Abstract
Immunosuppression and comorbidities might place solid organ transplant (SOT) recipients at higher risk from COVID-19, as suggested by recent case series. We compared 45 SOT vs. 2427 non-SOT patients who were admitted with COVID-19 to our health-care system (March 1, 2020 - August 21, 2020), evaluating hospital length-of-stay and inpatient mortality using competing-risks regression. We compared trajectories of WHO COVID-19 severity scale using mixed-effects ordinal logistic regression, adjusting for severity score at admission. SOT and non-SOT patients had comparable age, sex, and race, but SOT recipients were more likely to have diabetes (60% vs. 34%, p < .001), hypertension (69% vs. 44%, p = .001), HIV (7% vs. 1.4%, p = .024), and peripheral vascular disorders (19% vs. 8%, p = .018). There were no statistically significant differences between SOT and non-SOT in maximum illness severity score (p = .13), length-of-stay (sHR: 0.91.11.4, p = .5), or mortality (sHR: 0.10.41.6, p = .19), although the severity score on admission was slightly lower for SOT (median [IQR] 3 [3, 4]) than for non-SOT (median [IQR] 4 [3–4]) (p = .042) Despite a higher risk profile, SOT recipients had a faster decline in disease severity over time (OR = 0.760.810.86, p < .001) compared with non-SOT patients. These findings have implications for transplant decision-making during the COVID-19 pandemic, and insights about the impact of SARS-CoV-2 on immunosuppressed patients.
KEYWORDS: clinical research / practice, complication: infectious, infection and infectious agents - viral, infectious disease, organ transplantation in general
Abbreviations: AKI, acute kidney injury; ALC, absolute lymphocyte count; ALT, alanine transaminase; ANC, absolute neutrophil count; AST, aspartate transaminase; COVID-19, coronavirus infection 2019; Cr, creatinine; CRP, C-reactive protein; CRS, cytokine release syndrome; ICU, intensive care unit; IL-6, interleukin-6; JHM, Johns Hopkins Medical System; MMF, mycophenolate mofetil; NAAT, nucleic acid amplification test; SARS-CoV-2, severe acute respiratory syndrome – coronavirus-2; SOT, solid organ transplant; WHO, World Health Organization
1. INTRODUCTION
The COVID-19 pandemic has had an enormous impact on the field of transplantation.1 , 2 Early in the pandemic, there was a dramatic reduction in numbers of transplants performed,1 , 2 reflecting transplant clinicians’ concerns about risks to patients and living donors, as well as early reports of high mortality from COVID-19 in the solid organ transplant (SOT) population.3, 4, 5, 6, 7, 8, 9 Some subsequent studies have reported lower mortality,10 , 11 but many questions remain about the risk that COVID-19 poses to SOT recipients.
A recent large multicenter cohort study of 482 SOT recipients, which has augmented the information from previous single-center reports, reported a 20.5% mortality in hospitalized SOT recipients.12 However, few studies have directly compared outcomes between SOT and non-SOT patients with COVID-19. Chaudhry et al compared outcomes of 35 SOT recipients with 100 consecutive non-SOT patients who were hospitalized with COVID-19 at a single center, and found that a composite outcome (ICU transfer, mechanical ventilation, hospital mortality) was comparable between these two groups, although comorbidities and acute kidney injury were more common in the SOT group.13 Roberts et al compared outcomes of SOT inpatients who did and did not require inpatient admission, and compared these outcomes with unpublished data on non-SOT recipients in their hospitals as well as previously published data from the general population, and reported higher hospitalization rates, ICU admission rates, and mortality in SOT recipients.14 However, outcomes of SOT recipients and others have yet to be compared rigorously in a large database.
The aims of our study were to compare the presentations, clinical courses, and outcomes of COVID-19 infection in SOT recipients with those of all other inpatients with COVID-19, by leveraging data from a large COVID-19 database comprising five hospitals in the Johns Hopkins Medicine (JHM) health system.15 We compared in-hospital mortality, length of hospital stay, daily World Health Organization (WHO) disease scale,16 , 17 and laboratory test results (such as absolute lymphocyte counts [ALCs] and inflammatory markers) to elucidate differences between the SOT and non-SOT populations with COVID-19. It is important to understand the clinical trajectory of COVID-19 in SOT recipients against the backdrop of a large and diverse group of non-SOT inpatients with COVID-19, in order to identify best practices for SOT recipients.
2. MATERIALS AND METHODS
2.1. Data source
The primary data source was JH (Johns Hopkins) CROWN: The COVID-19 Precision Medicine Analytics Platform (PMAP) Registry, which utilizes the Hopkins Precision Medicine Analytics Platform, and encompasses five hospitals in Johns Hopkins Medicine (JHM), as previously described.15 The registry includes patient-level data such as demographics, medical history, inpatient diagnosis, oxygen therapy, medications, laboratory data, imaging, hospital admission and discharge time points, and inpatient deaths. The inpatient dataset included adults who were admitted to inpatient services or emergency observation within 1 week of a positive SARS-CoV-2 nucleic acid amplification (NAAT) test, who had been flagged as having COVID-19, or who had been diagnosed with suspected or confirmed COVID-19 at discharge. This study was approved by the Institutional Review Board at Johns Hopkins University School of Medicine.
2.2. Study population
We included all adult patients in the inpatient dataset who were admitted to one of the JHM hospitals between March 1, 2020 and August 21, 2020. The earliest inpatient admission was the index COVID-19 admission. Patients transferred between hospitals within JHM received a continuum of care, so both admissions were considered part of the index admission. Within the JH-CROWN Registry,15 we identified patients in the registry with a history of SOT using ICD-10 codes (Z94.0, Z94.1, Z94.2, Z94.3, Z94.4, Z94.82, Z94.83, Z48.21, Z48.22, Z48.23, Z48.24, Z48.28, T86.1, T86.2, T86.3, T86.4, T86.81), and “transplant” as keyword. Patients without an identifiable history of SOT were defined as non-SOT. Non-SOT patients with history of bone marrow transplant (T86.0, Z94.81, Z94.84, n = 6) and those with unspecified or ambiguous transplant (n = 3) were excluded. Patients with non-SOT procedures (e.g., corneal transplant) were classified in the non-SOT group. To minimize the risk of misclassification, this was cross-checked with a list of SOT patients with COVID-19 which had been maintained for clinical care purposes, and this was consistent with the SOT numbers derived from the database. Using this population, we recorded baseline characteristics: patient demographics (age, sex, race), body mass index (BMI), smoking status (never, past, current), comorbidities, and preadmission transfer from other hospitals stratified by history of SOT. Baseline patient characteristics were compared between non-SOT and SOT using Wilcoxon ranksum test for continuous variables, and Fischer’s exact test for categorical variables.
2.3. Oxygen therapy
We characterized oxygen therapy using a 4-point ordinal scale: room air (lowest); low-level support using nasal cannula; high-level support using noninvasive positive pressure ventilation (NIPPV) or high-flow oxygen; and intubation (highest). We compared oxygen therapy between SOT vs. non-SOT patients on admission using a Wilcoxon ranksum test. We compared trajectories of oxygen therapy over time between the two groups using multilevel mixed-effects ordinal logistic regression assuming proportional odds, adjusting for severity upon admission, with a patient-level random intercept, and an interaction between SOT status and time since admission. An odds ratio exceeding 1 indicates that SOT patients had higher odds of requiring higher level treatment as time went on. We also compared the duration of oxygen therapy using a Wilcoxon ranksum test; durations were converted from hours to days, allowing for noninteger durations.
2.4. WHO COVID-19 severity scale
We characterized severity of COVID-19 in SOT and non-SOT patients using the World Health Organization (WHO) COVID severity scale.16 , 17 The WHO severity scale is an 8-point scale for patients infected with COVID-19 comprising the following scores: 1 (ambulatory, no limitations); 2 (limitation on activities); 3 (admitted to hospital, not on oxygen); 4 (oxygen mask or nasal prong); 5 (high-flow oxygen or noninvasive positive pressure ventilation [NIPPV]); 6 (intubation); 7 (intubation and advanced life support); and 8 (death).
Therapies defined as advanced interventions included intravenous vasopressors (IVP), extra-corporeal membrane oxygenation (ECMO), and continuous renal-replacement therapy (CRRT). We compared WHO severity score between SOT vs. non-SOT patients on admission, and also the highest level of oxygen therapy required during the admission, using a Wilcoxon ranksum test as described above. Additionally, we compared trajectories of WHO severity score over time between SOT vs. non-SOT patients using multilevel ordinal logistic regression as described above after adjusting for severity upon admission, with a patient-level random intercept and an interaction between SOT status and time since admission.
2.5. Laboratory data
Laboratory data collected included the proportion of patients with a white blood cell count (WBC) lower than 1 or above 20 (k/mm3) at any time during the admission; absolute neutrophil count (ANC) at any time below 1 (k/mm3); the lowest ALC during the admission; and the peak levels of C-reactive protein (CRP), interleukin-6 (IL-6), erythrocyte sedimentation rate (ESR), ferritin, and D-dimer. Repeated measurements were tracked longitudinally and compared between SOT and non-SOT. Markers indicative of myocardial injury were reported as the proportion with abnormally high levels of creatine kinase (CK), troponin-I (TnI), or troponin-T (TnT). Transaminitis was reported as the proportion with AST above 74 U/L and ALT above 80 U/L (above 2x the upper limit of normal). Trajectories of WBC and ALC were compared between SOT vs. non-SOT patients using multilevel mixed-effects linear regression (after applying a log transform) with a patient-level random intercept. Other laboratory results were compared between SOT and non-SOT patients using Fisher’s exact test for proportions and Wilcoxon ranksum test for continuous values.
2.6. Medications
Use of a particular medication during the admission was reported as the proportion treated with that medication. We included maintenance immunosuppressive agents such as tacrolimus, mycophenolate mofetil (MMF), prednisone, as well as other agents used for targeted therapy of COVID-19 or its inflammatory phase, such as steroids (hydrocortisone, prednisone, methylprednisolone, and dexamethasone), hydroxychloroquine, tocilizumab, and remdesivir. Medication use was compared between SOT and non-SOT patients using Fisher’s exact test.
2.7. Hospital length-of-stay and in-hospital mortality
Hospital length-of-stay was defined as the duration between the date of the index admission, and the date of final discharge from the health system. Patients were followed from time of admission to discharge, in-hospital death, or administrative censorship on August 21, 2020; any outcomes which might have occurred after August 21, 2020 are not included in the analysis. Length-of-stay and in-hospital mortality were compared between SOT and non-SOT using Fine and Gray competing-risks regression. Competing-risks regression yields a subhazard ratio (sHR) analogous to the hazard ratio from Cox regression, representing relative hazard of the outcome of interest after accounting for competing risks. All time measurements were converted to days from hours.
2.8. Sensitivity analysis
Since the SOT and non-SOT populations were generally comparable with regard to age, race, and sex, the main analyses as described above were unadjusted. However, to account for potential confounding, as a sensitivity analysis we repeated the analyses of length-of-stay and mortality by calculating a propensity score for SOT based on age, race, sex, and oxygen therapy requirement on the date of admission, using the weighting-by-the-odds technique.18 , 19 Specifically, we calculated a propensity score for SOT using a logistic regression model with age, race, sex, and oxygen requirement on date of admission as covariates. We then performed weighted analyses, assigning the propensity score (converted to an odds scale, p/(1-p) as a weight to all non-SOT recipients, and a weight of 1 to all SOT recipients). This ensures comparable balance of age, race, sex, and oxygen requirement (yes/no) upon admission across both groups in the weighted populations.
2.9. Statistical analysis
Since the SOT and non-SOT populations were generally comparable with respect to age, race, and sex, all analyses were unadjusted, except for the sensitivity analyses described above. Since our goal was to compare COVID-19 in SOT recipients vs. all other inpatients with COVID-19, instead of SOT patients vs. patients with end-stage organ disease but no history of transplantation, we did not attempt to account for differences in history of organ failure, or sequelae such as anemia or fluid and electrolyte disorders. An α of 0.05 was used to determine statistical significance. All confidence intervals are 95%, and are reported as per the method of Louis and Zeger.20 All analyses were performed using Stata 16.1/MP for Linux (College Station, TX).
3. RESULTS
3.1. Patient demographics and baseline characteristics
Forty-five SOT and 2427 non-SOT adults with COVID-19 were included in the study population. There were no statistically significant differences in demographics comparing the two groups, including age (median [IQR] 59 [48,65] vs. 59 [43, 72]), female sex (46.7% vs. 48.1%), racial composition such as Black (55.6% vs. 36.3%), White (26.7% vs. 28.4%), and Hispanic (13.3% vs. 24.9%) ( Table 1). Compared to non-SOT, SOT patients were more likely to have diabetes (60.0% vs. 33.8%, p < .001), hypertension (68.9% vs. 44.3%, p = .001), peripheral vascular disorders (18.6% vs. 7.8%, p = .018), history of peptic ulcer disease (7.0% vs. 1.5%, p = .029), human immunodeficiency virus (HIV) (7.0% vs. 1.4%, p = .02), and any history of malignancy (23.3% vs. 10.9%, p = .023). Similarly, more SOT patients had indicators of organ failure (likely dating from the pretransplant time period rather than posttransplant complications) such as renal failure (83.7% vs. 19.4%, p < .001), liver disease (34.9% vs. 8.5%, p < .001), fluid and electrolyte disorders (74.4% vs. 52.7%, p = .005) and anemia (55.8% vs. 21.2%, p < .001) (Table 1). Two (4.4%) SOT and 265 (10.9%, p = .23) non-SOT patients had do-not-resuscitate or do-not-intubate status recorded on the first day of admission (Table 1).
TABLE 1.
Description of population, comparing SOT to non-SOT
| non-SOT (N = 2427) | SOT (N = 45) | p | |
|---|---|---|---|
| Age, median (IQR) | 59 (43, 72) | 59 (48, 65) | .61 |
| Female sex | 1168 (48.1%) | 21 (46.7%) | .88 |
| Race/ethnicity | .11 | ||
| White | 690 (28.5%) | 12 (26.7%) | |
| Black | 882 (36.4%) | 25 (55.6%) | |
| Asian | 131 (5.4%) | 1 (2.2%) | |
| Hispanic | 605 (24.9%) | 6 (13.3%) | |
| Others | 113 (4.7%) | 1 (2.2%) | |
| Unknown | 6 (0.2%) | 0 (0.0%) | |
| BMI, median (IQR) | 28.6 (24.4, 33.8)a | 27.3 (24.0, 29.9)b | .22 |
| Category of smoking status | .012 | ||
| Never smoked | 1390 (57.3%) | 23 (51.1%) | |
| Former smoker | 448 (18.5%) | 18 (40.0%) | |
| Current smoker | 134 (5.5%) | 1 (2.2%) | |
| Unknown | 455 (18.7%) | 3 (6.7%) | |
| Diabetes mellitus | 820 (33.8%) | 27 (60.0%) | <.001 |
| Hypertension | 1076 (44.3%) | 31 (68.9%) | .001 |
| Chronic pulmonary disease | 531 (22.6%) | 8 (18.6%) | .71 |
| Pulmonary circulation disorders | 195 (8.3%) | 3 (7.0%) | 1.0 |
| Congestive heart failure | 408 (17.4%) | 11 (25.6%) | .16 |
| Cardiac arrhythmias | 475 (19.6%) | 8 (17.8%) | 1.00 |
| Peripheral vascular disorders | 183 (7.8%) | 8 (18.6%) | .018 |
| Hypothyroidism | 260 (11.1%) | 8 (18.6%) | .14 |
| Fluid and electrolyte disorders | 1238 (52.7%) | 32 (74.4%) | .005 |
| Renal failure | 455 (19.4%) | 36 (83.7%) | <.001 |
| Liver disease | 200 (8.5%) | 15 (34.9%) | <.001 |
| Peptic ulcer disease excluding bleeding | 35 (1.5%) | 3 (7.0%) | .029 |
| Anemia, blood loss | 65 (2.5%) | 0 (0.0%) | .63 |
| Anemia, deficiency | 498 (21.2%) | 24 (55.8%) | <.001 |
| Coagulopathy | 314 (13.4%) | 9 (20.9%) | .17 |
| Lymphoma | 27 (1.1%) | 1 (2.3%) | .40 |
| Solid tumor without metastasis | 199 (8.5%) | 8 (18.6%) | .028 |
| Metastatic cancer | 114 (4.9%) | 4 (9.3%) | .16 |
| Solid tumor, metastasis, or lymphoma | 257 (10.9%) | 10 (23.3%) | .023 |
| HIV/AIDS | 32 (1.4%) | 3 (7.0%) | .024 |
| Rheumatoid arthritis/collagen Vascular diseases | 87 (3.7%) | 4 (9.3%) | .078 |
| Weight loss | 264 (11.2%) | 5 (11.6%) | .81 |
| Obesity | 745 (31.7%) | 15 (34.9%) | .62 |
| Depression | 385 (15.9%) | 5 (11.1%) | .54 |
| Psychosis | 155 (6.6%) | 0 (0.0%) | .11 |
| Alcohol abuse | 126 (5.4%) | 2 (4.7%) | 1.0 |
| Drug abuse | 111 (4.7%) | 4 (9.3%) | .15 |
| Paralysis | 80 (3.4%) | 2 (4.7%) | .66 |
| Other neurological disorders | 533 (22.7%) | 8 (18.6%) | .71 |
| Patients transferred from hospitals | 57 (2.3%) | 6 (13.3%) | <.001 |
| DNR/DNI at admission | 265 (10.9%) | 2 (4.4%) | .23 |
n = 1714.
n = 34.
3.2. Oxygen therapy
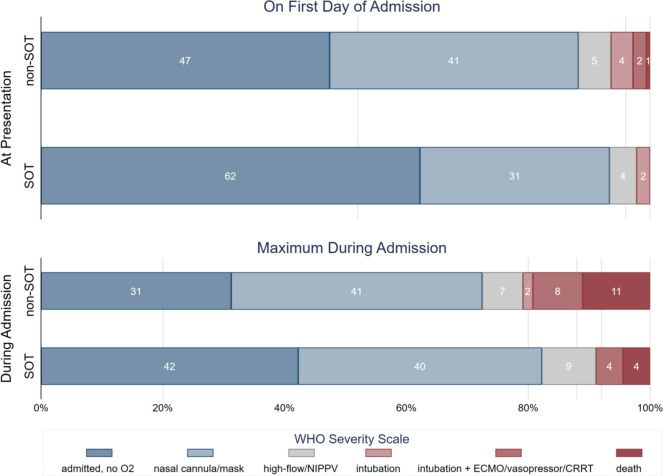
On the first admitted day, 28 (62.2%) SOT and 1153 (47.5%) non-SOT required no oxygen therapy (Fisher exact p = .035). Fourteen (31.1%) SOT and 995 (41.0%) non-SOT required low-level oxygen, two (4.4%) SOT and 132 (5.4%) non-SOT required high-level oxygen (noninvasive positive pressure ventilation [NIPPV] or high-flow) but not intubation, and one (2.2%) SOT and 147 (6.1%) non-SOT required intubation ( Table 2).
TABLE 2.
Inpatient oxygen treatment, advanced therapies, and mortality, comparing SOT to non-SOT
| Factor | Non-SOT | SOT | p |
|---|---|---|---|
| WHO severity scale on the day of admission, median (IQR) | 4 (3, 4) | 3 (3, 4) | .042 |
| Max WHO severity scale during admission, median (IQR) | 4 (3, 5) | 4 (3, 4) | .053 |
| Any oxygen treatment on the day of admission | 1274 (52.5%) | 17 (37.8%) | .035 |
| Any oxygen treatment during admission | 1663 (68.5%) | 26 (57.8%) | .15 |
| Duration on oxygen therapy, median (IQR) | 5.9 (2.4, 11.8)1 | 4.9 (2.7, 7.3)7 | .27 |
| Highest oxygen need on day 1 admission | .29 | ||
| Did not need O2 on admission | 1153 (47.5%) | 28 (62.2%) | - |
| Need oxygen, but not HI-FLOW or NIPPV | 995 (41.0%) | 14 (31.1%) | - |
| HI-FLOW or NIPPV | 132 (5.4%) | 2 (4.4%) | - |
| Ventilator/intubation | 147 (6.1%) | 1 (2.2%) | - |
| Highest level of O2 therapy during entire admission | .21 | ||
| No oxygen therapy | 763 (31.4%) | 19 (42.2%) | - |
| Low-level O2 therapy only | 1082 (44.6%) | 19 (42.2%) | - |
| NIPPV or high flow only, not ventilated | 187 (7.7%) | 4 (8.9%) | - |
| Ventilated | 395 (16.3%) | 3 (6.7%) | - |
| Any ventilation | 395 (16.3%) | 3 (6.7%) | .1 |
| Duration on ventilation, median days (IQR) | 10.3 (3.9, 21.4)2 | 3.5 (2.4, 13.1)8 | .31 |
| Any IV vasopressor | 356 (14.7%) | 4 (8.9%) | .39 |
| Duration on IV vasopressor, median days (IQR) | 6.1 (1.6, 16.2)3 | 2.7 (0.7, 6.8)9 | .20 |
| Any CRRT | 70 (2.9%) | 1 (2.2%) | 1.00 |
| Duration on CRRT therapy, median days (IQR) | 7.5 (2.7, 17.8)4 | 2.8 (2.8, 2.8)10 | .43 |
| Any inpatient dialysis | 86 (3.5%) | 5 (11.1%) | .023 |
| Duration on inpatient dialysis, median days (IQR) | 8.9 (3.8, 20.6)5 | 9.2 (7.3, 12.0)11 | .75 |
| Any ECMO | 17 (0.7%) | 0 (0%) | n/a |
| Duration on ECMO, median days (IQR) | 25.1 (13.0, 29.7)6 | n/a | n/a |
| Any advanced treatment: IV vasopressor, CRRT or ECMO | 357 (14.7%) | 4 (8.9%) | .39 |
| DNR/DNI during entire admission | 516 (21.3%) | 4 (8.9%) | .042 |
| Death at discharge | 269 (11.1%) | 2 (4.4%) | .23 |
1n = 1663,2 n = 395,3 n = 356,4 n = 70,5 n = 86,6 n = 16,7n = 26,8 n = 3,9 n = 4,10 n = 1,11 n = 5.
At some point during the admission, four (8.9%) SOT and 187 (7.7%) non-SOT required high-level oxygen as maximum oxygen therapy without being intubated, whereas three (6.7%) SOT and 395 (16.3%) non-SOT required intubation (Table 2). Maximum oxygen therapy of SOT vs. non-SOT patients during their hospital stay did not statistically significantly differ (ranksum p = .07). This continued to be true when restricted to patients who did not need high-level oxygen upon admission (ranksum p = .16).
3.3. WHO COVID-19 severity scores
Overall WHO severity score on admission was slightly lower for SOT patients (median [IQR] 3 [3, 4]) than not-SOT patients (median [IQR] 4 [3–4]) (p = .042) (Table 2). However, no SOT patients had a WHO severity score beyond 6 (intubation) on the date of admission. Among non-SOT patients, 89 (3.7%) had a score of 6 (intubation), 53 (2.2%) had a score of 7 (intubation +advanced life support), and 15 (0.6%) patients had a score of 8 by virtue of death on the date of admission ( Figure 1).
FIGURE 1.
Distribution of maximum WHO severity score at presentation and during the entire admission, comparing SOT to non-SOT
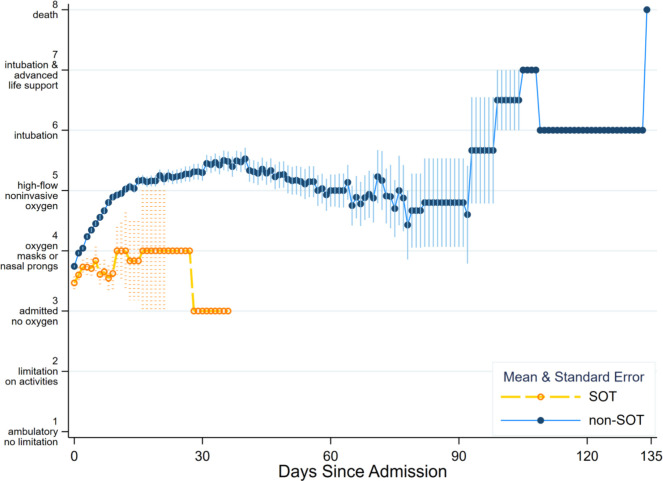
Maximum WHO severity score of SOT vs. non-SOT patients during their hospital stay did not statistically significantly differ (ranksum p = .053). This continued to be true when restricted to patients who did not need high-level oxygen upon admission (ranksum p = 0.15). The median duration on oxygen therapy was 4.9 (2.7–7.3) days in SOT, and 5.9 (2.4–11.8) days in non-SOT (p = .27) (Table 2). SOT status was associated with faster decline in WHO severity score, after adjusting for severity score upon admission (OR = 0.76 0.810.86, p < .001) ( Figure 2).
FIGURE 2.
Mean and standard error of daily WHO severity score throughout admission, comparing SOT to non-SOT
3.4. Laboratory data
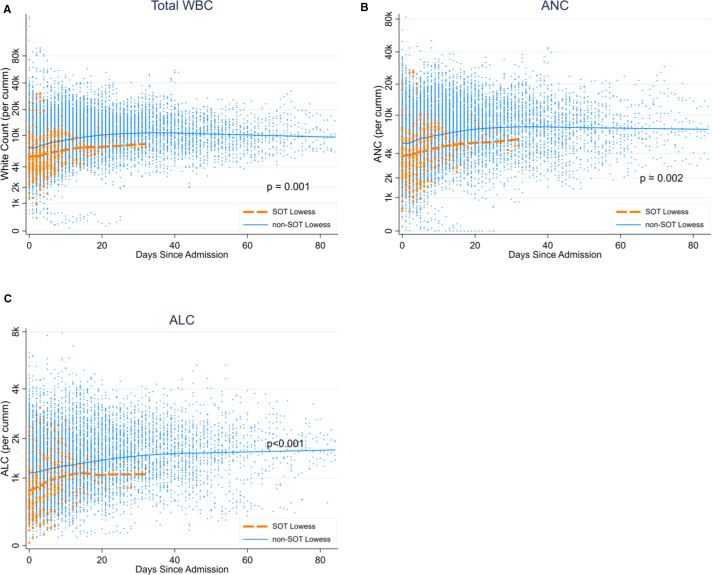
The proportion of patients in each group with an absolute neutrophil count lower than 1 k/mm3 at any point during the admission was not statistically significantly higher for the SOT group (SOT 3 [6.7%], non-SOT 46 [1.9%], p = .059) ( Table 3). However, the lowest ALC at any point during the admission was lower in the SOT group, with the median (IQR) 0.56 (0.32, 0.86) for SOT and 0.93 (0.63, 1.32) for non-SOT (p < .001) (Table 3). When including repeated measurements, there were statistically significant differences in WBC (0.730.820.93 p = .001) ( Figure 3A) and ALC levels (0.78 0.840.91 p < .001) (Figure 3C) compared to SOT to non-SOT patients, in that SOT patients were on average 18% lower in WBC and 16% lower in ALC compared to non-SOT patients. Peak C-reactive protein (CRP) of SOT patients during admission was lower compared to non-SOT (SOT 6.5 [2.6, 13.2] and non-SOT 12.9 [5.3, 30.7], p < .001). All the other laboratory data analyzed in this study were not different between SOT and non-SOT (Table 3).
TABLE 3.
Laboratory findings during admission, comparing SOT to non-SOT
| Factor | non-SOT | SOT | p |
|---|---|---|---|
| WBC any point <1 (k/mm3) | 8 (0.3%) | 1 (2.2%) | .15 |
| WBC any point >20 (k/mm3) | 249 (10.3%) | 2 (4.4%) | .32 |
| ANC any point <1 (k/mm3) | 46 (1.9%) | 3 (6.7%) | .059 |
| Lowest ALC, median (IQR) | 0.93 (0.63, 1.32)1 | 0.56 (0.32, 0.86)7 | <.001 |
| peak IL–6, median (IQR) | 54.0 (21.8, 137.0)2 | 42.5 (10.5, 104.1)8 | .26 |
| Peak CRP, median (IQR) | 12.9 (5.3, 30.7)3 | 6.5 (2.6, 13.2)9 | <.001 |
| Peak ESR, median (IQR) | 58 (34, 89)4 | 33.5 (16, 79.5)10 | .081 |
| Peak ferritin, median (IQR) | 706 (305, 1390)5 | 642 (267, 2188)11 | .75 |
| Peak D-dimer, median (IQR) | 1.33 (0.66, 3.26)6 | 0.97 (0.56, 2.09)12 | .067 |
| ALT>80 | 596 (24.8%) | 9 (20.0%) | .60 |
| AST>74 | 742 (30.8%) | 12 (26.7%) | .63 |
| Elevated CK | 550 (22.9%) | 8 (17.8%) | .48 |
| High Troponin-T | 266 (11.1%) | 5 (11.1%) | 1.00 |
| High Troponin-I | 317 (13.2%) | 8 (17.8%) | .37 |
1n = 2149,2n = 1057,3n = 2018,4n = 637,5n = 1999,6n = 1983,7n = 44,8n = 349n = 41,10n = 16,11n = 39,12n = 39.
FIGURE 3.
White blood cell count during COVID-19 admission, comparing SOT to non-SOT patients: (A) total WBC count, (B) absolute neutrophil count, (C) absolute lymphocyte count
3.5. Medications
SOT patients were more likely to receive tacrolimus (84.4% vs. 0%, p < .001), mycophenolate mofetil (MMF) (13.3% vs. 0.3%, p < .001), and prednisone (60.0% vs. 4.6%, p < .001) compared to non-SOT patients, which is consistent with posttransplant maintenance immunosuppression ( Table 4). Although this database does not contain information about medications prior to hospital admission, we found in a separate study, involving patient-level manual data extraction from the electronic medical record, that MMF was discontinued in 94% of SOT recipients who were receiving MMF on admission (unpublished data).
TABLE 4.
Use of maintenance immunosuppressive agents, targeted therapies, and immunomodulatory agents during admission
| Factor | Non-SOT | SOT | p |
|---|---|---|---|
| Prednisone | 111 (4.6%) | 27 (60.0%) | <.001 |
| Methylprednisolone | 111 (4.6%) | 3 (6.7%) | .46 |
| Hydrocortisone | 66 (2.7%) | 2 (4.4%) | .35 |
| Dexamethasone | 283 (11.7%) | 6 (13.3%) | .64 |
| Tacrolimus | 0 (<1%) | 38 (84.4%) | <.001 |
| Mycophenolate mofetil | 8 (0.3%) | 6 (13.3%) | <.001 |
| Tocilizumab | 88 (3.6%) | 6 (13.3%) | .006 |
| Remdesivir | 342 (14.2%) | 8 (17.8%) | .52 |
| Hydroxychloroquine | 395 (16.3%) | 13 (28.9%) | .040 |
In terms of therapies intended for treatment of COVID-19, six (13.3%) SOT and 88 (3.6%) non-SOT patients received tocilizumab (p = .006) (Table 4). Access to remdesivir at Johns Hopkins (other than clinical trials) was under the Emergency Use Authorization from the US FDA issued in May 2020, under drug distribution to hospitals through the allocation system established by the US Government21; therefore, remdesivir use in both groups was during the latter months of the observation period. None of the SOT patients who were admitted prior to June 17th received remdesivir, but from June 18 to August 21, eight (17.0%) received remdesivir. There were no statistically significant differences in the proportions who received methylprednisolone, hydrocortisone, and dexamethasone comparing SOT to non-SOT (Table 4).
3.6. Hospital length-of-stay and inpatient mortality
Two SOT recipients (4.4%) and 269 non-SOT patients (11.1%) died during the hospital admission (p = .23). When accounting for the competing risk of alive-at-discharge, there was no difference in risk of in-hospital mortality comparing SOT to non-SOT patients (sHR: 0.10.41.6, p = .19) ( Figure 4A). Similarly, accounting for the competing risk of in-hospital mortality, there was no difference in time to alive-at-discharge, that is, length-of-stay (sHR: 0.91.11.4, p = .55) (Figure 4B). The median length-of-stay for SOT and non-SOT patients were 7.0 (3.8, 9.3) and 5.3 (2.6, 10.6), respectively (p = .26). The 30-day cumulative incidence of mortality was 0.06 in SOT and 0.10 in non-SOT.
FIGURE 4.
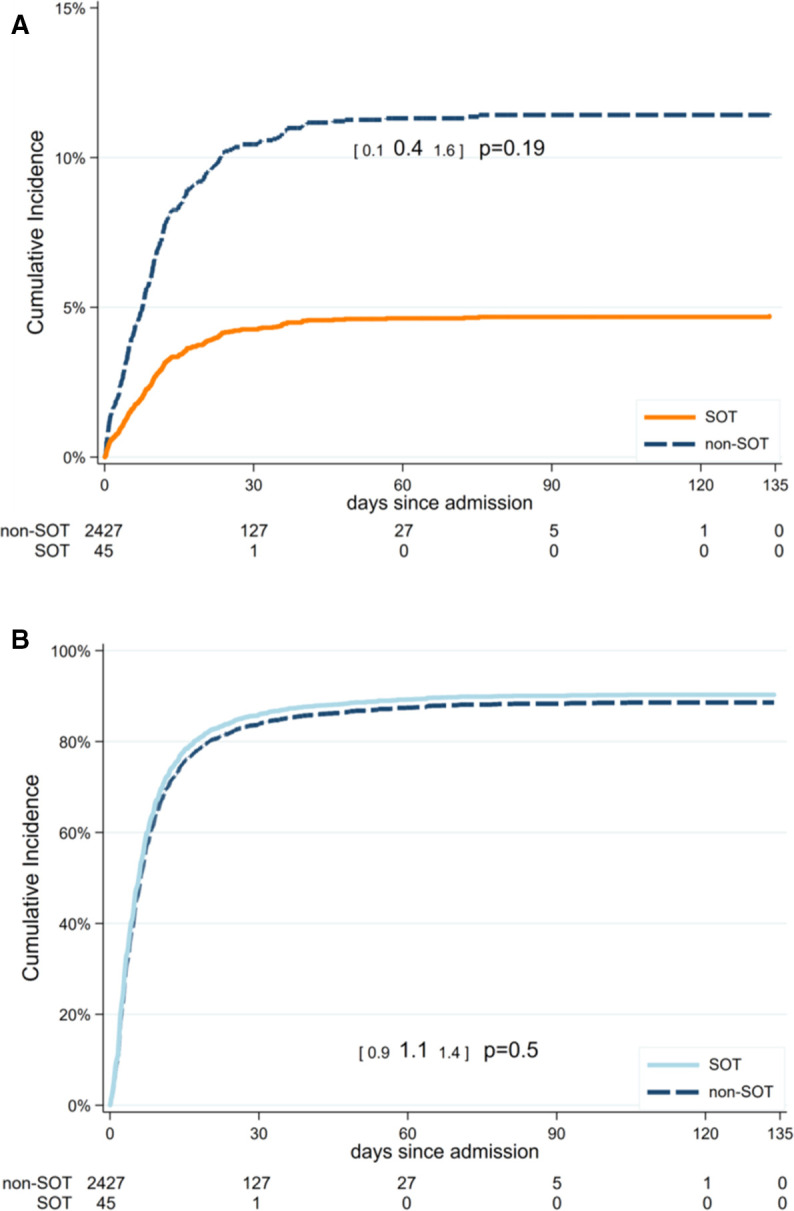
Cumulative incidence of inpatient mortality and alive-at-discharge comparing SOT to non-SOT patients, competing risks framework. (A) Cumulative incidence of inpatient mortality. (B) Cumulative incidence of alive-at-discharge
3.7. Sensitivity analysis
After weighting, the subhazard ratio of mortality (sHR: 0.160.662.66, p = .56) and length-of-stay (sHR: 0.700.921.20, p = 0.53) did not alter our conclusion. There were no differences in inpatient mortality and length-of-stay comparing SOT to non-SOT patients with or without weighting.
4. DISCUSSION
In this study of 45 adult SOT and 2427 non-SOT patients from a database of five hospitals within a single health system, we observed no differences in length-of-stay or risk of in-hospital mortality between SOT and non-SOT patients, despite more comorbidities (diabetes, hypertension, HIV, peripheral vascular disease) in the SOT group. Severity of illness was slightly less on the day of admission in the SOT group, but there were no statistically significant differences in the highest level of oxygen therapy, or highest WHO severity score reached, during the admission. Moreover, when comparing SOT and non-SOT in terms of longitudinal daily oxygen therapy and daily WHO severity score, and adjusting for severity score at the time of admission, SOT recipients had a more rapid decline in severity of illness. This more rapid improvement in disease severity in the SOT group occurred in the setting of ongoing transplant immunosuppressive medications (tacrolimus and prednisone; mycophenolate was usually discontinued) and comparable use of remdesivir and additional steroid therapies in both groups, as well as an ALC that, followed longitudinally, was statistically significantly lower in the SOT group. Peak CRP was also statistically significantly lower in the SOT group.
Our results are consistent with those of Chaudhry et al,13 in terms of comparable outcomes (mortality and severity of illness) between SOT and non-SOT recipients despite higher prevalence of comorbidities. However, our study also examined longitudinal evolution of clinical courses over time, and demonstrated a more rapid decline in severity of illness in the SOT group. Our results are in contrast to those of Roberts et al, who found a higher rate of hospitalization, ICU admission, mechanical ventilation, and death in the SOT group as compared with non-SOT recipients in their hospitals, and previously published data in the general population with COVID.14 These differences, as well as the lower mortality in our SOT group (4.4%) as compared with most previous published studies,3, 4, 5, 6, 7, 8, 9 , 12, 13, 14 might be accounted for by different patient populations, thresholds for hospital admission, resource availability, immunosuppression management and other therapeutic management, or other factors. In any event, we have observed that that SOT status per se is not associated with higher risk of mortality from COVID-19 in hospitalized patients, in this cohort study of patients from a single health-care system.
The SOT group in our study had a more rapid improvement in severity of illness, and lower peak CRP level, than the non-SOT group. In addition, a higher percentage of SOT patients received tocilizumab; these differences were statistically significant. Although recent results of a randomized trial suggest that tocilizumab may not confer benefit to the general population with COVID-19,22 our findings leave open the possibility that some SOT recipients may benefit, when tocilizumab is administered early in the inflammatory phase, consistent with other recent studies that suggest that some subsets of patients may benefit.23 Alternatively, it is possible that the host inflammatory response to COVID-19 in SOT recipients in this study was moderated by remaining on low-dose prednisone and tacrolimus, which were continued during their hospitalizations, while MMF was usually discontinued. Another possibility is suggested by studies in which the initial viral load was associated with the severity of illness.24 From early on, our transplant center had a major focus on patient education, and developed educational materials for SOT recipients. It is possible that safety measures may have resulted in a lower inoculum when SOT recipients had exposures, but we do not have viral load data to support this. While our results suggest that survival parity is achievable in hospitalized SOT and non-SOT recipients with COVID-19, we acknowledge that the factors which may have contributed to these outcomes are not known.
Limitations of this study include those related to obtaining data from a database derived from the electronic medical record without direct access to patient-level records; however, this affected both SOT and non-SOT groups. Ascertainment of SOT status may have been incomplete, especially for patients who had received transplants outside the Hopkins system, as this ascertainment was based on ICD-10 diagnoses at discharge or in the medical history, and was dependent on clinicians recording an SOT diagnosis. However, cross-referencing with a SOT COVID patient list developed for clinical care purposes confirmed the SOT numbers. Comorbidities listed in the database did not indicate a time frame, so some may have been pretransplant conditions rather than posttransplant complications. However, greater prevalence of organ disease and associated comorbidities would tend to bias in the direction of worse outcomes for SOT recipients. Information in the database on duration of oxygen therapy was incomplete or judged unreliable in 32 patients (3 SOT), whom we have therefore listed as not receiving oxygen therapy. This may have led to underestimation of the proportion receiving oxygen therapy in each group; however, the number of patients is small with respect to the entire cohort. We did not have data on clinical courses or treatments prior to transfer, for patients transferred into JHM from outside hospitals. However, if transferred patients represented a sicker group, the higher percentage of transferred patients in the SOT group would also bias in the direction of worse SOT outcomes. Data in this registry reflect the population of one region and one health-care system, and may not be generalizable to other geographic regions and health-care systems. However, our study did include five hospitals within this hospital system, potentially increasing generalizability of our findings. Finally, SOT recipients likely had more access to health care than some non-SOT recipients prior to admission, in that SOT recipients are typically followed closely by their transplant teams, and are encouraged to report symptoms.
5. CONCLUSIONS
Patients who have received a solid organ transplant did not experience worse outcomes than non-SOT patients hospitalized with COVID-19, in this cohort study of patients from a single health-care system. Despite having more comorbidities, and continuing on immunosuppressive agents including prednisone and tacrolimus (with discontinuation of mycophenolate mofetil), SOT patients had no increased mortality, and in fact had a more rapid decrease in illness severity. The use of longitudinal depictions of clinical courses to illustrate the tempo of the illness can yield insights beyond aggregated outcomes. The transplant community should be aware that SOT status does not necessarily portend an adverse outcome from COVID-19, a finding which has implications for transplant center policies during the ongoing pandemic.
ACKNOWLEDGMENTS
This work was supported by grant number K24DK101828 (Segev) and K01DK101677 (Massie) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). This research was made possible with generous support of the Ben-Dov family (Segev) and the Gary and Mary West Health Institute (Makary). The data utilized for this publication were part of the JH-CROWN: The COVID PMAP Registry, which is based on the contribution of many patients and clinicians. JH-CROWN received funding from Hopkins in Health, the Johns Hopkins Precision Medicine Program.
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. R. Avery: Study grant support from Aicuris, Astellas, Chimerix, Merck, Oxford Immunotec, Qiagen, Takeda/Shire. D. Segev: Consulting/speaking honoraria from Sanofi, Novartis, CSL Behring, and Veloxis. K.A. Marr: Consulting/advisory board income from Amplyx, Cidara, Merck, and Sfunga. Equity and licensing revenue from MycoMed Technologies. Research grant from Merck. N. Permpalung: Study grant support from Health Systems Research Institute, Ministry of Public Health, Thailand. D. C. Brennan: Consulting/speaking honoraria from Allovir, Amplyx, Argenyx, CareDx, Natera, Sanofi, Veloxis. Research Support to Johns Hopkins from CareDx. M.A. Makary: Advisor to Medregen. The other authors have no conflict of interests to disclose.
DATA AVAILABILITY STATEMENT
The data are not publicly available due to privacy and ethical restrictions and the terms and conditions of the Johns Hopkins CROWN Registry.
Funding information National Institute of Diabetes and Digestive and Kidney Diseases, Grant/ Award Number: K01DK101677 and K24DK101828
Footnotes
Robin K. Avery and Teresa Po-Yu Chiang contributed equally to this manuscript.
REFERENCES
- 1.Boyarsky BJ, Chiang TP-Y, Werbel WA, et al. Early impact of COVID-19 on transplant center practices and policies in the United States. Am J Transplant. 2020. 10.111/ajt/15915 [DOI] [PMC free article] [PubMed]
- 2.Massie AB, Boyarsky BJ, Werbel WA, et al. Identifying scenarios of benefit or harm from kidney transplantation during the COVID-19 pandemic: a stochastic simulation and machine learning study. Am J Transplant. 2020. 10.1111/ajt.16117 [DOI] [PMC free article] [PubMed]
- 3.Gandolfini I, Delsante M, Fiaccadori E, et al. COVID-19 in kidney transplant recipients. Am J Transplant. 2020;31 doi: 10.1111/ajt.15891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banerjee D, Popoola J, Shah S, et al. COVID-19 infection in kidney transplant recipients. Kidney Int. 2020. 10.1016/j.kint.2020.03.018 [DOI] [PMC free article] [PubMed]
- 5.Akalin E, Azzi Y, Bartash R, et al. COVID-19 and kidney transplantation. N Engl J Med. 2020. 10.1056/NEJMc2011117 [DOI] [PMC free article] [PubMed]
- 6.Pereira MR, Mohan S, Cohen DJ, et al. COVID-19 in solid organ transplant recipients; initial report from the US epicenter. Am J Transplant. 2020;20 doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez-Ruiz M, Andres A, Loinaz C, et al. COVID-19 in solid organ transplant recipients: a single-center case series from Spain. Am J Transplant. 2020;16 doi: 10.1111/ajt/15929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cravedi P, Suraj S, Azzi Y, et al. COVID-19 and Kidney Transplantation: Results from the TANGO International Transplant Consortium. Am J Transplant. 2020. 10.111/ajt.16185 [DOI] [PMC free article] [PubMed]
- 9.Bossini N, Alberici F, Delbarba E, et al. Kidney transplant patients with SARS-CoV-2 infection: the Brescia renal COVID task force experience. Am J Transplant. 2020. 10.1111/ajt.16176 [DOI] [PMC free article] [PubMed]
- 10.Tschopp J, L’Huillier AG, Mombelli M, et al. First experience of SARS-CoV-2 infections in solid organ transplant recipients in the Swiss Transplant Cohort Study. Am J Transplant. 2020. 10.1111/ajt.16062 [DOI] [PMC free article] [PubMed]
- 11.Yi SG, Rogers AW, Saharia A, et al. Early experience with COVID-19 and solid organ transplantation at a U. S. high-volume transplant center. Transplantation. 2020. 10.1097/TP.0000000000003339 [DOI] [PMC free article] [PubMed]
- 12.Kates O, Haydel BM, Florman SS, et al. COVID-19 in solid organ transplant: a multi-center cohort study. Clin Infect Dis. 2020. 10.1093/cid/ciaa1097 [DOI]
- 13.Chaudhry ZS, Williams JD, Vahia A, et al. Clinical characteristics and outcomes of COVID-19 in solid organ transplant recipients: a cohort study. Am J Transplant. 2020;12 doi: 10.1111/ajt.16188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts M, Izzy S, Tahir Z, Al Jarrah A, Fishman J, El Khoury J. COVID-19 in solid organ transplant recipients: dynamics of disease progression and inflammatory markers in ICU and non-ICU admitted patients. Transplant Infect Dis. 2020;2020 doi: 10.1111/tid.13407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garibaldi BT, Fiksel J, Muschelli J, et al. Patient trajectories among persons hospitalized for COVID-19: A Cohort Study. Ann Intern Med. 2020. 10.7326/M20-3905 [DOI] [PMC free article] [PubMed]
- 16.World Health Organization. COVID-19 Therapeutic Trial Synopsis. February 18, 2020, Geneva, Switzerland.
- 17.Cavalcanti AB, Zampieri FG, Rosa RG, et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N Engl J Med. 2020. 10.1056/NEJMoa2019014 [DOI] [PMC free article] [PubMed]
- 18.Ellis AR, Dusetzina SB, Hansen RA, Gaynes BN, Farley JF, Stürmer T. Investigating differences in treatment effect estimates between propensity score matching and weighting: a demonstration using STAR*D trial data. Pharmacoepidemiol Drug Saf. 2013;22(2):138–144. doi: 10.1002/pds.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu D, Guo F, Li F. Evaluating the causal effects of cellphone distraction on crash risk using propensity score methods. Accid Anal Prev. 2020;143:105579. doi: 10.1016/j.aap.2020.105579. [DOI] [PubMed] [Google Scholar]
- 20.Louis TA, Zeger SL. Effective communication of standard errors and confidence intervals. Biostatistics. 2009;1:1–2. doi: 10.1093/biostatistics/kxn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.FDA US Food and Drug Administration. Letter of authorization EUA for Veklury (remdesivir). https://www.fda.gov/media/137564/download
- 22.Furlow B. COVACTA trial raises questions about tocilizumab’s benefit in COVID-19. Lancet Rheumatology. 2020. 10.1016/S2665-9913(20)30313-1 [DOI] [PMC free article] [PubMed]
- 23.Biran N, Ip A, Ahn J, et al. Tocilizumab among patients with COVID-19 in the intensive care unit: a multicentre observational study. Lancet Rheumatology. 2020. 10.1016/S2665-9913(20)30227-0 [DOI] [PMC free article] [PubMed]
- 24.Piubelli C, Deiana M, Pomani E, et al. Overall decrease of SARS CoV-2 viral load and reduction of clinical burden: the experience of a Northern Italy hospital. Clin Microbiol Infect. 2020. 10.1016/j.cmi.2020.10.006 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are not publicly available due to privacy and ethical restrictions and the terms and conditions of the Johns Hopkins CROWN Registry.