Abstract
Objectives: In older lung cancer patients, polypharmacy and the use of potentially inappropriate medications (PIMs) are commonly reported, but no systematic review or meta-analysis has been carried out to ascertain the prevalence and risk variables in this group. This study aimed to identify the prevalence of polypharmacy, PIMs and associated risk variables in older lung cancer patients.
Methods: We searched for articles from the beginning to February 2022 in PubMed, Embase, and Web of Science that related the use of PIMs and polypharmacy by older lung cancer patients (PROSPERO Code No: CRD42022311603). Meta-analysis was performed on observational studies describing the prevalence and correlation of polypharmacy or PIMs in older patients with lung cancer.
Results: Of the 387 citations, 6 articles involving 16,890 patients were included in the final sample. In older lung cancer patients pooled by meta-analysis, 38% and 35% of PIMs and polypharmacy, respectively. The prevalence of PIMs was 43%, 49%, and 28%, respectively, according to the 2019 AGS Beers criteria, 2014 screening tool for older people’s prescriptions/screening tool for alerting to the proper therapy (STOPP/START criteria) criteria, and other criteria.
Conclusion: This systematic review and meta-analysis demonstrated a high prevalence of polypharmacy and PIMs among older lung cancer patients. Therefore, it is essential to take rational interventions for older lung cancer patients to receive reasonable pharmacotherapy.
Systematic Review Registration: [https://www.crd.york.ac.uk/PROSPERO/], identifier [CRD42022311603].
Keywords: polypharmacy, potentially inappropriate medication, older people, lung cancer, meta-analysis
Introduction
With over 1.8 million deaths from lung cancer in 2020 and 18% of all deaths from cancer, lung cancer is the leading cause of cancer fatalities worldwide (Global Cancer Observatory, 2020; World Health Organization, 2022). Cancer development is associated with older age and is influenced by biological factors, including DNA damage and telomere shortening over time. In the upcoming years, it is anticipated that the incidence of lung cancer in older individuals will increase even more as the population ages (Decoster and Schallier, 2019). In approximately 37% of cases, the patient is over 75 years old. Men and women with lung cancer generally present at a median age of 70 years. Men are more likely to develop lung cancer at a rate of 585.9 per 100,000 in their 85–89 years of age, but women are more likely to develop lung cancer at a rate of 365.8 per 100,000 in their 75–79 years of age (Torre et al., 2016).
Most senior lung cancer patients always have coexisting chronic diseases, which adds to the burden of having to take multiple medications (Grose et al., 2014; Nilsson et al., 2017; Ding et al., 2020). Older individuals, however, may be more susceptible to changes in pharmacokinetics and pharmacodynamics due to aging, which could increase the likelihood of medication interactions and drug-disease interactions (Fried et al., 2014; Payne, 2016). Because chemotherapy may increase the likelihood of medication-drug interactions and adverse drug reactions (ADRs), which may include chemotherapy-related toxicity, cancer patients are particularly vulnerable to unintended effects of adverse drug events (Maggiore et al., 2014; Woopen et al., 2016). According to some research, older cancer patients were more likely to suffer comorbid conditions, geriatric syndrome, and frailty, which increased the incidence of polypharmacy and PIMs [(Wildiers et al., 2014), (Koczwara et al., 2022), (LeBlanc et al., 2015)].
A growing body of evidence shows that polypharmacy can have detrimental effects. Polypharmacy is defined as the usage of more than five drugs. It is associated with the prescription of unsuitable medications (Field et al., 2001; Ferner and Aronson, 2006; Maddison et al., 2011; Weng et al., 2013). Potentially inappropriate medications (PIMs) are those that should not be used in older individuals due to the high risk of adverse drug responses and/or lack of adequate evidence of benefits when safer, equivalent, or more effective treatment options are available (Tian et al., 2021). As the first expert consensus on geriatric PIMs, the AGS Beers criteria are regularly updated and reviewed by the American Geriatrics Society, which are now in their sixth iteration (Beers et al., 1991; American Geriatrics Society AGS Beers Criteria® Update Expert Panel, 2019). The screening tool for older people’s prescriptions/screening tool for alerting to the proper therapy (STOPP/START criteria) was created at the University College Cork using a Delphi methodology, and the second edition was revised in 2014 (O'Mahony et al., 2015; O'Mahony, 2020). These two standards have been applied widely in drug application surveys in communities, clinics, and hospitals around the world.
These criteria have also been used in certain studies to investigate the prevalence of polypharmacy or PIMs among older lung cancer patients. To date, there have been no systematic reviews or meta-analyses about the use of PIMs and polypharmacy in older lung cancer patients. To overcome the shortcomings of past findings, we conducted the first systematic review on the prevalence of polypharmacy and PIMs in older lung cancer patients to provide pertinent evidence. The purpose of this meta-analysis is to increase sample size and indicate the direction for further research.
Methods
Search strategy
This study was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis Guidelines (Moher et al., 2015). This systematic review and meta-analysis was submitted to PROSPERO (CRD42022311603). We searched PubMed, Embase and Web of Science from inception to February 2022. For PubMed, the search items included (“Polypharmacy” [MeSH Terms] OR (“Potentially Inappropriate Medication” [Title/Abstract]) OR (“Potentially Inappropriate Prescription” [Title/Abstract]) OR (“Inappropriate Medication” [Title/Abstract]) OR (“Inappropriate Prescription” [Title/Abstract]) OR (“Inappropriate Prescribing” [Title/Abstract]) OR (“Inappropriate Drug Use” [Title/Abstract]) and (“Lung cancer” [Title/Abstract]). The prevalence of polypharmacy or PIMs was reported using any defined criteria in observational studies on older lung cancer patients who were published in the English language. Terms from both medical and non-medical topic headings were utilized in the search string. Additionally, to identify any potential studies, references to pertinent papers and reviews were made.
Selection criteria and data extraction
The studies satisfied the following criteria (World Health Organization, 2022): Observational study design (Global Cancer Observatory, 2020). The reported prevalence of PIMs or polypharmacy in older lung cancer patients (Decoster and Schallier, 2019). Medication use assessed using any PIMs-specific stated criteria (Torre et al., 2016). Documentation of any elements that increase the likelihood that older people would use PIMs or polypharmacy. Studies were excluded if they (World Health Organization, 2022) did not include older lung cancer populations or (Global Cancer Observatory, 2020) did not describe the prevalence of polypharmacy and PIMs usage in the older lung cancer population and (Decoster and Schallier, 2019) were duplicate studies, reviews, case reports, interventional studies, and meta-analyses.
Selection of studies
To ascertain whether each study complied with the predetermined inclusion criteria, two reviewers (FT and ZC) independently read the titles and abstracts of the studies. To determine whether further review was necessary, all 387 titles and abstracts were evaluated. The first 50 references were separately evaluated for quality control by a senior researcher (LM). The degree of agreement was 90%, with five inconsistencies that were discussed among the three reviewers to reach agreement. The two reviewer groups then conducted a second round of review on the remaining studies. The references of the retrieved articles were further searched in an effort to locate more appropriate articles.
Quality assessment
Utilizing cross-sectional data from the Agency for Healthcare Research and Quality (AHRQ), the methodological quality of the included studies was assessed (Hu et al., 2015). The AHRQ evaluates the representativeness of the information source, inclusion and exclusion criteria, the time period, whether subjects were sequential, whether assessors of the subjective portions of the research were masked to other aspects of the participants’ status, any evaluations made for quality assurance objectives, any patient exclusions from the analysis, how confounding was examined, how to deal with missing data in the analysis, summarize the degree of response of patients and the integrity of data collection, and clarify follow-up measures. AHRQ scores range from 0 (lowest level) to 11 (highest level). Research with a score of 8 or more was regarded as high quality, while research with a score of less than 4 was regarded as low quality.
Statistical analysis
STATA software was used to conduct the statistical analysis. The combined prevalence was expressed as percentages with a 95% confidence interval (95% CI), considering the population’s various real effect sizes. The random-effects model proposed by Der Simonian and Laird was used. To ascertain the relationship between different patient characteristics and the risk of polypharmacy and the use of PIMs, the pooled relative ratio was determined for each study.
Results
Basic information of the studies
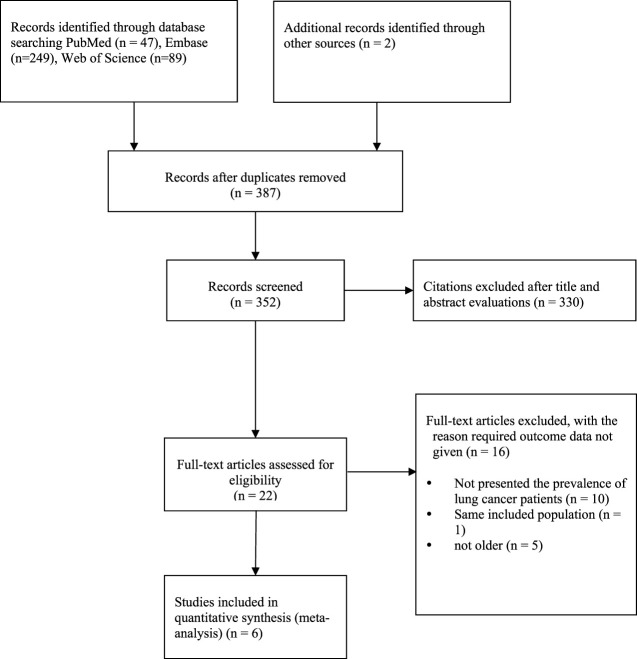
Through the use of resources such as PubMed, Embase, Web of Science, and others, 387 records in total were found. Using Endnote, 35 duplicates were eliminated. A total of 330 records were culled after title and abstract screening, leaving 22 full-text articles for additional analysis. Because the patients in ten of the papers were not lung cancer patients, the included population was the same across all trials, and the population was not old, these papers were not included in the analysis. Six papers (Lund et al., 2018; Hakozaki et al., 2020; Hakozaki et al., 2021; Mohamed et al., 2021; Ham et al., 2022; Tian et al., 2022) that satisfied the inclusion criteria were finally added to the study (Figure 1).
FIGURE 1.
Diagram of the literature selection.
Table 1 provides a summary of the features of the included studies. These 6 studies used an observational study design and included 16,890 patients in total, representing the older lung cancer population. Four studies involved inpatients, and two studies involved outpatients. One study was undertaken in Europe, two studies were undertaken in the United States, and three studies were undertaken in Asia. Three studies used the AGS Beers criteria, two studies used the STOPP/START criteria, and one study used the criteria for patients with cancer in the palliative phase. With reference to the AHRQ, the studies received an average score of 7, which denoted moderate quality.
TABLE 1.
Characteristics of included studies.
| Article | Country | Study design | Tumor type | Sample size | Setting | Male (%) | PIMs criteria applied | Prevalence (%) | Quality of studies | |
|---|---|---|---|---|---|---|---|---|---|---|
| Polypharmacy | PIMs | |||||||||
| Tian et al., 2022 [20] | China | Cross-sectional | Lung cancer | 1,275 | Outpatients | 56.55 | 2019 AGS Beers criteria | 17.88 | 42.67 | 7 |
| Hakozaki et al., 2021 [21] | Japan | Retrospective study | Advanced lung cancer | 232 | Inpatients | 25.86 | STOPP/START V2 | 38.4 | 31.9 | 7 |
| Ham et al., 2021 [22] | Netherland | Observational study | Lung cancer at the end of life | 7,864 | Outpatients | 67.43 | OncPal Deprescribing Guideline | 54.82 | 28.41 | 7 |
| Mohamed et al., 2021 [23] | America | Cross-sectional | Lung cancer with physical functional impairments | 125 | Inpatients | — | 2015 AGS Beers criteria | 48 | — | 7 |
| Hakozaki et al., 2020 [24] | Japan | Cross-sectional | Advanced non-small-cell Lung cancer | 157 | Inpatients | 63.69 | STOPP/START V2 | 59.87 | 38.22 | 7 |
| Lund et al., 2018 [25] | America | Cross-sectional | I–II Lung cancer | 7,237 | Inpatients | 42.48 | 2012 AGS Beers criteria | 33.30 | 37–45 | 7 |
Prevalence of polypharmacy and PIMs in the older Chinese population
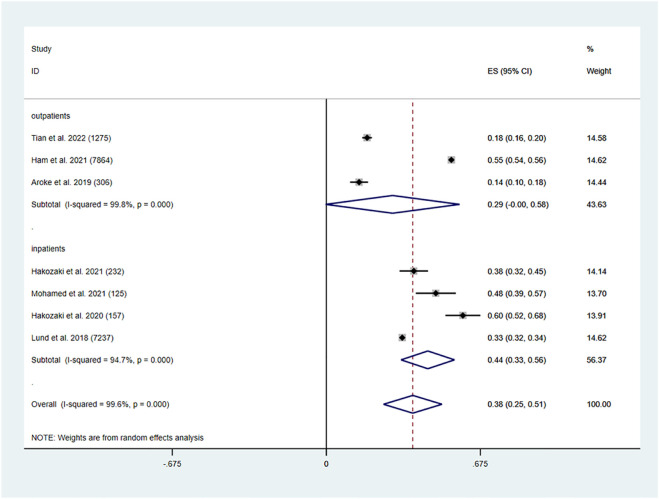
Six studies, including two outpatient studies and four inpatient studies, provided information on the prevalence of polypharmacy in older lung cancer patients. The overall prevalence of polypharmacy among older lung cancer patients was 38% (95% CI: 0.25, 0.51, p < 0.001). In older lung cancer outpatients, the pooled prevalence of polypharmacy was 29% (95% CI: 0.00, 0.58, p < 0.001). Older inpatients with lung cancer had a pooled polypharmacy prevalence of 44% (95% CI: 0.33, 0.56, p < 0.001) (Figure 2).
FIGURE 2.
Prevalence of polypharmacy in the older lung cancer population.
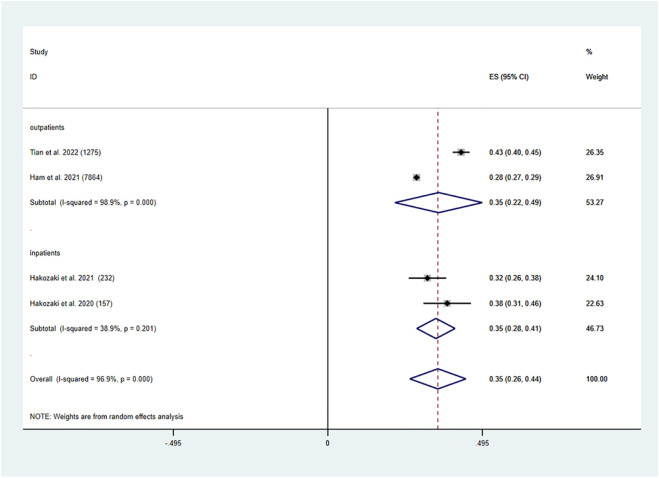
In older lung cancer patients, the pooled prevalence of PIMs was determined to be 35% (95% CI: 0.26, 0.44, P0.001). Both older lung cancer outpatients (95% CI: 0.22, 0.49, P0.001) and inpatients had a pooled prevalence of PIMs of 35% (95% CI: 0.28, 0.41, p < 0.001) (Figure 3).
FIGURE 3.
Prevalence of PIMs in the older lung cancer population.
Factors associated with the increased risk of polypharmacy
Age
Two studies focused on the correlation between the risk of polypharmacy and age change. Although the incidence of polypharmacy rose with age, there was no statistically significant difference in the risk for higher older age groups. The stratified meta-analysis revealed an increased risk (≥ 75 years old vs. 65–74 years old) of 9% polypharmacy exposure (RR: 1.09, 95% CI: 0.86,1.39, p = 0.486) (Table 2).
TABLE 2.
Factors associated with increased risk of polypharmacy and PIMs.
| Characteristics | Number of studies | Relative ratio, RR | 95% CI | p | |
|---|---|---|---|---|---|
| Polypharmacy | |||||
| Age | ≥ 75 vs. 65–74 | 2 | 1.09 | 0.86, 1.39 | 0.486 |
| Gender | 3 | 0.88 | 0.73, 1.04 | 0.137 | |
| Tumor type | |||||
| Adenocarcinoma vs. other | 2 | 1.02 | 0.75, 1.37 | 0.92 | |
| PIMs | |||||
| Gender | 2 | 1.04 | 0.99, 1.10 | 0.124 | |
| Tumor type | |||||
| NSCLC vs. SCLC | 2 | 1.04 | 0.96, 1.12 | 0.341 | |
| SCLC vs. other | 2 | 0.95 | 0.86,1.05 | 0.361 | |
| NSCLC vs. other | 2 | 0.98 | 0.91,1.05 | 0.536 | |
NSCLC: non-small cell lung cancer; SCLC: small cell lung cancer.
Gender
Three studies focused on the correlation between the risk of polypharmacy and gender change. According to the meta-analysis, polypharmacy in older lung cancer patients was not associated with sex differences (RR: 0.88, 95% CI: 0.73, 1.04, p = 0.137) (Table 2).
Tumor type
Two studies focused on the correlation between the risk of polypharmacy and tumor type change according to the meta-analysis, polypharmacy in older lung cancer patients was not associated with tumor type differences (RR: 1.02, 95% CI: 0.75, 1.37, p = 0.92) (Table 2).
Factors associated with the increased risk of PIMs
Gender
Two studies focused on the correlation between the risk of PIMs and gender change. According to the meta-analysis, PIMs in older lung cancer patients was not associated with sex differences (RR: 1.04, 95% CI: 0.99, 1.10, p = 0.124) (Table 2).
Tumor type
Two studies focused on the correlation between the risk of PIMs and tumor type change. According to the meta-analysis, PIMs in older lung cancer patients was not associated with tumor type differences (RR: 1.04, 95% CI: 0.96, 1.12, p = 0.341), (RR: 0.95, 95% CI: 0.86, 1.05, p = 0.361), (RR: 0.98, 95% CI: 0.91, 1.05, p = 0.536) (Table 2).
Different criteria
Five studies focused on the association of various criteria with the risk of PIMs. One study reported the 2019 AGS Beers criteria, two studies reported the STOPP/START criteria, one study reported the OncPal Deprescribing Guideline for patients with cancer in the palliative phase, and one study reported the 2012 AGS Beers criteria. According to the 2019 AGS Beers criteria (43%), the STOPP/START criteria (49%), the OncPal Deprescribing Guideline (28%), and the 2012 AGS Beers criteria (37%–45%), PIMs was prevalent.
Discussion
The simultaneous use of multiple medications, including prescription pharmaceuticals, over-the-counter medicines, and nutritional supplements, is known as polypharmacy. It is generally known that polypharmacy affects life quality and increases the risk of prescription mistakes, drug‒drug interactions, and adverse drug reactions (ADRs). Although the frequency of polypharmacy varies by demographic, it has been shown that older cancer patients had polypharmacy rates of 90%.
Even though the top number of new cases in 2020 worldwide was breast cancer (226, 1419, 11.7%), the number of deaths caused by lung cancer in 2020 was the highest (179, 6144, 18%) reported by the Global Cancer Observatory 2020 (Global Cancer Observatory, 2020). The highest lung cancer incidence, mortality and 5-year prevalence worldwide were in Asia. According to Global Cancer Statistics 2020, China has the most cancer fatalities and new cases worldwide (Sung et al., 2021). At present, the number of cancer fatalities and new cases in China continues to rise, and the medical cost caused by cancer exceeds 220 billion every year. The incidence (82.8 per 10,000) and mortality (65.7 per 10,000) of lung cancer both ranked first in China according to cancer incidence and mortality in China in 2016 (Zheng et al., 2022). With increasing age, this trend is further deepened. The peak number of new cancer cases in both men and women was 60–79 years old.
In a thorough assessment of the available research and a meta-analysis, we present in our study for the first time the pooled prevalence of polypharmacy and PIMs and the risks associated with these behaviors in the older lung cancer population. We concluded that 38% of older lung cancer patients had polypharmacy overall after analyzing six trials. The prevalence of polypharmacy ranged from 17.88% to 59.87% among older lung cancer patients from four different nations. The pooled prevalence of polypharmacy of older lung cancer outpatients was 29% and that of older lung cancer inpatients was 44%. A study that focused on the use of antitumor drugs in older metastatic colorectal cancer patients reported that the polypharmacy of patients accounted for 38.7% (Yekedüz et al., 2022). A study from America found that 26% of older breast cancer patients and 29% of older colorectal cancer patients took six or more drugs every day (Lund et al., 2018). One study found the prevalence of polypharmacy in older Indian patients with lung cancer was higher than other malignancies (Vanita et al., 2021). Some recent meta-analyses reported a pooled prevalence of PIMs in older cancer patients between 19.0% and 52.0% (Mohamed et al., 2020). The AGS Beers criteria were the most common criteria used to screen for PIMs in older cancer patients. According to the study, all three cancer cohorts (breast cancer, colon cancer, and lung cancer) had identical monthly prevalence rates of any PIMs before cancer diagnosis, which ranged between 37% and 40%. While PIMs prevalence increased significantly in the first few months after a colon or lung cancer diagnosis and gradually returned to prediagnosis levels over the next 23 months, PIMs prevalence among breast cancer patients steadily decreased across the period after diagnosis (Lund et al., 2018). Compared to patients with the other two malignancies, older lung cancer patients used PIMs more frequently. According to our meta-analysis, older lung cancer patients used PIMs at a rate of 35%. One study showed that concomitant conditions are present in at least half of lung cancer patients, increasing the likelihood that PIMs will be used (Pluchart et al., 2021). In addition, 16.3% of the 31 million cancer survivors in the United States reported using several psychiatric medications. Following survivors of breast cancer (17.8%), colorectal cancer (17.8%), and other gastrointestinal cancers (16.0%), survivors of lung cancer had the greatest rate of psychotropic polypharmacy (22.5%) (Vyas et al., 2020). This was also the cause of older lung cancer patients using PIMs more frequently than those with the other two cancers.
The results of the study confirmed population aging and rising trends in the risk of polypharmacy for higher age groups. Our meta-analysis showed that neither polypharmacy nor PIMs in the older lung cancer population was substantially related to sex differences. The STOPP/START criteria were more sensitive than the other criteria, which may be because the older lung cancer patients in these two studies were inpatients, and the prevalence of PIMs in older inpatients was originally higher than that in outpatients. There were no significant findings between different tumor types of the risk of PIMs and polypharmacy in our study.
The current research shows that interventions targeting PIMs might help older people’s health outcomes and reduce medication-related damage (Mekonnen et al., 2021). PIMs usage increased polypharmacy prevalence and resulted in significant morbidity and death in older persons (Achterhof et al., 2020). Deprescribing is a recognized management strategy to minimize the prevalence of polypharmacy and PIMs. polypharmacy and PIMs (Wu et al., 2021). There is little knowledge regarding the therapeutic effectiveness of reducing polypharmacy in older patients. Deprescribing intervention in older patients who unexpectedly enter the hospital or pass away is not without risks, but its benefits and long-term viability are unclear (Rieckert et al., 2020). According to one study, older cancer patients’ polypharmacy and PIMs seemed to be evaluated in relation to pharmacist-led drug evaluation deprescribing (Nightingale et al., 2015). In comparison to interdisciplinary team interventions, other studies showed that pharmacist-led deprescribing interventions in older patients may be more successful in lowering the use of unneeded drugs (Verrue et al., 2009; Tjia et al., 2013). As a result, it is necessary to create instruments that are standardized to define the elements that constitute the appropriate and inappropriate use of polypharmacy. Furthermore, an effective intervention and management of PIMs should be conducted in older cancer patients in the future, especially in older lung cancer patients.
Limitations
This systematic review and meta-analysis was conducted to consolidate quantitative evidence on the impact of polypharmacy and PIMs in older lung cancer patients. The study’s findings, however, have some limitations. First, there are only six studies included, so there is a certain risk of bias in the conclusions, the results might be impacted by variables including illness distribution, doctor diagnostic proficiency, and prescription practices that differ greatly between hospitals in other nations. Furthermore, most studies did not analyze the connection between polypharmacy and PIMs usage in older lung cancer patients. Third, the majority of the research that made up this systematic review and meta-analysis was carried out in North America, Europe, and Asia. As a result, the findings of this study could not accurately represent the circumstances in other nations.
Conclusion
In older lung cancer patients, this systematic review and meta-analysis found a high prevalence of polypharmacy (38%) and PIMs usage (35%). The findings of this study indicate that older lung cancer populations should undergo effective intervention and management of PIMs in the future. The incidence of polypharmacy, PIMs, and related risk factors in older patients with various malignancies should be the subject of further investigation.
Acknowledgments
The authors thank the participants.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Author contributions
FT: Developing design, literature search, manuscript writing. ZC and LM: Developing design, literature search, manuscript writing, and analysis of results. All authors read and approved the final manuscript.
Funding
This work was supported by the Sichuan Science and Technology Program (Project number: 2022JDR0326) and Sichuan Cadre Healthcare Program (Project number: 23PJYY0115).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Achterhof A. B., Rozsnyai Z., Reeve E., Jungo K. T., Floriani C., Poortvliet R. K. E., et al. (2020). Potentially inappropriate medication and attitudes of older adults towards deprescribing. PLoS One 15 (10), e0240463. 10.1371/journal.pone.0240463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Geriatrics Society AGS Beers Criteria® Update Expert Panel (2019). American geriatrics society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J. Am. Geriatr. Soc. 67, 674–694. 10.1111/jgs.15767 [DOI] [PubMed] [Google Scholar]
- Beers M. H., Ouslander J. G., Rollingher I., Reuben D. B., Brooks J., Beck J. C. (1991). Explicit criteria for determining inappropriate medication use in nursing home residents. UCLA Division of Geriatric Medicine. Archives Intern. Med. 151, 1825–1832. 10.1001/archinte.151.9.1825 [DOI] [PubMed] [Google Scholar]
- Decoster L., Schallier D. (2019). Treatment of older patients with advanced non-small-cell lung cancer: A challenge. J. Geriatr. Oncol. 10 (4), 528–533. 10.1016/j.jgo.2018.09.008 [DOI] [PubMed] [Google Scholar]
- Ding R., Zhu D., He P., Ma Y., Chen Z., Shi X. (2020). Comorbidity in lung cancer patients and its association with medical service cost and treatment choice in China. BMC Cancer 20 (1), 250. 10.1186/s12885-020-06759-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferner R. E., Aronson J. K. (2006). Communicating information about drug safety. BMJ 333 (7559), 143–145. 10.1136/bmj.333.7559.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T. S., Gurwitz J. H., Avorn J., McCormick D., Jain S., EcklerM., et al. (2001). Risk factors for adverse drug events among nursing home residents. Arch. Intern. Med. 161 (13), 1629–1634. 10.1001/archinte.161.13.1629 [DOI] [PubMed] [Google Scholar]
- Fried T. R., O'Leary J., Towle V., Goldstein M. K., Trentalange M., Martin D. K. (2014). Health outcomes associated with polypharmacy in community-dwelling older adults: A systematic review. J. Am. Geriatr. Soc. 62 (12), 2261–2272. 10.1111/jgs.13153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Cancer Observatory (2020). Cancer today international agency for research on cancer. Available at https://gco.iarc.fr/today/data/factsheets/cancers/15-Lung-fact-sheet.pdf (Accessed March 23, 2022).
- Grose D., Morrison D. S., Devereux G., Jones R., Sharma D., Selby C., et al. (2014). Comorbidities in lung cancer: Prevalence, severity and links with socioeconomic status and treatment. Postgrad. Med. J. 90 (1064), 305–310. 10.1136/postgradmedj-2013-132186 [DOI] [PubMed] [Google Scholar]
- Hakozaki T., Hosomi Y., Shimizu A., Kitadai R., Mirokuji K., Okuma Y. (2020). Polypharmacy as a prognostic factor in older patients with advanced non-small-cell lung cancer treated with anti-PD-1/PD-L1 antibody-based immunotherapy. J. Cancer Res. Clin. Oncol. 146 (10), 2659–2668. 10.1007/s00432-020-03252-4 [DOI] [PubMed] [Google Scholar]
- Hakozaki T., Matsuo T., Shimizu A., Ishihara Y., Hosomi Y. (2021). Polypharmacy among older advanced lung cancer patients taking EGFR tyrosine kinase inhibitors. J. Geriatr. Oncol. 12 (1), 64–71. 10.1016/j.jgo.2020.09.011 [DOI] [PubMed] [Google Scholar]
- Ham L., Geijteman E. C. T., Aarts M. J., Kuiper J. G., Kunst P. W. A., Raijmakers N. J. H., et al. (2022). Use of potentially inappropriate medication in older patients with lung cancer at the end of life. J. Geriatr. Oncol. 13 (1), 53–59. 10.1016/j.jgo.2021.07.009 [DOI] [PubMed] [Google Scholar]
- Hu J., Dong Y., Chen X., Liu Y., Ma D., Liu X., et al. (2015). Prevalence of suicide attempts among Chinese adolescents: A meta-analysis of cross-sectional studies. Compr. Psychiatry 61, 78–89. 10.1016/j.comppsych.2015.05.001 [DOI] [PubMed] [Google Scholar]
- Koczwara B., Deckx L., Ullah S., van den Akker M. (2022). Impact of comorbidities on physical function and survival of middle-aged, as compared to older, individuals with cancer. Support. Care Cancer 30 (2), 1625–1632. 10.1007/s00520-021-06567-1 [DOI] [PubMed] [Google Scholar]
- LeBlanc T. W., McNeil M. J., Kamal A. H., Currow D. C., Abernethy A. P. (2015). Polypharmacy in patients with advanced cancer and the role of medication discontinuation. Lancet. Oncol. 16 (7), e333–e341. 10.1016/S1470-2045(15)00080-7 [DOI] [PubMed] [Google Scholar]
- Lund J. L., Sanoff H. K., Peacock Hinton S., Muss H. B., Pate V., Sturmer T. (2018). Potential medication-related problems in older breast, colon, and lung cancer patients in the United States. Cancer Epidemiol. Biomarkers Prev. 27 (1), 41–49. 10.1158/1055-9965.EPI-17-0523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison A. R., Fisher J., Johnston G. (2011). Preventive medication use among persons with limited life expectancy. Prog. Palliat. Care 19 (1), 15–21. 10.1179/174329111X576698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggiore R. J., Dale W., Gross C. P., Feng T., Tew W. P., Mohile S. G., et al. (2014). Polypharmacy and potentially inappropriate medication use in older adults with cancer undergoing chemotherapy: Effect on chemotherapy-related toxicity and hospitalization during treatment. J. Am. Geriatr. Soc. 62 (8), 1505–1512. 10.1111/jgs.12942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekonnen A. B., Redley B., de Courten B., Manias E. (2021). Potentially inappropriate prescribing and its associations with health-related and system-related outcomes in hospitalised older adults: A systematic review and meta-analysis. Br. J. Clin. Pharmacol. 87 (11), 4150–4172. 10.1111/bcp.14870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed M. R., Ramsdale E., Loh K. P., Arastu A., Xu H., Obrecht S., et al. (2020). Associations of polypharmacy and inappropriate medications with adverse outcomes in older adults with cancer: A systematic review and meta-analysis. Oncologist 25 (1), e94–e108. 10.1634/theoncologist.2019-0406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed M. R., Ramsdale E., Loh K. P., Xu H., Patil A., Gilmore N., et al. (2021). Association of polypharmacy and potentially inappropriate medications with physical functional impairments in older adults with cancer. J. Natl. Compr. Canc. Netw. 19, 267–274. 10.6004/jnccn.2020.7628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., et al. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 4, 1. 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nightingale G., Hajjar E., Swartz K., Andrel-Sendecki J., Chapman A. (2015). Evaluation of a pharmacist-led medication assessment used to identify prevalence of and associations with polypharmacy and potentially inappropriate medication use among ambulatory senior adults with cancer. J. Clin. Oncol. 33 (13), 1453–1459. 10.1200/JCO.2014.58.7550 [DOI] [PubMed] [Google Scholar]
- Nilsson J., Berglund A., Bergström S., Bergqvist M., Lambe M. (2017). The role of comorbidity in the management and prognosis in nonsmall cell lung cancer: A population-based study. Acta Oncol. 56 (7), 949–956. 10.1080/0284186X.2017.1324213 [DOI] [PubMed] [Google Scholar]
- O'Mahony D., O'Sullivan D., Byrne S., O'Connor M. N., Ryan C., Gallagher P. (2015). STOPP/START criteria for potentially inappropriate prescribing in older people: Version 2. Age Ageing 44 (2), 213–218. 10.1093/ageing/afu145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mahony D. (2020). STOPP/START criteria for potentially inappropriate medications/potential prescribing omissions in older people: Origin and progress. Expert Rev. Clin. Pharmacol. 13 (1), 15–22. 10.1080/17512433.2020.1697676 [DOI] [PubMed] [Google Scholar]
- Payne R. A. (2016). The epidemiology of polypharmacy. Clin. Med. 16 (5), 465–469. 10.7861/clinmedicine.16-5-465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluchart H., Bailly S., Fauconnier J., Delafosse P., Chanoine S., Dumas I., et al. (2021). Study protocol to assess polypharmacy and comorbidities in lung cancer. Respir. Med. Res. 80, 100861. 10.1016/j.resmer.2021.100861 [DOI] [PubMed] [Google Scholar]
- Rieckert A., Reeves D., Altiner A., Drewelow E., Esmail A., Flamm M., et al. (2020). Use of an electronic decision support tool to reduce polypharmacy in elderly people with chronic diseases: Cluster randomised controlled trial. BMJ 369, m1822. 10.1136/bmj.m1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H., Ferlay J., Siegel R. L., Laversanne M., Soerjomataram I., Jemal A., et al. (2021). Global cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca. Cancer J. Clin. 71 (3), 209–249. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- Tian F., Liao S., Chen Z., Xu T. (2021). The prevalence and risk factors of potentially inappropriate medication use in older Chinese inpatients with multimorbidity and polypharmacy: A cross-sectional study. Ann. Transl. Med. 9 (18), 1483. 10.21037/atm-21-4238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian F., Yang R., Chen Z., Duan X., Yuan P. (2022). The prevalence and factors associated with potentially inappropriate medication use in Chinese older outpatients with cancer with multimorbidity. J. Geriatr. Oncol. 629 (22), 00021–00634. 10.1016/j.jgo.2022.02.006 [DOI] [PubMed] [Google Scholar]
- Tjia J., Velten S. J., Parsons C., Valluri S., Briesacher B. A. (2013). Studies to reduce unnecessary medication use in frail older adults: A systematic review. Drugs Aging 30 (5), 285–307. 10.1007/s40266-013-0064-1 [DOI] [PubMed] [Google Scholar]
- Torre L. A., Siegel R. L., Jemal A. (2016). Lung cancer Statistics. Adv. Exp. Med. Biol. 893, 1–19. 10.1007/978-3-319-24223-1_1 [DOI] [PubMed] [Google Scholar]
- Vanita Noronha, Anant Ramaswamy, Chandrashekhar Gattani Shreya, Renita Castelino, Nookala Krishnamurthy Manjunath, Menon Nandini, et al. (2021). Polypharmacy and potentially inappropriate medication use in older Indian patients with cancer: A prospective observational study. Cancer Res. Stat. Treat. 4 (4), 67–73. 10.4103/crst.crst_50_21 [DOI] [Google Scholar]
- Verrue C. L., Petrovic M., Mehuys E., Remon J. P., Vander Stichele R. (2009). Pharmacists' interventions for optimization of medication use in nursing homes : A systematic review. Drugs Aging 26 (1), 37–49. 10.2165/0002512-200926010-00003 [DOI] [PubMed] [Google Scholar]
- Vyas A., Alghaith G., Hufstader-Gabriel M. (2020). Psychotropic polypharmacy and its association with health-related quality of life among cancer survivors in the USA: A population-level analysis. Qual. Life Res. 29 (8), 2029–2037. 10.1007/s11136-020-02478-6 [DOI] [PubMed] [Google Scholar]
- Weng M. C., Tsai C. F., Sheu K. L., Lee Y. T., Lee H. C., Tzeng S. L., et al. (2013). The impact of number of drugs prescribed on the risk of potentially inappropriate medication among outpatient older adults with chronic diseases. QJM 106 (11), 1009–1015. 10.1093/qjmed/hct141 [DOI] [PubMed] [Google Scholar]
- Wildiers H., Heeren P., Puts M., Topinkova E., Janssen-Heijnen M. L. G., Extermann M., et al. (2014). International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J. Clin. Oncol. 32 (24), 2595–2603. 10.1200/JCO.2013.54.8347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woopen H., Richter R., Ismaeel F., ChekeRov R., Roots I., Siepmann T., et al. (2016). The influence of polypharmacy on grade III/IV toxicity, prior discontinuation of chemotherapy and overall survival in ovarian cancer. Gynecol. Oncol. 140 (3), 554–558. 10.1016/j.ygyno.2016.01.012 [DOI] [PubMed] [Google Scholar]
- World Health Organization (2022). Cancer. Available at https://www.who.int/news-room/fact-sheets/detail/cancer (Accessed March 23, 2022).
- Wu H., Kouladjian O'Donnell L., Fujita K., Masnoon N., Hilmer S. N. (2021). Deprescribing in the older patient: A narrative review of challenges and solutions. Int. J. Gen. Med. 14, 3793–3807. 10.2147/ijgm.s253177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yekedüz E., Aktaş E. G., Köksoy E. B., Doğan N., Urun Y., Utkan G. (2022). The prognostic role of polypharmacy in metastatic colorectal cancer patients treated with regorafenib. Future Oncol. 18 (9), 1067–1076. 10.2217/fon-2021-1182 [DOI] [PubMed] [Google Scholar]
- Zheng R., Zhang S., Zeng H., Wang S., Sun K., Chen R., et al. (2022). Cancer incidence and mortality in China. JNCC 2 (1), 1–9. 10.1016/j.jncc.2022.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.