Abstract
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), a novel coronavirus responsible for a worldwide pandemic has forced drastic changes in medical practice in an alarmingly short period of time. Caregivers must modify their strategies as well as optimize the utilization of resources to ensure public and patient safety. For organ transplantation, in particular, the loss of lifesaving organs for transplantation could lead to increased waitlist mortality. The priority is to select uninfected donors to transplant uninfected recipients while maintaining safety for health care systems in the backdrop of a virulent pandemic. We do not yet have a standard approach to evaluating donors and recipients with possible SARS-CoV-2 infection. Our current communication shares a protocol for donor and transplant recipient selection during the coronavirus disease 2019 (COVID-19) pandemic to continue lifesaving solid organ transplantation for heart, lung, liver, and kidney recipients. The initial results using this protocol are presented here and meant to encourage dialogue between providers, offering ideas to improve safety in solid organ transplantation with limited health care resources. This protocol was created utilizing the guidelines of various organizations and from the clinical experience of the authors and will continue to evolve as more is understood about SARS-CoV-2 and how it affects organ donors and transplant recipients.
KEYWORDS: clinical decision-making, clinical research/practice, donors and donation: donor evaluation, health services and outcomes research, infection and infectious agents – viral, infectious disease, organ allocation, organ procurement and allocation, organ transplantation in general, waitlist management
Abbreviations: ASTS, American Society of Transplant Surgeons; CMS, Centers for Medicare & Medicaid Services; COVID-19, coronavirus disease 2019; CT, computed tomography; CXR, chest X-ray; LRTI, lower respiratory tract infection; NP, nasopharyngeal; PUI, person under investigation; RT-PCR, reverse transcriptase polymerase chain reaction assay; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2
1. INTRODUCTION
The world faces an unprecedented pandemic in modern times wrought by the novel coronavirus. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) was first reported in Wuhan, China in late December 2019 and has since spread worldwide, including the United States. This virus is particularly contagious, with each infected person estimated to infect 1-3 others with a 20% rate of hospitalization and a case fatality rate of 1% up to as high as 16.4%.1 , 2 The World Health Organization declared an international pandemic on March 11, 2020, which led to a proclamation of national emergency in the United States on March 12, 2020 and a local stay-at-home order by March 24, 2020, all in an effort to prevent widespread nosocomial and community spread. At the time of this submission, 1 339 819 positive cases are documented in the United States, among over 4 152 670 positive cases worldwide.3
At this time there are 112 207 patients on the United Network for Organ Sharing waitlist with end-stage organ disease facing the competing risk of waitlist mortality.4 In the effort to navigate this pandemic in a pragmatic way, while mitigating the risks to our hospital inpatients and staff, and ultimately maximizing lifesaving transplantation, we have devised a protocol implemented at our institution on March 24, 2020 to select donors and transplant candidates for solid organ transplantation during the coronavirus disease 2019 (COVID-19) pandemic. This has allowed the continued possibility of transplantation for those who do not have the option to wait, adhering to the Centers for Medicare & Medicaid Services (CMS) “Adult Elective Surgery and Procedures Recommendations,” which designated transplants as “tier 3b” (high acuity surgery/unhealthy patients) with the consecutive action directive to not postpone surgery.5
The American Society of Transplant Surgeons (ASTS) COVID-19 Strike Force Guidance published online on March 24, 2020 advised against transplanting from donors infected with SARS-CoV-2.6 , 7 As such, this protocol was devised on the premise that SARS-CoV-2 testing be performed on all donors and transplant candidates universally, whether symptomatic or not. This is especially relevant to the pandemic where asymptomatic individuals can shed the virus and continue to infect others.8 This transplant protocol had 3 priorities: (1) avoiding infected deceased donor allografts, (2) avoiding admission of potentially infected transplant candidates, and (3) avoiding transplantation of currently hospitalized infected transplant candidates. This protocol combines the history, physical exam, SARS-CoV-2 reverse transcriptase polymerase chain reaction assay (RT-PCR) testing, radiographic imaging of the chest using computed tomography (CT), and transplant infectious disease consultation that allows for informed and consistent decisions that minimize risks to all of our patients and health care workers.
The fundamental questions that our protocol was designed to address were the following (as adapted from Chen et al9):
-
1
Is the donor infected with SARS-CoV-2?
-
2
Can we obtain adequate donor screening, that is, assessment of exposure risk and clinical risk?
-
3
Is the transplant candidate infected with SARS-CoV-2?
-
4
Can we prevent the potential of bringing a transplant candidate infected with SARS-CoV-2 into the hospital, increasing the likelihood of infecting other patients and health care workers?
-
5
Can we assure that the transplant procedure would not expose our health care staff (eg, procurement team, anesthesiologists, surgeons, operating room staff, and intensive care unit staff) to unnecessary risk?
This protocol is timely because there is no established policy to assess donors and transplant candidates for transplantation. We developed this screening tool based on the data available regarding the pathophysiology of the virus and professional task forces assembled to confront the pandemic.6 , 7 , 10, 11, 12 Given the constraints of screening questionnaires, especially as it pertains to deceased donors, and the limited sensitivity of the nasopharyngeal swab test available at this time,13 we have incorporated radiographic imaging of the chest using CT of both potential donors and transplant candidates to improve sensitivity. This is based on the understanding that over half of asymptomatic patients will show changes on CT and the sensitivity of CT chest findings increases as the COVID-19 progresses.14, 15, 16, 17, 18, 19, 20, 21 Based on the data available, a noncontrast chest CT used for diagnosis of COVID-19 may serve as an important complement to RT-PCR testing, as published data have found that CT imaging of the chest may show changes associated with COVID-19 before or coinciding with onset of infection. This is described by Ai et al in a report of 1014 cases in China, which describes sensitivity of chest CT imaging for COVID-19 at 97% for those suspected of having COVID-19, a higher sensitivity than the RT-PCR testing (71%). Notably, in the subgroup initially found negative on RT-PCR who then converted to positive on serial testing, 67% were found to show initial CT imaging suggestive of COVID-19 before the initial negative RT-PCR test and 93% had positive CT imaging suggestive of COVID-19 preceding or in parallel to the eventual positive RT-PCR by a median of 8 days.22 Although these data are based on symptomatic patients, an eventual meta-analysis suggests that although chest CT scans have a low positive predictive value (PPV) range of 1.5%-30.7%, the negative predictive value was 95.4%-99.8%.23 That is, the probability that an individual with a negative test has a very high likelihood of not actually having the disease. Added to this finding is the fact that nasopharyngeal (NP) swabs taken for RT-PCR of SARS-CoV-2 have varying sensitivity and specificity within the clinical setting, depending on how the swab was acquired.24 A well-collected NP swab is essential to ensure a reliable test and is necessary in that it will dictate eligibility of surgery and inform administration of immunosuppressive therapy, as prescribed by the Infectious Disease Society of America and its Guidelines on the Diagnosis of COVID-19.24 , 25 Notwithstanding the limitations of our current testing strategies to screen and detect SARS-CoV-2, those reasons serve as the rationale for including CT chest imaging to complement RT-PCR testing in our algorithm. Furthermore, it is an easily obtainable, efficient study that nearly all of our donors have already undergone during their donor workup. These practicalities are predicated on our intent to prevent transplantation with a donor organ infected with SARS-CoV-2 or transplantation of a candidate with COVID-19 during the COVID-19 pandemic, which shows no signs of abating in the near future. Although the risk of declining a donor or deferring a transplant for a patient who does not actually have COVID-19 exists, transplanting a donor organ or transplant recipient with SARS-CoV-2 could be devastating for the recipient, the transplant program, and the donor family. This protocol combines the history, physical exam, SARS-CoV-2 RT-PCR testing, CT chest imaging, and transplant infectious disease consultation that allows for informed and consistent decisions that minimize risks to all of our patients and health care workers.
2. METHODS
2.1. Donor assessment
Donor assessment consists of 2 evaluations. First, the organ procurement organization assesses for exposure history, to include travel history, and known contact with others with known or suspected COVID-19. Second, the donor’s clinical risk is assessed, utilizing clinical history, RT-PCR, and imaging modalities.
2.1.1. Donor exposure assessment
Our stratification criteria for assessing potential donor exposures to SARS-CoV-2 are outlined in Table 1. Category 0 represents a low-risk donor who we deem unlikely to have been exposed and infected. Categories 1-3 represent moderate to high-risk donors who have a greater likelihood of being infected.
TABLE 1.
Donor exposure assessment for COVID-19
| Category 0 | The donor has not traveled to CDC high-risk area (level 2-3) in past 21 d and has had no contact with either PUI or confirmed case |
| Category 1 | The donor has been in an area deemed as a CDC high-risk area (level 2-3) in the preceding 21 d, and exposures are unknown |
| Category 2 | The donor had direct unprotected contact with a PUI for COVID-19 in the last 21 d |
| Category 3 | The donor had direct unprotected contact with a confirmed case of COVID-19 in the last 28 d |
Abbreviations: CDC, Centers for Disease Control and prevention; COVID-19, coronavirus disease 2019; PUI, person under investigation.
2.1.2. Donor clinical risk assessment
We also outlined criteria for assessing clinical risk of donors based on lower respiratory tract infection symptoms (LRTI = cough, shortness of breath or dyspnea), fever status, presence of anosmia, SARS-CoV-2 RT-PCR testing, noncontrast CT of the chest, and chest X-ray (CXR) results. These criteria are outlined in Table 2. All donors are tested for SARS-CoV-2 with nasopharyngeal swabs as well as undergo a noncontrast cCT of the chest to rule out evidence of COVID-19. If they are a lung donor, they will have a bronchoscopy and a lower respiratory tract sample for RT-PCR testing as part of their evaluation.
TABLE 2.
Donor clinical risk assessment for COVID-19a
| Category 0 | Asymptomatic, negative RT-PCR for SARS-CoV-2, and CXR and CT chest without signs of COVID-19 |
| Category 1 | Symptomatic (LRTI, fever, anosmia) with negative RT-PCR for SARS-CoV-2, and CXR and CT chest without signs of COVID-19 |
| Category 2 | Symptomatic (LRTI, fever, anosmia) with negative RT-PCR for SARS-CoV-2 and CXR or CT chest concerning for COVID-19 |
| Category 3 | Symptomatic (LRTI, fever, anosmia) with positive RT-PCR for SARS-CoV-2 and CXR or CT chest concerning for COVID-19 |
Abbreviations: COVID-19, coronavirus disease 2019; CT, computed tomography; CXR, chest X-ray; LRTI, lower respiratory tract infection symptoms; RT-PCR, reverse transcriptase polymerase chain reaction assay; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2.
For the Clinical Categories 0-3—Request SARS-CoV-2 RT-PCR for ALL donors. Request a noncontrast CT imaging of the chest on all donors to evaluate for evidence of COVID-19, regardless of CXR result. All lung donors will have a bronchoscopy as part of their evaluation, to include a lower respiratory tract specimen for RT-PCR testing. In heart, liver, or kidney donors, a bronchoscopy is NOT required for the sole purpose of obtaining a sample to test for SARS-CoV-2, as a nasopharyngeal swab is adequate.
These exposure and clinical risk assessments are then fed into a decision-making algorithm for acceptance or rejection of donor organs.
2.1.3. Donor risk categorization
We currently separate algorithms for lung donors ( Table 3) and heart, liver, and kidney donors ( Table 4) because of the potentially higher risk of viral transmission from lung donors.
TABLE 3.
COVID-19 risk categorization for lung donors
| Risk category | Exposure category | Clinical categorya | Decision |
|---|---|---|---|
| Low | 0-1 | 0 | Accept |
| Moderate | 2 | 0 | Consider case by case, if CT imaging of the chest and bronchoscopy is without signs of COVID-19 |
| Moderate - high | 3 | 0 | Consider case by case; likely reject, unless transplant candidate risk/benefit considerations support transplant |
| High | 0-3 | 1-3 | Reject |
Note: ONLY lung donors will have a bronchoscopy as part of their evaluation, to include a lower respiratory tract specimen for RT-PCR testing. In heart, liver, or kidney donors, a bronchoscopy is NOT required to obtain a sample to test for SARS-CoV-2, as a nasopharyngeal swab is adequate.
Abbreviations: COVID-19, Coronavirus Disease 2019; CT, computed tomography; CXR, chest X-ray; NP, nasopharyngeal; RT-PCR, reverse transcriptase polymerase chain reaction assay; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2.
For the clinical categories 0-3—Request SARS-CoV-2 RT-PCR for ALL donors (NP swab for heart, liver, and kidney donors, lower respiratory tract sample for lung donors). Request a noncontrast CT of the chest for ALL donors to evaluate for evidence of COVID-19, regardless of CXR result.
TABLE 4.
COVID-19 risk categorization for heart, liver, or kidney donors
| Risk category | Exposure category | Clinical category | Decision |
|---|---|---|---|
| Low | 0-3 | 0 | Accept |
| Moderate | 0-3 | 1-2 | Consider (if negative SARS-CoV-2 RT- PCR, CT chest without signs of COVID-19 and based on risk/benefit) |
| High | 0-3 | 3 | Reject |
Abbreviations: COVID-19, coronavirus disease 2019; CT, computed tomography; CXR, chest X-ray; NP, nasopharyngeal; RT-PCR, reverse transcriptase polymerase chain reaction assay; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2.
a For the Clinical Categories 0-3—Request SARS-CoV-2 RT-PCR for ALL donors (NP swab for heart, liver, and kidney donors). Request a noncontrast CT of the chest for ALL donors to evaluate for evidence of COVID-19, regardless of CXR result.
Lung donor risk categorization
Our decision criteria for lung transplantation, outlined in Table 3, utilize the donor exposure and clinical risk assessment scores described previously. In order to best protect providers and organ transplant candidates, all donors who test positive for COVID-19 will be rejected. Additionally, donors with unexplained respiratory failure leading to death as well as those who meet federally mandated exclusion criteria will be rejected. Any donor who is symptomatic (Clinical Risk Categories 1-3) is considered high risk and will be rejected, regardless of SARS-CoV-2 RT-PCR results, bronchoscopy, or CT chest findings.
Asymptomatic donors in Exposure Category 1 (unknown exposure while ongoing community transmission) must be tested for SARS-CoV-2 by RT-PCR (only lower respiratory tract samples obtained via bronchoscopy are acceptable) and will be accepted if there are no concerning symptoms, a negative SARS-CoV-2 RT-PCR assay, and noncontrast CT imaging of the chest without signs of COVID-19.
Asymptomatic donors in exposure categories 2 or 3 who test negative for SARS-CoV-2 in addition to a normal CXR, noncontrast CT imaging of the chest without signs of COVID-19, and normal bronchoscopy may be accepted based on clinical judgment. However, it must be noted that donors with a lower risk of exposure are preferred, and donors in Exposure Category 3 should likely be rejected unless risk-benefit considerations strongly support transplantation.
Heart/liver/kidney donor risk categorization
Our decision criteria for heart, liver, and kidney transplantation, outlined in Table 4, utilize the donor exposure and clinical risk scores described later.
Asymptomatic donors with negative RT-PCR and CT imaging of the chest without evidence of COVID-19 (Clinical Category 0) may be accepted. Symptomatic donors with negative RT-PCR and CT imaging of the chest without evidence of COVID-19 (Clinical Category 1) may also be accepted, though the risk-benefit of acceptance of symptomatic donors with exposure to a confirmed case of COVID-19 (Exposure Category 3) should be carefully considered and should include CT imaging of the chest without evidence of COVID-19.
Symptomatic donors who test negative for SARS-CoV-2 with a CT imaging of the chest concerning for evidence of COVID-19 (Clinical Category 2) ought to be critically evaluated based on risk-benefit to the transplant candidate, and level of exposure must be included in this consideration.
2.2. Transplant candidate assessment
Based on ASTS guidance, it is presumed that patients with active or recent COVID-19 who are transplanted will likely lead to adverse outcomes.6 , 7 Therefore, upon organ allocation to the specific transplant candidate, both inpatient and outpatient transplant candidates with active or recent COVID-19 will be deferred from transplantation. It is worth noting that once a transplant candidate-donor match has been identified and appropriately screened, the medical director of transplantation will confirm availability of health care resources with the chief medical officer before transplantation. Furthermore, at the recommendation of multiple international transplant societies, the ASTS, CMS, and our hospital network, the living donor programs and nonurgent deceased donor transplantations have been temporarily suspended at the time this manuscript was submitted.5 , 12 Deceased donor kidney transplants were evaluated on a case-by-case basis, understanding end-stage renal disease was not immediately life threatening, though our institution continued to evaluate donors for candidates with high reactivity or who had limited vascular access, which accounts for the lower volume of kidney transplant recipients in this cohort.
2.2.1. Outpatient transplant candidate exposure assessment
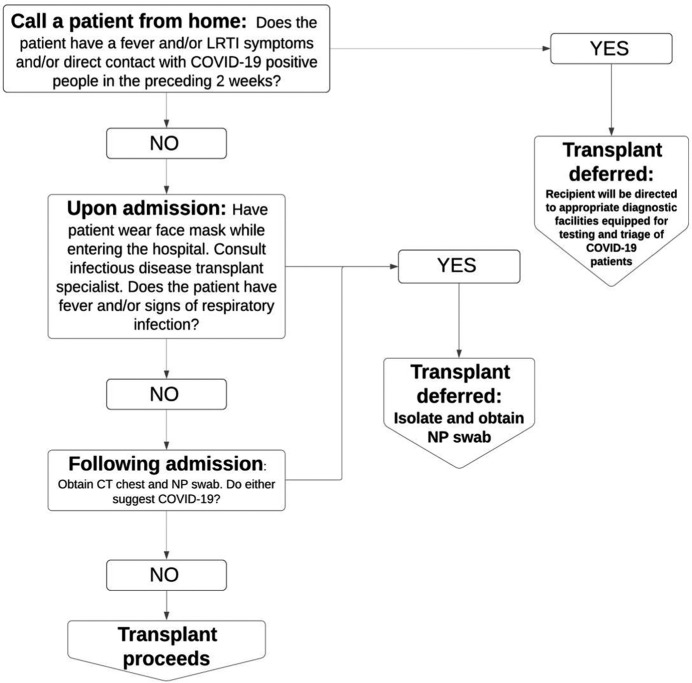
Initially and for patients who are not hospitalized, potential transplant candidates are screened for exposure history and clinical symptoms by the transplant coordinator over the telephone prior to being admitted into the hospital. If the candidate screens positive for recent travel greater than 150 miles from our transplant center or to a Centers for Disease Control and Prevention high-risk area (level 2-3), had exposure to a person under investigation (PUI) in the last 21 days, or had exposure to a confirmed case in the last 28 days and/or has the presence of lower respiratory tract symptoms (cough, shortness of breath, chest pain) with or without fever, with or without malaise, with or without anosmia will be deferred for transplantation at the time of this organ allocation. This arm of the protocol is especially important as it prevents the admission of a potentially SARS-CoV-2 infected patient into the hospital system, exposing the inpatients and health care workers to greater risk of infection. As outlined in Figure 1, if screening is positive, the transplant will be deferred, and the transplant candidate will be directed to appropriate diagnostic facilities equipped for testing and triage of COVID-19 patients. If screening is negative, the patient will be admitted for further workup that will aid decision making in risk-benefit analysis of transplant vs waitlist mortality. Upon arrival to the hospital, the transplant candidate will wear a mask24 and undergo nasopharyngeal swab for SARS-CoV-2 RT-PCR as well as noncontrast CT imaging of the chest immediately to facilitate expedited results. Our laboratory uses the Cepheid Xpert Xpress SARS-CoV-2 assay and is able to return a RT-PCR result in 3-6 hours. Furthermore, during this pretransplant workup, all potential transplant candidates also receive a transplant infectious disease consultation (Figure 1).
FIGURE 1.
Outpatient COVID-19 Transplant Candidate Screening and Assessment Algorithm. Algorithm used for screening outpatient transplant candidates for heart, lung, liver, and kidney transplants during the COVID-19 pandemic. LRTI, lower respiratory tract infection symptoms; NP, nasopharyngeal; CT, computed tomography; COVID-19, coronavirus disease 2019
2.2.2. Inpatient transplant candidate clinical assessment
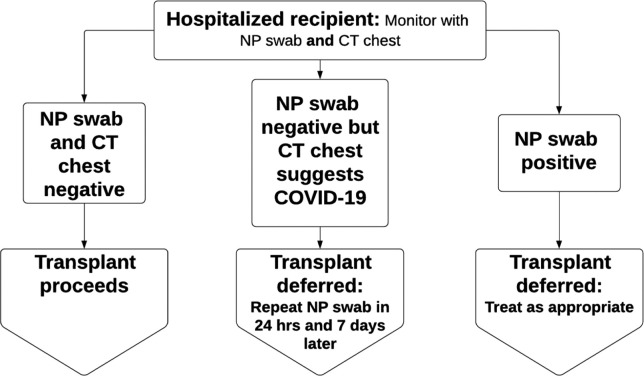
All inpatient candidates must be subject to a SARS-CoV-2 RT-PCR test from nasopharyngeal swab if they have not been tested and found to be negative within the previous 48 hours. A noncontrast CT imaging of the chest will be obtained on all candidate transplant candidates within 7 days of anticipated transplant date, if feasible. Exceptions may be granted to cardiac patients with mechanical circulatory support who are immobilized and the risk of movement to a CT scanner is not justified. Asymptomatic SARS-CoV-2 PCR negative candidates with CT imaging of the chest without evidence of COVID-19 may be considered for transplant if they have no known exposure to PUI in the previous 21 days or confirmed cases in the previous 28 days ( Figure 2).
FIGURE 2.
Inpatient COVID-19 Transplant Candidate Assessment and Screening Algorithm. Algorithm used for screening inpatient transplant candidates for heart, lung, liver, and kidney transplants during the COVID-19 pandemic. Our institution uses the Cepheid Xpert Xpress severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2 assay). NP, nasopharyngeal; CT, computed tomography; COVID-19, coronavirus disease 2019
Of note, any inpatient transplant candidates found to have signs and symptoms concerning for COVID-19, including fevers, anosmia, and/or respiratory symptoms, regardless of SARS-CoV-2 test result, will have their transplant deferred. These patients will need to have complete resolution of symptoms in addition to 2 negative SARS-CoV-2 PCR tests with CT imaging of the chest without evidence of COVID-19 before being reconsidered for transplantation.
Previously symptomatic COVID-19 positive candidates must have 2 subsequent negative SARS-CoV-2 PCR tests at least 1 week apart and CT imaging of the chest without evidence of COVID-19 to be reconsidered for transplant.
3. RESULTS
A prospective chart review was performed on all primary organ allocations, where clinical suitability has already been evaluated and deemed clinical suitable, utilizing this Transplant Donor and Transplant Candidate COVID-19 Screening and Assessment Protocol. Because of the declaration of a pandemic,1 we developed this protocol and implemented it on March 24, 2020, which is the same day the local stay-at-home order was issued. In tabulated form, we present the results of all solid organ primary offers we intended to accept for our candidate at our center starting March 24, 2020 until May 11, 2020. Organ offers that were otherwise not clinically suitable for our transplant candidate were not included in this summary as they did not require utilization of this protocol.
To proceed with the protocol, we first complete the Donor Exposure Assessment using Table 1, followed by the Donor Clinical Risk Assessment using Table 2. Table 3 is designed specifically to score the risk of lung transplant donors. Lung donors potentially have greater risk in transmitting SARS-CoV-2 and therefore require bronchoscopy and lower respiratory tract samples for RT-PCR. Separately, Table 4 summarizes the risk for heart, liver, and kidney donors by using the exposure and clinical risk categorizations of Tables 1 and 2. If the donors are deemed low or moderate risk based on the assessment categories, the transplant candidate will then go through the Transplant Candidate Screening algorithms outlined in Figure 1 (Outpatient Assessment) and Figure 2 (Inpatient Assessment), which will inform the decision to proceed to transplant. Transplant candidates were educated on the unknown risk of transplantation in the COVID-19 pandemic both prior to admission by telephone and after admission in person.
As demonstrated in Table 5, 32 potential transplant candidate matches were identified during this time, all utilizing the Donor and Transplant Candidate COVID-19 Screening and Assessment Protocol to inform the decision to proceed with transplantation during the COVID-19 pandemic. 5 transplants were deferred. Twenty-seven of the 32 donor-candidate matches have been transplanted. Ten primary heart offers were considered of which 7 were transplanted (one patient received a heart/lung and another received a heart/kidney). Three lung and 2 kidney primary offers were considered, of which all were transplanted. Seventeen primary liver offers were considered of which 15 were transplanted. All of these donors and candidates had met prespecified criteria to proceed with transplant, and thus only those primary organ offers utilizing the algorithm during the study time are reported. Of note, 4 donors donated 2 organs or more each (totaling 10 transplants of our cohort of 27 transplantations)—1 donated a heart/lung/liver, 1 donated a heart/liver/kidney, 1 donated a heart and liver, and 1 donated lungs and a kidney to this cohort. Transplant dates were omitted out of respect for patient privacy. Five transplant candidates were not transplanted because of a positive screening categorization that precluded them from proceeding to transplantation. Two donors were found to have moderate-high risk and thus their organs were not utilized for transplantation. One was a heart donor who had an indeterminate SARS-CoV-2 PCR test. The other was a liver donor who was screened and found to be of moderate risk because they were admitted for respiratory failure after a known COVID exposure. Two of those 5 canceled transplants were cardiac candidates with symptoms concerning for COVID-19; one had LRTI symptoms who screened positive during the preadmission telephone interview, as dictated by the Outpatient Transplant Candidate Assessment and Screening Algorithm in Figure 1, and the other potential cardiac transplant candidate had LRTI symptoms with a CT chest suggestive of COVID-19. And the last transplant that was deferred was a potential liver candidate who was found to have ground glass opacities bilaterally on CT imaging of the chest, concerning for both infectious and noninfectious etiologies. This transplant was deferred. The candidate underwent repeated RT-PCRs, as per the protocol, which were negative as well as repeat imaging, which was stable. The candidate subsequently underwent transplantation with a different donor liver. At the time of this submission, all of the 27 patients transplanted have survived, and none in this cohort have tested positive for SARS-CoV-2 since transplantation with the implementation of this algorithm.
TABLE 5.
Transplant outcomes utilizing the transplant donor and transplant candidate COVID-19 screening and assessment protocol
| Decision | Exposure category | Clinical risk category | Risk category | Comment | ||
|---|---|---|---|---|---|---|
| Heart | ||||||
| 7 primary offers | Transplanted | Donor | 0 | 0 | Low | Of note, 1 candidate received a heart/lung, and 1 candidate received a heart/kidney |
| Candidate | ✓ | ✓ | ||||
| 1 primary offer | Not transplanted | Donor | 0 | 3 | High | Donor was found to have an indeterminate SARS-CoV-2 RT-PCR |
| Candidate | ✓ | ✓ | ||||
| 2 primary offers | Not transplanted | Donor | 0 | 0 | Low | One transplant candidate was found to have a positive telephone screen (malaise and cough). The other transplant candidate had profound dyspnea on exam and a CT Chest suggestive of COVID-19. Both candidates tested negative for SARS-CoV-2 |
| Candidate | ✗ | ✗ | ||||
| Lung | ||||||
| 3 primary offers | Transplanted | Donor | 0 | 0 | Low | |
| Candidate | ✓ | ✓ | ||||
| Kidney | ||||||
| 2 primary offers | Transplanted | Donor | 0 | 0 | Low | |
| Candidate | ✓ | ✓ | ||||
| Liver | ||||||
| 15 primary offers | Transplanted | Donor | 0 | 0-1 | Low | One donor was febrile, but clinical risk score was low and therefore transplant proceeded |
| Candidate | ✓ | ✓ | ||||
| 1 primary offer | Not transplanted | Donor | 3 | 1 | Moderate | Donor had symptoms of respiratory distress and known exposure to COVID-19 positive people |
| Candidate | ✓ | ✓ | ||||
| 1 primary offer | Not transplanted | Donor | 0 | 0 | Low | Candidate was found to have CT Chest imaging with ground glass opacities concerning for infectious etiology. Transplant was deferred per protocol, and repeated RT-PCRs were negative. The candidate later proceeded to transplant with a different donor organ. |
| Candidate | ✓ | ✗ | ||||
Note: Ten primary heart offers were considered of which 7 were transplanted. Three lung and 2 kidney primary offers were considered, of which all were transplanted. Seventeen primary liver offers were considered of which 15 were transplanted. All of these donors and candidates had met prespecified criteria to proceed with transplant, and thus only those utilizing the algorithm during the study time are reported. At the time of this submission, there are no recipients in this cohort who have tested positive for SARS-CoV-2 since transplantation.
✓, candidate met criteria to proceed with transplant, no evidence of COVID-19 infection; ✗, candidate had clinical symptoms and/or signs of COVID-19 infection, transplant deferred—see comments
4. DISCUSSION
Balancing the risks and competing demands using the most current data available, we created a practical protocol that allows us to continue treating life-threatening end-organ disease safely for our patients in the context of a virulent pandemic that can infect our donors, health care workers, and our transplant candidates. Using guidance from various international transplant societies, the ASTS, US Department of Defense, and Centers for Disease Control and Prevention, and the clinical expertise of the authors, we have created the Transplant Donor and Transplant Candidate COVID-19 Screening and Assessment Protocol to ensure the safety of and minimization of risks to our transplant candidates and our health care system at large in the midst of a pandemic. The vetting of our donors and transplant candidates is paramount considering the true risk of donor-derived transmission of SARS-CoV-2 remains unclear. Data are limited at this time, both in amount and sample size but suggests that patients with COVID-19 who undergo surgery suffer a significant mortality.26 Extrapolating these data to our transplant population would suggest a much higher mortality that would be catastrophic if there were no mechanism to screen for and prevent further transmission. Considering the extraordinary amount of resources involved in every single transplantation, including intensive care beds, ventilators, blood products, personal protective equipment, nurses, intensivists, surgeons, and other health care workers and of course the donor organs themselves, we are obligated as professionals to perform due diligence and proper stewardship to protect our precious few donors, their organs, and the patients they would serve.
Limitations of this work include the constantly evolving understanding of the transmission and pathophysiology of this virus and its effects on our patients, varying degrees of negative predictive value of chest CT imaging based on the prevalence of disease, and our sample size. There is also a risk that we may be especially selective at this point, considering the low positive predictive value of chest CT imaging and the varying sensitivity of RT-PCR dependent on its collection,24 there may be some donors and candidates who are deferred for transplantation. However, we feel that this is the most pragmatic way to approach donor and candidate selection, in the backdrop of limited medical supplies, personnel, hospital resources, and evolving understanding of this virulent disease. A working transplant protocol is important because the careful selection of donors and transplant candidates is necessary in the COVID-19 pandemic to attenuate risk and ensure appropriate utilization of resources, and ultimately to continue life-saving transplantation for our patients with end-organ disease who face very real and well-documented waitlist mortality. This is especially true considering the public health consensus is that SARS-COV-2 will require a coordinated and pragmatic framework including widespread testing that will take this pandemic well into the future27 during which time our patients continue to face a significant waitlist mortality. In preparing this manuscript, a review of Transplant Society Recommendation Consensus was published that outlined the recommendations of 22 transplant societies internationally.12 This consensus supports our algorithm in requiring clinical screening, donor testing, excluding those infected or suspected of SARS-CoV-2 infection, candidate RT-PCR testing, resolution of symptoms with negative RT-PCR assay before transplantation, and a CT imaging of the chest of candidates prior to transplantation.12
We have performed 7 heart (including 1 heart/lung and 1 heart/kidney patient), 3 lung, 2 kidney, and 15 liver transplantations between March 24, 2020 and May 11, 2020 utilizing this protocol with 100% survival and no evidence of SARS-CoV-2 infection in our recipients at the time of this submission. Our hope is that this protocol will serve as the foundation for further discussion on working transplant protocols to continue transplantation in the midst of a modern worldwide pandemic.
Acknowledgments
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1.Aslam S, Mehra MR. COVID-19: yet another coronavirus challenge in transplantation. J Heart Lung Transplant. 2020;39(5):408–409. doi: 10.1016/j.healun.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mortality Analyses. Johns Hopkins Coronavirus Resource Center. 2020. https://coronavirus.jhu.edu/data/mortality. Accessed May 12, 2020.
- 3.COVID-19 Map. Johns Hopkins Coronavirus Resource Center. 2020. https://coronavirus.jhu.edu/map.html. Accessed April 14, 2020.
- 4.Transplant trends. UNOS. 2020. https://unos.org/data/transplant-trends/. Accessed April 14, 2020
- 5.Adult Elective Surgery and Procedures Recommendations. CMS. 2020. https://www.cms.gov/files/document/cms-non-emergent-elective-medical-recommendations.pdf. Accessed May 12, 2020.
- 6.Organ Retrieval for Transplantation in the COVID-19 Era. ASTS. 2020. https://asts.org/advocacy/covid-19-resources/asts-covid-19-strike-force/asts-covid-19-strike-force-organ-retrieval-guidance. Accessed April 14, 2020
- 7.ASTS COVID 19 Strike Force Guidance to Members on the Evolving Pandemic. ASTS. 2020. https://asts.org/advocacy/covid-19-resources/asts-covid-19-strike-force/asts-covid-19-strike-force-initial-guidance. Accessed April 14, 2020
- 8.Le TQM, Takemura T, Moi ML, et al. Severe acute respiratory syndrome coronavirus 2 shedding by Travelers, Vietnam, 2020. [published online ahead of print 2020] Emerg Infect Dis. 2020;26(7) doi: 10.3201/eid2607.200591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C-Y, Chen SF, Hollander SA, et al. Donor heart selection during the COVID-19 pandemic: a case study. J Heart Lung Transplant. 2020;39(5):497–498. doi: 10.1016/j.healun.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lt Col Matos RI, Col Chung KK. DoD COVID-19 Practice Management Guide. 2020. https://www.health.mil/Reference-Center/Technical-Documents/2020/03/24/DoD-COVID-19-Practice-Management-Guide. Accessed April 14, 2020
- 11.National Center for Immunization and Respiratory Diseases (NCIRD) DoVD. Coronavirus Disease 2019 (COVID-19) in the U.S. Centers for Disease Control and Prevention. 2020. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed April 14, 2020
- 12.Ritschl PV, Nevermann N, Wiering L, et al. Solid organ transplantation programs facing lack of empiric evidence in the COVID-19 pandemic: a by-proxy Society Recommendation Consensus approach. Am J Transplant. 2020;00:1–12. doi: 10.1111/ajt.15933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zitek T. The appropriate use of testing for COVID-19. West J Emerg Med. 2020;21(3):470–472. doi: 10.5811/westjem.2020.4.47370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung M, Bernheim A, Mei X, et al. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295(1):202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang P, Liu T, Huang L, et al. Use of chest CT in combination with negative RT-PCR assay for the 2019 novel coronavirus but high clinical suspicion. Radiology. 2020;295(1):22–23. doi: 10.1148/radiol.2020200330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inui S, Fujikawa A, Jitsu M, et al. Chest CT findings in cases from the Cruise Ship “Diamond Princess” with coronavirus disease 2019 (COVID-19) Radiol Cardiothorac Imaging. 2020;2(2):e200110. doi: 10.1148/ryct.2020200110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nair A, Rodrigues J, Hare S, et al. A British Society of Thoracic Imaging statement: considerations in designing local imaging diagnostic algorithms for the COVID-19 pandemic. Clin Radiol. 2020;75(5):329–334. doi: 10.1016/j.crad.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan F, Ye T, Sun P, et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020. 10.1148/radiol.2020200370 [DOI] [PMC free article] [PubMed]
- 19.Rodrigues J, Hare SS, Edey A, et al. An update on COVID-19 for the radiologist - a British Society of Thoracic Imaging Statement. Clin Radiol. 2020;75(5):323–325. doi: 10.1016/j.crad.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye Z, Zhang Y, Wang Y, et al. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review [published online ahead of print 2020]. Eur Radiol. 10.1007/s00330-020-06801-0. [DOI] [PMC free article] [PubMed]
- 21.Yoon SH, Lee KH, Kim JY, et al. Chest radiographic and CT findings of the 2019 novel coronavirus disease (COVID-19): analysis of nine patients treated in Korea. Korean J Radiol. 2020;21(4):494–500. doi: 10.3348/kjr.2020.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020:200642. 10.1148/radiol.2020200642 [DOI] [PMC free article] [PubMed]
- 23.Kim H. Outbreak of novel coronavirus (COVID-19): what is the role of radiologists? Eur Radiol. 2020;30(6):3266–3267. doi: 10.1007/s00330-020-06748-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zou L, Ruan F, Huang M, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanson KE, Arias CA, Englund JA, et al. IDSA; 2020. Infectious Diseases Society of America Guidelines on the Diagnosis of COVID-19. https://www.idsociety.org/practice-guideline/covid-19-guideline-diagnostics/. Accessed April 14, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lei S, Jiang F, Su W, et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection [published online ahead of print 2020] EClinicalMedicine. 2020;21:100331. doi: 10.1016/j.eclinm.2020.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pettit SD, Jerome KR, Rouquie D, et al. ‘All In’: a pragmatic framework for COVID-19 testing and action on a global scale [published online ahead of print 2020] EMBO Mol Med. 2020;12(6):e12634. doi: 10.15252/emmm.202012634. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.