Abstract
Introduction
The coronavirus disease-2019 (COVID-19) pandemic has substantially affected the delivery of healthcare globally. The purpose of this study was to evaluate the association of this era with the timeline of care in esophageal cancer patients.
Methods
We performed a retrospective chart-review of patients presenting to a single high-volume tertiary care center with the diagnosis of esophageal cancer. COVID era was defined as March 2020-December 2020 and compared with the year before (3/2019-12/2019).
Results
In total, 117 patients presented in the COVID-era versus 190 in pre-COVID. Stage 3 + 4 disease was found in 77.8% of the patients in the COVID-era compared to 68.9% in the pre-COVID era (P = 0.34). Diagnoses through emergency department admission were 35.5% in the COVID versus 26.7% in the pre-COVID group (P = 0.15). In the COVID era it took a median of 78 d to visit primary care provider (versus 52 d, P = 0.12 in pre-COVID), 45 d to endoscopy (versus 18 d, P = 0.004) and 38 d to treatment initiation (versus 36 d, P = 0.48). Thirty-five percent of the patients underwent esophagectomy compared to 26% in the pre-COVID-era. Median days of intensive-care-unit (ICU) (2 versus 3, P = 0.16) and hospital stay (14 versus 15, P = 0.28) were similar in both groups as well as postoperative 30-day morbidities (63 versus 63%, P = 0.48). One-year follow-up showed 83.7% (95% confidence interval [CI]: 73.8%-90.1%) survival in the COVID-group compared to 76.4% (95% CI: 66.9%-83.5%) in the pre-COVID-group (P = 0.58). Only three patients had a positive COVID result.
Conclusions
Our institution treated fewer esophageal cancer patients during COVID-19 accompanied by a delay in endoscopic diagnosis. Postoperative outcomes and 1-year survival remained similar.
Keywords: Cancer, COVID-19, Delay, Esophagus, Outcomes, Pandemic
Introduction
The onset of the coronavirus disease-2019 (COVID-19) pandemic in March 2020 and its ongoing persistence has affected everyone across the globe, with more than 420 million cases recorded by February 2022.1 As the pandemic continues to evolve, hospitals seek to comply with ever-changing public-health policies to minimize the spread of the virus while simultaneously recommencing essential healthcare operations to their fullest capacity. This has dramatically changed the delivery of healthcare both in the United States and across the world. Oncologic patients are at a higher susceptibility to COVID-19 because of their immunosuppressive state and under a greater threat for poor clinical outcomes due to the disruption in healthcare.2
Patt et al. reported that screening rates for various cancers decreased by as much as 85% during the peak of the pandemic in April 2020.3 This resulted in reduced diagnosis and delayed treatment.3 , 4 Esophageal cancer patients represent a particularly vulnerable population, as they are suffering from one of the deadliest malignancies worldwide, with a 5-year survival of 15%-20% and ranking 6th in cancer-related-mortality.5 , 6 For resectable esophageal cancer, neoadjuvant chemoradiation followed by surgical resection is the standard-of-care, and delays in esophagectomy have been shown to decrease long-term survival.7 , 8 However, early pandemic data across all types of surgeries reported a 50% postoperative pulmonary complication and 23% mortality rate in patients with perioperative COVID-19 infection.9 This led to recommendations for postponing all nonemergent cases and delaying operative management where possible.9 At our institution, resources were significantly diverted towards battling the pandemic; however, priority was given to high-risk cancer patients as well. The purpose of this study was to analyze trends in esophageal cancer care before and during the COVID-19 pandemic at our institution to assess any delays and differences in outcomes.
Materials & Methods
Data source
This is a retrospective cohort study that utilized our institution's electronic database, EPIC (Epic Systems Corporation, Verona, WI). International classification of diseases–10 (ICD-10) code C15 was used to identify esophageal cancer patients who presented from 2019 to 2020. We used date of histopathological diagnosis to categorize patients into pre-COVID (3/2019-12/2019) and COVID (3/2020-12/2020) cohorts. Inclusion criteria were all patients who received care relevant to esophageal cancer at our institution. Exclusion criteria were patients who presented with recurrence instead of index diagnosis. A waiver of informed consent was obtained from the Institutional Review Board (IRB) at The Johns Hopkins Hospital before beginning the study.
Study variables
Chart review was conducted to gather data on demographic—age, gender, race, body mass index (BMI) and histopathological variables—date of biopsy, tumor, nodes, and metastasis (TNM) stage, tumor histology, tumor location and American Joint Committee on Cancer (AJCC) 8th Ed stage. Data were obtained on the following comorbidities at presentation: hypertension, Barrett's esophagus, atrial fibrillation, diabetes, congestive heart failure, coronary heart disease, history of smoking, chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), and liver disease. Subjective weight loss reported by patients on their initial visit to their primary care provider (PCP) was also recorded. Date of initial patient visit (in person or virtual) to their PCP for esophageal symptoms, date of upper gastrointestinal (GI) endoscopy diagnosing esophageal cancer, and date of treatment initiation—including neoadjuvant chemotherapy, radiotherapy or chemoradiotherapy—were all noted. Information was also recorded on whether esophageal cancer diagnosis resulted from the patient presenting acutely at an emergency department (ED) with subsequent hospital admission. Data was abstracted on the treatment received, and for patients undergoing surgery at our institution, postoperative outcomes including length of intensive care unit (ICU) or hospital stay, 30-day morbidity, 30- and 90-day mortality were noted. For survival analysis, we used 1-year follow up for all patients. For patients in the COVID era, data were collected on any positive COVID test received throughout their entire care.
Statistical analysis
Continuous data were evaluated for normality using the Jarque-Bera goodness of fit test. Normal data were presented in the form of mean with standard deviation whereas data that were not normally distributed was summarized using median with interquartile ranges. The Chi-square test was used to assess for difference between two categorical variables whereas the t-test or the Mann–Whitney test was performed to assess difference between two means. A P-value was <0.05 was used for statistical significance. RedCap (Nashville, TN) and Microsoft Excel (Redmond, WA) were used for data collection whereas R Core Team (Version 4.1.1 Vienna, Austria) was used for analyses.
Results
We identified a total of 117 COVID-era and 190 pre-COVID patients, demonstrating a 38% reduction in patient volume following COVID onset. The mean age was 66.3 y (standard deviation (SD) ± 10.8) with 85.5% males and 77.8% Whites during COVID. There was no difference in comorbidities between both groups (Table 1 ).
Table 1.
Patient characteristics.
| Variables | COVID-n = 117 (%) | Pre-COVID-n = 190 (%) | P-value |
|---|---|---|---|
| Mean age (SD) | 66.3 (10.8) | 65.1 (11.3) | 0.18 |
| Gender | 0.11 | ||
| Males | 100 (85.5) | 147 (77.4) | |
| Females | 17 (14.5) | 43 (22.6) | |
| Race | 0.06 | ||
| White | 91 (77.8) | 169 (88.9) | |
| Black | 11 (9.4) | 9 (4.7) | |
| Asian | 6 (5.1) | 6 (3.2) | |
| Other | 9 (7.7) | 6 (3.2) | |
| Histopathology | 0.49 | ||
| Adenocarcinoma | 84 (71.8) | 146 (76.8) | |
| Squamous cell | 29 (24.8) | 36 (18.9) | |
| Carcinoma unspecified | 4 (3.4) | 8 (4.2) | |
| Location of tumor | 0.52 | ||
| Upper-third | 8 (6.8) | 12 (5.9) | |
| Middle-third | 13 (11.0) | 34 (16.6) | |
| Lower-third | 58 (49.2) | 89 (43.4) | |
| GE-Junction | 39 (33.1) | 70 (34.1) | |
| BMI (median-IQR) | 27.1 (24.5-29.8) | 27.1 (23.2-31.3) | 0.92 |
| Weight loss-lbs. (median-IQR) | 11 (0-30) | 8 (0-20) | 0.14 |
| Comorbidities | |||
| Hypertension | 84 (71.8) | 133 (70.0) | 0.79 |
| Barrett's | 26 (22.2) | 63 (33.2) | 0.05 |
| Afib | 26 (22.2) | 55 (28.9) | 0.23 |
| Other Cancer | 18 (15.4) | 37 (19.5) | 0.44 |
| Diabetes | 33 (28.2) | 44 (23.2) | 0.34 |
| CHF | 4 (3.4) | 15 (7.9) | 0.14 |
| CAD | 24 (20.5) | 38 (20.0) | 1.0 |
| COPD | 12 (10.3) | 20 (10.5) | 1.0 |
| Smoking History | 68 (58.1) | 127 (66.8) | 0.14 |
| CKD | 12 (10.3) | 30 (15.8) | 0.23 |
| Liver disease | 6 (5.1) | 17 (8.9) | 0.26 |
| AJCC staging 8th ed | 0.34 | ||
| Stage 0 | 1 (0.9) | 1 (0.5) | |
| Stage 1 | 10 (8.5) | 28 (14.7) | |
| Stage 2 | 12 (10.3) | 28 (14.7) | |
| Stage 3 | 46 (39.3) | 61 (32.1) | |
| Stage 4 | 45 (38.5) | 70 (36.8) | |
| Unknown | 3 (2.6) | 2 (1) | |
| Visit type leading to diagnosis | 0.14 | ||
| Emergency Department | 35 (35.5) | 36 (26.7) | |
| Elective visit | 61 (63.5) | 99 (73.3) | |
| Covid test result | |||
| Positive | 3 (3.3) | ||
| Negative | 88 (96.7) | N/A |
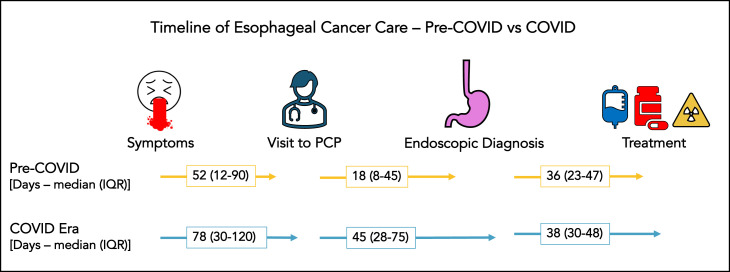
COVID-era patients reported a slightly higher median weight loss of 11 (interquartile range: IQR 0-30) lbs. versus 8 (0-20) lbs. in the pre-COVID group (P = 0.14) despite comparable BMI at initial presentation (27.1 versus 27.1 kg/m2). Up to 35.5% of COVID-era patients received their esophageal cancer diagnosis during an emergent visit to the hospital compared to 26.7% in the pre-COVID era (P = 0.15). Of the remaining patients who had an elective endoscopic diagnosis, it took 78.5 (IQR: 30-120) d from start of symptoms to initial PCP visit during COVID compared to 52.5 (IQR: 12-90) d the year prior (P = 0.12). Moreover, during COVID there was a significantly longer duration of 45 (IQR: 28.8-75.3) d from initial PCP visit to endoscopic diagnosis compared to 18 d (IQR: 8-45) during pre-COVID (P = 0.004). The period from histopathologic diagnosis to initiation of treatment was 38.5 (IQR: 30.5-48) d in COVID era compared to 36 (IQR: 23-47.5) d in pre-COVID (P = 0.48) (Fig. ). Only three patients (3.3%) had a positive COVID test during their entire treatment course.
Fig.
Timeline of care across various phases depicted between COVID and pre-COVID era.
Stage I was present in 8.5% versus 14.7%, stage II in 10.3% versus 14.7%, stage III in 39.3% versus 32.1% and stage IV in 38.5% versus 36.8% in the COVID group compared to pre-COVID group (P = 0.34). Adenocarcinoma was present in more than 70% in both groups and lower third of esophagus was the most common site of tumor in both groups. Initial treatment received was neoadjuvant chemoradiation in 73.6% of the patients during COVID and 70.6% during pre-COVID era (P = 0.88).
Forty-one (35%) COVID-era patients underwent esophagectomy at our institution compared to 49 pre-COVID patients (25.8%). Median length of ICU stay was 2 (1-4) versus 3 (1-6) d (P = 0.16) and median length of hospital stay was 14 (11-17) versus 15 (11-22) d (P = 0.28) for COVID versus pre-COVID groups. The 30-day rate of any-morbidity was equal in both groups (63 versus 63%, P = 0.48). Thirty-day readmission (9.8 versus 8.2%, P = 0.79), 30-day mortality (0 versus 1), and 90-day mortality (0 versus 2) were similar between COVID and pre-COVID era. When all patients in both cohorts were evaluated, 1-year follow-up showed 83.7% (95% CI: 73.8-90.1%) overall survival in the COVID-group compared to 76.4% (95% CI: 66.9-83.5%) in the pre-COVID group (P = 0.58) (Table 2 ).
Table 2.
Treatment characteristics.
| Variables | COVID-n = 117 (%) | Pre-COVID-n = 190 (%) | P-value |
|---|---|---|---|
| Days symptoms to provider (Median-IQR) | 78.5 (30-120) | 52.5 (12-90) | 0.12 |
| Days provider to EGD (Median-IQR) | 45 (28.8-75.3) | 18 (8-45) | 0.004 |
| Days EGD to treatment (Median-IQR) | 38.5 (30.5-48) | 36 (23-47.5) | 0.48 |
| Initial treatment offered | 0.88 | ||
| Chemo-Radiation | 67 (73.6) | 89 (70.6) | |
| Chemotherapy only | 20 (22.0) | 32 (25.4) | |
| Radiation only | 4 (4.4) | 5 (4.0) | |
| Type of surgical intervention | |||
| Ivor-Lewis esophagectomy | 35 | 45 | |
| Trans-hiatal esophagectomy | 5 | 7 | |
| 3-incision esophagectomy | 4 | 4 | |
| EMR/RFA | 2 | 6 | |
| Endoscopic stenting | 0 | 4 | |
| PEG/J-tube | 6 | 12 | |
| Esophagectomies at our institute | 41/117 (35%) | 49/190 (25.8%) | 0.10 |
| Length of ICU stay-days (median-IQR) | 2 (1-4) | 3 (1-6) | 0.16 |
| Length of hospital stay-days (median-IQR) | 14 (11-17) | 15 (11-22) | 0.28 |
| 30-day post-operative morbidity | 0.48 | ||
| Any Morbidity | 26 (63.4%) | 31 (63.2%) | |
| None | 15 | 18 | |
| Afib/Vtach/BBB | 11 | 12 | |
| PNA | 7 | 9 | |
| Reintubation | 3 | 7 | |
| PE/DVT | 4 | 4 | |
| C.Diff | 1 | 2 | |
| SSI | 6 | 5 | |
| Splenic Infarct | 0 | 1 | |
| GI Bleed | 0 | 1 | |
| UTI | 0 | 4 | |
| Vocal cord dysfunction | 1 | 1 | |
| Anastomotic Leak | 1 | 5 | |
| Chyle Leak | 6 | 5 | |
| Reoperation | 1 | 3 | |
| 30-day readmission | 4 (9.8%) | 4 (8.2%) | 0.79 |
| 30-day mortality | 0 | 1 | |
| 90-day mortality | 0 | 2 | |
| 1-year overall survival (95% CI) | 83.7% (73.8-90.1) | 76.4% (66.9-83.5) | 0.58 |
Discussion
This study analyzed diagnoses and treatment trends for patients with esophageal cancer during COVID-19 and compared it with the year before evaluate associations between the COVID era and the delivery of esophageal cancer care. We found that there was a significant delay from PCP visit to endoscopy. We noticed no statistical difference in the stage of tumors or proportion of emergent cases during COVID. Postoperative outcomes including 30-day morbidity and 30/90-day mortality were equal, if not better, for the COVID group. Finally, a very small proportion of the patients in the COVID era had a positive COVID test during their entire treatment course.
Almost 40% reduction in esophageal cancer patients during the pandemic was noted. Our findings are concordant with other related studies, such as by Kaufman et al., who reported that weekly number of newly diagnosed cancer cases in the United States decreased by 46% during the pandemic.10 Trinidade et al. reviewed a large US claims database and reported that during the early pandemic (April-June 2020), newly diagnosed Barrett's esophagus went down by 68.3% (P = 0.0009) whereas newly diagnosed esophageal cancer went down by 21.8% (P = 0.19) when compared to similar times the year before.11 Since our institution is a tertiary care center, one of the reasons why fewer patients were treated might be because of a decrease in referrals as seen in other countries as well.12
Although delays at all phases of care were noticed for patients with elective diagnosis, they were only significant when endoscopic intervention was required. Perhaps, this was due to concerns of virus aerosolization during intubation in the early phase of the pandemic.7 Interestingly, once histopathologic diagnosis was made, the delay in care between the two groups was the shortest. Dolan et al. also reported that the wait-time from neoadjuvant treatment to surgery was similar in COVID compared to the year before.7 However, Perkons et al. noticed that the disruption of healthcare delivery for gastrointestinal cancer patients was seen broadly from new patient visits to radiology encounters and infusion visits.13 In other parts of the world, a delay in surgery was noticed primarily due to shortage of intensive care facilities.14 At our institution, there was no complete shutdown of the operating rooms and high-risk patients—such as those with esophageal cancer—were prioritized with appropriate resources allocated to them even in the initial few months of the pandemic. These measures proved successful in achieving optimal outcomes.
Timely intervention is essential for optimal outcomes in cancer patients.15 Literature that originated early in the pandemic raised valid concerns about the potential increase in cancer-related deaths primarily due to a suspension of cancer screening, delay in diagnostic interventions, and a decrease in health-seeking behavior.12 , 16 However, at that time, the risk of COVID-19 infection had to be weighed against disease progression with watchful waiting. Predictive models for early-stage esophageal cancer recommended delayed approach when the perioperative COVID-19 infection risk became 7% or higher to obtain superior 5-year survival.17 At our institution, a total of 3% patients contracted the virus in their preoperative to 1-year follow-up period. This suggests that institutional and state-wide policies in COVID prevention were effective. A multicenter study across Europe reported no increase in the risk of respiratory failure or pulmonary complications during COVID-19 pandemic and in fact noted that of the 139 patients studied, none contracted the virus.18
The COVID cohort in our study showed no statistical difference in the proportion of higher stage esophageal cancer. This finding was observed in other studies as well7 although Japanese institutes noticed an increased proportion of higher-stage tumors during the pandemic.19 Although nonsignificant, we witnessed that during the pandemic a higher proportion of patients presented to the emergency department in critical condition leading to immediate endoscopic diagnosis (35% versus 26%). One rationale to interpret this finding may be that some patients opted to stay at home longer than expected due to the fear of contracting COVID-19 virus in the healthcare setting. Despite this, the number of esophagectomies at our institution did not decrease. This finding is supported by Purdy et al., who evaluated retrospective data from more than 500 hospitals in the U.S. and reported significantly reduced volume of all common gastrointestinal operations (24%-98%) in April 2020 except esophagectomies and gastrectomies for cancer.20
The slightly shorter length of ICU and hospital stay during COVID, although nonsignificant, is likely due to the emphasis placed on early discharge to mitigate the risk of contracting COVID-19. This was accompanied by a 30-day readmission rate that was similar for both groups and presents an opportunity for improvement in quality of care and discharge efficiency as Weksler suggests.21 Postoperative mortality and 1-year overall survival for the entire cohort was also equivalent in both groups, although there is a selection bias as not all patients presented for care. Indeed, with a 40% reduction in patients, it is plausible that some might have passed away at home from the disease or from COVID-19 because of an immune system that was already weakened. Thus, true estimates of survival outcomes are difficult to ascertain as of now.
There are a number of limitations that must be kept in mind while interpreting the results. This is a single-center study with a small sample size and a retrospective design that can only suggest associations or trends rather than causation. Variables recorded such as weight loss at presentation or duration of symptoms at home before visiting a PCP are subjective and prone to bias. Often patients had been visiting their PCP for unrelated symptoms (e.g. lethargy) that led to delay in diagnosis. Such a delay is not a system error and introduces bias when trying to understand delays associated with the COVID-19 pandemic. However, such bias was presumed to be equal for both pre-COVID and COVID groups. We also recognize the fact that most of our results did not reach the targeted statistical value of P < 0.05. However, we believe that our analysis is underpowered due to a small sample size coupled with wide ranges and regardless identifies important trends highlighting delay of care at the prehospital level. Further investigation with multi-institutional, state-wide, or national databases that can afford a larger sample size is warranted to explore some of the trends identified.
At our institution, in accordance with Centers for Disease Control and Prevention (CDC) guidelines, a number of measures were taken to curb the spread of the coronavirus while providing care to high-risk cancer patients as follows: 1) COVID testing was performed 3 d before surgery, and patients were asked to socially isolate in those 3 d, 2) In-hospital visitation was limited, 3) Operating rooms, ICU and surgical wards were separate for COVID and non-COVID patients, 4) COVID testing was performed every 3 d for admitted patients, 5) There were COVID-specific healthcare teams that did not interact with non-COVID patients, and 6) Strict masking and social distancing policies were enacted.
How the SARS-CoV2 (severe acute respiratory syndrome coronavirus 2) affects the pathophysiology of esophageal cancer is yet to be elucidated. Kostoff et al. identified more than 60 contributing factors that were common to both gastro-intestinal cancers and COVID-19, a number of which contributed to disease pathogenesis via immune dysfunction and were modifiable.22 As data matures on this topic, we will have a clearer understanding of what to expect regarding these patients and how a change in the treatment or preventive paradigm is needed. Majority of the literature arising from European studies reports that with careful planning, esophageal cancer surgery can be performed safely with excellent outcomes.23, 24, 25 Similar data are now also reported from the U.S. Despite the fact that U.S. Food & Drug Administration (FDA) authorized vaccines are now available for more than a year, globally we are still battling the pandemic with the introduction of new variants of the coronavirus. Fortunately, with implementation of appropriate protocols and COVID-19 risk mitigation strategies, optimal oncologic outcomes can be achieved.
Conclusions
More than 2 y later, the coronavirus pandemic continues to affect our healthcare system. The introduction of vaccines has allowed for some normalcy as we try to deliver effective healthcare while mitigating the spread of the virus. This study identified a significant delay in the diagnosis of esophageal cancer; however, such findings were not associated with worse postoperative or 1-year outcomes. As long-term data accrue, further research with a larger sample size is warranted. In the meantime, focus must be placed on appropriate delivery of healthcare to vulnerable patients.
Study Type
Retrospective Cohort Study.
Level of Evidence
Level IV.
Supplementary Materials
None.
Author Contributions
HK, CJ, JM, PH were involved in data acquisition and analysis, HK, JM, RB, SY, SL, VL, JH were involved in conceptualization, design and methodology. HK, CJ, JM, RB, SY, SL, PH, VL, and JH were involved in manuscript preparation, edition, and revision.
Acknowledgments
The authors would like to thank Oscar Li, B.S. for his contributions toward the cover image.
Disclosure
None declared.
Funding
This work was supported by the NIH-T32 Grant (T32 CA126607–Clinical and Laboratory Research Training for Surgical Oncologists) awarded to Hamza Khan.
Meeting Presentation
A modified version of this article was presented as an abstract at the Association of Academic Surgery (AAS) Annual Conference–2022, Florida. The current abstract has been presented at the Maryland Chapter of the American College of Surgeons 2022 Annual Meeting.
References
- 1.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang L., Chai P., Yu J., Fan X. Effects of cancer on patients with COVID-19: a systematic review and meta-analysis of 63,019 participants. Cancer Biol Med. 2021;18:298. doi: 10.20892/j.issn.2095-3941.2020.0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patt D., Gordan L., Diaz M., et al. Impact of COVID-19 on cancer care: how the pandemic is delaying cancer diagnosis and treatment for American seniors. JCO Clin Cancer Inform. 2020;4:1059–1071. doi: 10.1200/CCI.20.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Englum B.R., Prasad N.K., Lake R.E., et al. Impact of the COVID-19 pandemic on diagnosis of new cancers: a national multicenter study of the Veterans Affairs Healthcare System. Cancer. 2021;128:1048–1056. doi: 10.1002/cncr.34011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang J., Koulaouzidis A., Marlicz W., et al. Global burden, risk factors, and trends of esophageal cancer: an analysis of cancer registries from 48 countries. Cancers (Basel) 2021;13:141. doi: 10.3390/cancers13010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Short M.W., Burgers K., Fry V. Esophageal cancer. Am Fam Physician. 2017;95:22–28. [PubMed] [Google Scholar]
- 7.Dolan D.P., Swanson S.J., Lee D.N., et al. Esophagectomy for Esophageal Cancer Performed During the Early Phase of the COVID-19 Pandemic. Semin Thorac Cardiovasc Surg. 2021;34:1075–1080. doi: 10.1053/j.semtcvs.2021.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qin Q., Xu H., Liu J., et al. Does timing of esophagectomy following neoadjuvant chemoradiation affect outcomes? A meta-analysis. Int J Surg. 2018;59:11–18. doi: 10.1016/j.ijsu.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Nepogodiev D., Bhangu A., Glasbey J.C., et al. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396:27–38. doi: 10.1016/S0140-6736(20)31182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaufman H.W., Chen Z., Niles J., Fesko Y. Changes in the number of US patients with newly identified cancer before and during the coronavirus disease 2019 (COVID-19) pandemic. JAMA Netw Open. 2020;3:e2017267. doi: 10.1001/jamanetworkopen.2020.17267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trindade A.J., Zhang J., Hauschild J., Leggett C.L., Iyer P.G. Impact of coronavirus disease 2019 on the diagnosis and therapy for Barrett’s esophagus and esophageal cancer in the United States. Gastroenterology. 2021;162:978–980.e6. doi: 10.1053/j.gastro.2021.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maringe C., Spicer J., Morris M., et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21:1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perkons N.R., Kim C., Boedec C., et al. Quantifying the impact of the COVID-19 pandemic on gastrointestinal cancer care delivery. Cancer Rep. 2021;5:e1427. doi: 10.1002/cnr2.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rebecchi F., Arolfo S., Ugliono E., et al. Impact of COVID-19 outbreak on esophageal cancer surgery in Northern Italy: lessons learned from a multicentric snapshot. Dis Esophagus. 2021;34:doaa124. doi: 10.1093/dote/doaa124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neal R., Tharmanathan P., France B., et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112:S92–S107. doi: 10.1038/bjc.2015.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sud A., Jones M.E., Broggio J., et al. Collateral damage: the impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann Oncol. 2020;31:1065–1074. doi: 10.1016/j.annonc.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shipe M.E., Baechle J.J., Deppen S.A., Gillaspie E.A., Grogan E.L. Modeling the impact of delaying surgery for early esophageal cancer in the era of COVID-19. Surg Endosc. 2021;35:6081–6088. doi: 10.1007/s00464-020-08101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borgstein A.B., Brunner S., Hayami M., et al. Safety of esophageal cancer surgery during the first wave of the COVID-19 pandemic in Europe: a multicenter study. Ann Surg Oncol. 2021;28:4805–4813. doi: 10.1245/s10434-021-09886-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuzuu K., Misawa N., Ashikari K., et al. Gastrointestinal cancer stage at diagnosis before and during the COVID-19 pandemic in Japan. JAMA Netw Open. 2021;4:e2126334. doi: 10.1001/jamanetworkopen.2021.26334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Purdy A.C., Smith B.R., Hohmann S.F., Nguyen N.T. The impact of the novel coronavirus pandemic on gastrointestinal operative volume in the United States. Surg Endosc. 2021;36:1943–1949. doi: 10.1007/s00464-021-08477-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weksler B. Commentary: SARS-CoV-2 and esophagectomy for esophageal cancer: timely operations and good outcomes. Semin Thorac Cardiovasc Surg. 2021;34:1081–1082. doi: 10.1053/j.semtcvs.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kostoff R.N., Briggs M.B., Kanduc D., et al. Contributing factors common to COVID-19 and gastrointestinal cancer. Oncol Rep. 2022;47:1–16. doi: 10.3892/or.2021.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan D., MacCormick A., Rashid B., et al. Short-term outcomes after Ivor Lewis oesophagectomy for cancer during the COVID-19 pandemic. Ann R Coll Surg Engl. 2021;103:134–137. doi: 10.1308/rcsann.2020.7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wahed S., Chmelo J., Navidi M., Hayes N., Phillips A., Immanuel A. Delivering esophago-gastric cancer care during the COVID-19 pandemic in the United Kingdom: a surgical perspective. Dis Esophagus. 2020;33:doaa091. doi: 10.1093/dote/doaa091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bolger J.C., Donlon N.E., Butt W., et al. Successful maintenance of process and outcomes for oesophageal cancer surgery in Ireland during the first wave of the COVID-19 pandemic. Ir J Med Sci. 2021;191:831–837. doi: 10.1007/s11845-021-02597-0. [DOI] [PMC free article] [PubMed] [Google Scholar]