Abstract
Susceptibility of BALB/c mice to Leishmania major depends on the early production of IL-4 by CD4+ T cells which react to the parasite LACK antigen. Here, we show that LACK-specific cells are rapidly recruited to the site of infection and favor the early dissemination of L. major to the internal organs.
In contrast to mice from most strains which develop a Th1 protective immune response when infected with Leishmania major, susceptible BALB/c mice mount a counterprotective Th2 response which is characterized by the expansion of interleukin-4 (IL-4)-secreting CD4+ T cells (12). In agreement with a critical role of IL-4 in promoting Th2 responses, L. major induces the rapid production of IL-4 mRNA in the draining lymph nodes (LN) of infected BALB/c mice (2, 9, 10), and immunological or genetic manipulations aimed at neutralizing IL-4 prevent the development of Th2 cells and induce the development of a Th1 protective response (4, 13). The cells which rapidly secrete IL-4 in mice infected with L. major are class II-restricted CD4+ T cells which react to the parasite LACK antigen and use the T-cell receptor Vα8 and Vβ4 regions (5, 6, 10, 11). As a further demonstration of the critical role of LACK-specific cells in susceptibility to L. major, BALB/c mice that were made tolerant to LACK by the transgenic expression of LACK in the thymus or by the deletion of Vβ4+ T cells exhibited an impaired Th2 response and eventually healed (5, 10). In addition, treatment of Vβ4-deficient BALB/c mice with IL-4 during the first 64 h after infection led to Th2 cell development and susceptibility to infection (3). Thus, the critical role of the LACK-specific T cells in susceptibility to L. major is likely to result from their unusual ability to rapidly secrete IL-4 in infected mice.
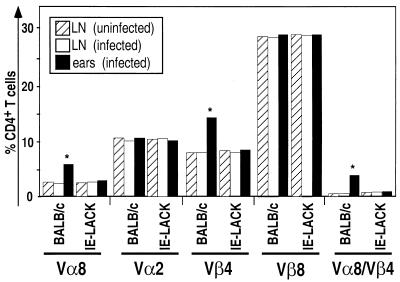
While LACK-specific T cells play a critical role in susceptibility, it was not known how rapidly these cells are recruited to the inflammatory site. To answer this question, BALB/c mice were injected with 2 × 106 L. major promastigotes into the dermis of the ears. Cells were recovered from the ears and the draining LN 20 h later and then analyzed by flow cytometry for the expression of Vα8 and Vβ4 (Fig. 1). While the proportions of Vα8+, Vβ4+, and Vα8+ Vβ4+ cells were similar in the draining LN of infected mice and in those of naive animals, these cells were more frequent at the site of infection. Thus, the frequency of Vα8+ Vβ4+ CD4+ T cells was 20- to 30-fold higher in the ears of infected BALB/c mice than in the draining LN. In striking contrast, no increase in the proportion of Vα8+ Vβ4+ cells was found in the ears of LACK-tolerant BALB/c-derived IE-LACK transgenic mice (Fig. 2). Thus, most of the Vα8 Vβ4 cells which were recovered from the ears of infected BALB/c mice most likely reacted to LACK.
FIG. 1.
Vα8+ Vβ4+ CD4+ T cells are rapidly recruited to the site of parasite injection in susceptible BALB/c mice. BALB/c-derived IE-LACK transgenic mice (5) and BALB/c mice were infected with 2 × 106 stationary-phase L. major promastigotes in the dermis of the ear as previously described (1). At 20 h after infection, cells were recovered from the draining LN of uninfected (dashed bars) or infected (open bars) mice and from the ears of infected mice (solid bars). To recover cells from the ears, the ears were collected, separated into ventral and dorsal leaflets, and transferred, dermal site down, to culture medium into a six-well hydrophobic bottom plate. Non adherent cells were recovered 18 h later and filtered through a 70-μm (pore-size) nylon cell strainer. Viable cells were further purified by passage through a Ficoll-Hypaque gradient (d = 1.083) and stained with fluorochrome-conjugated anti-CD4 (GK1.5), and anti-Vβ4 (KT4.1), anti-Vα2 (B20.1), anti-Vβ8 (F23.1), or anti-Vα8 (B21.14) monoclonal antibodies. Viable lymphocytes (106 cells/ sample) were analyzed on a FACScan flow cytometer using CellQuest software. The results show the percentages of cells stained with the indicated antibodies after gating on CD4+ T cells. The values given are the results of a representative experiment (out of four).
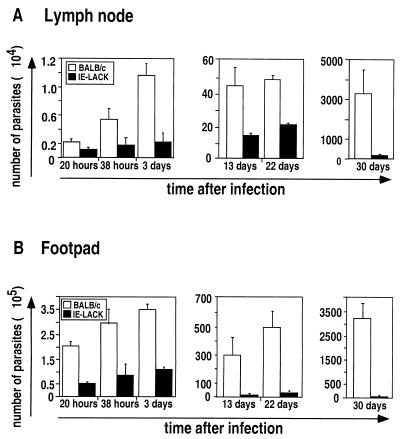
FIG. 2.
Early parasite containment in IE-LACK transgenic mice. IE-LACK transgenic mice (solid bars) and BALB/c mice (open bars) were infected with 2 × 106 stationary-phase L. major promastigotes into both footpads. At different times after infection, parasite numbers were determined in the draining LN (A) and the footpad (B) by limiting dilution assay as described elsewhere (15). For each time point at least two animals per group were analyzed, and a mean of at least two independent experiments is represented. To determine the number of parasites in the site of infection and the draining LN, serial dilutions of the tissue homogenate were prepared. Briefly, footpads were cut above the heel and then into several pieces and left in 4 ml of complete M199 medium supplemented with collagenase A (1 mg/ml) at 37°C for 1 h. Afterward, the footpads were ground vigorously using a glass tissue homogenizer. LN were gently crushed between two glass slides in order to get single-cell suspensions in 2 ml of complete M199 medium. Then, serial 10-fold dilutions were prepared for both footpad and LN samples, and 100 μl of each dilution was distributed into previously prepared 96-well plates containing 50 μl of SeaPlaque agarose (7% in complete M199). For each dilution, at least 32 replicate wells were analyzed. The assay was read by scoring the number of wells as positive for parasite growth using inverted microscope, and the number of parasites was determined applying Poisson statistics.
LACK-specific CD4+ T cells rapidly secrete IL-4 in BALB/c mice infected with L. major (2, 3, 6, 9, 10). In addition, we have recently found that these cells also secrete IL-10 (7). Since IL-4 and IL-10 prevent the killing of Leishmania parasites by macrophages (16) and since LACK-specific T cells were found in the inflammatory sites of BALB/c mice 20 h after infection, we sought to determine whether these cells could prevent the early control of parasite burden at the site of infection. To do this, we infected BALB/c and IE-LACK mice with L. major, and the numbers of parasites in the infected footpads and in the draining LN were measured by limiting dilution assays. In agreement with a critical role of the LACK-specific T cells in promoting susceptibility to L. major, the footpads and the draining LN of BALB/c mice contained more parasites than those from IE-LACK mice at all times after infection. Thus, the footpads of BALB/c mice contained four- to sixfold more parasites than those of IE-LACK transgenic mice by 20, 38, and 72 h after infection. Likewise, the LN of BALB/c mice contained more parasites than those from IE-LACK mice, even at early time points.
Early studies have shown that L. major rapidly disseminate in susceptible BALB/c mice but not in resistant animals (8). While parasite containment in resistant strains was dependent on natural killer cells but not on T cells, it was not clear whether T cells contributed to parasite dissemination in susceptible strains. To determine if LACK-specific T cells promoted the early dissemination of L. major in BALB/c mice, BALB/c and IE-LACK mice were injected with 2 × 106 promastigotes into the footpads. At different times after infection, DNA was prepared from the spleen and the liver and then analyzed by PCR using L. major-specific primers (Table 1). Parasite DNA was detected in the spleen and in the liver from seven (out of nine) and five (out of nine) BALB/c mice 38 h after infection. In contrast, none of the nine IE-LACK transgenic mice which were analyzed at that time contained parasite DNA in their liver, and only one of the nine animals contained L. major DNA in its spleen.
TABLE 1.
Parasite dissemination in IE-LACK transgenic mice during the early phase of infection
| Organ | No. of positive mice/no. of mice testedb at:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 20 h
|
38 h
|
3 days
|
6 days
|
|||||
| IE-LACK | BALB/c | IE-LACK | BALB/c | IE-LACK | BALB/c | IE-LACK | BALB/c | |
| Spleen | 0/2 | 1/2 | 1/9 | 7/9 | 1/2 | 2/2 | 2/2 | 2/2 |
| Liver | 0/2 | 0/2 | 0/9 | 5/9 | 0/2 | 1/2 | 1/2 | 2/2 |
IE-LACK transgenic mice and BALB/c mice were infected with 2 × 106 stationary-phase L. major promastigotes subcutaneously into both footpads. At different times after infection, spleens and livers from individual mice were harvested and analyzed for the presence of parasites using PCR as described earlier (8). Briefly, DNA was isolated from tissue homogenates using the InstaGene DNA purification matrix. To detect Leishmania DNA from live parasites by PCR, two primers (primer 1, 5′-GTGGGGAGGGGGCGTTCT-3′; primer 2, 5′-ATTTTACACCAACCCCCAGTT-3′) were used for amplification of the 120-bp fragment of the minicircle kinetoplast DNA of L. major. Briefly, 20 μl of extracted DNA were mixed with 0.5 pmol of each primer, a 200 μM concentration of each deoxynucleoside triphosphate, 50 mM KCl, 1.5 mM MgCl2, 10 mM Tris-HCl (pH 9.0), and 0.1 U of Taq DNA polymerase in a total volume of 100 μl. The sample was submitted to 27 cycles of amplification (94°C for 30 s, 50°C for 45 s, 72°C for 60 s) in an automated DNA, thermal cycler (Perkin-Elmer Cetus). PCR products were analyzed by gel electrophoresis in a 2% agarose gel.
That is, the number of mice in which L. major was detected by PCR/the total number of tested animals.
We have shown here that the inflammatory sites of recently infected BALB/c mice contained a higher proportion of Vα8+ Vβ4+ T CD4+ T cells than the LN of infected or naive animals. Since this was not the case in IE-LACK mice, most of these T cells were likely to react to LACK. Our results are in agreement with studies showing that LACK-specific T cells are rapidly activated to secrete IL-4 in BALB/c mice. While the surface phenotype of these cells could not be analyzed due to the lack of appropriate reagents, they were shown to express CD69 as early as 16 h after infection (10). Thus, LACK-specific T cells are rapidly activated in the draining LN and eventually migrate to the site of infection, possibly as the result of changes in the expression of chemokine receptors (14).
As IL-4 is required for the differentiation of Th2 cells, LACK-specific T cells are likely to play an important role in L. major-infected mice by acting directly on naive T cells through an IL-4-dependent mechanism. We have shown here that LACK-specific T cells not only act in the draining LN but also at the site of infection by promoting the rapid replication of the parasite and its dissemination to the internal organs. Several mechanisms can account for this latter phenomenon. First, IL-4 and IL-10, which are rapidly produced by LACK-specific T cells, could prevent killing of Leishmania parasites by infected macrophages. Alternatively, these cytokines could promote increased phagocytosis or alter inflammation leading to enhanced infection.
We thank G. Milon for advice.
S.S. was supported by a fellowship from the Boehringer Ingelheim Funds. This work was supported by grants from the Association François Aupetit, the Ministère de l'Education Nationale, de la Recherche, et de l'Enseignement Supérieur (MENRT), and the DRET (contract 500098) and by EU contract QLGI-CT-1999-00050.
REFERENCES
- 1.Belkaid Y, Jouin H, Milon G. A method to recover, enumerate and identify lymphomyeloid cells present in an inflammatory dermal site: a study in laboratory mice. J Immunol Methods. 1996;199:5–25. doi: 10.1016/s0022-1759(96)00117-2. [DOI] [PubMed] [Google Scholar]
- 2.Himmelrich H, Parra-Lopez C, Tacchini-Cottier F, Louis J A, Launois P. The IL-4 rapidly produced in BALB/c mice after infection with Leishmania major down-regulates IL-12 receptor beta 2-chain expression on CD4+ T cells resulting in a state of unresponsiveness to IL-12. J Immunol. 1998;161:6156–6163. [PubMed] [Google Scholar]
- 3.Himmelrich H, Launois P, Maillard I, Biedermann T, Tacchini-Cottier F, Locksley R M, Rocken M, Louis J A. In BALB/c mice, IL-4 production during the initial phase of infection with Leishmania major is necessary and sufficient to instruct Th2 cell development resulting in progressive disease. J Immunol. 2000;164:4819–4825. doi: 10.4049/jimmunol.164.9.4819. [DOI] [PubMed] [Google Scholar]
- 4.Kopf M, Brombacher F, Kohler G, Kienzle G, Widmann K H, Lefrang K, Humborg C, Ledermann B, Solbach W. IL-4-deficient BALB/c mice resist infection with Leishmania major. J Exp Med. 1996;184:1127–1136. doi: 10.1084/jem.184.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Julia V, Rassoulzadegan M, Glaichenhaus N. Resistance to Leishmania major induced by tolerance to a single antigen. Science. 1996;274:421–423. doi: 10.1126/science.274.5286.421. [DOI] [PubMed] [Google Scholar]
- 6.Julia V, Glaichenhaus N. CD4+ T cells which react to the leishmania major LACK antigen rapidly secrete interleukin-4 and are detrimental to the host in resistant B10.D2 mice. Infect Immun. 1999;67:3641–3644. doi: 10.1128/iai.67.7.3641-3644.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Julia V, McSorley S, Malherbe L, Breittmayer J-P, Girard-Pipau F, Beck A, Glaichenhaus N. Priming by microbial antigens from the intestinal florza determines the ability of CD4+ T cells to rapidly secrete IL-4 in BALB/c mice infected with Leishmania major. J Immunol. 2000;165:5637–5645. doi: 10.4049/jimmunol.165.10.5637. [DOI] [PubMed] [Google Scholar]
- 8.Laskay T, Diefenbach A, Rollinghoff M, Solbach W. Early parasite containment is decisive for resistance to Leishmania major infection. Eur J Immunol. 1995;25:2220–2227. doi: 10.1002/eji.1830250816. [DOI] [PubMed] [Google Scholar]
- 9.Launois P, Ohteki T, Swihart K, MacDonald H R, Louis J A. In susceptible mice, Leishmania major induce very rapid interleukin-4 production by CD4+ T cells which are NK1.1. Eur J Immunol. 1995;25:3298–3307. doi: 10.1002/eji.1830251215. [DOI] [PubMed] [Google Scholar]
- 10.Launois P, Maillard I, Pingel S, Swihart K G, Xenarios I, Acha-Orbea H, Diggelmann H, Locksley R M, MacDonald H R, Louis J A. IL-4 rapidly produced by Vβ4+ Vα8+ CD4+ T cells instructs Th2 development and susceptibility to Leishmania major in BALB/c mice. Immunity. 1997;6:541–549. doi: 10.1016/s1074-7613(00)80342-8. [DOI] [PubMed] [Google Scholar]
- 11.Mougneau E, Altare F, Wakil A E, Zheng S, Coppola T, Wang Z E, Waldmann R, Locksley R M, Glaichenhaus N. Expression cloning of a protective Leishmania antigen. Science. 1995;268:563–566. doi: 10.1126/science.7725103. [DOI] [PubMed] [Google Scholar]
- 12.Reiner S L, Locksley R M. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 13.Sadick M D, Heinzel F P, Holaday B J, Pu R T, Dawkins R S, Locksley R M. Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell-dependent, interferon gamma-independent mechanism. J Exp Med. 1990;171:115–127. doi: 10.1084/jem.171.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sallusto F, Palermo B, Hoy A, Lanzavecchia A. The role of chemokine receptors in directing traffic of naive, type 1 and type 2 T cells. Curr Top Microbiol Immunol. 1999;246:123–128. doi: 10.1007/978-3-642-60162-0_16. [DOI] [PubMed] [Google Scholar]
- 15.Stenger S, Donhauser N, Thuring H, Rollinghoff M, Bogdan C. Reactivation of latent leishmaniasis by inhibition of inducible nitric oxide synthase. J Exp Med. 1996;183:1501–1514. doi: 10.1084/jem.183.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vouldoukis I, Becherel P A, Riveros-Moreno V, Arock M, daSilva O, Debre P, Maizer D, Mossalayi M D. Interleukin-10 and interleukin-4 inhibit intracellular killing of Leishmania infantum and Leishmania major by human macrophages by decreasing nitric oxide generation. Eur J Immunol. 1997;27:860–865. doi: 10.1002/eji.1830270409. [DOI] [PubMed] [Google Scholar]