Abstract
The zygapophyseal joints of the spine, also known as the facet joints, are paired diarthrodial joints posterior to the intervertebral disc and neural elements. The pathophysiology of facet osteoarthritis, as well as crosstalk between the disc and facets, remains largely understudied compared to disc degeneration. The purpose of this study was to characterize alterations to human facet cartilage and subchondral bone across a spectrum of degeneration and to investigate correlations between disc and facet degeneration. Human lumbar facet articular surfaces from 6 independent donors were subject to creep indentation mechanical testing to quantify cartilage mechanical properties, followed by μCT analyses for subchondral bone morphometry. The degenerative state of each articular surface was assessed via macroscopic scoring and via OARSI histopathology scoring. Our data suggest reduced facet cartilage compressive and tensile moduli and increased permeability with increasing degenerative grade, particularly at the lower levels of the spine. μCT analyses revealed spinal level-dependent alterations to the subchondral bone, with an increase in trabecular bone at the L4-L5 level, but a decrease at the upper levels of the lumbar spine with increasing degenerative grade. Cortical bone volume fraction was generally decreased with increasing degenerative grade across spinal levels. Correlation analysis revealed several associations between quantitative measures of disc degeneration and facet osteoarthritis.This work showed that alterations in the mechanical properties of facet cartilage and in the structural properties of facet subchondral bone correlated with aspects of disc degeneration and were highly dependent on spinal level.
Keywords: zygapophyseal joint, intervertebral disc degeneration, biomechanics, osteoarthritis, indentation
Introduction
Back pain is one of the most common causes of disability worldwide, resulting in more than 200 billion dollars in yearly healthcare costs in the US alone1.The causes of back pain are complex and multifactorial, and degeneration of the musculoskeletal components of the spine can be a frequent source of pain. The anterior column of the spine is composed of bony vertebral bodies with intervening intervertebral discs. Each intervertebral disc forms a fibrocartilaginous joint (or symphysis) and consists of a central nucleus pulposus, composed primarily of proteoglycans,surrounded by the lamellar annulus fibrosus, which consists mainly of type I collagen2. These elements interact to provide the disc with tension-resisting properties similar to a ligament and compression-resisting properties as seen in articular cartilage3. Posterior to each intervertebral disc are paired diarthrodial facet joints, also known as zygapophyseal joints.Each facet consists of a superior articular process (SAP) of the inferior vertebra, which faces dorsomediallyand articulates with the inferior articular process (IAP) of the superior vertebra, whichfaces ventrolaterally3. The facet joint shares features that are characteristic of other synovial joints in the body, such that cartilage covers each articular surface atop a layer of subchondral bone, with the entire articulation immersed in synovial fluid and surrounded by a joint capsule.
The spinal motion segment is often referred to as a three-joint complex, with the intervertebral discs anteriorly bearing about 75% of the axial loads through the spine and the facets bearing the remaining 25% of axial loads4.In addition, facets play a role in providing torsional stiffness, resistance to shear, lateral and antero-posterior vertebral translation, and joint distraction5,6.Thus, both the anterior and posterior column are integral to the stability of the motion segment. With aging or following spinal trauma, degeneration of both the discs and facets can occur, the downstream consequences of whichcan include spinal instability, nerve compression, pain, and motor deficits. Disc degeneration and concomitant facet osteoarthritis are frequently observed in humans; however, the temporal relationship between the two processes is not well defined7,8.
In an effort to further understand spinal pathologies contributing to back pain, many have focused onstudying disc degeneration itself9.Compared to the numerous studies on the pathophysiology of disc degeneration reported in the literature, the structure-function relationships associated with facet degeneration are largely understudied. Research suggests that facet degeneration develops in patients as young as 15 years old, with almost two-thirds of individuals affected by facet arthritis by 30 years of age10.Facet degeneration has been most commonly reported atL4-L5, with no detectable difference between right versus left facet joints11.Pathology of the facet cartilage during OA is characterized by progressive cartilage fibrillation, thinning and loss of proteoglycan content8,12. The effect of these structural alterations on the mechanical properties of the facet cartilage has not been studied, but in other synovial joints OA is associated with dramatic alterations in poroviscoelastic mechanical properties of the articular cartilage13.In other synovial joints such as the knee, it is also accepted that degeneration is not only associated with compromised cartilage structure and mechanics but also with changes in the underlying subchondral bone14–16.Previous work comparing human osteoarthritic and healthy facets suggests that subchondral cortical plate thickness is reduced while trabecular bone volume fraction is increased.17 It has also been shown that patients with back pain have higher faet subchondral bone mineral density18.
The morphological, biomechanical and histological changes across facet bone and cartilage with degenerationas well as across levels of the human lumbar spine remain poorly understood. Therefore, the goal of this study was to not only examine the facet joint cartilage and subchondral bone structure-function properties with advanced degeneration but also to further elucidate correlations between facet and disc degeneration in human spines across a range of degenerativestates. We hypothesized that alterations in facet cartilage biomechanical properties and subchondral bone morphometry with increasing OA severity would be most evident at the lower levels of the spine, and that structure-function metrics of facet OA and disc degeneration would correlate.
Methods
Donor Demographics and Specimen Isolation
Six human donor lumbar spines were purchased fresh-frozen from the National Disease Research Interchange and Science Care, yielding 24 total spinal motion segments for analysis. Donor demographics included 4 male donors (54yo, 65yo, 67yo, 69yo) and 2 female donors (54yo, 66yo). Deidentified patient demographics and the spinal levels utilized from each donor are summarized in Figure 1A and 1B. When requesting donor tissue, a history of spine surgery or implants was an exclusion criterion; however, any clinical history of back pain in the donors is unknown.Spines were stored at −20°C until dissection.
Figure 1.
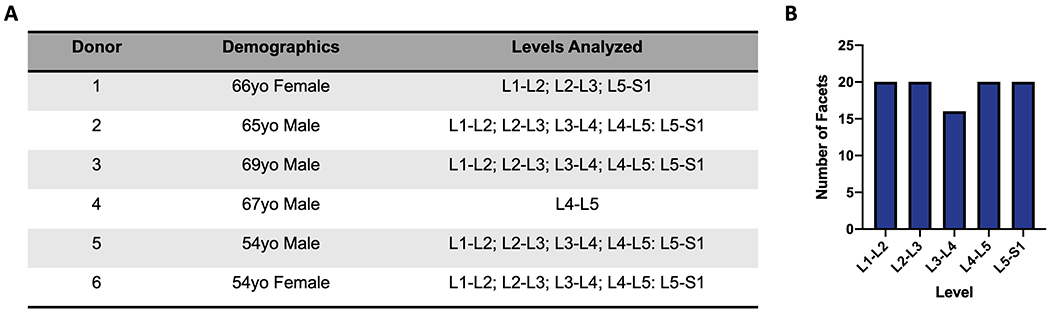
A). Donor Demographics and levels analyzed per donor. B). Number of facets evaluated corresponding to each lumbar spine level.
Each spine was carefully dissected to isolate anterior bone—intervertebral disc—bone motion segments as well as the posterior elements at each level. For the posterior elements, the facet articulation was carefully disrupted by dissection through the facet joint capsule, and 4 facet articular surfaces were isolated from each level. Following dissection, each facet or articular process was photographed and macroscopically scored on a scale of 0 (healthy) to 4 (most degenerative) by 2 blinded, independent graders according to established methods19(Figure 2A). In instances where graders did not agree, the reasoning behind each score was discussed and a consensus score was determined.
Figure 2.
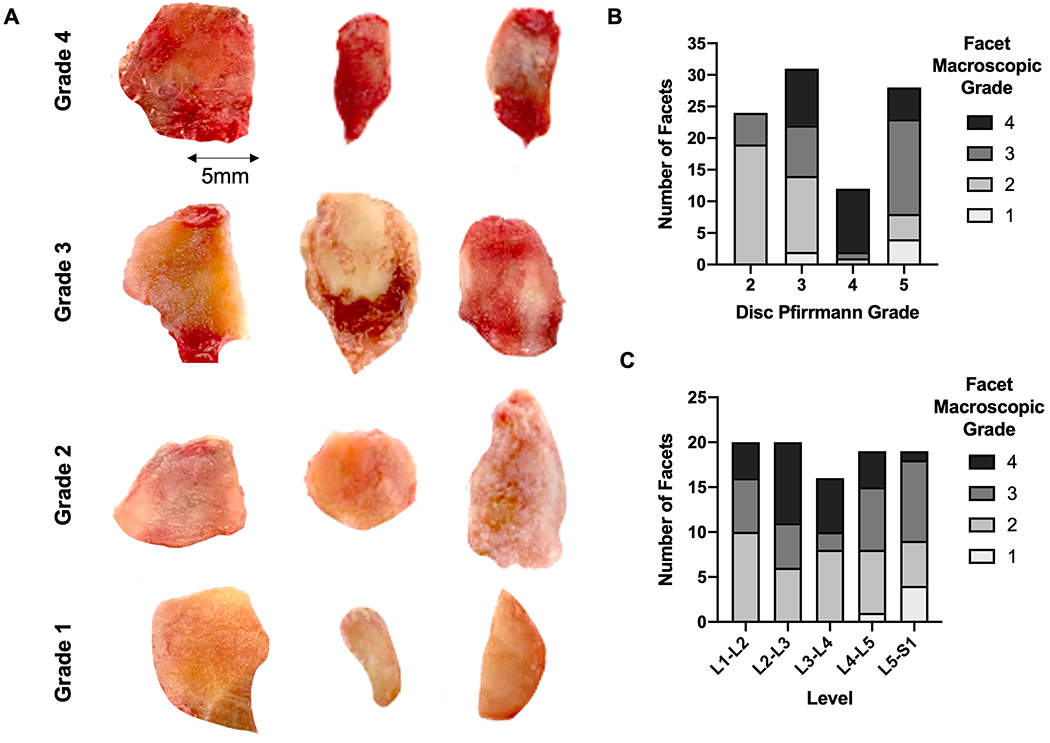
A). Sample macroscopic images of individual facets for each grade from different donors. Graded according to prior study by Li et al; (Grade 1: surface fibrillation; Grade 2: fissuring; Grade 3: erosion of 30% or less of the articular surface; Grade 4: erosion of more than 30% of the articular surface). B). Distribution of number of facets from each macroscopic grade stratified by disc Pfirrmann grade. C). Distribution of number of facets from each macroscopic grade stratified by spinal level.
Intervertebral Disc Motion Segment Analyses
The compressive mechanical properties, NP T2 relaxation times, and disc height of the anterior portion of the motion segments were analyzed as described in our prior work, which characterized changes in these parameters over a spectrum of degeneration20. Briefly, after thawing, each donor spine underwent MRI scanning at 3T to acquire mid-sagittal T2-weighted images to measure disc height and for Pfirrmann grading21, as well as a sequence of images for quantitative T2 mapping. Isolated motion segments were then subject to a compressive mechanical testing protocol consisting of 20 cycles of compression (0 to −750N) at 0.05 Hz in a bath of phosphate buffered saline (PBS) with protease inhibitors. Mechanical properties (toe and linear region moduli and transition and maximal strains) normalized to disc area and height obtained from the MR images were calculated from the 20th cycle of compression, as previously described20,22.
Facet Articular Cartilage Mechanical Testing
Following dissection, to quantify changes in facet articular cartilage properties with degeneration, each articular surface was subjected to a biomechanical indentation testing protocol. Facets were potted using a low melting temperature indium casting alloy. Next, a custom indentation testing rigcoupled with an Instron 5948 electromechanical test frame was used to apply a creep load of −0.1N for 15 minutes through a 1mm diameter spherical indenter while simultaneously measuring deformation(Figure 4A)23. Indentation testing was conducted in a PBS bath with protease inhibitors at room temperature. Four points on each facet surface, each 3mm apart in a “diamond” configuration (Figure4B), were tested. Mechanical properties of the facet cartilage including the hydraulic permeability (ko), nonlinear strain-dependent flow-limited constant(M), the tensile modulus (EY+), and the compressive modulus (EY−) were then calculated by fitting the displacement vs. time curves to a Hertzian biphasic creep model24(Figure 4D). Data from each of the four indentation locations were averaged for each facet. Indentation tests that resulted in an R2 value of less than 0.95 in the creep model fit, and had a total deformation less than 0.02 mm, were excluded from the above analysis. These samples did not exhibit the characteristic viscoelastic creep behavior of cartilage and likely represented regions of severe cartilage erosion where the indentation queried the underlying bone, as only macroscopic grades 3 (two samples) and 4 (five samples) facets were excluded from the creep analysis.
Figure 4.
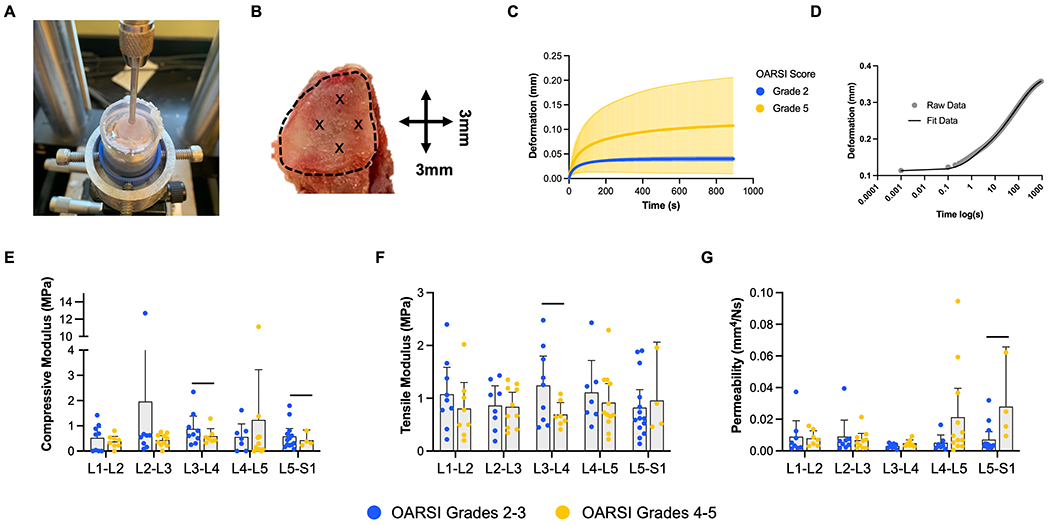
A). Photograph of cartilage indentation testing setup with 1mm indenter and potted facet sample. B). “Diamond” configuration demonstrating the 4 individual points, each 3mm apart, where cartilage creep testing was performed. The dashed line delineates the articular surface.C). Mean (dark colored line) and standard deviation (shaded area) deformation versus time curves from the creep indentation testingfor OARSI grade 2 and 5 facets. D). Individual sample deformation versus time (on a logarithmic scale) showing the Hertzian biphasictheory model fit to the raw data from indentation testing to determine compressive modulus, tensile modulus,K0,and M of the facet cartilage24. E). Compressive modulus, F). tensile modulus and G). permeability stratified by OARSI grade and spinal level. Graphs depict mean plus the 95% confidence interval, and bars denote significant differences between groups.
Assessment of Facet Subchondral Bone via Microcomputed Tomography (μCT)
Following cartilage indentation testing, all samples were fixed in 10% neutral buffered formalin and imaged using μCT (Scanco μCT50) at an isotropic 10.3 μm resolution to characterize the underlying cortical and trabecular subchondral bone as in our previous studies25,26. For each articular surface, the cortical bone was manually contoured and the bone volume fraction (BV/TV) within the central 4 mm of the jointquantified (Figure 5A).Additional bone morphometry parameters (BV/TV, trabecular number, trabecular thickness, and trabecular spacing) were determinedin the adjacent trabecular bone within a rectangular region of interest for each sample (superior facet: 8mm x 1.5mm, inferior facet: 3.5mm x 1.5mm) using Scanco software. Different sized regions of interest were utilized due to the smaller size of the inferior facet.
Figure 5.
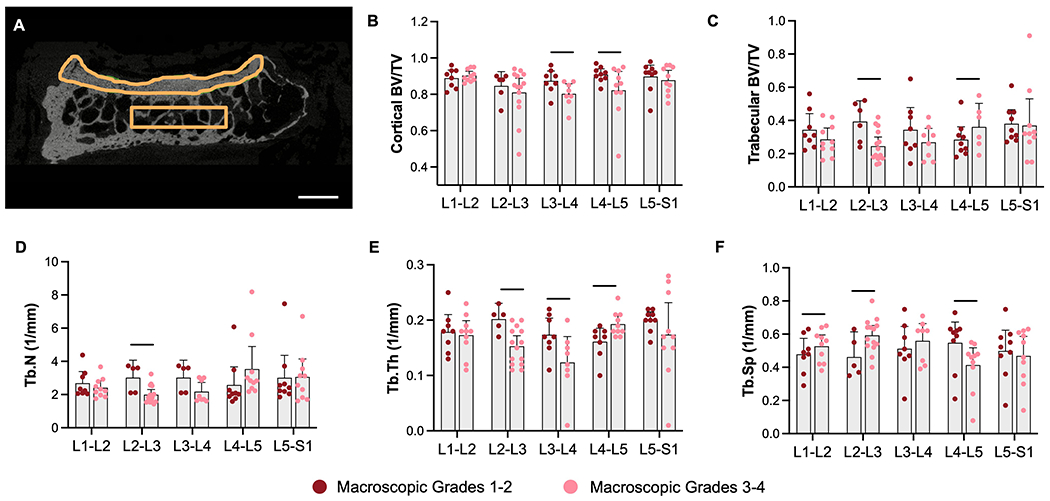
A). Example contours of facet cortical and trabecular bone for μCT analysis (scale = 3mm)of B). cortical bone volume fraction (BV/TV), C). trabecular bone volume fraction (BV/TV), D). trabecular number, E). trabecular thickness, and F). trabecular spacing, stratified by spinal level and macroscopic grade. Graphs depict mean plus the 95% confidence interval, and bars denote significant differences between groups.
Facet Histology and OARSI Scoring
To examine the facet cartilage at a microscopic scale, each articular process was decalcified and processed through paraffin for histology. A single10 μm thick mid-sagittal section for each facetwas stained with Safranin-O and Fast Green, and slides were imaged at 20x using an Aperio Slide Scanner. Each sample was scored according to the OARSI (Osteoarthritis Research Society International) Cartilage OA (osteoarthritis) histopathology grading system previously developed for human knee cartilage27. OARSI scoring was conducted by 3 independent graders, and the scores were averaged for each sample.
Statistical Analysis
Statistical analyses were conducted using GraphPad Prism 9 and R (R-project.org). The two-sided p value of 0.05 was used as the statistical significance cutoff. Standard descriptive statistics were used to summarize the patient characteristics and the macroscopic and histology scores, e.g., mean and standard deviation (SD) for continuous variables, and count and percent for categorical variables. A paired t-test was applied to compare the within-subject quantitative outcomes (cartilage mechanics and subchondral bone morphometry) between superior and inferior across all levels. In addition, linear regression was used to test whether the difference between superior and inferior macroscopic scores depended on level or grade. Linear regressions were also used to evaluate any correlation between the macroscopic and corresponding histology scores. Generalized estimating equations (GEE) were used to assess the effect of the interaction between spinallevel and grade (macroscopic or OARSI) on all quantitative outcome metrics of facet cartilage mechanics or subchondral bone morphometry. GEE accounts for the within-subject correlation due to multiple measures taken on the same subject by using a sandwich estimator of the standard error of the regression coefficients.For OARSI scoring, the intraclass correlation coefficient (ICC) was calculated to assess inter-rater reliability. To assess relationships between disc and facet degeneration, Pearson correlation coefficients were calculated for all combinations of measured variables from disc and facet pairs.To generate the Pearson correlation matrix, quantitative data were averaged across all facets at each spinal level such that each disc was paired with a single value for each facet outcome.
Results
For each level of the lumbar spine, four facets were dissected (the SAP and IAP from each laterality), and 96 articular surfaces isolated from 24 spinal levels (Figure 1A and 1B). One of the 96 samples was incidentally damaged during dissection. The remaining 95 were scored macroscopically using the above mentioned criteria revealing: 6 grade 1, 36 grade 2, 29 grade 3, and 24 grade 4 facets19. Combining this analysis with previous MRI grading of discs from these motion segments revealed no strong association between facet macroscopic grade and disc Pfirrmann grade (linear regression r2 = 0.06, p=0.01). In addition, there was no clear qualitative association between spinal level and macroscopic grade (Figure 2B and 2C).
OARSI histopathology scoring of a single mid-sagittal section from all facets revealed 26 grade 2, 23 grade 3, 21 grade 4, and 26 Grade 5 facets. There was very good agreement between the three independent graders, with an ICC of 0.89 (95% CI: 0.85, 0.92). Examples of each grade from different donors are represented in Figure 3A, demonstrating the structural variability between facets within the same grade. With regard to spinal level, the distribution of facet OARSI score was relatively even (Figure 3B). There also were no obvious associations between disc Pfirrmann grade and facet OARSI score (Figures 3C)—severely degenerative facets (OARSI scores of 4 and 5) were found adjacent to discs of all Pfirrmann grades.
Figure 3.
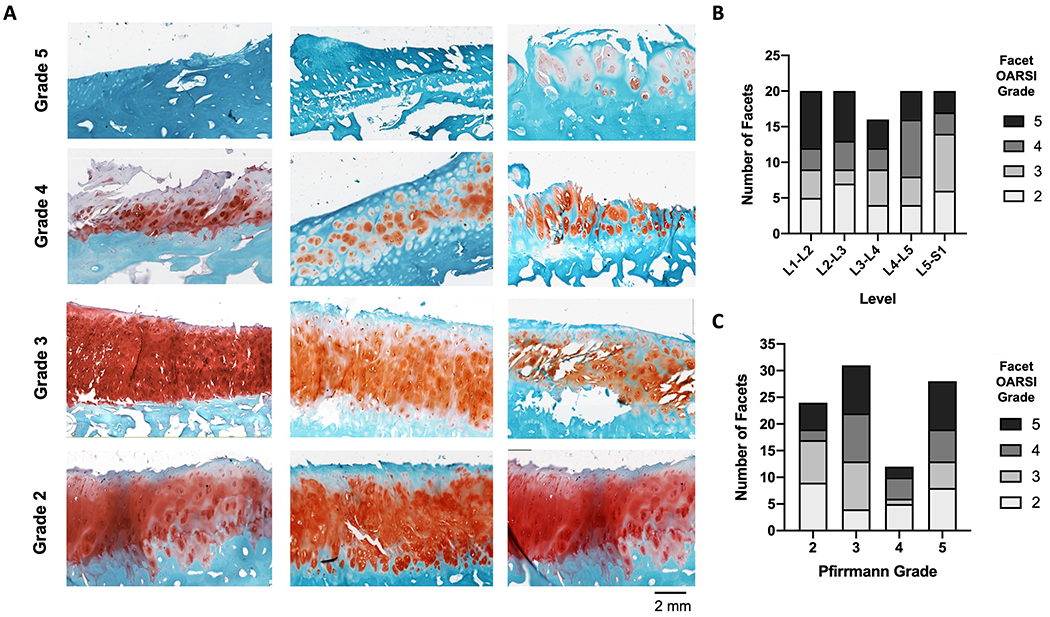
A). Safranin-O and Fast Green-stained histological sections of human facets demonstrating the sample variation from different donors within the same OARSI score (Scale = 2 mm). The number of facets from each OARSI score was stratified by B). spinal level and C). disc Pfirrmann grade.
For quantitative outcomes inclusive of facet cartilage mechanical properties and subchondral bone morphometry, paired t-tests revealed no statistically significant differences between superior and inferior facet joints. Due to the sample size limitations of the data set, we therefore did not include superior/inferior as a factor in the GEE model. OARSI grades 2-3, and 4-5 were combined for this statistical analysis, as were macroscopic grades 1-2 and 3-4. The GEE model identified significant interactions between spinal level and OARSI score only for cartilage mechanical testing outcomes, and between spinal level and macroscopic grade only for subchondral bone morphometry variables.Cartilage mechanical properties stratified by level and macroscopic score and subchondral bone morphometric parameters stratified by level and OARSI score are shown in Supplemental Figures 1 and 2.
Facet cartilage creep indentation testing demonstrated increasing heterogeneity in the creep deformation curves with increasing OARSI score (Figure 4C).Facet cartilage compressive modulus decreased with increasing OARSI score by 33% and 26% at L3-L4 and L5-S1, respectively (Figure 4E). Facet cartilage tensile modulus was also significantly reduced by 44% in OARSI scores 4-5 at the L3-L4 spinal level (Figure 4F). There was a concomitant 293% increase in facet cartilage permeability at the L5-S1 spinal level with increasing OARSI scores (Figure 4G).
Facet subchondral cortical bone volume fraction was significantly reduced by 8% and 10% at L3-L4 and L4-L5, respectively, with more severe facet OA characterized by higher macroscopic degeneration grades (Figure 5A–B). Facet trabecular bone volume fraction was also reduced by 38% at L2-L3, driven primarily by reductions in trabecular number and thickness and increases in trabecular spacing at L2-L3 and other upper spinal levels. Interestingly, a significant 27% increase in trabecular bone volume fraction was observed at L4-L5 with increasing macroscopic grade, driven by increases in trabecular thickness and reductions in trabecular spacing (Figure 5 C–F).
To elucidate any associations between disc and facet degeneration in our paired sample set, a Pearson correlation coefficient matrixof all combinations of facet mechanical properties, μCT parameters, facet OARSI and macroscopic scores, disc mechanical properties, disc height, and disc NP T2 relaxation times was calculated(Table 1). Facet trabecular thickness negatively correlated with disc compressive modulus (r= −0.34, p= 0.021), while facet trabecular number positively correlated with disc transition and maximum strains (r=0.35, p=0.018; r=0.34, p=0.020 respectively). Facet macroscopic grade correlated with several parameters, including positive correlations with disc compressive modulus and negative correlations with disc transition and maximum strains. With regards to OARSI score, there was a trending negative correlation with disc height (r= −0.31, p=0.071) and a positive correlation with disc compressive modulus (r= 0.25, p=0.039).
Table 1.
Results from the Pearson correlation matrix between quantitative facet and disc outcome metrics.
| Parameter 1 | Parameter 2 | Pearson Correlation Coefficient (r) | P Value |
|---|---|---|---|
| Facet Tb.Th | Disc Compressive Modulus | −0.34 | 0.021 |
| Facet Tb. N | Disc Transition Strain | 0.35 | 0.018 |
| Disc Max Strain | 0.34 | 0.020 | |
| Facet Macroscopic Grade | Disc Transition Strain | −0.45 | 0.002 |
| Disc Max Strain | −0.43 | 0.003 | |
| Disc Compressive Modulus | 0.30 | 0.043 | |
| Facet OARSI Score | Disc Height | −0.31 | 0.071 |
| Disc Compressive Modulus | 0.26 | 0.039 |
Discussion
This study sought to evaluate facet joint cartilage and subchondral bone structure-function properties across a spectrum of spine degeneration and to investigate their correlations with adjacent disc degeneration in the human lumbar spine. Our results suggest that with advancing stages of degeneration, alterations to facet cartilage mechanics and subchondral bone structure occur in a highly level dependent manner. These spinal level dependent changes were not immediately apparent when simply identifying the number of facets of higher macroscopic/OARSI scores at each spinal level but became evident when stratifying structure-function properties by spinal level. Significant differences in facet cartilage mechanics or subchondral bone morphometry were most frequently observed at the lower levels of the spine (L3 to S1), in agreement with prior studies that have demonstrated higher rates of degeneration at L4-L519.This may be related to theincreased mechanical stress and contact loads at lower regions of the spine leading to more advanced degeneration in these areas28.
Facet cartilage biomechanical properties measured by creep indentation testing were significantly impacted by degeneration and generally included reductions in compressive and tensile modulus and increases in cartilage permeability (Figure 4). These observed alterations in facet cartilage mechanicsdemonstrate a loss of integrity of the articular cartilage, which is to be expected with advanced degeneration13. This is consistent with previous work in other human synovial joints, where permeability is increased and compressive modulus is decreased with higher OARSI scores13,29.We also noted increased variability in the creep deformation response (Figure 4C) with increasing severity of degeneration, likely related to heterogeneity of human samples especially with more severe stages of cartilage wear4.The average values for facet cartilage compressive modulus quantified in this study ranged from 0.87 MPa (OARSI grades 2-3) to 0.68 MPa (OARSI grades 4-5). This is within the range aggregate moduli previously reported from creep testing of healthy human knee cartilage (0.6-0.7 MPa), and human knee cartilage from OARSI grades 2 through 5 (0.25-1MPa)29,30, suggesting that facet and knee cartilage in humans have roughly similar mechanical properties. This is in contrast with quadrupedal species, in which facet cartilage aggregate modulus is approximately four times lower than knee cartilage in the same species31.
Substantial alterations to the facet subchondral bone across spinal levels and with increasing degree of macroscopic degeneration were also observed in this study (Figure 5).Generally cortical and trabecular bone volume fraction were reduced with increasing degree of degeneration, particularly in the mid-lumbar spine. Interestingly, an increase in trabecular bone volume fraction was observed only at the L4-L5 spinal level, driven by increases in trabecular thickness and reductions in trabecular spacing. These overall trends, with exception to those at L4-L5, are consistent with prior studies involving knee OA models that showed greater subchondral and cortical bone resorption with increased porosity and a concurrent loss of bone mineral density with advancing stages of degeneration32–36.A previous study of subchondral boney changes in human osteoarthritic and healthy facets demonstrated increases in both cortical and trabecular bone in osteoarthritic facets, but the study did not account for level dependent differences and examined a broader age range of donors than the current study includes17.Ourfindings at L4-L5 could be related to bony changes specific to that level and may require further study with greater sample sizes. Osteoporosis could be a confounding factor which may account for the loss ofcortical bone, especially with advanced age37. Finally, previous work has suggested overall facet bone density is higher in patients with back pain versus asymptomatic individuals18. Future work should aim to expand characterization of these facet cartilage and bone structure-function relationships to patient populations with back pain.Nevertheless, our study demonstrates that, similar to the knee and other joints of the body, facet osteoarthritis is a degenerative disease of both bone and cartilage16,38.
The correlation analysis between quantitative metrics of facet and disc health and mechanical function revealed statistically significant correlations between these two spinal tissues, similar to prior studies11. Our data suggests that facet boney sclerosis (increased trabecular thickness and number) isassociated with increased adjacent disc strains and reduced disc compressive moduli, which are mechanical changes characteristic of disc degeneration.Facet macroscopic grade and OARSI score correlated with different parameters. Our statistical analysis matched these correlations, showing significant differences by level for cartilage mechanics only for OARSI score and for subchondral bone morphometry only for macroscopic grade. This is likely due to heterogeneity in cartilage erosion patterns across the facet articular surface (as captured in macroscopic scoring) that may not be well represented by a single 10-micron histological section in the mid-plane of the facet.Additionally, facet cartilage mechanical properties and disc mechanical properties were not correlated, in agreement with a recent in vivo study which found no association between disc and facet biomechanics under diurnal loading39.Future studies may need to quantify whole facet cartilage volumes (using contrast enhanced μCT40) to improve such correlations.
Due to the cross-sectional nature of this study in cadaveric human tissues, any causal relationships between disc and facet degeneration cannot be determined. Prior studies, including those by Butler, Vernon-Roberts, and Bywaters have postulated that disc degeneration occurs before facet OA, given their observation of preserved facet joints in degenerated discs, as assessed by MRI28,41,42. However, cadaveric studies have noted significant facet degeneration in the absence of disc degeneration, and other work has proposed that disc and facet degeneration occur concurrently43,19. Many of these prior studies rely solely on clinically available imaging, such as MRI or CT to detect facet degeneration, methods that may be less sensitive in detecting the early stages of OA. Further work will be necessary to elucidate temporal relationships between disc and facet degeneration, potentially in animal models where degeneration of each spinal substructure can be induced independent of one another44,45.
Limitations to this study include the number of donors, especially given the variability and heterogeneity in some outcomes, particularly indentation testing of the cartilage.A priori data were not available for human facet cartilage mechanical properties.However a power analysis was conducted using previously published data from Netzer et al.17 on subchondral bone morphometry in healthy and osteoarthritic facets. Assuming a conservative 18.4% standard deviation in trabecular bone volume fraction at our sample size (approximately 6 vs. 18 facets at each grade strata), we can detect a difference of 19.2% using GEE with an intraclass correlation of 0.5, 80% power, and a significance level of 0.05. This detectable difference is smaller than we have observed in our study for trabecular bone parameters (where percent changes in trabecular bone morphometry range from 27-38%), suggesting that our study is adequately powered at least with respect to bone morphometry.
In the current study, it is also difficult to discern any effect of sex on facet degeneration, due to the limited number of female donors.Future studies should involve a greater number of donors with more balanced age and sex distributions. We were also unable to measure lumbar lordosis or facet alignment, which can contribute to facet OAand should be investigated in future work46.Overall, this study characterized alterations in the mechanical properties of facet cartilage and structural properties of facet subchondral bone over a spectrum of spine degeneration which were highly dependent on spinal level.We found facet OA to be characterized by heterogenous and progressive cartilage erosion, which culminated in reduced cartilage mechanical properties. Our results suggest an overall loss of subchondral bone with increasing degeneration, except at the L4-L5 level where boney sclerosis was observed in the trabecular compartment. Facet boney sclerosis, macroscopic grade and OARSI score were significantly correlated with disc mechanical properties, suggesting a significant interrelationship between these adjacent tissues and highlighting the importance of considering the contribution of the facet joints in the study of disc degeneration and repair.
Supplementary Material
Supplemental Figure 2. Facet cartilage mechanical properties stratified by macroscopic grade. Graphs depict mean plus the 95% confidence interval.
Supplemental Figure 1. Facet A). cartilage mechanical properties and B-F). subchondral bone morphometry stratified by OARSI grade and spinal level. Graphs depict mean plus the 95% confidence interval, and bars denote significant differences between groups.
Acknowledgements
This work was supported by the Penn Center for Musculoskeletal Disorders (National Institutes of Health, P30 AR069619) and the Department of Veterans Affairs (IK2 RX003118, I01 RX002274, IK6 RX003416). The study sponsors had no role in the study design, collection, analysis and interpretation of data, writing of the manuscript or the decision to submit the manuscript for publication.
Footnotes
Conflict of Interest
Robert L. Mauck is a Co-Editor in Chief of JOR Spine.
References
- 1.Casiano VE, Dydyk AM, Varacallo M. 2021. Back Pain. In: StatPearls. Treasure Island (FL): StatPearls Publishing; [cited 2021 Dec 10] Available from: http://www.ncbi.nlm.nih.gov/books/NBK538173/. [Google Scholar]
- 2.Humzah MD, Soames RW. 1988. Human intervertebral disc: structure and function. The Anatomical record 220(4):337–56. [DOI] [PubMed] [Google Scholar]
- 3.Varlotta GP, Lefkowitz TR, Schweitzer M, et al. 2011. The lumbar facet joint: a review of current knowledge: part 1: anatomy, biomechanics, and grading. Skeletal radiology 40(1):13–23. [DOI] [PubMed] [Google Scholar]
- 4.Gellhorn AC, Katz JN, Suri P. 2013. Osteoarthritis of the spine: the facet joints. Nature reviews. Rheumatology 9(4):216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaumard NV, Welch WC, Winkelstein BA. 2011. Spinal facet joint biomechanics and mechanotransduction in normal, injury and degenerative conditions. Journal of biomechanical engineering 133(7):71010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raynor RB, Pugh J, Shapiro I. 1985. Cervical facetectomy and its effect on spine strength. Journal of neurosurgery 63(2):278–282. [DOI] [PubMed] [Google Scholar]
- 7.Suri P, Miyakoshi A, Hunter DJ, et al. 2011. Does lumbar spinal degeneration begin with the anterior structures? A study of the observed epidemiology in a community-based population. BMC Musculoskelet Disord 12(1):202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Muehleman C, Abe Y, Masuda K. 2011. Prevalence of facet joint degeneration in association with intervertebral joint degeneration in a sample of organ donors. J Orthop Res 29(8):1267–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taher F, Essig D, Lebl DR, et al. 2012. Lumbar degenerative disc disease: current and future concepts of diagnosis and management. Advances in orthopedics 2012:970752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eubanks JD, Lee MJ, Cassinelli E, Ahn NU. 2007. Prevalence of lumbar facet arthrosis and its relationship to age, sex, and race: an anatomic study of cadaveric specimens. Spine 32(19):2058–2062. [DOI] [PubMed] [Google Scholar]
- 11.O’Leary SA, Paschos NK, Link JM, et al. 2018. Facet Joints of the Spine: Structure-Function Relationships, Problems and Treatments, and the Potential for Regeneration. Annual Review of Biomedical Engineering . [DOI] [PubMed] [Google Scholar]
- 12.Kim J-S, Ali MH, Wydra F, et al. 2015. Characterization of degenerative human facet joints and facet joint capsular tissues. Osteoarthritis Cartilage 23(12):2242–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Setton LA, Elliott DM, Mow VC. 1999. Altered mechanics of cartilage with osteoarthritis: human osteoarthritis and an experimental model of joint degeneration. Osteoarthritis and cartilage 7(1):2–14. [DOI] [PubMed] [Google Scholar]
- 14.Anderson-MacKenzie JM, Quasnichka HL, Starr RL, et al. 2005. Fundamental subchondral bone changes in spontaneous knee osteoarthritis. The international journal of biochemistry & cell biology 37(1):224–236. [DOI] [PubMed] [Google Scholar]
- 15.Thomsen JS, Straarup TS, Danielsen CC, et al. 2011. Relationship between articular cartilage damage and subchondral bone properties and meniscal ossification in the Dunkin Hartley guinea pig model of osteoarthritis. Scandinavian journal of rheumatology 40(5):391–399. [DOI] [PubMed] [Google Scholar]
- 16.Quasnichka HL, Anderson-MacKenzie JM, Bailey AJ. 2006. Subchondral bone and ligament changes precede cartilage degradation in guinea pig osteoarthritis. Biorheology 43(3,4):389–397. [PubMed] [Google Scholar]
- 17.Netzer C, Distel P, Wolfram U, et al. 2018. Comparative Analysis of Bone Structural Parameters Reveals Subchondral Cortical Plate Resorption and Increased Trabecular Bone Remodeling in Human Facet Joint Osteoarthritis. International Journal of Molecular Sciences 19:845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan C-C, Simon P, Espinoza Orías AA, et al. 2020. Lumbar facet joint subchondral bone density in low back pain and asymptomatic subjects. Skeletal Radiol 49(4):571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Muehleman C, Abe Y, Masuda K. 2011. Prevalence of facet joint degeneration in association with intervertebral joint degeneration in a sample of organ donors. Journal of orthopaedic research : official publication of the Orthopaedic Research Society 29(8):1267–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashinsky BG, Gullbrand SE, Wang C, et al. 2021. Degeneration alters structure-function relationships at multiple length-scales and across interfaces in human intervertebral discs. Journal of Anatomy 238(4):986–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfirrmann CWA, Metzdorf A, Zanetti M, et al. 2001. Magnetic Resonance Classification of Lumbar Intervertebral Disc Degeneration. Spine 26(17):1873–1878. [DOI] [PubMed] [Google Scholar]
- 22.Martin JT, Gorth DJ, Beattie EE, et al. 2013. Needle puncture injury causes acute and long-term mechanical deficiency in a mouse model of intervertebral disc degeneration. Journal of Orthopaedic Research 31(8):1276–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meloni GR, Fisher MB, Stoeckl BD, et al. 2017. Biphasic Finite Element Modeling Reconciles Mechanical Properties of Tissue-Engineered Cartilage Constructs Across Testing Platforms. Tissue engineering. Part A 23(13–14):663–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore AC, DeLucca JF, Elliott DM, Burris DL. 2016. Quantifying Cartilage Contact Modulus, Tension Modulus, and Permeability With Hertzian Biphasic Creep. Journal of tribology 138(4):414051–414057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashinsky BG, Bonnevie ED, Mandalapu SA, et al. 2020. Intervertebral Disc Degeneration Is Associated With Aberrant Endplate Remodeling and Reduced Small Molecule Transport. Journal of Bone and Mineral Research 35(8):1572–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gullbrand SE, Ashinsky BG, Bonnevie ED, et al. 2018. Long-term mechanical function and integration of an implanted tissue-engineered intervertebral disc. Science Translational Medicine 10(468):eaau0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pritzker KPH, Gay S, Jimenez SA, et al. 2006. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis and cartilage 14(1):13–29. [DOI] [PubMed] [Google Scholar]
- 28.Butler D, Trafimow JH, Andersson GB, et al. 1990. Discs degenerate before facets. Spine 15(2):111–113. [DOI] [PubMed] [Google Scholar]
- 29.Waldstein W, Perino G, Gilbert SL, et al. 2016. OARSI osteoarthritis cartilage histopathology assessment system: A biomechanical evaluation in the human knee. Journal of Orthopaedic Research 34(1):135–140. [DOI] [PubMed] [Google Scholar]
- 30.Athanasiou KA, Rosenwasser MP, Buckwalter JA, et al. 1991. Interspecies comparisons of in situ intrinsic mechanical properties of distal femoral cartilage. Journal of Orthopaedic Research 9(3):330–340. [DOI] [PubMed] [Google Scholar]
- 31.O’Leary SA, Link JM, Klineberg EO, et al. 2017. Characterization of facet joint cartilage properties in the human and interspecies comparisons. Acta Biomaterialia 54:367–376. [DOI] [PubMed] [Google Scholar]
- 32.Pauly HM, Larson BE, Coatney GA, et al. 2015. Assessment of cortical and trabecular bone changes in two models of post-traumatic osteoarthritis. Journal of orthopaedic research : official publication of the Orthopaedic Research Society 33(12):1835–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwan Tat S, Lajeunesse D, Pelletier J-P, Martel-Pelletier J. 2010. Targeting subchondral bone for treating osteoarthritis: what is the evidence? Best practice & research. Clinical rheumatology 24(1):51–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li B, Aspden RM. 1997. Mechanical and material properties of the subchondral bone plate from the femoral head of patients with osteoarthritis or osteoporosis. Annals of the Rheumatic Diseases 56(4):247 LP–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tokgöz MA, Atik OŞ, Esendağlı G, et al. 2018. Is it possible that the pathogenesis of osteoarthritis could start with subchondral trabecular bone loss like osteoporosis? Eklem hastaliklari ve cerrahisi = Joint diseases & related surgery 29(3):152–158. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y, Hu Y, Yu YE, et al. 2018. Subchondral Trabecular Rod Loss and Plate Thickening in the Development of Osteoarthritis. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 33(2):316–327. [DOI] [PubMed] [Google Scholar]
- 37.Osterhoff G, Morgan EF, Shefelbine SJ, et al. 2016. Bone mechanical properties and changes with osteoporosis. Injury 47 Suppl 2(Suppl 2):S11–S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu Y, Chen X, Wang S, et al. 2021. Subchondral bone microenvironment in osteoarthritis and pain. Bone Research 9(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oldweiler AB, Martin JT. 2021. In vivo relationships between lumbar facet joint and intervertebral disc composition and diurnal deformation. Clinical Biomechanics 88:105425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin ASP, Salazar-Noratto GE, Guldberg RE. 2015. EPIC-μCT Imaging of Articular Cartilage. In: Westendorf JJ, van Wijnen AJ, editors. Osteoporosis and Osteoarthritis. New York, NY: Springer. p 131–140 [cited 2021 Dec 10] Available from: 10.1007/978-1-4939-1619-1_11. [DOI] [Google Scholar]
- 41.Moore RJ, Crotti TN, Osti OL, et al. 1999. Osteoarthrosis of the facet joints resulting from anular rim lesions in sheep lumbar discs. Spine 24(6):519–525. [DOI] [PubMed] [Google Scholar]
- 42.Fujiwara A, Tamai K, Yamato M, et al. 1999. The relationship between facet joint osteoarthritis and disc degeneration of the lumbar spine: an MRI study. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society 8(5):396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suri P, Rainville J, Fitzmaurice GM, et al. 2011. Acute low back pain is marked by variability: An internet-based pilot study. BMC musculoskeletal disorders 12:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gullbrand SE, Malhotra NR, Schaer TP, et al. 2017. A large animal model that recapitulates the spectrum of human intervertebral disc degeneration. Osteoarthritis and Cartilage 25(1):146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim J-S, Kroin JS, Buvanendran A, et al. 2011. Characterization of a new animal model for evaluation and treatment of back pain due to lumbar facet joint osteoarthritis. Arthritis and rheumatism 63(10):2966–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jentzsch T, Geiger J, König MA, Werner CML. 2017. Hyperlordosis is Associated With Facet Joint Pathology at the Lower Lumbar Spine. Clinical Spine Surgery 30(3):129–135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 2. Facet cartilage mechanical properties stratified by macroscopic grade. Graphs depict mean plus the 95% confidence interval.
Supplemental Figure 1. Facet A). cartilage mechanical properties and B-F). subchondral bone morphometry stratified by OARSI grade and spinal level. Graphs depict mean plus the 95% confidence interval, and bars denote significant differences between groups.


