Abstract
Comparisons of immunoglobulin G (IgG) subclass responses to the major polymorphic region and to a conserved region of MSP-1 in three cohorts of African villagers exposed to Plasmodium falciparum revealed that responses to Block 2 are predominantly IgG3 whereas antibodies to MSP-119 are mainly IgG1. The striking dominance of IgG3 to Block 2 may explain the short duration of this response and also the requirement for continuous stimulation by malaria infection to maintain clinical immunity.
Effective human immunity to blood forms of Plasmodium falciparum involves the acquisition of anti-parasite antibodies (Abs) of the cytophilic immunoglobulin G (IgG) subclasses 1 and 3, targeted at surface antigens of the invasive extracellular merozoite stage of P. falciparum (3, 4). The absence of parasite-specific IgG3 Abs can be associated with poor clinical prognosis of malaria (32). Although IgG1 and/or IgG3 Abs specific for merozoite and/or schizont antigens are relevant to clinical protection, the identity of the target antigens remains to be elucidated.
Merozoite surface protein 1 (MSP-1) is the precursor of most merozoite surface antigens (20, 27). The MSP-1 gene of P. falciparum encodes conserved, dimorphic, and polymorphic regions of the protein (28, 35). There are two major families of MSP-1, based on the dimorphic sequences (35). Polymorphism in the Block 2 region is more extensive, but all Block 2 sequences belong to one or another of only three main types represented by variants originally described in the K1, MAD20, and RO33 isolates (9, 28, 35).
Abs to the conserved MSP-119 region, found in most malaria-exposed individuals (7, 33), have been correlated with protection from clinical symptoms of malaria in some but not all studies (1, 11, 30). Abs to polymorphic and/or dimorphic sequences located outside MSP-119 may also play a role in immunity (7, 17, 29). Recently, a novel approach combining population genetics with an immunoepidemiological prospective cohort study has identified Block 2 as a major target of human immunity to clinical malaria (10).
Antibody isotype distributions of IgG responses to Block 2 and MSP-119 regions were compared in individuals from areas with different levels of malaria transmission. Plasma samples were selected for IgG subclass analysis from larger sets of samples on the basis of a single criterion, the presence of substantial amounts of Block 2-specific total IgG (optical density [OD] > 0.9 at a 1/500 dilution). Plasma samples were from three cohorts of donors. In the village of Daraweesh, Sudan, 28 donors (age 5 to 35 years) were from a cohort of 52, with plasma samples taken during or following documented malaria infections. In Koka, eastern Sudan, 29 donors (age 3 to 65 years) were from a cohort of 70 individuals who were blood film positive for P. falciparum. From 200 people surveyed in the area around Kilifi, Kenya, a month after malaria transmission peak, 29 donors (age 0.5 to 76 years) were selected; 15 of these were blood film positive and 14 tested blood film negative for P. falciparum. Malaria epidemiology at these sites has been described elsewhere (5, 14, 34). All blood samples were obtained after informed consent of donors or their parents.
Nine different recombinant glutathione S-transferase fusion proteins of MSP-1 Block 2, representative of all three known Block 2 types, were used (7, 8). Sequences of all these proteins have been published (7, 8). MSP-119 fusion protein was as described previously (6). Plasma samples were tested by enzyme-linked immunosorbent assay (ELISA) for total IgG and all IgG subclasses able to recognize the 10 different recombinant MSP-1 antigens as described elsewhere (7, 8, 16). The antigens were coated separately onto parallel columns of wells on 96-well plates (Immunlon 4; Dynex Technologies) at 50 ng per well. Each plasma sample, diluted 1:500, was added to two rows of antigen-coated wells on a plate and held at 4°C overnight. Thus, any one plasma sample was tested in duplicate against all different antigens on the same plate at the same time. Identical sets of antigen-coated plates were prepared for the determination of total IgG, IgG1, IgG2, IgG3, or IgG4 in assays performed with any one plasma sample in parallel on the same day. Plasma samples from any one location were all tested on the same day. Next, wells were incubated for 3 h with 100 μl per well of horseradish peroxidase (HRP)-conjugated polyclonal sheep Abs specific for human IgG1 (1:1,000), IgG2 (1:1,000), IgG3 (1:6,000), or IgG4 (1:3,000) (The Binding Site, Birmingham, United Kingdom) or HRP-conjugated polyclonal rabbit Abs against human total IgG specific for gamma chains (1:5,000; Dako). The wells were washed with 100 μl of substrate (0.1 mg of o-phenylenediamine per ml 0.012% H2O2) in development buffer before being incubated (7, 8). The reactions were stopped by addition of 20 μl of 2 M H2SO4, and the OD492 was measured. For each plasma sample, IgG subclass, and antigen combination, corrected OD492 values were calculated by subtracting a mean OD value of two wells containing the control GST protein alone from a mean OD value of duplicates obtained with each combination.
MSP-1-specific IgG subclass concentrations were estimated by an indirect standardization method (38). Purified human myeloma proteins IgG1, IgG2, IgG3, and IgG4 were coated on ELISA plates at threefold dilutions from 10 to 0.00457 μg ml−1. HRP-conjugated Abs to each IgG subclass (at the same dilutions as used for plasma samples) were reacted with the myeloma proteins and developed as above. Standard curves were produced for each IgG subclass in each experiment. The amounts of anti-MSP-1 Abs in each plasma sample were estimated for each antigen-IgG subclass combination by converting OD492 readings to Ab concentrations in micrograms per milliliter by interpolation from the standard curves using polynomial regression. The concentrations of Block 2- and of MSP-119-specific IgG subclass Abs were determined and compared in one experiment for all individuals in each of the cohorts tested.
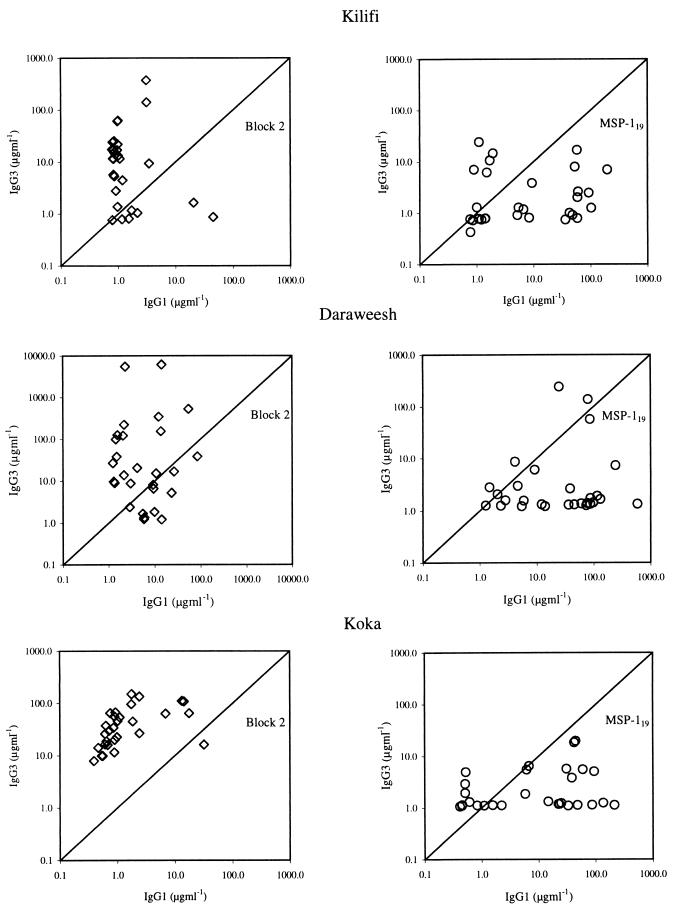
The results are summarized in Figure 1. IgG1 and IgG3 levels are shown for Block 2- or MSP-119-specific Abs in separate panels. Individuals had IgG subclasses recognizing Block 2 antigens in a type-specific manner, i.e., most samples contained IgG specific for only one of the three Block 2 types (data not shown). The levels of Block 2-specific IgG1 and IgG3 from any one individual are shown as a single data point representing the maximum detected with any one of the antigens used. Results for all three Block 2 types were similar and are combined in Fig. 1 for simplicity. In each donor cohort, the predominant response to Block 2 was of the IgG3 subclass, with a few individuals having both IgG3 and IgG1 (Fig. 1). In contrast, in the same donors, the predominant response to the MSP-119 region was of the IgG1 subclass, with some samples also containing IgG3. No IgG2 or IgG4 to any Block 2 type or to MSP-119 were detected in any donor (data not shown). Each cohort illustrated the same striking bias toward IgG3 responses to Block 2 and towards IgG1 responses to MSP-119. These distinct subclass biases of the responses to different regions of MSP-1 were statistically significant in all three cohorts of donors when analyzed by the two-tailed Wilcoxon matched-pairs ranked sign test. IgG3 was the predominant subclass against Block 2 at all three sites (z = −3.319, P < 0.0001 [Kilifi]; z = −2.5048, P < 0.0124 [Daraweesh]; and z = −4.552, P < 0.00006 [Koka]). In contrast, IgG1 was the predominant Ab subclass directed against MSP-119 (z = −2.9948, P < 0.0028 [Kilifi]; z = −3.2335, P < 0.0014 [Daraweesh]; z = −3.254, P < 0.0014 [Koka]).
FIG. 1.
Comparisons of IgG subclass levels to Block 2 and MSP-119 regions. Each point shows Ab levels (in micrograms per milliliter) of IgG1 and IgG3 in an undiluted plasma sample from one individual. Ab levels specific for the Block 2 and MSP-119 regions of MSP-1 in the same sets of plasma samples are plotted on adjacent graphs. Results are shown separately for cohorts of donors from Kilifi (Kenya), Daraweesh (Sudan), and Koka (Sudan).
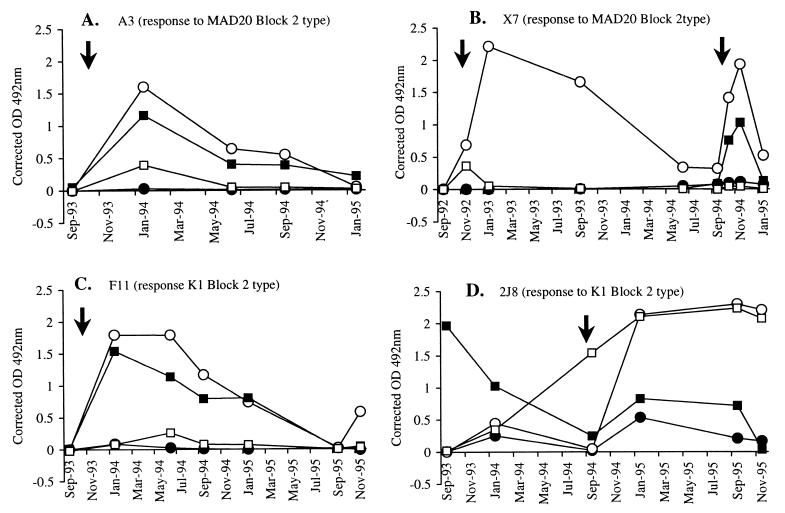
Plasma samples from individuals in Daraweesh were from a longitudinal study of immune responses to malaria conducted since 1990 (7, 18). Therefore, we tested whether changes in IgG subclass response profiles occurred in individuals over 3 to 4 years. Longitudinal series of plasma samples from eight individuals (5 to 11 samples each) were assessed for the IgG subclass composition of Abs to Block 2 and MSP-119 in successive transmission seasons. Seven of the individuals consistently produced IgG3 to Block 2 and, equally consistently, IgG1 to MSP-119 in response to their clinical malaria infections. IgG subclass profiles of individuals A3, X7, and F11 are shown as examples in Fig. 2A to C. Among the eight individuals tested longitudinally, the one notable exception to the general pattern was the response of individual 2J8 to MSP-119, which shifted from the usual IgG1 subclass in 1993 to the IgG3 subclass following a clinical infection in 1994 (Fig. 2D). The anti-Block 2 response of this individual was IgG3, as usual.
FIG. 2.
Longitudinal patterns of IgG subclass responses to Block 2 and MSP-119 in four donors from Daraweesh (Sudan). Solid symbols indicate IgG1 responses, and open symbols represent IgG3 responses. Squares indicate responses to MSP-119 while circles indicate responses to Block 2. Arrows indicate documented clinical malaria episodes. (A) Individual A3 (IgG3 response to MAD20 Block 2 and IgG1 response to MSP-119). (B) Individual X7 (IgG3 response to MAD20 type Block 2 and IgG1 response to MSP-119). (C) Individual F11 (IgG3 response to K1 type Block 2 and IgG1 response to MSP-119). (D) Individual 2J8 (IgG3 response to K1 type Block 2 and a shift from IgG1 to IgG3 in response to MSP-119).
This study presents the first direct evidence for strikingly distinct subclass preferences of Ab responses to two different regions of one protein, the Block 2 and the MSP-119 regions of MSP-1. It poses general questions about the regulation of human isotype responses and specific questions about the consequences of such responses in malaria. Strong IgG subclass biases to either IgG1 or IgG3 are a feature of human responses to different protein antigens of P. falciparum merozoites. Similar to the IgG3 bias of responses to Block 2, IgG3 is the main subclass in response to another merozoite surface protein (MSP-2) in Gambians (36) and in Solomon Islanders (31). Similar to the IgG1 bias to MSP-119 (13), responses to RAP-1, another antigen of P. falciparum merozoites, are also biased to the IgG1 subclass (16).
Protein antigens of most bacteria and viruses induce both IgG1 and IgG3 antibodies, with IgG1 generally being the dominant antiviral subclass (15, 19, 25). Virus-specific IgG3 correlates with current infection by nonlatent viruses and is normally a short-lived response (26). Persistence of IgG3 is usually seen only in asymptomatic latent viral infections, indicating that the presence of viral Ag is required for maintenance of the IgG3 responses (24). Like these antiviral responses, the generalized bias to IgG3 of Block 2-specific responses could be because most of the donors tested in this study were infected at the time of or shortly before plasma sample collection. However, an IgG3 bias would also be expected in the responses of these donors to MSP-119, but this was not observed. Thus, there must be other explanations for the differential subclass bias of responses to different regions of MSP-1.
Responses to multimeric antigens can be distinctly different from responses to less organized, soluble proteins. Repetitiveness of antigen structures on surfaces of cells or viral particles is sensed by the degree of cross-linking of surface Ig on B cells, which are thought to recognize antigen organization as a general marker of foreignness (2). Epitopes from viral proteins can elicit rapid T-cell-independent Ab responses, if expressed as part of a surface protein on recombinant bacteria, whereas the same epitopes elicit delayed, T-cell-dependent Ab responses when expressed as soluble, internal antigens (23). MSP-119, believed to be attached to the merozoite surface, could be recognized as a repetitively arranged surface antigen on the relatively large merozoite (1 μm). In contrast, Block 2 of MSP-1 is located on the MSP-183 fragment, a component of a soluble protein complex shed from the surface of the parasite. The uptake and processing by antigen-presenting cells of Block 2 and MSP-119 may therefore be different and is likely to influence the type of antibody produced. MSP-119 on merozoites probably would be phagocytosed for presentation by macrophages. In comparison, the soluble Block 2 is likely to be taken up by different antigen-presenting cells, e.g., follicular dendritic cells.
The significance of the IgG3 bias in the response to the polymorphic Block 2 of MSP-1 in malaria immunity is not yet known. Merozoite-specific antibodies of the IgG1 and IgG3 subclasses can trigger the release of soluble anti-parasite mediators from monocytes in vitro (12), but whether there are differences in the effectiveness of these two subclasses is not known. Soluble immune complexes of antigen and specific IgG3 interact differently with immune effector cells than do complexes formed by other IgG subclasses (22, 37). IgG3-containing complexes could mediate beneficial effects, such as Ab-dependent cellular cytotoxicity and phagocytosis of the parasite, or pathological inflammation. Kelly proposed the hypothesis that in a population with a high frequency of a mutant allotype of IgG3, a chronic presence of circulating immune complexes of unknown malaria antigens with IgG3 contributes to hyperreactive malarious splenomegaly, a chronic complication of malaria associated with up to 60% mortality (21). However, anti-Block 2 Abs, shown here to be mostly IgG3, are strongly associated with protection against clinical malaria, with protective efficacy in the range of 22 to 46% for type-specific Abs against types of Block 2 that are most prevalent in natural populations of P. falciparum (10). Further work is necessary to elucidate the protective and/or pathological potential of different IgG subclass responses against distinct portions of P. falciparum MSP-1.
Acknowledgments
We thank all donors for their participation in this study. We are grateful to Jane Robinson for her excellent technical assistance with ELISA and to Angus Buckling and Andrew Read for statistical advice.
This work was supported by grants from the Wellcome Trust (057270/z/99/z and 013163/z/94), the Danish International Development Agency, the Danish biotechnology programme (DANIDA), and the KEMRI. The manuscript is submitted with the permission of the Director, KEMRI.
REFERENCES
- 1.al-Yaman F, Genton B, Kramer K J, Chang S P, Hui G S, Baisor M, Alpers M P. Assessment of the role of naturally acquired antibody levels to Plasmodium falciparum merozoite surface protein-1 in protecting Papua New Guinean children from malaria morbidity. Am J Trop Med Hyg. 1996;54:443–448. doi: 10.4269/ajtmh.1996.54.443. [DOI] [PubMed] [Google Scholar]
- 2.Bachmann M F, Zinkernagel R M. Neutralizing antiviral B cell responses. Annu Rev Immunol. 1997;15:235–270. doi: 10.1146/annurev.immunol.15.1.235. [DOI] [PubMed] [Google Scholar]
- 3.Bouharoun-Tayoun H, Druilhe P. Plasmodium falciparum malaria: evidence for an isotype imbalance which may be responsible for delayed acquisition of protective immunity. Infect Immun. 1992;60:1473–1481. doi: 10.1128/iai.60.4.1473-1481.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouharoun-Tayoun H, Oeuvray C, Lunel F, Druilhe P. Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J Exp Med. 1995;182:409–418. doi: 10.1084/jem.182.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bull P C, Lowe B S, Kortok M, Molyneux C S, Newbold C I, Marsh K. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat Med. 1998;4:358–360. doi: 10.1038/nm0398-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burghaus P A, Holder A A. Expression of the 19-kilodalton carboxy-terminal fragment of the Plasmodium falciparum merozoite surface protein-1 in Escherichia coli as a correctly folded protein. Mol Biochem Parasitol. 1994;64:165–169. doi: 10.1016/0166-6851(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 7.Cavanagh D R, Elhassan I M, Roper C, Robinson V J, Giha H, Holder A A, Hviid L, Theander T G, Arnot D E, McBride J S. A longitudinal study of type-specific antibody responses to Plasmodium falciparum merozoite surface protein-1 in an area of unstable malaria in Sudan. J Immunol. 1998;161:347–359. [PubMed] [Google Scholar]
- 8.Cavanagh D R, McBride J S. Antigenicity of recombinant proteins derived from Plasmodium falciparum merozoite surface protein 1. Mol Biochem Parasitol. 1997;85:197–211. doi: 10.1016/s0166-6851(96)02826-5. [DOI] [PubMed] [Google Scholar]
- 9.Certa U, Rotmann D, Matile H, Reber-Liske R. A naturally occurring gene encoding the major surface antigen precursor p190 of Plasmodium falciparum lacks tripeptide repeats. EMBO J. 1987;6:4137–4142. doi: 10.1002/j.1460-2075.1987.tb02759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conway D J, Cavanagh D R, Tanabe K, Roper C, Mikes Z S, Sakihama N, Bojang K A, Oduola A M J, Kremsner P G, Arnot D E, Greenwood B M, McBride J S. A principal target of immunity to malaria identified by molecular population genetic and immunological analysis. Nat Med. 2000;6:689–692. doi: 10.1038/76272. [DOI] [PubMed] [Google Scholar]
- 11.Dodoo D, Theander T G, Kurtzhals J A, Koram K, Riley E, Akanmori B D, Nkrumah F K, Hviid L. Levels of antibody to conserved parts of Plasmodium falciparum merozoite surface protein 1 in Ghanaian children are not associated with protection from clinical malaria. Infect Immun. 1999;67:2131–2137. doi: 10.1128/iai.67.5.2131-2137.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Druilhe P, Perignon J-L. A hypothesis about the chronicity of malaria infection. Parasitol Today. 1997;13:353–357. doi: 10.1016/s0169-4758(97)01095-8. [DOI] [PubMed] [Google Scholar]
- 13.Egan A F, Chappel J A, Burghaus P A, Morris J S, McBride J, Holder A A, Kaslow D C, Riley E M. Serum antibodies from malaria-exposed people recognize conserved epitopes formed by the two epidermal growth factor motifs of MSP1(19), the carboxy-terminal fragment of the major merozoite surface protein of Plasmodium falciparum. Infect Immun. 1995;63:456–466. doi: 10.1128/iai.63.2.456-466.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elhassan I M, Hviid L, Jakobsen P H, Giha H, Satti G M, Arnot D E, Jensen J B, Theander T G. High proportion of subclinical Plasmodium falciparum infections in an area of seasonal and unstable malaria in Sudan. Am J Trop Med Hyg. 1995;53:78–83. [PubMed] [Google Scholar]
- 15.Ferrante A, Beard L J, Feldman R G. IgG subclass distribution of antibodies to bacterial and viral antigens. Pediatr Infect Dis J. 1990;9:S16–S24. [PubMed] [Google Scholar]
- 16.Fonjungo P N, Elhassan I M, Cavanagh D R, Theander T G, Hviid L, Roper C, Arnot D E, McBride J S. A longitudinal study of human antibody responses to Plasmodium falciparum rhoptry-associated protein 1 in a region of seasonal and unstable malaria transmission. Infect Immun. 1999;67:2975–2985. doi: 10.1128/iai.67.6.2975-2985.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fruh K, Doumbo O, Muller H M, Koita O, McBride J, Crisanti A, Toure Y, Bujard H. Human antibody response to the major merozoite surface antigen of Plasmodium falciparum is strain specific and short-lived. Infect Immun. 1991;59:1319–1324. doi: 10.1128/iai.59.4.1319-1324.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giha H A, Theander T G, Staalso T, Roper C, Elhassan I M, Babiker H, Satti G M, Arnot D E, Hviid L. Seasonal variation in agglutination of Plasmodium falciparum-infected erythrocytes. Am J Trop Med Hyg. 1998;58:399–405. doi: 10.4269/ajtmh.1998.58.399. [DOI] [PubMed] [Google Scholar]
- 19.Hammarstrom L, Smith C I. IgG subclasses in bacterial infections. Monogr Allergy. 1986;19:122–133. [PubMed] [Google Scholar]
- 20.Holder A A, Freeman R R. The three major antigens on the surface of Plasmodium falciparum merozoites are derived from a single high molecular weight precursor. J Exp Med. 1984;160:624–629. doi: 10.1084/jem.160.2.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly K M. IGHG3 G and the pathogenesis of hyperreactive malarious splenomegaly. Med Hypotheses. 1996;46:135–139. doi: 10.1016/s0306-9877(96)90013-4. [DOI] [PubMed] [Google Scholar]
- 22.Kocher M, Siegel M E, Edberg J C, Kimberly R P. Cross-linking of Fc gamma receptor IIa and Fc gamma receptor IIIb induces different proadhesive phenotypes on human neutrophils. J Immunol. 1997;159:3940–3948. [PubMed] [Google Scholar]
- 23.Leclerc C, Charbit A, Martineau P, Deriaud E, Hofnung M. The cellular location of a foreign B cell epitope expressed by recombinant bacteria determines its T cell-independent or T cell-dependent characteristics. J Immunol. 1991;147:3545–3552. [PubMed] [Google Scholar]
- 24.Linde A, Andersson J, Lundgren G, Wahren B. Subclass reactivity to Epstein-Barr virus capsid antigen in primary and reactivated EBV infections. J Med Virol. 1987;21:109–121. doi: 10.1002/jmv.1890210203. [DOI] [PubMed] [Google Scholar]
- 25.Linde A, Sundqvist V A, Mathiesen T, Wahren B. IgG subclasses to subviral components. Monogr Allergy. 1988;23:27–32. [PubMed] [Google Scholar]
- 26.Linde G A. Subclass distribution of rubella virus-specific immunoglobulin G. J Clin Microbiol. 1985;21:117–121. doi: 10.1128/jcm.21.1.117-121.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McBride J S, Heidrich H G. Fragments of the polymorphic Mr 185,000 glycoprotein from the surface of isolated Plasmodium falciparum merozoites form an antigenic complex. Mol Biochem Parasitol. 1987;23:71–84. doi: 10.1016/0166-6851(87)90189-7. [DOI] [PubMed] [Google Scholar]
- 28.Miller L H, Roberts T, Shahabuddin M, McCutchan T F. Analysis of sequence diversity in the Plasmodium falciparum merozoite surface protein-1 (MSP-1) Mol Biochem Parasitol. 1993;59:1–14. doi: 10.1016/0166-6851(93)90002-f. [DOI] [PubMed] [Google Scholar]
- 29.Muller H M, Fruh K, von Brunn A, Esposito F, Lombardi S, Crisanti A, Bujard H. Development of the human immune response against the major surface protein (gp190) of Plasmodium falciparum. Infect Immun. 1989;57:3765–3769. doi: 10.1128/iai.57.12.3765-3769.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riley E M, Allen S J, Wheeler J G, Blackman M J, Bennett S, Takacs B, Schonfeld H J, Holder A A, Greenwood B M. Naturally acquired cellular and humoral immune responses to the major merozoite surface antigen (PfMSP1) of Plasmodium falciparum are associated with reduced malaria morbidity. Parasite Immunol. 1992;14:321–337. doi: 10.1111/j.1365-3024.1992.tb00471.x. [DOI] [PubMed] [Google Scholar]
- 31.Rzepczyk C M, Hale K, Woodroffe N, Bobogare A, Csurhes P, Ishii A, Ferrante A. Humoral immune responses of Solomon Islanders to the merozoite surface antigen 2 of Plasmodium falciparum show pronounced skewing towards antibodies of the immunoglobulin G3 subclass. Infect Immun. 1997;65:1098–1100. doi: 10.1128/iai.65.3.1098-1100.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarthou J L, Angel G, Aribot G, Rogier C, Dieye A, Toure B A, Diatta B, Seignot P, Roussilhon C. Prognostic value of anti-Plasmodium falciparum-specific immunoglobulin G3, cytokines, and their soluble receptors in West African patients with severe malaria. Infect Immun. 1997;65:3271–3276. doi: 10.1128/iai.65.8.3271-3276.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi Y P, Sayed U, Qari S H, Roberts J M, Udhayakumar V, Oloo A J, Hawley W A, Kaslow D C, Nahlen B L, Lai A A. Natural immune response to the C-terminal 19-kilodalton domain of Plasmodium falciparum merozoite surface protein 1. Infect Immun. 1996;64:2716–2723. doi: 10.1128/iai.64.7.2716-2723.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Staalso T, Khalil E A, Elhassan I M, Zijlstra E E, Elhassan A M, Giha H A, Theander T G, Jakobsen P H. Antibody reactivity to conserved linear epitopes of Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) Immunol Lett. 1998;60:121–126. doi: 10.1016/s0165-2478(97)00143-0. [DOI] [PubMed] [Google Scholar]
- 35.Tanabe K, Mackay M, Goman M, Scaife J G. Allelic dimorphism in a surface antigen gene of the malaria parasite Plasmodium falciparum. J Mol Biol. 1987;195:273–287. doi: 10.1016/0022-2836(87)90649-8. [DOI] [PubMed] [Google Scholar]
- 36.Taylor R R, Smith D B, Robinson V J, McBride J S, Riley E M. Human antibody response to Plasmodium falciparum merozoite surface protein 2 is serogroup specific and predominantly of the immunoglobulin G3 subclass. Infect Immun. 1995;63:4382–4388. doi: 10.1128/iai.63.11.4382-4388.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voice J K, Lachmann P J. Neutrophil Fc gamma and complement receptors involved in binding soluble IgG immune complexes and in specific granule release induced by soluble IgG immune complexes. Eur J Immunol. 1997;27:2514–2523. doi: 10.1002/eji.1830271008. [DOI] [PubMed] [Google Scholar]
- 38.Wahlgren M, Berzins K, Perlmann P, Persson M. Characterization of the humoral immune response in Plasmodium falciparum malaria. II. IgG subclass levels of anti-P. falciparum antibodies in different sera. Clin Exp Immunol. 1983;54:135–142. [PMC free article] [PubMed] [Google Scholar]