
| 2003 – 2009 | [Reference] | |||
| 2010 – 2014 | 1.09 (0.70 – 1.70) | 0.71 | ||
| 2015 – 2019 | 0.88 (0.54 – 1.44) | 0.60 | ||
HR, hazard ratio
Harrell’s C index for multivariable model = 0.85/0.82a
Multivariable model run without Karnofsky performance status given limitations in its reliability in the UNOS registry
Adjusted for UNOS region of listing
LT outcomes
From 2003 to 2019, 55.6% (n = 479) of patients on the waiting list because of HH underwent LT. The mean MELD score at LT was 21.2 (SD, 10.2), with a median time to LT of 61 days (IQR, 196 days). The mean follow-up time after LT was 5.0 years (SD, 4.4). Overall, the 1-, 3-, and 5-year post-LT survival rates in patients with HH were 88.7% (95% CI, 85.4%–91.4%), 82.3% (95% CI, 78.2%–85.7%), and 77.5% (95% CI, 72.8%–81.4%), respectively. Figure 1 compares survival outcomes in patients with HH with a comparable cohort of recipients with CLD propensity matched for recipient and donor demographics, MELD score at LT, transplant year, LDRI parameters, HCC, and living donation (Table S1). Compared with LT recipients with HH (n = 479), matched recipients with CLD (n = 484) experienced no difference in post-LT survival (log-rank p value = 0.96), with 1- and 5-year post-LT survival rates of 89.4% (95% CI, 86.2%–91.9%) and 75.7% (95% CI, 70.9%–79.8%), respectively.
FIGURE 1.
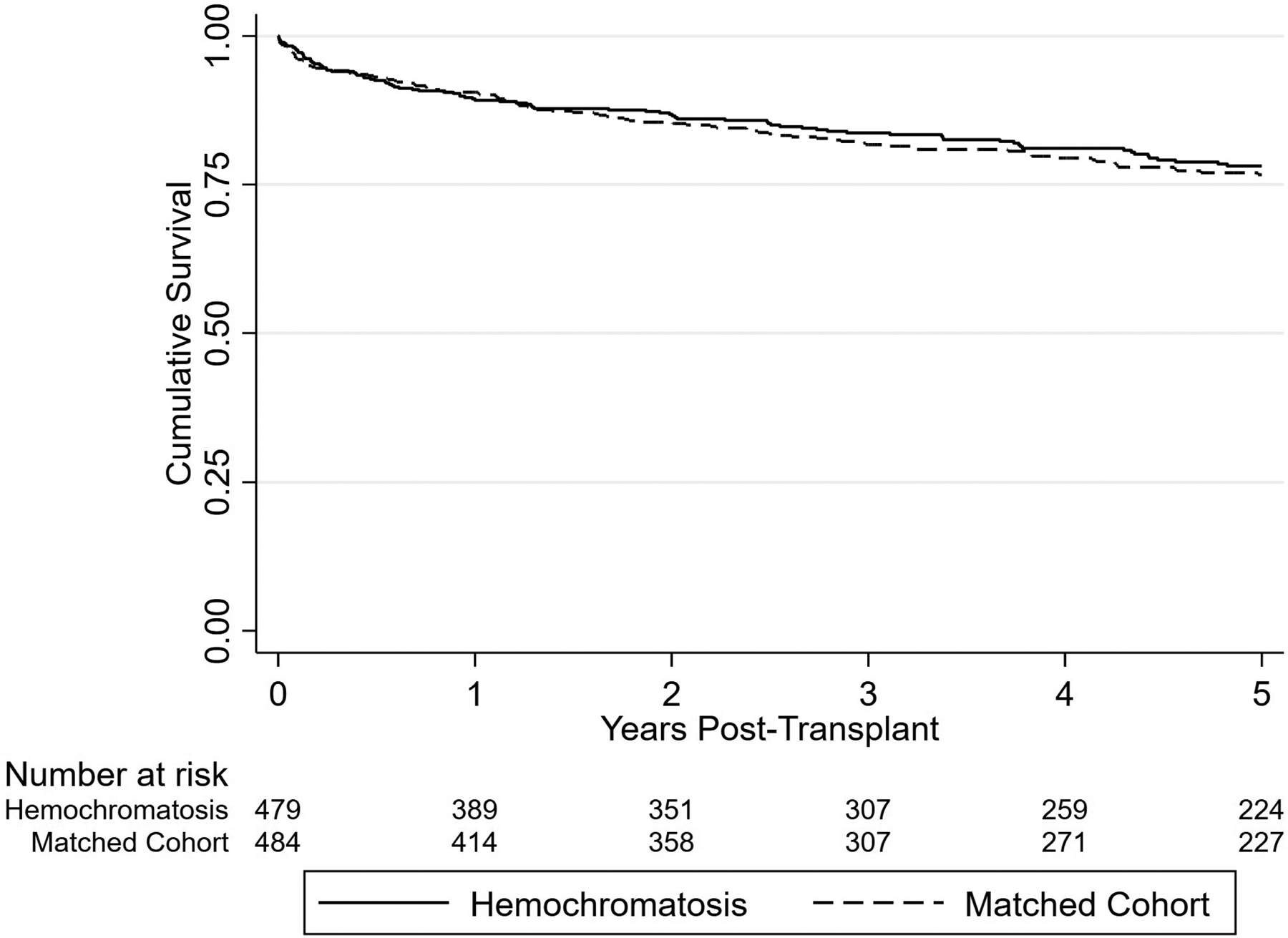
Kaplan–Meier curve plot demonstrating survival outcomes after LT for patients with HH compared with a propensity-matched cohort with CLD. Log-rank p value = 0.96
Separately, in an unadjusted survival analysis, recipients with HH were compared with recipients with WD, and there were a comparable number of LTs performed during the study period (n = 365). As shown in Figure 2, recipients with WD had significantly better post-LT survival than those with HH, with 1- and 5-year survival rates of 94.7% (95% CI, 91.7%–96.6%) and 89.2% (95% CI, 85.0%–92.2%), respectively. However, recipients with WD were significantly younger than recipients with HH (mean age, 35.3 years vs. 58.2 years; p < 0.01). Differences in characteristics between these cohorts are shown in Table S2.
FIGURE 2.
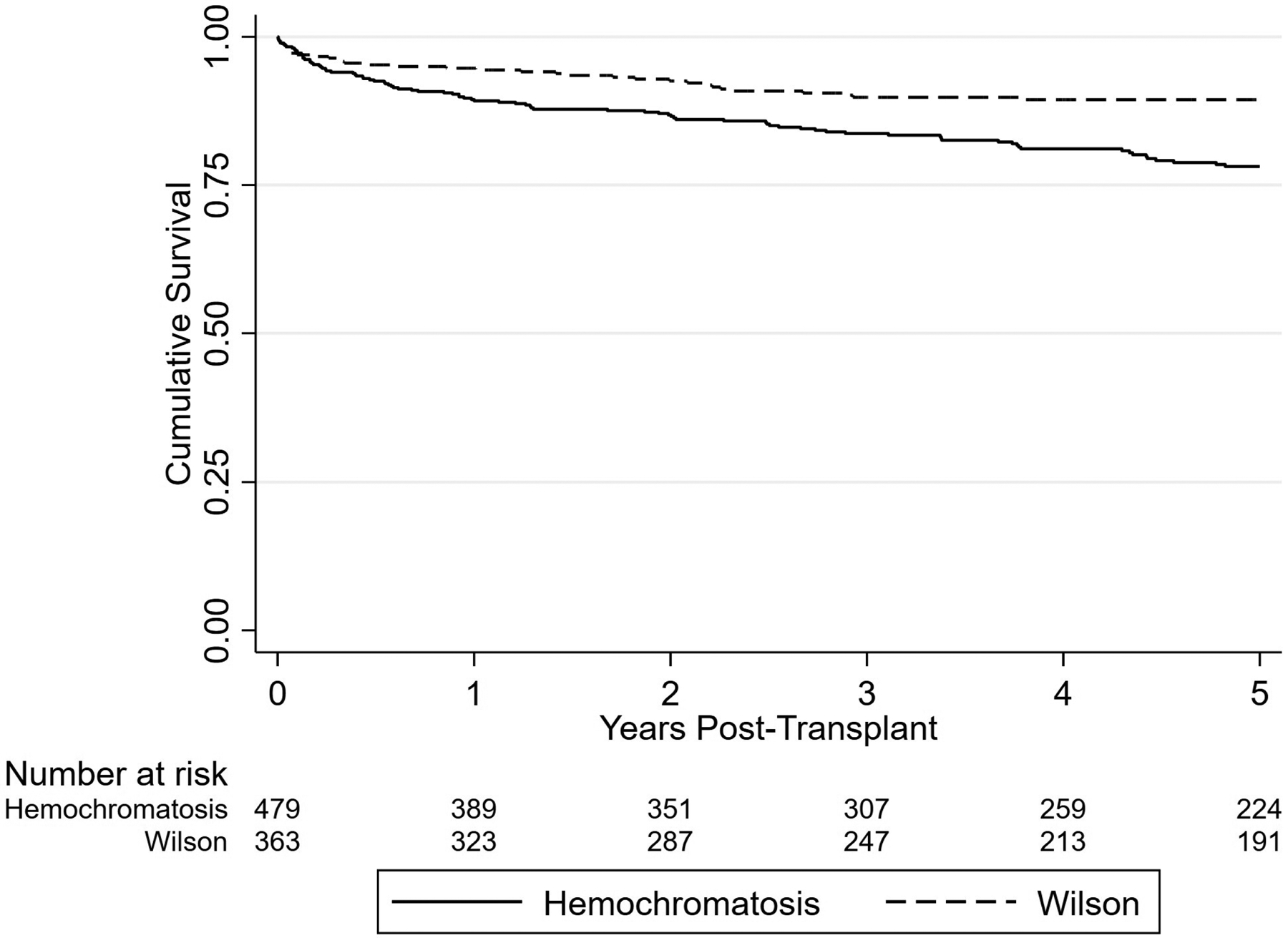
Kaplan–Meier curve plot demonstrating survival outcomes after LT for patients with HH versus patients with WD. Log-rank p value <0.01
Clinical predictors for long-term (5-year) post-LT survival in recipients with HH are shown in Table 3. The multivariable analysis showed that the presence of portal vein thrombosis (PVT; HR, 1.96; 95% CI, 1.07–3.58) and obesity measurements greater than Class II (HR, 1.98; 95% CI, 1.16–3.39) at LT were associated with an increased risk for long-term mortality. Recipients with increasing KPS (HR, 0.98; 95% CI, 0.97–0.99) had a decreased risk for mortality. Harrell’s C index for the long-term risk model was 0.71/0.70 (with and without KPS). Although there was no statistically significant difference in post-LT survival with transplant year or era (4-year era cohorts: 2003–2006, 2007–2010, and 2011–2014), survival was observed to improve over time as shown in Figure S1.
Table 3:
Univariable and multivariable predictors of post-LT 5-year mortality
| Post-LT Mortality Cox Proportional Hazard Ratios | ||||
|---|---|---|---|---|
| Post-LT 5-year mortality Univariable Cox proportional HR (95% CI) | Multivariable Cox proportional HR (95% CI) | |||
| Recipient characteristics | HR (95% CI) | p value | HR (95% CI) | p value |
| Age at transplant | 1.02 (0.99 – 1.04) | 0.28 | 1.03 (1.00 – 1.06)/1.02 (0.99 – 1.05)a | 0.09/0.23a |
| Female | 0.89 (0.46 – 1.72) | 0.73 | 0.63 (0.30 – 1.31)/0.73 (0.37 – 1.46)a | 0.21/0.38a |
| Race/ethnicity | ||||
| Non-Hispanic white | [Reference] | [Reference] | ||
| Black | 2.71 (0.85 – 8.59) | 0.09 | 3.02 (0.81 – 11.22)/2.42 (0.66 – 8.90)a | 0.10/0.18a |
| Hispanic | 1.22 (0.53 – 2.79) | 0.64 | 1.55 (0.64 – 3.77)/1.25 (0.52 – 3.00)a | 0.33/0.61a |
| Obtained associate or bachelor degree | 0.98 (0.62 – 1.55) | 0.94 | ||
| Employed at transplant | 0.85 (0.49 – 1.48) | 0.57 | ||
| Karnofsky performance status at transplant | 0.98 (0.97 – 0.99) | < 0.01 | 0.98 (0.97 – 0.99) | < 0.01 |
| Albumin at transplant | 0.91 (0.69 – 1.20) | 0.50 | ||
| Previous abdominal surgery at transplant | 0.87 (0.56 – 1.35) | 0.54 | ||
| BMI at transplant, kg/m2 | ||||
| < 18.5 | 2.98 (0.67 – 12.83) | 0.14 | ||
| 18.5 – 24.9 | [Reference] | [Reference] | ||
| 25 – 29.9 | 1.29 (0.73 – 2.27) | 0.38 | ||
| 30 – 34.9 | 0.71 (0.35 – 1.45) | 0.35 | ||
| ≥ 35 | 2.04 (1.06 – 3.92) | 0.03 | 1.58 (0.87 – 2.88)/1.98 (1.16 – 3.39)a | 0.13/0.01a |
| Diabetes mellitus at listing | 1.07 (0.69 – 1.66) | 0.77 | ||
| Ascites at transplant | 0.90 (0.56 – 1.46) | 0.68 | ||
| Encephalopathy at transplant | 0.97 (0.68 – 1.38) | 0.87 | ||
| Spontaneous bacterial peritonitis at listing | 1.37 (0.69 – 2.74) | 0.37 | ||
| Portal vein thrombosis at transplant | 1.96 (1.10 – 3.48) | 0.02 | 2.03 (1.09 – 3.80)/1.96 (1.07 – 3.58) a | 0.03/0.03 a |
| MELD score at transplant | 1.02 (1.00 – 1.04) | 0.05 | ||
| HCC at listing | 1.14 (0.62 – 2.09) | 0.68 | ||
| TIPS at transplant | 1.12 (0.52 – 2.43) | 0.77 | ||
| On life support at transplant | 2.40 (1.11 – 5.21) | 0.03 | 1.13 (0.38 – 3.36)/1.54 (0.52 – 4.58)a | 0.83/0.43a |
| Dialysis at transplant | 2.35 (1.25 – 4.43) | < 0.01 | 1.83 (0.75 – 4.47)/2.31 (0.98 – 5.46)a | 0.18/0.06a |
| Wait time to transplant | 1.00 (0.99 – 1.01) | 0.75 | ||
| Year of transplant | ||||
| 2003 – 2009 | [Reference] | |||
| 2010 – 2014 | 0.83 (0.51 – 1.36) | 0.47 | ||
| 2015 – 2019 | 0.59 (0.34 – 1.05) | 0.07 | ||
| Retransplant | 3.22 (1.18 – 8.78) | 0.02 | 1.56 (0.44 – 5.62)/2.73 (0.92 – 8.14)a | 0.49/0.07a |
| 5-year | ||||
| Univariable | Multivariable | |||
| Donor characteristics | HR (95% CI) | P value | HR (95% CI) | P value |
| Age | 1.01 (1.00 – 1.03) | 0.06 | 1.02 (1.01 – 1.04)/1.00 (0.99 – 1.02)a | 0.04/0.62a |
| Female | 1.19 (0.76 – 1.84) | 0.45 | 0.97 (0.60 – 1.59)/1.08 (0.69 – 1.71)a | 0.91/0.73a |
| Race/ethnicity | ||||
| Non-Hispanic white | [Reference] | [Reference] | ||
| Black | 1.52 (0.92 – 2.50) | 0.10 | 1.31 (0.74 – 2.29)/1.35 (0.80 – 2.29)a | 0.35/0.26a |
| Hispanic | 0.94 (0.45 – 1.98) | 0.88 | 1.10 (0.46 – 2.60)/1.13 (0.53 – 2.39)a | 0.84/0.76a |
| BMI, kg/m2 | ||||
| < 18.5 | 1.70 (0.59 – 4.89) | 0.33 | ||
| 18.5 – 24.9 | [Reference] | |||
| 25 – 29.9 | 1.38 (0.82 – 2.31) | 0.23 | ||
| 30 – 34.9 | 0.95 (0.49 – 1.84) | 0.88 | ||
| ≥ 35 | 0.90 (0.40 – 2.00) | 0.79 | ||
| Cold ischemia time, h | ||||
| < 3 | [Reference] | |||
| 3 – 5.9 | 1.71 (0.67 – 4.35) | 0.26 | ||
| 6 – 8.9 | 1.43 (0.55 – 3.67) | 0.46 | ||
| ≥ 9 | 1.42 (0.50 – 3.97) | 0.51 | ||
| Cause of death | ||||
| Anoxia | 0.91 (0.54 – 1.53) | 0.72 | ||
| CVA | 1.93 (1.26 – 2.94) | < 0.01 | 1.18 (0.64 – 2.18)/1.50 (0.85 – 2.63)a | 0.59/0.16a |
| Trauma | 0.49 (0.28 – 0.84) | < 0.01 | 0.83 (0.42 – 1.66)/0.72 (0.37 – 1.41)a | 0.60/0.34a |
| Organ location | ||||
| Local | [Reference] | |||
| Regional | 0.86 (0.50 – 1.46) | 0.57 | ||
| National | 0.83 (0.26 – 2.63) | 0.74 | ||
| Donation after cardiac death | 1.52 (0.66 – 3.48) | 0.33 | ||
| Macrovesicular steatosis > 5% | 1.21 (0.67 – 2.17) | 0.54 | ||
| History of hypertension | 1.39 (0.91 – 2.12) | 0.13 | ||
| History of diabetes | 1.41 (0.81 – 2.46) | 0.23 | ||
| High risk for blood-borne disease transmission | 1.43 (0.79 – 2.58) | 0.24 | ||
| Positive for anti-HBc | 0.84 (0.31 – 2.29) | 0.73 | ||
Note: Harrell’s C index for the 5-year multivariable model = 0.71/0.70.
Bold table values indicate statistically significant predictors in the univariate and multivariate analyses, respectively.
Abbreviations: anti-HBc, hepatitis B core antibody; BMI, body mass index; CI, confidence interval; HCC, hepatocellular carcinoma; CVA, cerebrovascular accident; HR, hazard ratio; KPS, Karnofsky performance status; LT, liver transplantation; MELD, Model for End-Stage Liver Disease; PVT, portal vein thrombosis; SBP, spontaneous bacterial peritonitis; TIPS, transjugular intrahepatic portosystemic shunt; UNOS, United Network for Organ Sharing.
Multivariable model run without KPS given limitations in its reliability in the UNOS registry and adjusted for UNOS region of transplant.
Cause of death
Of the 145 total documented deaths among LT recipients with HH, 105 (72.4%) had a specified primary cause of death in the UNOS registry as shown in Table 4. Overall, the three most common causes of death in order were malignancy (25.5%), sepsis (24.1%), and cardiovascular disease (8.3%), respectively. The median time to death for recipients secondary to malignancy was 3.7 years (IQR, 6.4 years). After stratifying the recipients’ year of death, the most common causes of post-LT death from 2003 to 2008 were sepsis (30.4%), malignancy (17.4%), and graft failure (13.0%). From 2009 to 2014, sepsis (33.3%), malignancy (26.3%), and cardiovascular disease (7.0%) were the most frequent causes of death, whereas malignancy (27.7%), sepsis (13.8%), and cardiovascular disease (10.8%) were the leading causes of death from 2015 to 2020.
Table 4:
Primary causes of death post-LT for patients with HH
| Causes of Post-LT Death | Overall | 2003 – 2008 | 2009 – 2014 | 2015 – 2020 |
|---|---|---|---|---|
| n = 145 | n = 23 | n = 57 | n = 65 | |
| Malignancy, No. (%) | 37 (25.5) | 4 (17.4) | 15 (26.3) | 18 (27.7) |
| Median time to death, days (IQR) | 1365 (2343) | - | - | - |
| Sepsis, No. (%) | 35 (24.1) | 7 (30.4) | 19 (33.3) | 9 (13.8) |
| Cardiovascular, No. (%) | 12 (8.3) | 1 (4.3) | 4 (7.0) | 7 (10.8) |
| Graft failure, No. (%) | 7 (4.8) | 3 (13.0) | 2 (3.5) | 2 (3.1) |
| Renal failure, No. (%) | 5 (3.4) | - | 3 (5.3) | 2 (3.1) |
| Respiratory failure, No. (%) | 4 (2.8) | - | 2 (3.5) | 2 (3.1) |
| Cerebrovascular, No. (%) | 3 (2.1) | - | 1 (1.8) | 2 (3.1) |
| Hemorrhage, No. (%) | 1 (0.7) | 1 (4.3) | - | - |
| Non-immunosuppressive drug related, No. (%) | 1 (0.7) | - | - | 1 (1.5) |
| Unknown, No. (%) | 40 (27.6) | 7 (30.4) | 11 (19.3) | 22 (33.8) |
Abbreviations: CVA, cerebrovascular accident; HH, hereditary hemochromatosis; IQR, interquartile range; LT, liver transplantation.
DISCUSSION
Although HH is the most common hereditary metabolic liver disease, it remains an uncommon cause for LT. Outcomes for patients with HH undergoing LT remain unclear, without reported outcomes in this population in more than a decade. We demonstrated that post-LT survival for patients with HH is excellent and, more important, comparable with outcomes for other causes of CLD. Survival for HH was shown to be lower than WD, possibly as a result of older age and the longer disease duration to transplant evaluation seen in HH.[20,21] PVT, obesity, and poor functional status at the time of LT were significant predictors of long-term post-LT mortality.
With the adoption of the MELD score for LT allocation along with other dynamic changes in policies and candidate selection, previously reported outcomes may not be relevant to the current landscape in LT.[9,22] Prior studies that reported poor post-LT outcomes for HH likely included patients with HCC outside of the CT2 criteria that would not qualify for an HCC exception with the current policies. For example, Crawford et al. revealed that four of 22 recipients with HH had large tumors ranging from 7 to 12 cm in diameter at the time of LT, and all died from recurrent HCC.[9] In a study analyzing post-LT survival from 1997 to 2006, Yu and Ioannou showed that survival for HH was higher than previously reported, and their study demonstrated excellent average post-LT survival rates with no difference compared with all other LT recipients.[12] However, when comparing HH, an uncommon indication (0.5%) for LT, with all other recipients, this may have contributed to a null effect found in survival outcomes in that analysis. Rather, in our cohort propensity matched for relevant recipient and donor characteristics, we provide evidence that post-LT survival is indeed comparable with other recipients in the MELD era.
Waitlist survival for HH was found to be comparable with that of the overall waiting list.[23,24] Patients with HH who were employed at listing had better waitlist outcomes, and those with diminished KPS had worse outcomes, indicating that functional status may be a key determinant in outcomes in HH. Hypoalbuminemia, another surrogate for functional status, was also found to be a strong predictor of waitlist mortality in the univariable analysis. As seen in Table 1, there was a significant decrease in mean KPS from waiting list to transplant (65.2 vs. 56.0; p < 0.01). Frailty has been shown to be a significant factor in outcomes of waitlist and post-LT survival, even for those with low MELD scores.[25] Furthermore, PVT, obesity, and low KPS were significant predictors for post-LT mortality and could suggest that closer management of intrahepatic and extrahepatic manifestations of HH-induced cirrhosis at the time of and following LT can improve outcomes. Risk associated with PVT had the highest magnitude of risk within the first year after LT, and decreased with time, albeit significant. This suggests that the mortality risk with PVT was likely from early post-LT complications, but the plausibility of underlying hypercoagulable conditions playing a significant role should be explored further. Retransplant, another surgical parameter, was found to be a significant predictor of post-LT mortality in the univariable analysis. Although statistical significance for this surgical predictor was lost in the multivariable analysis, this could be attributed to the small number of events in this study cohort. In contrast to a prior study, we also found malignancy, not cardiovascular disease, to be the leading cause of death in recipients.[12] Previously, most post-LT deaths were attributable to cardiac-related deaths in the perioperative period from inadequate removal of excess iron stores prior to LT. Less than 10% of recipients died as a result of cardiovascular causes, suggesting improvement in waitlist management over time. Because of limitations in reporting in the UNOS registry, we were unable to determine the type of malignancy that resulted in post-LT death. Cancer risk in patients with HH has been well studied, with a 20-fold increased risk of HCC and minimal increased risk of all other cancers[26]; the risk for cancer in HH following LT is not well described. Recurrent HCC was listed as the most common cause of post-LT death in a small study of 22 patients with HH.[9] A multicenter retrospective study assessing malignancy risk following LT is needed and can help provide information on determining post-LT malignancy surveillance in this population.
The limitations of our study are related to the inherent drawbacks of using large data sets, such as the UNOS registry. We identified patients with HH via the diagnosis codes reported to UNOS by transplant centers. We were unable to independently confirm the diagnosis with hepatic iron concentrations, potential effect of secondary iron overload, and HFE/non-HFE genotypes because most transplant centers do not have HFE genotyping information. As HH is a genetic diagnosis restricted to the Caucasian population, another limitation of our data set–based study is that we cannot definitively conclude that 8.9% of waitlisted patients were non-White. Our understanding of genotypes/phenotypes in HH continues to evolve and is integral in future prognostic studies for HH. In addition, because of the risk of misclassification of exposure and reporting bias as well as the inability to accurately determine the interaction between etiologies, we did not evaluate the effects on patients with HH or iron overload who had concomitant liver disease. It is possible that HCC could contribute to selection bias between cohorts given that patients with HH with a primary diagnosis of HCC were included in this study and that malignancy was the most prevalent cause of death. We attempted to address this limitation by comparing post-LT survival of the HH cohort to that of a CLD cohort propensity matched for several factors, including HCC status. Cause of death was missing in approximately 28% of recipients, a significant percentage; however, this is a lower percentage of missing data than reported in other studies.[12] There are limited systematic studies evaluating obesity and functional status in patients with HH compared with other etiologies of CLD. These underlying causes may explain the mortality associated with patients with HH at the time of LT.[27,28]
In summary, our study shows that patients with HH listed for LT from 2003 to 2019 in the United States had excellent survival outcomes comparable with all other LT recipients. Our findings also suggest that HH alone does not predict poor outcomes after LT, as transplant centers have improved preoperative and post-LT management. PVT, obesity, and poor functional status at the time of LT are significant predictors of post-LT mortality and are potential areas to improve outcomes in this population. Together, these factors should be taken into consideration when evaluating candidates with HH for LT.
Supplementary Material
Funding information
Cancer Prevention and Research Institute of Texas, Grant/Award Number: RP150587; National Cancer Institute, Grant/Award Number: R01 CA256977 and U01 CA230997
Abbreviations:
- anti-HBc
hepatitis B core antibody
- BMI
body mass index
- CI
confidence interval
- CLD
chronic liver disease
- CVA
cerebrovascular accident
- HCC
hepatocellular carcinoma
- HH
hereditary hemochromatosis
- HR
hazard ratio
- IQR
interquartile range
- KPS
Karnofsky performance status
- LDRI
liver donor risk index
- LT
liver transplantation
- MELD
Model for End-Stage Liver Disease
- OPTN
Organ Procurement and Transplantation Network
- PVT
portal vein thrombosis
- SBP
spontaneous bacterial peritonitis
- SD
standard deviation
- TIPS
transjugular intrahepatic portosystemic shunt
- UNOS
United Network for Organ Sharing
- WD
Wilson’s disease
Footnotes
CONFLICT OF INTEREST
Nothing to report.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Brissot P, Pietrangelo A, Adams PC, de Graaff B, McLaren CE, Loréal O. Haemochromatosis. Nat Rev Dis Primers. 2018;4:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feder JN, Gnirke A, Thomas W, Tsuchihashi Z, Ruddy DA, Basava A, et al. A novel MHC class I–like gene is mutated in patients with hereditary haemochromatosis. Nat Genet. 1996;13:399–408. [DOI] [PubMed] [Google Scholar]
- 3.Kowdley KV, Brown KE, Ahn J, Sundaram V. ACG clinical guideline: hereditary hemochromatosis. Am J Gastroenterol. 2019;114:1202–18. [DOI] [PubMed] [Google Scholar]
- 4.Grosse SD, Gurrin LC, Bertalli NA, Allen KJ. Clinical penetrance in hereditary hemochromatosis: estimates of the cumulative incidence of severe liver disease among HFE C282Y homozygotes. Genet Med. 2018;20:383–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarigianni M, Liakos A, Vlachaki E, Paschos P, Athanisiadou E, Montori VM, et al. Accuracy of magnetic resonance imaging in diagnosis of liver iron overload: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2015;13:55–63. [DOI] [PubMed] [Google Scholar]
- 6.Brandhagen DJ. Liver transplantation for hereditary hemochromatosis. Liver Transpl. 2001;7:663–72. [DOI] [PubMed] [Google Scholar]
- 7.Kilpe VE, Krakauer H, Wren RE. An analysis of liver transplant experience from 37 transplant centers as reported to Medicare. Transplantation. 1993;56:554–61. [DOI] [PubMed] [Google Scholar]
- 8.Brandhagen DJ, Alvarez W, Therneau TM, Kruckeberg KE, Thibodeau SN, Ludwig J, et al. Iron overload in cirrhosis—HFE genotypes and outcome after liver transplantation. Hepatology. 2000;31:456–60. [DOI] [PubMed] [Google Scholar]
- 9.Crawford DHG, Fletcher LM, Hubscher SG, Stuart KA, Gane E, Angus PW, et al. Patient and graft survival after liver transplantation for hereditary hemochromatosis: implications for pathogenesis. Hepatology. 2004;39:1655–62. [DOI] [PubMed] [Google Scholar]
- 10.Kowdley KV, Brandhagen DJ, Gish RG, Bass NM, Weinstein J, Schilsky ML, et al. Survival after liver transplantation in patients with hepatic iron overload: the national hemochromatosis transplant registry. Gastroenterology. 2005;129:494–503. [DOI] [PubMed] [Google Scholar]
- 11.Dobrindt EM, Keshi E, Neulichedl J, Schöning W, Ollinger R, Pratschke ED. Long-term outcome of orthotopic liver transplantation in patients with hemochromatosis: a summary of a 30-year transplant program. Transplant Direct. 2020;6:e560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu L, Ioannou GN. Survival of liver transplant recipients with hemochromatosis in the United States. Gastroenterology. 2007;133:489–95. [DOI] [PubMed] [Google Scholar]
- 13.Dar FS, Faraj W, Zaman MB, Bartlett A, Bomford A, O’Sullivan A, et al. Outcome of liver transplantation in hereditary hemochromatosis. Transpl Int. 2009;22:717–24. [DOI] [PubMed] [Google Scholar]
- 14.United Network for Organ Sharing. [cited 2021 Feb 10]. Available from: https://unos.org/data/data-collection/
- 15.Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783–90. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 17.Cox DR. Regression models and life-tables. J R Stat Soc Series B Stat Methodol. 1972;34:187–202. [Google Scholar]
- 18.Wang CW, Lai JC. Reporting functional status in UNOS: the weakness of the Karnofsky performance status scale. Clin Transplant. 2017;31:e13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrell FE, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982;247:2543–6. [PubMed] [Google Scholar]
- 20.Catana AM, Medici V. Liver transplantation for Wilson disease. World J Hepatol. 2012;4:5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schilsky ML. Liver transplantation for Wilson’s disease. Ann N Y Acad Sci. 2014;1315:45–9. [DOI] [PubMed] [Google Scholar]
- 22.Farrell FJ, Nguyen M, Woodley S, Imperial JC, Garcia-Kennedy R, Man K, et al. Outcome of liver transplantation in patients with hemochromatosis. Hepatology. 1994;20:404–10. [PubMed] [Google Scholar]
- 23.Kitajima T, Moonka D, Yeddula S, Rizzari M, Collins K, Yoshida A, et al. Liver transplant waitlist outcomes in alcoholic hepatitis compared with other liver diseases: an analysis of UNOS registry. Clin Transplant. 2020;34:e13837. [DOI] [PubMed] [Google Scholar]
- 24.Fink MA, Berry SR, Gow PJ, Angus PW, Wang B, Muralidharan V, et al. Risk factors for liver transplantation waiting list mortality. J Gastroenterol Hepatol. 2007;22:119–24. [DOI] [PubMed] [Google Scholar]
- 25.Klein CG, Malamutmann E, Latuske J, Tagay S, Dörri N, Teufel M, et al. Frailty as a predictive factor for survival after liver transplantation, especially for patients with MELD≤15-a prospective study. Langenbecks Arch Surg. 2021;406:1963–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elmberg M, Hultcrantz R, Ekbom A, Brandt L, Olsson S, Olsson R, et al. Cancer risk in patients with hereditary hemochromatosis and in their first-degree relatives. Gastroenterology. 2003;125:1733–41. [DOI] [PubMed] [Google Scholar]
- 27.Powell EE, Ali A, Clouston AD, Dixon JL, Lincoln DJ, Purdi DM, et al. Steatosis is a cofactor in liver injury in hemochromatosis. Gastroenterology. 2005;129:1937–43. [DOI] [PubMed] [Google Scholar]
- 28.Udani K, Chris-Olaiya A, Ohadugha C, Malik A, Sansbury J, Paari D. Cardiovascular manifestations in hospitalized patients with hemochromatosis in the United States. Int J Cardiol. 2021;342:117–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


