Abstract
Tumor drug resistance is a multifactorial and heterogenous condition that poses a serious burden in clinical oncology. Given the increasing incidence of resistant tumors, further understanding of the mechanisms that make tumor cells able to escape anticancer drug effects is pivotal for developing new effective treatments. Neutrophils constitute a considerable proportion of tumor infiltrated immune cells, and studies have linked elevated neutrophil counts with poor prognosis. Tumor-associated neutrophils (TANs) can acquire in fact immunoregulatory capabilities, thus regulating tumor progression and resistance, or response to therapy. In this review, we will describe TANs’ actions in the tumor microenvironment, with emphasis on the analysis of the role of interleukin-8 (IL-8) and extracellular vesicles (EVs) as crucial modulators and mediators of TANs biology and function in tumors. We will then discuss the main mechanisms through which TANs can induce drug resistance, finally reporting emerging therapeutic approaches that target these mechanisms and can thus be potentially used to reduce or overcome neutrophil-mediated tumor drug resistance.
Keywords: drug resistance, neutrophil, interleukin-8, extracellular vesicles, Tumor-associated neutrophils, NETosis
1. Introduction
During the past decades, huge progress has been made in the field of cancer genetics, immunology and pathology for the identification of new markers and methods for diagnosis and treatments (1, 2). Despite these achievements, resistance to classical chemotherapeutic agents or to novel drugs is one of the major causes of therapy failure and death in cancer, still representing a crucial limiting factor in the treatment of cancer patients (3).
The mechanisms through which cancer cells get resistant or acquire resistance to drug therapies are numerous, and sometimes tumors can be resistant to multiple therapies and display, simultaneously or subsequently, different mechanisms of drug resistance. In this context, it has been proposed that the mechanisms of drug resistance in tumors can be both active (cell-autonomous) or adaptive (non-cell-autonomous): the firsts depend on cancer intracellular responses, which include, for example, genetic or epigenetic alterations that promote cell survival (4–7), while the seconds result from tumor interactions with the surrounding tumor microenvironment (TME) (8, 9) that is shaped to favor tumor growth, expansion and drug resistance.
Together with myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages (TAMs), tumor-associated neutrophils (TANs) represent the most abundant population (10, 11) of immune cells infiltrated in the TME, and many studies so far have highlighted the link between elevated TAN counts and increased risk of metastasis, drug resistance and poor prognosis (12–17). In TME, neutrophils can acquire immunoregulatory capabilities, facilitating tumor progression (18) and drug resistance through a number of different mechanisms. In this context, interleukin-8 (IL-8, aka CXCL-8), a member of the CXC chemokine family that is highly produced by neoplastic cells (19), is an important chemoattractant and activator for neutrophils and is a key mediator of their biology, behavior and actions inside the tumor. On the other hand, increasing evidence is highlighting the crucial role of extracellular vesicles (EVs) in both the mediation and regulation of neutrophils’ response within the TME. EVs, produced both by tumor cells and by TANs, or by other immune or stromal cells, function in fact as intercellular mediators of the communication within the TME and beyond, and can ultimately promote neutrophil-mediated tumor drug resistance (20).
In this review, we will describe the role of IL-8 and EVs in the regulation and mediation of neutrophil biology and function in the TME, promoting pro-tumoral functions of these cells, ultimately leading to neutrophil-mediated tumor drug resistance through the production of neutrophil extracellular traps (NETs) and the secretion of neutrophil-derived EVs and other factors. Finally, we will discuss emerging therapies that, targeting IL-8, EVs and neutrophil functions, could be considered as potential therapeutic tools to reduce or overcome neutrophil-mediated tumor drug resistance.
2. Neutrophils in cancer
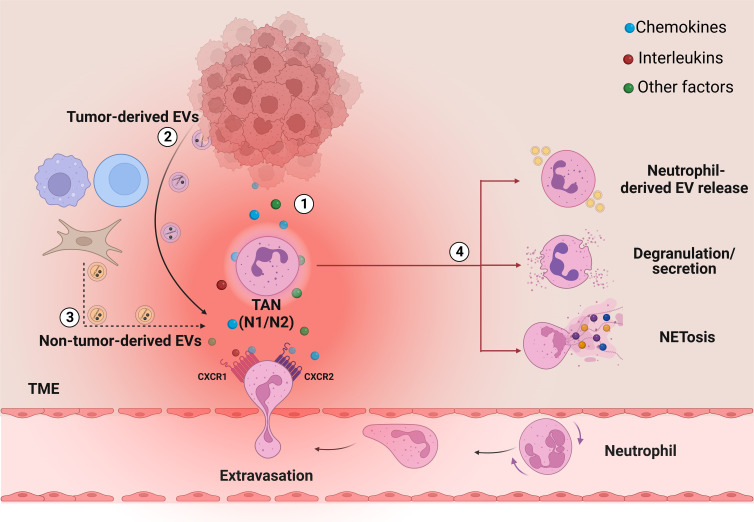
Neutrophils are the most abundant leukocytes in the circulation, representing around 70% of all white blood cells (21). Produced in the bone marrow (BM) through the granulopoiesis (i.e., progressive maturation) of hematopoietic progenitors, neutrophils are then released into the blood stream, ready to respond to a plethora of stimuli released by inflamed tissues (22). In tumors, several chemotactic and inflammatory factors, as well as EVs, are released from tumoral and non-tumoral cells and can attract mature neutrophils, which thus migrate from blood stream and infiltrate into the TME (23–25) ( Figure 1 ).
Figure 1.
The role of secreted factors and EVs in neutrophil recruitment and activation into the TME. In TME, cancer cells can regulate neutrophil biology through the secretion of several factors, among which (1) interleukins and chemokines, such as IL-8, and (2) tumor-derived EVs. These factors regulate the recruitment of the neutrophils from the bloodstream to the tumor and the generation of TANs, which in turn promote cancer progression, metastasis and drug resistance through different cell mechanisms: release of neutrophil-derived EVs, degranulation/secretion and NETosis (4). Together with cancer cells, also other cell types in the TME can release vesicles, which are also potentially able to act on neutrophils (3, dashed arrows). Image created with biorender.com.
Neutrophil recruitment into the tumor site from circulation is a multi-step process that involves several factors, but seems to be mainly regulated by two G protein-coupled receptors (GPCRs): CXCR4 and CXCR2 (26). CXCR4 is a neutrophil homing marker in the bone marrow, while CXCR2 activation by its ligands (i.e., CXCL-1, CXCL-2, CXCL-3, CXCL-5, CXCL-6, CXCL-7 and CXCL-8) induces the release of neutrophils into circulation and their recruitment into the TME (27–29). Indeed, the diverse cell types in the TME (e.g., tumor cells, immune cells, fibroblasts) release large quantities of CXCR2 ligands, forming a chemotactic gradient that attracts the neutrophils from the bloodstream (29). Among the chemokines that can influence neutrophil functions, IL-8 is the master regulator of neutrophil biology and one of the most characterized chemokine in cancer as it has been found overexpressed in several tumors (30–43). Once into the TME, neutrophils turn into TANs, a plastic and dynamic population that can rapidly switch between two forms: N1 TANs with anti-tumoral functions, and N2 TANs, with pro-tumoral effects (44–46). N1 TANs are mature and short-living cells, which exert their highly cytotoxic and immune-stimulating activities by producing reactive oxygen species (ROS) and other cytotoxic substances, and by recruiting and activating other immune cells (17). On the other hand, N2 TANs are immature and long-living cells, which can produce and release cytokines, chemokines and other factors to favor pro-angiogenic, pro-metastatic and immune-suppressive activities (47). TANs polarization towards one of the two sub-populations is crucially regulated by multiple TME factors including, among others, cytokines and chemokines, such as IL-8, and also EVs, released by tumor, stromal and immune cells (17, 48, 49). In addition, TANs can also regulate cancer progression through NETosis, a process by which neutrophils extrude a sort of web-like structures called NETs (50–52). NETs are formed by DNA fibers decorated with cytotoxic enzymes, such as neutrophil elastase (NE), myeloperoxidase (MPO) and matrix metalloproteinases-9 (MMP-9) and are released by activated neutrophils into the extracellular space as mechanism of defense against pathogen micro-organisms (53). In tumors, NETs have been identified as factors that can significantly contribute to carcinogenesis and metastasis (11, 54) in several ways, as by inducing the degradation of the extracellular matrix which promotes the extravasation of cancer cells (50), trapping circulating tumor cells (CTCs) (55, 56) or deactivating thrombospondin-1 (TSP-1), a potent inhibitor of angiogenesis and tumor progression (57, 58).
Regulated by different factors and acting through several mechanisms, neutrophils thus play a key role in hijacking the immune system response against the tumor, ultimately promoting cancer progression and tumor drug resistance (59, 60).
3. IL-8 and EVs crucially regulate and mediate the biology and functions of TANs in the TME
3.1. IL-8 and TANs
Tumor cells produce several factors, such as cytokines, chemokines, lipids, and growth factors that, not only increase their growth and survival in an autocrine manner (61), but also increase the number of circulating neutrophils by stimulating granulopoiesis in the bone marrow and promote their recruitment to TME in a paracrine manner (62–64) ( Figure 1 ). In particular, among other factors, IL-8 has demonstrated to be crucial in tumor progression (19, 65–67), since it was found to be overexpressed in several tumors, where induces angiogenesis and is involved in the maintenance of cancer stem cells (CSCs) (68, 69). Also, a direct correlation between IL-8 and poor prognosis has been reported (70–73). IL-8 exists as a monomer or dimer and exerts its activity by binding its two receptors: CXCR1 and CXCR2 (74). It is a well-known chemoattractant able to recruit leukocytes and in particular neutrophils, which express a substantial number of IL-8 receptors on their surface (75, 76). During carcinogenesis, the IL-8 released by neoplastic cells promotes the activation of both the phosphatidylinositol-3-kinase (PI3K) and the mitogen-activated protein kinase (MAPK) signal pathways via CXCR2, thus leading to cell migration and survival (77–79). In addition, IL-8 mediates the formation of NETs, through the binding to CXCR1 and CXCR2 (18, 80) ( Figure 1 ). These mechanisms help to dampen the anti-tumor immune responses and cause disfunctions of cytotoxic immune cells, thus crucially contributing to tumor growth and progression (81).
3.2. Extracellular vesicles and TANs
In addition to IL-8, EVs are other factors that are crucially involved in the regulation and mediation of TANs’ pro-tumoral functions in the TME (82). EVs are heterogenous lipid bilayer structures secreted by cells that can carry a plethora of cargoes, including lipids, proteins and nucleic acids (83–86). In the past they were divided in three subtypes (microvesicles (MVs), exosomes and apoptotic bodies) depending on their biogenesis, release pathways, size, content and functions (84, 87, 88). However, since it is not easy to clearly determine EVs biogenesis pathway, the last MISEV guidelines (MISEV 2018) suggest to classify EV subtype referring to 1) physical characteristics of EVs, such as size or density; 2) biochemical composition; or 3) descriptions of conditions or cell of origin (89). One of the main functions of EVs is to facilitate the exchanges of cellular components, acting as an intercellular communication system in both physiological and pathological conditions (88, 90, 91). The EV-mediated intercellular communication is achieved in two manners: by delivering cargoes that are within the vesicles in the target cells (92, 93), or by using EVs surface markers without requiring vesicle internalization (94). In tumors, EVs are important components of the TME and promote the crosstalk between cancer and cancer-associated cells (e.g. fibroblasts, endothelial and immune cells), creating a favorable niche that supports and nourishes the tumor, promoting its growth and progression, and also regulating tumor drug resistance.
Tumor-derived EVs are nanoscale membrane vesicles (95) that contain tumor-specific functional biomolecules both in their lumen, such as cytokines, growth factors, proteases and enzymes, as well as on their surface, including receptors/ligands, adherent molecules, or tetraspanins (96, 97). Tumor-derived EVs work in both autocrine and paracrine way to favor local invasion of tumor cells and spreading of metastasis and to induce the reprogramming of recipient cells (82, 98–101). They can also promote immune-modulation by attenuating the cytotoxic activity of T and NK cells, prompting the recruitment of regulatory B cells and Tregs and inducing the differentiation of M2 macrophages and N2 immune-suppressive sub-population of tumor-associated macrophages (TAMs) and TANs, respectively (102, 103), thereby creating a pro-tumorigenesis environment for tumor progression (104–106).
Among innate immune cells, neutrophils may be especially prone to stimulation from tumor-derived EVs (107); for example, they can promote TAN polarization into the anti-inflammatory N2 tumorigenic subtype ( Figure 1 ). Although the underlying mechanisms remain poorly understood, Zhang and colleagues have recently started analyzing tumor-derived EVs induced N2 neutrophil polarization in gastric cancer, demonstrating that gastric cancer-derived EVs can induce the expression of programmed death-ligand 1 (PD-L1) on neutrophils, which in turn polarizes their differentiation through the N2 phenotype and suppresses T cell-mediated immunity (108, 109). On the other hand, tumor-derived EVs from murine colorectal CSCs have been shown to prolong bone marrow-derived neutrophil life-span through the activation of the NF-κB signaling, which in turn induces the expression of interleukin-1β (IL-1β) in neutrophils, thus promoting their pro-tumoral phenotype (110). Besides inducing N2 polarization, tumor-derived EVs can also modulate other properties of neutrophils’ biology. Tumor-derived EVs from a metastatic human melanoma cell line (MV3), for example, have been shown to induce neutrophil chemotaxis through the CXCR2/PI3K-Akt axis and to promote the formation of NETs (103) ( Figure 1 ), which play a crucial role in inducing cancer-associated thrombosis (111–113) and tumor drug resistance (114). Similar results have been obtained in a mouse model of breast cancer, where 4T1-derived exosomes induced NETs formation in neutrophils derived from G-CSF-treated mice and accelerated venous thrombus formation in tumor-free neutrophilic mice (115). Also the EVs released from a human cell line of breast carcinoma (MDA-EVs) induced neutrophil activation (i.e., increased chemotaxis and secretion of IL-8 and MMP-9), N2-like phenotype and increase of ROS production, which were followed by augmented NETosis (116). Finally, a recent report also showed that exosomes can transfer mutant KRAS from DKO-1 colorectal cancer cells to neutrophils, resulting in increased IL-8 production, neutrophil recruitment and NETs formation, ultimately promoting tumor growth and metastasis. Interestingly, these effects were abolished by an anti-IL-8 treatment (117).
Although tumor-derived EVs represent the majority of vesicles secreted in the TME, studies have shown that EVs can be released also by other cells within the TME, such as cancer-associated stromal cells (CASCs), including fibroblasts, immune cells, endothelial cells and neurons (118) ( Figure 1 ). These EVs can influence many aspects of tumor biology, but their direct role in the regulation of neutrophil biology has not been fully addressed yet. For example, cancer associated fibroblasts (CAFs) can secrete EVs which act on cancer cells to enhance their metastatic potential by delivering bioactive molecules, such as extracellular matrix proteins and remodeling enzymes (118). Ji et al. demonstrated that primary colorectal cancer cells can secrete integrin beta-like 1 (ITGBL1)-bearing EVs which enter the circulation, reach distant organs, and activate fibroblasts via the TNFAIP3-mediated NF-κB signaling (119). In addition to fibroblasts, also immune cells can release EVs within the TME ultimately exerting anti-cancer effects as for natural killer (NK) cells, or pro-cancer effects in the case of regulatory T cells (Tregs) (120). NK cell-derived EVs are released by resting and activated NK cells and both can exert cytotoxic activity on activated but not resting immune cells (121), but also exhibit immune-modulatory activity by stimulating other immune cells via paracrine action or through the circulatory system (122).
Further studies are needed to better understand if this subset of non-tumor-derived EVs may have a direct role in the regulation of neutrophil biology in the context of tumor progression.
4. TAN-mediated tumor drug resistance
The involvement of neutrophils in tumor drug resistance is determined by the interplay of several factors. Among others, IL-8 and EVs are key modulators of neutrophil biology and functions within the TME. They act on neutrophils to promote tumor drug resistance which is exerted through different mechanisms, such as release of neutrophil-derived EVs, secretion of specific molecules/factors and NETosis ( Figure 1 ). These mechanisms can act in a concerted way to promote tumor drug resistance by reducing the availability or stability of administered therapeutics, inducing ROS production or alterations of DNA damage repair pathways, and modulating antitumor immunity (123).
4.1. Tumor drug resistance promoted by neutrophil-derived EVs
Like tumor cells, neutrophils can also produce and release EVs in response to intracellular metabolic changes and/or extracellular environmental stress. As reviewed by Rubenich and colleagues, the genetic and molecular composition of neutrophil-derived EVs reflects that of the mother cell and varies depending on the existing physiological or pathological conditions (20, 124). Depending on the context, neutrophils polarize into inflammatory N1 or regenerative N2 subtypes, which are thought to be able to release two different kinds of EVs: the N1-derived and the N2-derived EVs, respectively (124).
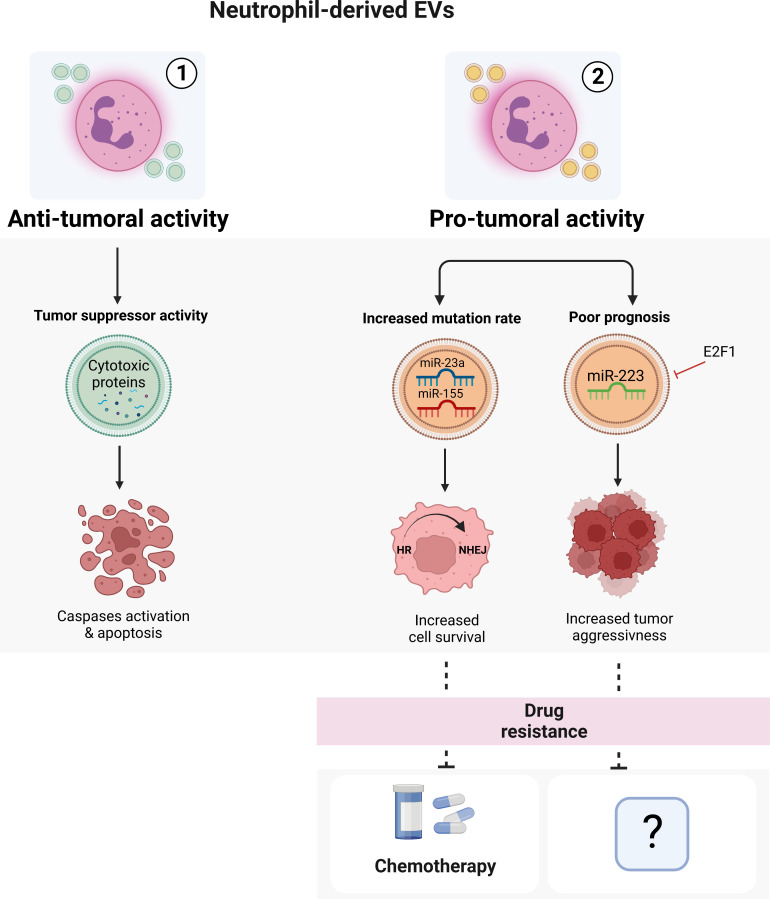
During cancer progression, the role of neutrophil-derived EVs seems to be important for the prediction of disease outcome, although the underlying mechanisms are still unclear (125). Even if few, the available evidences on neutrophil-derived EVs isolated from tumoral contexts seem to mainly suggest a role for these vesicles in mediating cancer progression and drug resistance. On the other hand, EVs produced by neutrophils from healthy donors may possess a tumor suppressive activity both in vitro and in vivo (126). As recently demonstrated in fact, EVs from healthy neutrophils contain cytotoxin proteins that are able to activate the caspases signaling pathway and then promote tumor cell apoptosis (126) ( Figure 2 ).
Figure 2.
Neutrophil-derived EVs. Neutrophil-derived EVs can exert both anti-tumoral (1) or pro-tumoral (2) activities in a context-dependent manner. The EVs isolated from healthy neutrophils can induce apoptosis of cancer cells through the activation of caspases pathway. On the other hand, tumoral neutrophil-derived EVs seem to promote cancer spreading, progression and drug resistance. Image created with biorender.com.
The role of neutrophil-derived EVs in drug resistance has been demonstrated by a recent work from Butin-Israeli and colleagues (127). Using samples from inflammatory bowel disease (IBD) patients, who are more prone to develop colitis-associated colorectal cancer and have an important neutrophil infiltrate in the intestinal mucosa, they demonstrated that neutrophil-derived EVs containing miR-23a and miR-155 inhibited Homologous Recombination (HR) repair by targeting the main HR regulators RAD51 while promoting non-homologous DNA end joining (NHEJ), ultimately leading to the formation of highly mutagenic DNA Double-Strand Breaks (DSBs) (127). This switch from HR to NHEJ may result in the acquisition of drug resistance in tumors (128–131) as observed in colorectal cancer, in which neutrophil-mediated NHEJ induced resistance to a lethal dose of topo-isomerase I inhibitor Camptothecin (CMPT) as tumor cells effectively resolved CMPT-induced DSBs and entered normally into cell cycle (132) ( Figure 2 ). Other evidence for the role of neutrophil-derived EVs in cancer, suggest that they can act either as an onco-suppressor (133–136) or as an onco-promoter (137–139) in a context-dependent manner. For example, neutrophil-derived EVs containing miR-223, a miRNA essential for the development of cells of the myeloid lineage and the mobilization of neutrophils from the bone marrow (140–142), have been described to be able to both sustain and inhibit tumor growth (135, 137–139, 143). In both acute myeloid leukemia and breast cancer for instance, E2F1-dependent downregulation of EVs-transported miR-223 is associated with tumor aggressiveness and poor prognosis (135, 143). Of note, a clear role of neutrophil-derived EVs carrying miR-223 in drug resistance still remains unknown ( Figure 2 ).
Interestingly, in addition to regulate tumor progression and drug resistance, neutrophil-derived EV have recently also been engineered to efficiently deliver anti-cancer drugs at the tumor site (126), thus not only demonstrating the intricate complexity of the processes regulating neutrophil-derived EVs content and secretion but also showing the therapeutic potential of these vesicles.
4.2. Tumor drug resistance promoted by TAN-released factors and NETosis
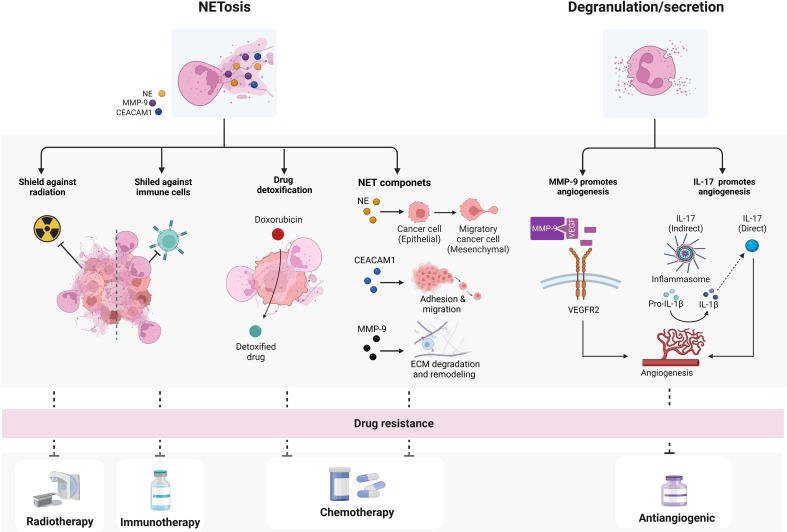
Attracted to the tumor site and regulated by the action of IL-8, EVs and other chemotactic factors, TANs can interfere with different antitumoral treatments not only by releasing EVs but also by secreting specific factors as well as by undergoing NETosis. During degranulation and NETosis, TANs can for example increase the secretion of matrix metalloproteinases (MMPs), such as MMP-2 and MMP-9, thus counteracting the effects of anti-angiogenic therapies. MMP-9, the production of which is also directly induced by IL-8 through CXCR2 receptor (144), can in fact cleave matrix-bound isoforms of VEGF-A into soluble fragments that are able to elicit VEGFR2 receptor activation and induce angiogenesis with a higher potential than uncleaved protein (145, 146) ( Figure 3 ). In addition, TANs can directly secrete the pro-angiogenic cytokine IL-17 (147) or induce the activation of cathepsin B/NLRP3 inflammasome followed by IL-1β overproduction, with consequent increase of IL-17 secretion (148, 149) ( Figure 3 ).
Figure 3.
Mechanisms by which TANs may confer drugs resistance. TANs can promote drug resistance through two main mechanisms: NETosis and degranulation/secretion. NETs or NETs components can mediate resistance to immune- radio- and chemotherapy, while neutrophil-secreted factors have been shown to mainly influence angiogenesis and interfere with angiogenic therapies. biorender.com.
Besides secreting factors in the TME, TANs can mediate drug resistance also through the formation of NETs or through the activities of several NET-associated components ( Figure 3 ). In agreement with this, increased levels of cell free cell free DNA (cfDNA), which is at least in part derived from NETs, predict limited response to chemo- and immune-therapy in several tumors (150–152). NET components, including NE, MMP-9, Cathepsin G (CG), the carcinoembryonic antigen cell adhesion molecule 1 (CEACAM1), and other factors, have been shown to promote resistance to chemotherapy through different mechanisms (55, 153–156). Preclinical studies suggest that NE can promote malignancy and resistance to chemo- and immune-therapy by inducing cell epithelial-mesenchymal transition (EMT) (153, 154, 157). Evidence emerged to support the infiltration of neutrophils into TME as a driver of EMT through NE activity (158–160). On the other hand, MMP-9 and CG, associated with NETs, mediate the degradation and remodeling of the extracellular matrix and, as discussed above, promote angiogenesis, so that their presence has been associated with tumor progression and poor response to chemotherapy (155, 161). Finally, CEACAM1 protein, that decorates NETs and facilitates NET-dependent pro-metastatic interactions by improving neoplastic cells adhesion and migration, is potentially involved also in mediating cancer response to therapy (156) ( Figure 3 ).
Increased NETosis promotes tumor resistance also to radiation-therapy (RT) (162). In a syngeneic bladder cancer model, RT increased NET deposition and, notably, when NETosis was inhibited by DNase I or neutrophil elastase inhibitor, the overall radiation response improved. Consistently with these data, NETs have been also observed in bladder tumors of patients who did not respond to RT and had persistent post-RT relapse (163, 164) ( Figure 3 ). In addition, tumor-associated NETs can also support metastatic cells to evade immune response by creating a physical shield from cytotoxic immune cells, such as cytotoxic CD8+ T and natural killer cells (NKs), thus preventing interactions between tumor and effector immune cells (165, 166) ( Figure 3 ). In line with this, NETs formation has also been shown to mediate the resistance to checkpoint blockade, thus reducing responses to immunotherapy (18, 167–169). NETs can also have a role in detoxifying tetracycline drugs, such as doxorubicin ( Figure 3 ), and degradation of NETs through DNase treatment restored chemosensitivity in animal models, demonstrating a functional role for NETs in chemo-resistance (166). Although this finding has yet to be corroborated in other tumors, this emerging evidence is notable since it raises NETs as therapeutic targets for the improvement of chemotherapy response.
Pharmacological NETosis inhibition has been shown to synergize with immunotherapies, such as anti-PD-1 and anti-CTLA-4 mAbs (18, 170), possibly by favoring cytotoxic effector T cell response against cancer cells following checkpoint inhibition. As further confirmation of the role of NETosis in immunotherapy resistance, it has been demonstrated that hPMNs recruited by IL-17 in pancreatic ductal adenocarcinoma undergo NETosis, and when NETosis is abrogated, the tumor acquires an immunotherapy-sensitive phenotype (171).
In conclusion, TANs and related regulatory factors and mediators (i.e., IL-8, EVs, and other secreted factors) represent potential targets for novel therapeutic approaches aiming to target cancer cells and reduce drug resistance.
5. Therapeutic strategies to inhibit neutrophils in cancer progression and cancer drug resistance
5.1. Investigational drugs
5.1.1. Targeting CXCR1/2 and neutrophils
With the aim to overcome the deleterious effects of neutrophils in cancer, the IL-8 and CXCR1/CXCR2 inhibition could reduce neutrophils migration to the tumor, thus avoiding NETs formation and eventually preventing drug resistance. In this section, we briefly report an overview of investigational drugs targeting IL-8 and its receptors CXCR1/CXCR2, and discuss their therapeutic potential in the field of cancer resistance ( Table 1 ).
Table 1.
Summary of the main CXCL8-CXCR1/2 inhibitors for cancer therapy.
| Drug | Therapeutic combination |
Indication | Trial phase/Study type | Recruitment status | NCT number |
| Nivolumab (anti PD-1) | Advanced solid tumor | Phase 1 | Completed | NCT02536469 | |
| Cabiralizumab (anti CSF1R) | Head and neck squamous cell carcinoma | Phase 2 | Recruiting | NCT04848116 | |
| Humax IL8 | Nivolumab (anti PD-1) | Prostate cancer | Phase 1 | Recruiting | NCT03689699 |
| Nivolumab (anti PD-1) | Adenocarcinoma of the prostate | Phase 2 | Recruiting | NCT03689699 | |
| Nivolumab (anti PD-1) | Pancreatic cancer | Phase 2 | Recruiting | NCT02451982 | |
| Navarixin | Pembrolizumab (anti PD-1) | Metastatic solid tumor | Phase 2 | Completed | NCT03473925 |
| Durvalumab (anti PD-L1) | Metastatic pancreatic ductal carcinoma | Phase 1/2 | Completed | NCT02583477 | |
| AZD5069 | Durvalumab (anti PD-L1) | Advanced solid tumor and squamous cell carcinoma of head and neck | Phase 1/2 | Active, not recruiting | NCT02499328 |
| Monotherapy | Myelodysplastic syndrome | Phase1 | Recruiting | NCT04245397 | |
| SX-682 | Pembrolizumab (anti PD-L1) | Metastatic melanoma | Phase 1 | Recruiting | NCT03161431 |
| Nivolumab (anti PD-1) | Metastatic colorectal cancer | Phase 1/2 | Recruiting | NCT04599140 | |
| Nivolumab (anti PD-1) | Metastatic pancreatic ductal adenocarcinoma | Phase 1 | Recruiting | NCT04477343 | |
| Reparixin | Monotherapy | Fatigue | Phase 2 | Not yet recruiting | NCT05212701 |
| Locally advance or metastatic breast cancer | |||||
| Paclitaxel (antineoplastic agent) | Metastatic breast cancer | Phase 1 | Completed | NCT02001974 | |
| Paclitaxel (antineoplastic agent) | Metastatic breast cancer | Phase 2 | Completed | NCT02370238 | |
| Monotherapy | Breast cancer | Phase 2 | Terminated | NCT01861054 | |
| RP-72 | Monotherapy or combination with gemcitabine | Pancreatic cancer | Phase 1 | Recruiting | NCT04338763 |
HuMax-IL8, also known as BMS-986253, is a fully human monoclonal antibody inhibitor of the IL-8 pathway. Humax-IL-8 was shown to block tumor progression (172), immune escape, EMT and MDSCs recruitment (173) in humans, thus pushing further new investigations in cancer resistance (172). HuMax-IL8 was developed for the treatment of patients with advanced solid tumors in combination with nivolumab, an anti-PD-1 monoclonal antibody immune check point inhibitor (NCT02536469), and it is currently under clinical evaluation for the treatment in several other tumors, including advanced solid tumors (NCT03400332), non-small cell lung cancer (NSCLC) (NCT04123379), advanced melanoma and metastatic renal cell carcinoma (NCT04050462), pancreatic cancer (NCT02451982), and head and neck squamous cell carcinoma (NCT04848116). In addition, HuMax IL8 is currently in phase 1b/2 trial in combination with nivolumab for treatment of men with hormone-sensitive prostate cancer (NCT03689699).
Navarixin is a CXCR1/CXCR2 receptor antagonist that impairs neutrophils recruitment (174), and that was shown to repress tumor cells metastasis and angiogenesis in preclinical models (175, 176). The molecule was shown to suppress CXCR2 signaling by decreasing MAPK/AKT pathway phosphorylation, resulting in sensitization of colorectal cancer cells to oxaliplatin treatment (177). Navarixin was assessed for its efficacy and safety in combination with pembrolizumab, an anti-PD-1 monoclonal antibody, in a phase 2 clinical trial of three types of solid tumors: programmed death-ligand 1 (PD-L1) positive refractory non-small cell lung cancer (NSCLC), castration resistant prostate cancer (CRPC) or microsatellite stable (MSS) colorectal cancer (CRC) (NCT03473925).
AZD5069 is a reversible CXCR2 antagonist that was shown to inhibit IL-8 or GRO-α-induced cytosolic calcium increase, CD11b surface expression, adhesion and chemotaxis in neutrophils (178, 179). The molecule was developed as part of combination therapies with durvalumab, an anti PD-L1 monoclonal antibody, in cancer indications including metastatic squamous cell carcinoma of the head and neck (SCCHN) (NCT02499328), and pancreatic ductal adenocarcinoma (NCT02583477).
SX-682 is a CXCR1/CXCR2 antagonist with potential anticancer activities. It exhibited significant activity in solid tumor models, where it reversed chemoresistance and extended overall survival. In syngeneic and genetically engineered mouse (GEM) melanoma models, it potently synergized with anti-PD1 therapy inducing complete remissions (180). In addition, it enhanced both PD-1 immune check point blockade, reduced MDSCs in the TME, and increased natural killer (NK) and T cells infiltration into the tumor site in animal models of head and neck tumor (181). The molecule is currently under active development as monotherapy or in combination with anti PD-1 molecules for the treatment of myelodysplastic syndrome (MDS) (NCT04245397), melanoma (NCT03161431), metastatic colon adenocarcinoma or colorectal carcinoma (NCT04599140) and metastatic pancreatic adenocarcinoma (NCT04477343).
Reparixin is an antagonist of IL-8 that binds CXCR1 and CXCR2 receptors to prevent neutrophil chemotaxis, thus avoiding graft tissue damage in organ transplantation and cancer, including breast cancer (182, 183). The combination of reparixin with antineoplastic agent docetaxel reduced the tumor size in a model of human breast cancer cell lines and breast cancer patient-derived xenografts (184) demonstrating that reparixin is able to reduce in vivo the tumor-initiating ability of breast cancer cells by affecting the CSC population; in fact, in tumor-bearing mice treated with reparixin alone or in combination with chemotherapy, the CSCs proportion was far lower than in tumor from mice receiving chemotherapy alone. Additional preclinical evidence highlighted the antitumor and antistemness activity of reparixin in epithelial thyroid cancer (185) and pancreatic cancer (186). Several clinical trials were conducted to assess the efficacy of reparixin in combination with taxanes or in monotherapy in metastatic breast cancer (NCT02001974, NCT02371238, NCT0161054). A new phase 2 clinical trial (NCT05212701) has started to evaluate the efficacy of reparixin in the treatment of oncological fatigue in locally or advanced metastatic breast cancer, a highly disabling condition, very common in cancer patients.
Danirixin is a CXCR2 antagonist originally developed for the potential oral treatment of chronic pulmonary disease (COPD). The molecule is able to strongly reduce the CD11b upregulation mediated by IL-8 or GRO-α agonists in healthy donor neutrophils, thus making the molecule a potential therapeutic agent for diseases characterized by neutrophil hyperactivation (187). In addition, Danirixin was found to block migration, invasion and EMT events mediated by TAMs and IL-8 in a preclinical in vitro model of breast cancer (188).
RP-72 is a 72 amino-acid recombinant protein that blocks the activation of IL-8-mediated signaling transduction pathways by decreasing proliferation of susceptible pancreatic cancer cells. The protein is under a Phase 1 clinical trial development for the potential intravenous treatment of metastatic pancreatic cancer in monotherapy or in combination with antiangiogenic gemcitabine (NCT04338763).
5.1.2. Targeting EVs
Targeting EVs in cancer progression could also represent a good strategy to counteract tumor drug resistance ( Table 2 ). In this context, promising results were obtained in an in vitro model of ovarian cancer, in which the treatment with heparin, amiloride and dynasore inhibited EV release after treatment with cisplatin (189) known as mechanism responsible for cancer resistance to the therapy. Similar results were obtained in another model of ovarian cancer, in which the phospholipase inhibitor GW4869 was shown to inhibit the exosomal DNA methyltransferase 1 (DNMT1)-mediated cisplatin resistance in cells, and to increase apoptosis (190). These findings suggest that the combination of cisplatin with EV inhibitors can potentially overcome the drug resistance. In a melanoma model, the same GW4869 inhibited exosome secretion that caused the induction of tumor cell proliferation and apoptosis (191). A similar effect was observed in a model of prostate cancer where treatment with GW4869 effectively reduced cancer cell viability associated to exosome secretion (192). In aggressive B-cell lymphomas, suppression of exosomal drug resistance with indometacin increased efficacy of doxorubicin therapy (193). Finally, in a tumor mice model the treatment with dimethyl amiloride (DMA), known to reduce exosome release into the bloodstream, given in combination with the chemotherapeutic drug cyclophosphamide, halted the tumor growth by 50% or more, if compared to the untreated controls (192).
Table 2.
Summary of the main anti-EV agents in cancer and cancer drug resistance in preclinical models.
| Drug | Antitumor therapy | Mechanism of targeted or cancer therapy resistance | In vitro model | Reference |
|---|---|---|---|---|
| Heparin | cisplatin | EV uptake inhibitor | Ovarian cancer | Samuel P et al., 2018 (189) |
| Amiloride | cisplatin | EV uptake inhibitor | Ovarian cancer | Samuel P et al., 2018 (189) |
| Dynasore | cisplatin | EV uptake inhibitor | Ovarian cancer | Samuel P et al., 2018 (189) |
| GW4869 | cisplatin | EV inhibitor | Ovarian cancer | Cao Y et al., 2017 (190) |
| EV inhibitor | Melanoma | Matsumoto A et al., 2017 (191) | ||
| EV inhibitor | Prostate cancer | Panigrahi GK et al., 2018 (192) | ||
| Indomethacin | doxorubicin/pixantrone | EV inhibitor | Lymphoma | Koch R et al., 2016 (193) |
Thus, new interest is arising for the development of EV/exosome pathway inhibitors. The combined use of IL-8 biological activity inhibitors that modulate the hyperactivation of neutrophils could represent a new strategy to mitigate cancer drug resistance induced by EVs release. A first example of such approach is represented by the combined blockade of IL-8 and IL-6 in osteosarcoma. Starting from data showing that osteosarcoma tumor-secreted EVs can induce a pro-metastatic phenotype by strongly inducing IL-6 production in mesenchymal stem cells (MSCs), it has been demonstrated that EVs from aggressive cancer cell lines can induce MSCs to express inflammatory cytokines and chemokines, among which IL-8 was the most upregulated one, and that this was due to tumor EV-associated non-coding RNAs. The blockade of IL-8 signaling with ladarixin (an allosteric inhibitor of CXCR1 and CXCR2) and, even more strikingly, its combination with tocilizumab (an anti-IL-6 receptor antibody) reduced lung metastasis formation in a xenograft mouse model of osteosarcoma and, notably, prevented the occurrence of MSC-induced tumor resistance to antimetastatic drugs (abstract submitted to the ASCO 2022 meeting).
6. Conclusions
TANs play a key role in tumor drug resistance, and their activities in this context are regulated and mediated by different factors. Among these, EVs and IL-8, produced either by tumoral cells or by neutrophils themselves, crucially function to both control and mediate the pro-tumoral functions of neutrophils in the TME. The role of both EVs and IL-8 is crucial for neutrophil-mediated tumor drug resistance, which is mainly due to the induction of NETs formation and the secretion of pro-tumoral factors, including neutrophil-derived EVs. Growing evidence has highlighted the close association between high levels of IL-8, EVs production, NETosis, and limited therapeutic response in a variety of malignancies, thus paving the way to investigations on the therapeutic potential of combination treatments either of IL-8 activity blockers, or anti-EVs drugs, or NETosis inhibitors with standard antitumoral therapies, to reduce or counteract tumor drug resistance (162, 193).
In conclusion, IL-8 and EVs represent key potential targets for the development of novel therapeutic options aimed to target neutrophil-mediated tumor drug resistance.
Author contributions
MZ, AR, FR, and MSM performed data collection (literature reviewing) and prepared the original draft of the manuscript. RN revised and wrote the final version of the manuscript. MA and MCC revised the manuscript for critically important intellectual content. PGA conceptualized the study and revised and wrote the final version of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors are employees of Dompé farmaceutici S.p.A.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Hiam-Galvez KJ, Allen BM, Spitzer MH. Systemic immunity in cancer. Nat Rev Cancer. (2021) 21(6):345–59. doi: 10.1038/s41568-021-00347-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stower H. Understanding cancer genetics through a global collaboration. Nat Med (2020) 26(3):312. doi: 10.1038/s41591-020-0809-4 [DOI] [PubMed] [Google Scholar]
- 3. Vasan N, Baselga J, Hyman DM. A view on drug resistance in cancer. Nature (2019) 575(7782):299–309. doi: 10.1038/s41586-019-1730-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheng P, Levesque MP, Dummer R, Mangana J. Targeting complex, adaptive responses in melanoma therapy. Cancer Treat Rev (2020) 86:101997. doi: 10.1016/j.ctrv.2020.101997 [DOI] [PubMed] [Google Scholar]
- 5. Jiang W, Xia J, Xie S, Zou R, Pan S, Wang Z-W, et al. Long non-coding RNAs as a determinant of cancer drug resistance: Towards the overcoming of chemoresistance via modulation of lncRNAs. Drug Resistance Updates (2020) 50:100683. doi: 10.1016/j.drup.2020.100683 [DOI] [PubMed] [Google Scholar]
- 6. Long L, Assaraf YG, Lei ZN, Peng H, Yang L, Chen ZS, et al. Genetic biomarkers of drug resistance: A compass of prognosis and targeted therapy in acute myeloid leukemia. Drug Resist Updat. (2020) 52:100703. doi: 10.1016/j.drup.2020.100703 [DOI] [PubMed] [Google Scholar]
- 7. Wang Y, Fang Z, Hong M, Yang D, Xie W. Long-noncoding RNAs (lncRNAs) in drug metabolism and disposition, implications in cancer chemo-resistance. Acta Pharm Sin B (2020) 10(1):105–12. doi: 10.1016/j.apsb.2019.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu P, Gao W, Su M, Nice EC, Zhang W, Lin J, et al. Adaptive mechanisms of tumor therapy resistance driven by tumor microenvironment. Front Cell Dev Biol (2021) 9:641469. doi: 10.3389/fcell.2021.641469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hanahan D. Rethinking the war on cancer. Lancet (2014) 383(9916):558–63. doi: 10.1016/S0140-6736(13)62226-6 [DOI] [PubMed] [Google Scholar]
- 10. Alfaro C, Teijeira A, Onate C, Perez G, Sanmamed MF, Andueza MP, et al. Tumor-produced interleukin-8 attracts human myeloid-derived suppressor cells and elicits extrusion of neutrophil extracellular traps (NETs). Clin Cancer Res (2016) 22(15):3924–36. doi: 10.1158/1078-0432.CCR-15-2463 [DOI] [PubMed] [Google Scholar]
- 11. Gonzalez-Aparicio M, Alfaro C. Influence of interleukin-8 and neutrophil extracellular trap (NET) formation in the tumor microenvironment: Is there a pathogenic role? J Immunol Res (2019) 2019:6252138. doi: 10.1155/2019/6252138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moschetta M, Uccello M, Kasenda B, Mak G, McClelland A, Boussios S, et al. Dynamics of neutrophils-to-lymphocyte ratio predict outcomes of PD-1/PD-L1 blockade. BioMed Res Int (2017) 2017. doi: 10.1155/2017/1506824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nakaya A, Kurata T, Yoshioka H, Takeyasu Y, Niki M, Kibata K, et al. Neutrophil-to-lymphocyte ratio as an early marker of outcomes in patients with advanced non-small-cell lung cancer treated with nivolumab. Int J Clin Oncol (2018) 23(4):634–40. doi: 10.1007/s10147-018-1250-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Romano A, Parrinello NL, Vetro C, Chiarenza A, Cerchione C, Ippolito M, et al. Prognostic meaning of neutrophil to lymphocyte ratio (NLR) and lymphocyte to monocyte ration (LMR) in newly diagnosed Hodgkin lymphoma patients treated upfront with a PET-2 based strategy. Ann hematol (2018) 97(6):1009–18. doi: 10.1007/s00277-018-3276-y [DOI] [PubMed] [Google Scholar]
- 15. Ferrucci PF, Ascierto PA, Pigozzo J, Del Vecchio M, Maio M, Antonini Cappellini GC, et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol (2018) 29(2):524. doi: 10.1093/annonc/mdx059 [DOI] [PubMed] [Google Scholar]
- 16. McCluney SJ, Giakoustidis A, Segler A, Bissel J, Valente R, Hutchins RR, et al. Neutrophil: Lymphocyte ratio as a method of predicting complications following hepatic resection for colorectal liver metastasis. J Surg Oncol (2018) 117(5):1058–65. doi: 10.1002/jso.24996 [DOI] [PubMed] [Google Scholar]
- 17. Masucci MT, Minopoli M, Carriero MV. Tumor associated neutrophils. their role in tumorigenesis, metastasis, prognosis and therapy. Front Oncol (2019) 9:1146. doi: 10.3389/fonc.2019.01146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Teijeira A, Garasa S, Ochoa MC, Villalba M, Olivera I, Cirella A, et al. IL8, neutrophils, and NETs in a collusion against cancer immunity and immunotherapy. Clin Cancer Res (2021) 27(9):2383–93. doi: 10.1158/1078-0432.CCR-20-1319 [DOI] [PubMed] [Google Scholar]
- 19. Yuan A, Chen JJ, Yao PL, Yang PC. The role of interleukin-8 in cancer cells and microenvironment interaction. Front Biosci (2005) 10:853–65. doi: 10.2741/1579 [DOI] [PubMed] [Google Scholar]
- 20. Rubenich DS, Omizzollo N, Szczepanski MJ, Reichert TE, Whiteside TL, Ludwig N, et al. Small extracellular vesicle-mediated bidirectional crosstalk between neutrophils and tumor cells. Cytokine Growth Factor Rev (2021) 61:16–26. doi: 10.1016/j.cytogfr.2021.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rosales C. Neutrophil: A cell with many roles in inflammation or several cell types? Front Physiol (2018) 9:113. doi: 10.3389/fphys.2018.00113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cossio I, Lucas D, Hidalgo A. Neutrophils as regulators of the hematopoietic niche. Blood (2019) 133(20):2140–8. doi: 10.1182/blood-2018-10-844571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wculek SK, Malanchi I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature (2015) 528(7582):413–7. doi: 10.1038/nature16140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee W, Naora H. Neutrophils fertilize the pre-metastatic niche. Aging (Albany NY). (2019) 11(17):6624–5. doi: 10.18632/aging.102258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang S, Chen L, Zong Y, Li Q, Zhu K, Li Z, et al. Research progress of tumor-derived extracellular vesicles in the treatment of malignant pleural effusion. Cancer Med. doi: 10.1002/cam4.5005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martin C, Burdon PC, Bridger G, Gutierrez-Ramos JC, Williams TJ, Rankin SM. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity (2003) 19(4):583–93. doi: 10.1016/S1074-7613(03)00263-2 [DOI] [PubMed] [Google Scholar]
- 27. Sody S, Uddin M, Grüneboom A, Görgens A, Giebel B, Gunzer M, et al. Distinct spatio-temporal dynamics of tumor-associated neutrophils in small tumor lesions. Front Immunol (2019) 10:1419. doi: 10.3389/fimmu.2019.01419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sharma B, Nawandar DM, Nannuru KC, Varney ML, Singh RK. Targeting CXCR2 enhances chemotherapeutic response, inhibits mammary tumor growth, angiogenesis, and lung metastasis. Mol Cancer Ther (2013) 12(5):799–808. doi: 10.1158/1535-7163.MCT-12-0529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Girbl T, Lenn T, Perez L, Rolas L, Barkaway A, Thiriot A, et al. Distinct compartmentalization of the chemokines CXCL1 and CXCL2 and the atypical receptor ACKR1 determine discrete stages of neutrophil diapedesis. Immunity (2018) 49(6):1062–76.e6. doi: 10.1016/j.immuni.2018.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shi Q, Abbruzzese JL, Huang S, Fidler IJ, Xiong Q, Xie K. Constitutive and inducible interleukin 8 expression by hypoxia and acidosis renders human pancreatic cancer cells more tumorigenic and metastatic. Clin Cancer Res (1999) 5(11):3711–21. doi: 10.1089/107999099312821 [DOI] [PubMed] [Google Scholar]
- 31. Shi Q, Le X, Wang B, Xiong Q, Abbruzzese JL, Xie K. Regulation of interleukin-8 expression by cellular pH in human pancreatic adenocarcinoma cells. J Interferon Cytokine Res (2000) 20(11):1023–8. doi: 10.1089/10799900050198471 [DOI] [PubMed] [Google Scholar]
- 32. Le X, Shi Q, Wang B, Xiong Q, Qian C, Peng Z, et al. Molecular regulation of constitutive expression of interleukin-8 in human pancreatic adenocarcinoma. J Interferon Cytokine Res (2000) 20(11):935–46. doi: 10.1089/10799900050198372 [DOI] [PubMed] [Google Scholar]
- 33. Shi Q, Le X, Abbruzzese JL, Wang B, Mujaida N, Matsushima K, et al. Cooperation between transcription factor AP-1 and NF-kappaB in the induction of interleukin-8 in human pancreatic adenocarcinoma cells by hypoxia. J Interferon Cytokine Res (1999) 19(12):1363–71. doi: 10.1089/107999099312821 [DOI] [PubMed] [Google Scholar]
- 34. Schönbohn H, Schuler M, Kolbe K, Peschel C, Huber C, Bemb W, et al. Plasma levels of IL-1, TNF alpha, IL-6, IL-8, G-CSF, and IL1-RA during febrile neutropenia: results of a prospective study in patients undergoing chemotherapy for acute myelogenous leukemia. Ann hematol (1995) 71(4):161–8. doi: 10.1007/BF01910312 [DOI] [PubMed] [Google Scholar]
- 35. di Celle PF, Carbone A, Marchis D, Zhou D, Sozzani S, Zupo S, et al. Cytokine gene expression in b-cell chronic lymphocytic leukemia: evidence of constitutive interleukin-8 (IL-8) mRNA expression and secretion of biologically active IL-8 protein. Blood (1994) 84(1):220–8. doi: 10.1182/blood.V84.1.220.220 [DOI] [PubMed] [Google Scholar]
- 36. Morita M, Kasahara T, Mukaida N, Matsushima K, Nagashima T, Nishizawa M, et al. Induction and regulation of IL-8 and MCAF production in human brain tumor cell lines and brain tumor tissues. Eur Cytokine Netw (1993) 4(5):351–8. [PubMed] [Google Scholar]
- 37. Green AR, Green VL, White MC, Speirs V. Expression of cytokine messenger RNA in normal and neoplastic human breast tissue: identification of interleukin-8 as a potential regulatory factor in breast tumours. Int J Cancer. (1997) 72(6):937–41. doi: [DOI] [PubMed] [Google Scholar]
- 38. Luciani MG, Stoppacciaro A, Peri G, Mantovani A, Ruco LP. The monocyte chemotactic protein a (MCP-1) and interleukin 8 (IL-8) in hodgkin's disease and in solid tumours. Mol Pathol (1998) 51(5):273–6. doi: 10.1136/mp.51.5.273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tjiong MY, van der Vange N, ten Kate FJ, Tjong AHSP, ter Schegget J, Burger MP, et al. Increased IL-6 and IL-8 levels in cervicovaginal secretions of patients with cervical cancer. Gynecolo Oncol (1999) 73(2):285–91. doi: 10.1006/gyno.1999.5358 [DOI] [PubMed] [Google Scholar]
- 40. Kitadai Y, Takahashi Y, Haruma K, Naka K, Sumii K, Yokozaki H, et al. Transfection of interleukin-8 increases angiogenesis and tumorigenesis of human gastric carcinoma cells in nude mice. Br J Cancer (1999) 81(4):647–53. doi: 10.1038/sj.bjc.6690742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gruss HJ, Brach MA, Drexler HG, Bonifer R, Mertelsmann RH, Herrmann F. Expression of cytokine genes, cytokine receptor genes, and transcription factors in cultured Hodgkin and reed-sternberg cells. Cancer Res (1992) 52(12):3353–60. [PubMed] [Google Scholar]
- 42. Smith DR, Polverini PJ, Kunkel SL, Orringer MB, Whyte RI, Burdick MD, et al. Inhibition of interleukin 8 attenuates angiogenesis in bronchogenic carcinoma. J Exp Med (1994) 179(5):1409–15. doi: 10.1084/jem.179.5.1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Scheibenbogen C, Möhler T, Haefele J, Hunstein W, Keilholz U. Serum interleukin-8 (IL-8) is elevated in patients with metastatic melanoma and correlates with tumour load. Melanoma Res (1995) 5(3):179–81. doi: 10.1097/00008390-199506000-00006 [DOI] [PubMed] [Google Scholar]
- 44. Fridlender ZG, Albelda SM. Tumor-associated neutrophils: Friend or foe? Carcinogenesis (2012) 33(5):949–55. doi: 10.1093/carcin/bgs123 [DOI] [PubMed] [Google Scholar]
- 45. Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN. Cancer Cell (2009) 16(3):183–94. doi: 10.1016/j.ccr.2009.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci U.S.A. (2006) 103(33):12493–8. doi: 10.1073/pnas.0601807103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. (2016) 16(7):431–46. doi: 10.1038/nrc.2016.52 [DOI] [PubMed] [Google Scholar]
- 48. Raftopoulou S, Valadez-Cosmes P, Mihalic ZN, Schicho R, Kargl J. Tumor-mediated neutrophil polarization and therapeutic implications. Int J Mol Sci (2022) 23(6). doi: 10.3390/ijms23063218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hou PP, Chen HZ. Extracellular vesicles in the tumor immune microenvironment. Cancer Lett (2021) 516:48–56. doi: 10.1016/j.canlet.2021.05.032 [DOI] [PubMed] [Google Scholar]
- 50. Park J, Wysocki RW, Amoozgar Z, Maiorino L, Fein MR, Jorns J, et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci Transl Med (2016) 8(361):361ra138. doi: 10.1126/scitranslmed.aag1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Oklu R, Sheth RA, Wong KHK, Jahromi AH, Albadawi H. Neutrophil extracellular traps are increased in cancer patients but does not associate with venous thrombosis. Cardiovasc diagnosis Ther (2017) 7(Suppl 3):S140–s9. doi: 10.21037/cdt.2017.08.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li Y, Yang Y, Gan T, Zhou J, Hu F, Hao N, et al. Extracellular RNAs from lung cancer cells activate epithelial cells and induce neutrophil extracellular traps. Int J Oncol (2019) 55(1):69–80. doi: 10.3892/ijo.2019.4808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science (2004) 303(5663):1532–5. doi: 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]
- 54. Masucci MT, Minopoli M, Del Vecchio S, Carriero MV. The emerging role of neutrophil extracellular traps (NETs) in tumor progression and metastasis. Front Immunol (2020) 11:1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest (2013) 123(8):3446–58. doi: 10.1172/JCI67484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Najmeh S, Cools-Lartigue J, Rayes RF, Gowing S, Vourtzoumis P, Bourdeau F, et al. Neutrophil extracellular traps sequester circulating tumor cells via beta1-integrin mediated interactions. Int J Cancer (2017) 140(10):2321–30. doi: 10.1002/ijc.30635 [DOI] [PubMed] [Google Scholar]
- 57. Xiao Y, Cong M, Li J, He D, Wu Q, Tian P, et al. Cathepsin c promotes breast cancer lung metastasis by modulating neutrophil infiltration and neutrophil extracellular trap formation. Cancer Cell (2021) 39(3):423–37.e7. doi: 10.1016/j.ccell.2020.12.012 [DOI] [PubMed] [Google Scholar]
- 58. Catena R, Bhattacharya N, El Rayes T, Wang S, Choi H, Gao D, et al. Bone marrow-derived Gr1+ cells can generate a metastasis-resistant microenvironment via induced secretion of thrombospondin-1. Cancer Discovery (2013) 3(5):578–89. doi: 10.1158/2159-8290.CD-12-0476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Galdiero MR, Varricchi G, Loffredo S, Mantovani A, Marone G. Roles of neutrophils in cancer growth and progression. J Leukoc Biol (2018) 103(3):457–64. doi: 10.1002/JLB.3MR0717-292R [DOI] [PubMed] [Google Scholar]
- 60. Shaul ME, Fridlender ZG. Tumour-associated neutrophils in patients with cancer. Nat Rev Clin Oncol (2019) 16(10):601–20. doi: 10.1038/s41571-019-0222-4 [DOI] [PubMed] [Google Scholar]
- 61. Scheel C, Eaton EN, Li SH, Chaffer CL, Reinhardt F, Kah KJ, et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell (2011) 145(6):926–40. doi: 10.1016/j.cell.2011.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Waight JD, Hu Q, Miller A, Liu S, Abrams SI. Tumor-derived G-CSF facilitates neoplastic growth through a granulocytic myeloid-derived suppressor cell-dependent mechanism. PloS One (2011) 6(11):e27690. doi: 10.1371/journal.pone.0027690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS, et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature (2015) 522(7556):345–8. doi: 10.1038/nature14282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Casbon AJ, Reynaud D, Park C, Khuc E, Gan DD, Schepers K, et al. Invasive breast cancer reprograms early myeloid differentiation in the bone marrow to generate immunosuppressive neutrophils. Proc Natl Acad Sci U.S.A. (2015) 112(6):E566–75. doi: 10.1073/pnas.1424927112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Xie K. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev (2001) 12(4):375–91. doi: 10.1016/S1359-6101(01)00016-8 [DOI] [PubMed] [Google Scholar]
- 66. Brat DJ, Bellail AC, Van Meir EG. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro-oncology (2005) 7(2):122–33. doi: 10.1215/S1152851704001061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fousek K, Horn LA, Palena C. Interleukin-8: A chemokine at the intersection of cancer plasticity, angiogenesis, and immune suppression. Pharmacol Ther (2021) 219:107692. doi: 10.1016/j.pharmthera.2020.107692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ginestier C, Charafe-Jauffret E, Birnbaum D. Targeting breast cancer stem cells: fishing season open! Breast Cancer Res (2010) 12(5):312. doi: 10.1186/bcr2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Xiong X, Liao X, Qiu S, Xu H, Zhang S, Wang S, et al. CXCL8 in tumor biology and its implications for clinical translation. Front Mol Biosci (2022) 9:723846. doi: 10.3389/fmolb.2022.723846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Merogi AJ, Marrogi AJ, Ramesh R, Robinson WR, Fermin CD, Freeman SM. Tumor-host interaction: analysis of cytokines, growth factors, and tumor-infiltrating lymphocytes in ovarian carcinomas. Hum Pathol (1997) 28(3):321–31. doi: 10.1016/S0046-8177(97)90131-3 [DOI] [PubMed] [Google Scholar]
- 71. Nürnberg W, Tobias D, Otto F, Henz BM, Schadendorf D. Expression of interleukin-8 detected by in situ hybridization correlates with worse prognosis in primary cutaneous melanoma. J Pathol (1999) 189(4):546–51. doi: [DOI] [PubMed] [Google Scholar]
- 72. Veltri RW, Miller MC, Zhao G, Ng A, Marley GM, Wright GL, Jr., et al. Interleukin-8 serum levels in patients with benign prostatic hyperplasia and prostate cancer. Urology (1999) 53(1):139–47. doi: 10.1016/S0090-4295(98)00455-5 [DOI] [PubMed] [Google Scholar]
- 73. Ferrer FA, Miller LJ, Andrawis RI, Kurtzman SH, Albertsen PC, Laudone VP, et al. Angiogenesis and prostate cancer: In vivo and in vitro expression of angiogenesis factors by prostate cancer cells. Urology (1998) 51(1):161–7. doi: 10.1016/S0090-4295(97)00491-3 [DOI] [PubMed] [Google Scholar]
- 74. Nasser MW, Raghuwanshi SK, Grant DJ, Jala VR, Rajarathnam K, Richardson RM. Differential activation and regulation of CXCR1 and CXCR2 by CXCL8 monomer and dimer. J Immunol (2009) 183(5):3425–32. doi: 10.4049/jimmunol.0900305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Matsushima K, Baldwin ET, Mukaida N. Interleukin-8 and MCAF: novel leukocyte recruitment and activating cytokines. Chem Immunol (1992) 51:236–65. doi: 10.1159/000420761 [DOI] [PubMed] [Google Scholar]
- 76. Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest (1989) 84(4):1045–9. doi: 10.1172/JCI114265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hassan B, Akcakanat A, Holder AM, Meric-Bernstam F. Targeting the PI3-kinase/Akt/mTOR signaling pathway. Surg Oncol Clinics North America (2013) 22(4):641–64. doi: 10.1016/j.soc.2013.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ding M, van der Kwast TH, Vellanki RN, Foltz WD, McKee TD, Sonenberg N, et al. The mTOR targets 4E-BP1/2 restrain tumor growth and promote hypoxia tolerance in PTEN-driven prostate cancer. Mol Cancer Res (2018) 16(4):682–95. doi: 10.1158/1541-7786.MCR-17-0696 [DOI] [PubMed] [Google Scholar]
- 79. Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene (2007) 26(22):3279–90. doi: 10.1038/sj.onc.1210421 [DOI] [PubMed] [Google Scholar]
- 80. Zha C, Meng X, Li L, Mi S, Qian D, Li Z, et al. Neutrophil extracellular traps mediate the crosstalk between glioma progression and the tumor microenvironment via the HMGB1/RAGE/IL-8 axis. Cancer Biol Med (2020) 17(1):154–68. doi: 10.20892/j.issn.2095-3941.2019.0353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. SenGupta S, Hein LE, Parent CA. The recruitment of neutrophils to the tumor microenvironment is regulated by multiple mediators. Front Immunol (2021) 12:734188. doi: 10.3389/fimmu.2021.734188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. Extracellular vesicles in cancer: Cell-to-Cell mediators of metastasis. Cancer Cell (2016) 30(6):836–48. doi: 10.1016/j.ccell.2016.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Dang VD, Jella KK, Ragheb RRT, Denslow ND, Alli AA. Lipidomic and proteomic analysis of exosomes from mouse cortical collecting duct cells. FASEB J (2017) 31(12):5399–408. doi: 10.1096/fj.201700417R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells (2019) 8(7). doi: 10.3390/cells8070727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ridder K, Keller S, Dams M, Rupp AK, Schlaudraff J, Del Turco D, et al. Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PloS Biol (2014) 12(6):e1001874. doi: 10.1371/journal.pbio.1001874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Nederveen JP, Warnier G, Di Carlo A, Nilsson MI, Tarnopolsky MA. Extracellular vesicles and exosomes: Insights from exercise science. Front Physiol (2020) 11:604274. doi: 10.3389/fphys.2020.604274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zaborowski MP, Balaj L, Breakefield XO, Lai CP. Extracellular vesicles: Composition, biological relevance, and methods of study. Bioscience (2015) 65(8):783–97. doi: 10.1093/biosci/biv084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles (2015) 4:27066. doi: 10.3402/jev.v4.27066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J extracellular vesicles (2018) 7(1):1535750. doi: 10.1080/20013078.2018.1535750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol (2014) 30:255–89. doi: 10.1146/annurev-cellbio-101512-122326 [DOI] [PubMed] [Google Scholar]
- 91. van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol (2018) 19(4):213–28. doi: 10.1038/nrm.2017.125 [DOI] [PubMed] [Google Scholar]
- 92. Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles (2014) 3. doi: 10.3402/jev.v3.24641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Raposo G, Stoorvogel W. Extracellular vesicles: Exosomes, microvesicles, and friends. J Cell Biol (2013) 200(4):373–83. doi: 10.1083/jcb.201211138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhang DX, Vu LT, Ismail NN, Le MTN, Grimson A. Landscape of extracellular vesicles in the tumour microenvironment: Interactions with stromal cells and with non-cell components, and impacts on metabolic reprogramming, horizontal transfer of neoplastic traits, and the emergence of therapeutic resistance. Semin Cancer Biol (2021) 74:24–44. doi: 10.1016/j.semcancer.2021.01.007 [DOI] [PubMed] [Google Scholar]
- 95. Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringnér M, et al. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci U.S.A. (2013) 110(18):7312–7. doi: 10.1073/pnas.1220998110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Cui S, Cheng Z, Qin W, Jiang L. Exosomes as a liquid biopsy for lung cancer. Lung Cancer (Amsterdam Netherlands) (2018) 116:46–54. doi: 10.1016/j.lungcan.2017.12.012 [DOI] [PubMed] [Google Scholar]
- 97. Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol (2008) 10(12):1470–6. doi: 10.1038/ncb1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol (2015) 17(6):816–26. doi: 10.1038/ncb3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Peinado H, Alec kovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, et al. Corrigendum: Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med (2016) 22(12):1502. doi: 10.1038/nm1216-1502b [DOI] [PubMed] [Google Scholar]
- 100. Aleckovic M, Kang Y. Regulation of cancer metastasis by cell-free miRNAs. Biochim Biophys Acta (2015) 1855(1):24–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ortiz A, Gui J, Zahedi F, Yu P, Cho C, Bhattacharya S, et al. An interferon-driven oxysterol-based defense against tumor-derived extracellular vesicles. Cancer Cell (2019) 35(1):33–45.e6. doi: 10.1016/j.ccell.2018.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Han Q, Zhao H, Jiang Y, Yin C, Zhang J. HCC-derived exosomes: Critical player and target for cancer immune escape. Cells (2019) 8(6). doi: 10.3390/cells8060558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Guimaraes-Bastos D, Frony AC, Barja-Fidalgo C, Moraes JA. Melanoma-derived extracellular vesicles skew neutrophils into a pro-tumor phenotype. J Leukoc Biol (2022) 111(3):585–96. doi: 10.1002/JLB.3A0120-050RR [DOI] [PubMed] [Google Scholar]
- 104. Ohue Y, Nishikawa H. Regulatory T (Treg) cells in cancer: Can treg cells be a new therapeutic target? Cancer Sci (2019) 110(7):2080–9. doi: 10.1111/cas.14069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Whiteside TL. Human regulatory T cells (Treg) and their response to cancer. Expert Rev Precis Med Drug Dev (2019) 4(4):215–28. doi: 10.1080/23808993.2019.1634471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Mrizak D, Martin N, Barjon C, Jimenez-Pailhes AS, Mustapha R, Niki T, et al. Effect of nasopharyngeal carcinoma-derived exosomes on human regulatory T cells. J Natl Cancer Institute (2015) 107(1):363. doi: 10.1093/jnci/dju363 [DOI] [PubMed] [Google Scholar]
- 107. Snoderly HT, Boone BA, Bennewitz MF. Neutrophil extracellular traps in breast cancer and beyond: current perspectives on NET stimuli, thrombosis and metastasis, and clinical utility for diagnosis and treatment. Breast Cancer Res (2019) 21(1):145. doi: 10.1186/s13058-019-1237-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zhang X, Shi H, Yuan X, Jiang P, Qian H, Xu W. Tumor-derived exosomes induce N2 polarization of neutrophils to promote gastric cancer cell migration. Mol Cancer (2018) 17(1):146. doi: 10.1186/s12943-018-0898-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Shi Y, Zhang J, Mao Z, Jiang H, Liu W, Shi H, et al. Extracellular vesicles from gastric cancer cells induce PD-L1 expression on neutrophils to suppress T-cell immunity. Front Oncol (2020) 10:629. doi: 10.3389/fonc.2020.00629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Hwang WL, Lan HY, Cheng WC, Huang SC, Yang MH. Tumor stem-like cell-derived exosomal RNAs prime neutrophils for facilitating tumorigenesis of colon cancer. J Hematol Oncol (2019) 12(1):10. doi: 10.1186/s13045-019-0699-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Demers M, Wagner DD. NETosis: a new factor in tumor progression and cancer-associated thrombosis. Semin Thromb Hemost (2014) 40(3):277–83. doi: 10.1055/s-0034-1370765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Demers M, Krause DS, Schatzberg D, Martinod K, Voorhees JR, Fuchs TA, et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci United States America (2012) 109(32):13076–81. doi: 10.1073/pnas.1200419109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Abdol Razak N, Elaskalani O, Metharom P. Pancreatic cancer-induced neutrophil extracellular traps: A potential contributor to cancer-associated thrombosis. Int J Mol Sci (2017) 18(3). doi: 10.3390/ijms18030487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Shahzad MH, Feng L, Su X, Brassard A, Dhoparee-Doomah I, Ferri LE, et al. Neutrophil extracellular traps in cancer therapy resistance. Cancers (2022) 14(5):1359. doi: 10.3390/cancers14051359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Leal AC, Mizurini DM, Gomes T, Rochael NC, Saraiva EM, Dias MS, et al. Tumor-derived exosomes induce the formation of neutrophil extracellular traps: Implications for the establishment of cancer-associated thrombosis. Sci Rep (2017) 7(1):6438. doi: 10.1038/s41598-017-06893-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Amorim C, Docasar CL, Guimarães-Bastos D, Frony AC, Barja-Fidalgo C, Renovato-Martins M, et al. Extracellular vesicles derived from MDA-MB-231 cells trigger neutrophils to a pro-tumor profile. Cells (2022) 11(12). doi: 10.3390/cells11121875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Shang A, Gu C, Zhou C, Yang Y, Chen C, Zeng B, et al. Exosomal KRAS mutation promotes the formation of tumor-associated neutrophil extracellular traps and causes deterioration of colorectal cancer by inducing IL-8 expression. Cell commun Signaling (2020) 18(1):52. doi: 10.1186/s12964-020-0517-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ji Q, Zhou L, Sui H, Yang L, Wu X, Song Q, et al. Primary tumors release ITGBL1-rich extracellular vesicles to promote distal metastatic tumor growth through fibroblast-niche formation. Nat Commun (2020) 11(1):1211. doi: 10.1038/s41467-020-14869-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Choi SJ, Cho H, Yea K, Baek MC. Immune cell-derived small extracellular vesicles in cancer treatment. BMB Rep (2022) 55(1):48–56. doi: 10.5483/BMBRep.2022.55.1.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Federici C, Shahaj E, Cecchetti S, Camerini S, Casella M, Iessi E, et al. Natural-Killer-Derived extracellular vesicles: Immune sensors and interactors. Front Immunol (2020) 11. doi: 10.3389/fimmu.2020.00262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Fais S. NK cell-released exosomes: Natural nanobullets against tumors. Oncoimmunology (2013) 2(1):e22337. doi: 10.4161/onci.22337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Bui TM, Yalom LK, Sumagin R. Tumor-associated neutrophils: orchestrating cancer pathobiology and therapeutic resistance. Expert Opin Ther Targets (2021) 25(7):573–83. doi: 10.1080/14728222.2021.1954162 [DOI] [PubMed] [Google Scholar]
- 123. Kolonics F, Szeifert V, Timar CI, Ligeti E, Lorincz AM. The functional heterogeneity of neutrophil-derived extracellular vesicles reflects the status of the parent cell. Cells (2020) 9(12). doi: 10.3390/cells9122718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Liu T, Wang J, Li T, Cui P, Hou B, Zhuang C, et al. Predicting disease progression in advanced non-small cell lung cancer with circulating neutrophil-derived and platelet-derived microparticles. BMC Cancer (2021) 21(1):939. doi: 10.1186/s12885-021-08628-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Zhang J, Ji C, Zhang H, Shi H, Mao F, Qian H, et al. Engineered neutrophil-derived exosome-like vesicles for targeted cancer therapy. Sci Adv (2022) 8(2):eabj8207. doi: 10.1126/sciadv.abj8207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Butin-Israeli V, Bui TM, Wiesolek HL, Mascarenhas L, Lee JJ, Mehl LC, et al. Neutrophil-induced genomic instability impedes resolution of inflammation and wound healing. J Clin Invest (2019) 129(2):712–26. doi: 10.1172/JCI122085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Helleday T, Petermann E, Lundin C, Hodgson B, Sharma RA. DNA Repair pathways as targets for cancer therapy. Nat Rev Cancer (2008) 8(3):193–204. doi: 10.1038/nrc2342 [DOI] [PubMed] [Google Scholar]
- 128. Pearl LH, Schierz AC, Ward SE, Al-Lazikani B, Pearl FM. Therapeutic opportunities within the DNA damage response. Nat Rev Cancer (2015) 15(3):166–80. doi: 10.1038/nrc3891 [DOI] [PubMed] [Google Scholar]
- 129. Yang S, Wang XQ. XLF-mediated NHEJ activity in hepatocellular carcinoma therapy resistance. BMC Cancer (2017) 17(1):344. doi: 10.1186/s12885-017-3345-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Edwards SL, Brough R, Lord CJ, Natrajan R, Vatcheva R, Levine DA, et al. Resistance to therapy caused by intragenic deletion in BRCA2. Nature (2008) 451(7182):1111–5. doi: 10.1038/nature06548 [DOI] [PubMed] [Google Scholar]
- 131. Bui TM, Butin-Israeli V, Wiesolek HL, Zhou M, Rehring JF, Wiesmuller L, et al. Neutrophils alter DNA repair landscape to impact survival and shape distinct therapeutic phenotypes of colorectal cancer. Gastroenterology (2021) 161(1):225–38.e15. doi: 10.1053/j.gastro.2021.03.027 [DOI] [PubMed] [Google Scholar]
- 132. Dong Z, Qi R, Guo X, Zhao X, Li Y, Zeng Z, et al. MiR-223 modulates hepatocellular carcinoma cell proliferation through promoting apoptosis via the Rab1-mediated mTOR activation. Biochem Biophys Res Commun (2017) 483(1):630–7. doi: 10.1016/j.bbrc.2016.12.091 [DOI] [PubMed] [Google Scholar]
- 133. Pinatel EM, Orso F, Penna E, Cimino D, Elia AR, Circosta P, et al. miR-223 is a coordinator of breast cancer progression as revealed by bioinformatics predictions. PloS One (2014) 9(1):e84859. doi: 10.1371/journal.pone.0084859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Pulikkan JA, Dengler V, Peramangalam PS, Peer Zada AA, Müller-Tidow C, Bohlander SK, et al. Cell-cycle regulator E2F1 and microRNA-223 comprise an autoregulatory negative feedback loop in acute myeloid leukemia. Blood (2010) 115(9):1768–78. doi: 10.1182/blood-2009-08-240101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Stamatopoulos B, Meuleman N, Haibe-Kains B, Saussoy P, Van Den Neste E, Michaux L, et al. microRNA-29c and microRNA-223 down-regulation has in vivo significance in chronic lymphocytic leukemia and improves disease risk stratification. Blood (2009) 113(21):5237–45. doi: 10.1182/blood-2008-11-189407 [DOI] [PubMed] [Google Scholar]
- 136. Masciarelli S, Fontemaggi G, Di Agostino S, Donzelli S, Carcarino E, Strano S, et al. Gain-of-function mutant p53 downregulates miR-223 contributing to chemoresistance of cultured tumor cells. Oncogene (2014) 33(12):1601–8. doi: 10.1038/onc.2013.106 [DOI] [PubMed] [Google Scholar]
- 137. Fassan M, Saraggi D, Balsamo L, Realdon S, Scarpa M, Castoro C, et al. Early miR-223 upregulation in gastroesophageal carcinogenesis. Am J Clin Pathol (2017) 147(3):301–8. doi: 10.1093/ajcp/aqx004 [DOI] [PubMed] [Google Scholar]
- 138. Streppel MM, Pai S, Campbell NR, Hu C, Yabuuchi S, Canto MI, et al. MicroRNA 223 is upregulated in the multistep progression of barrett's esophagus and modulates sensitivity to chemotherapy by targeting PARP1. Clin Cancer Res (2013) 19(15):4067–78. doi: 10.1158/1078-0432.CCR-13-0601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Neudecker V, Brodsky KS, Clambey ET, Schmidt EP, Packard TA, Davenport B, et al. Neutrophil transfer of miR-223 to lung epithelial cells dampens acute lung injury in mice. Sci Transl Med (2017) 9(408). doi: 10.1126/scitranslmed.aah5360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Brook AC, Jenkins RH, Clayton A, Kift-Morgan A, Raby AC, Shephard AP, et al. Neutrophil-derived miR-223 as local biomarker of bacterial peritonitis. Sci Rep (2019) 9(1):10136. doi: 10.1038/s41598-019-46585-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Fazi F, Rosa A, Fatica A, Gelmetti V, De Marchis ML, Nervi C, et al. A minicircuitry comprised of microRNA-223 and transcription factors NFI-a and C/EBPalpha regulates human granulopoiesis. Cell (2005) 123(5):819–31. doi: 10.1016/j.cell.2005.09.023 [DOI] [PubMed] [Google Scholar]
- 142. Citron F, Segatto I, Vinciguerra GLR, Musco L, Russo F, Mungo G, et al. Downregulation of miR-223 expression is an early event during mammary transformation and confers resistance to CDK4/6 inhibitors in luminal breast cancer. Cancer Res (2020) 80(5):1064–77. doi: 10.1158/0008-5472.CAN-19-1793 [DOI] [PubMed] [Google Scholar]
- 143. Chakrabarti S, Patel KD. Regulation of matrix metalloproteinase-9 release from IL-8-stimulated human neutrophils. J Leukoc Biol (2005) 78(1):279–88. doi: 10.1189/jlb.1004612 [DOI] [PubMed] [Google Scholar]
- 144. Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-a by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol (2005) 169(4):681–91. doi: 10.1083/jcb.200409115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Massena S, Christoffersson G, Vagesjo E, Seignez C, Gustafsson K, Binet F, et al. Identification and characterization of VEGF-a-responsive neutrophils expressing CD49d, VEGFR1, and CXCR4 in mice and humans. Blood (2015) 126(17):2016–26. doi: 10.1182/blood-2015-03-631572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Pan B, Shen J, Cao J, Zhou Y, Shang L, Jin S, et al. Interleukin-17 promotes angiogenesis by stimulating VEGF production of cancer cells via the STAT3/GIV signaling pathway in non-small-cell lung cancer. Sci Rep (2015) 5:16053. doi: 10.1038/srep16053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Chung AS, Wu X, Zhuang G, Ngu H, Kasman I, Zhang J, et al. An interleukin-17-mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nat Med (2013) 19(9):1114–23. doi: 10.1038/nm.3291 [DOI] [PubMed] [Google Scholar]
- 148. Bruchard M, Mignot G, Derangere V, Chalmin F, Chevriaux A, Vegran F, et al. Chemotherapy-triggered cathepsin b release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med (2013) 19(1):57–64. doi: 10.1038/nm.2999 [DOI] [PubMed] [Google Scholar]
- 149. Capizzi E, Gabusi E, Grigioni AD, De Iaco P, Rosati M, Zamagni C, et al. Quantification of free plasma DNA before and after chemotherapy in patients with advanced epithelial ovarian cancer. Diagn Mol Pathol (2008) 17(1):34–8. doi: 10.1097/PDM.0b013e3181359e1f [DOI] [PubMed] [Google Scholar]
- 150. Cabel L, Proudhon C, Mariani P, Tzanis D, Beinse G, Bieche I, et al. Circulating tumor cells and circulating tumor DNA: What surgical oncologists need to know? Eur J Surg Oncol (2017) 43(5):949–62. doi: 10.1016/j.ejso.2017.01.010 [DOI] [PubMed] [Google Scholar]
- 151. Mondelo-Macia P, Castro-Santos P, Castillo-Garcia A, Muinelo-Romay L, Diaz-Pena R. Circulating free DNA and its emerging role in autoimmune diseases. J Pers Med (2021) 11(2). doi: 10.3390/jpm11020151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Martins-Cardoso K, Almeida VH, Bagri KM, Rossi MID, Mermelstein CS, Konig S, et al. Neutrophil extracellular traps (NETs) promote pro-metastatic phenotype in human breast cancer cells through epithelial-mesenchymal transition. Cancers (Basel) (2020) 12(6). doi: 10.3390/cancers12061542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Zhu T, Wang L, Feng Y, Sun G, Sun X. Classical active ingredients and extracts of Chinese herbal medicines: Pharmacokinetics, pharmacodynamics, and molecular mechanisms for ischemic stroke. Oxid Med Cell Longev (2021) 2021:8868941. doi: 10.1155/2021/8868941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Gao H, Lan X, Li S, Xue Y. Relationships of MMP-9, e-cadherin, and VEGF expression with clinicopathological features and response to chemosensitivity in gastric cancer. Tumour Biol (2017) 39(5):1010428317698368. doi: 10.1177/1010428317698368 [DOI] [PubMed] [Google Scholar]
- 155. Rayes RF, Vourtzoumis P, Bou Rjeily M, Seth R, Bourdeau F, Giannias B, et al. Neutrophil extracellular trap-associated CEACAM1 as a putative therapeutic target to prevent metastatic progression of colon carcinoma. J Immunol (2020) 204(8):2285–94. doi: 10.4049/jimmunol.1900240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Lu W, Kang Y. Epithelial-mesenchymal plasticity in cancer progression and metastasis. Dev Cell (2019) 49(3):361–74. doi: 10.1016/j.devcel.2019.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Huang H, Zhang H, Onuma AE, Tsung A. Neutrophil elastase and neutrophil extracellular traps in the tumor microenvironment. Adv Exp Med Biol (2020) 1263:13–23. doi: 10.1007/978-3-030-44518-8_2 [DOI] [PubMed] [Google Scholar]
- 158. Lerman I, Hammes SR. Neutrophil elastase in the tumor microenvironment. Steroids (2018) 133:96–101. doi: 10.1016/j.steroids.2017.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Deryugina E, Carre A, Ardi V, Muramatsu T, Schmidt J, Pham C, et al. Neutrophil elastase facilitates tumor cell intravasation and early metastatic events. iScience (2020) 23(12):101799. doi: 10.1016/j.isci.2020.101799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Hawinkels LJ, Zuidwijk K, Verspaget HW, de Jonge-Muller ES, van Duijn W, Ferreira V, et al. VEGF release by MMP-9 mediated heparan sulphate cleavage induces colorectal cancer angiogenesis. Eur J Cancer (2008) 44(13):1904–13. doi: 10.1016/j.ejca.2008.06.031 [DOI] [PubMed] [Google Scholar]
- 161. Wisdom AJ, Hong CS, Lin AJ, Xiang Y, Cooper DE, Zhang J, et al. Neutrophils promote tumor resistance to radiation therapy. Proc Natl Acad Sci U.S.A. (2019) 116(37):18584–9. doi: 10.1073/pnas.1901562116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Shinde-Jadhav S, Mansure JJ, Rayes RF, Marcq G, Ayoub M, Skowronski R, et al. Role of neutrophil extracellular traps in radiation resistance of invasive bladder cancer. Nat Commun (2021) 12(1):2776. doi: 10.1038/s41467-021-23086-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Homa-Mlak I, Majdan A, Mlak R, Malecka-Massalska T. Metastatic potential of NET in neoplastic disease. Postepy Hig Med Dosw (Online) (2016) 70(0):887–95. doi: 10.5604/17322693.1216275 [DOI] [PubMed] [Google Scholar]
- 164. Teijeira A, Garasa S, Gato M, Alfaro C, Migueliz I, Cirella A, et al. CXCR1 and CXCR2 chemokine receptor agonists produced by tumors induce neutrophil extracellular traps that interfere with immune cytotoxicity. Immunity (2020) 52(5):856–71.e8. doi: 10.1016/j.immuni.2020.03.001 [DOI] [PubMed] [Google Scholar]
- 165. Ferrucci PF, Ascierto PA, Pigozzo J, Del Vecchio M, Maio M, Antonini Cappellini GC, et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol (2016) 27(4):732–8. doi: 10.1093/annonc/mdw016 [DOI] [PubMed] [Google Scholar]
- 166. Hopkins AM, Rowland A, Kichenadasse G, Wiese MD, Gurney H, McKinnon RA, et al. Predicting response and toxicity to immune checkpoint inhibitors using routinely available blood and clinical markers. Br J Cancer (2017) 117(7):913–20. doi: 10.1038/bjc.2017.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Ogata T, Satake H, Ogata M, Hatachi Y, Inoue K, Hamada M, et al. Neutrophil-to-lymphocyte ratio as a predictive or prognostic factor for gastric cancer treated with nivolumab: A multicenter retrospective study. Oncotarget (2018) 9(77):34520–7. doi: 10.18632/oncotarget.26145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med (2019) 381(16):1535–46. doi: 10.1056/NEJMoa1910836 [DOI] [PubMed] [Google Scholar]
- 169. Zhang Y, Chandra V, Riquelme Sanchez E, Dutta P, Quesada PR, Rakoski A, et al. Interleukin-17-induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer. J Exp Med (2020) 217(12). doi: 10.1084/jem.20190354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Bilusic M, Heery CR, Collins JM, Donahue RN, Palena C, Madan RA, et al. Phase I trial of HuMax-IL8 (BMS-986253), an anti-IL-8 monoclonal antibody, in patients with metastatic or unresectable solid tumors. J Immunother Cancer (2019) 7(1):240. doi: 10.1186/s40425-019-0706-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Dominguez C, McCampbell KK, David JM, Palena C. Neutralization of IL-8 decreases tumor PMN-MDSCs and reduces mesenchymalization of claudin-low triple-negative breast cancer. JCI Insight (2017) 2(21). doi: 10.1172/jci.insight.94296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Chapman RW, Minnicozzi M, Celly CS, Phillips JE, Kung TT, Hipkin RW, et al. A novel, orally active CXCR1/2 receptor antagonist, Sch527123, inhibits neutrophil recruitment, mucus production, and goblet cell hyperplasia in animal models of pulmonary inflammation. J Pharmacol Exp Ther (2007) 322(2):486–93. doi: 10.1124/jpet.106.119040 [DOI] [PubMed] [Google Scholar]
- 173. Fu S, Lin J. Blocking interleukin-6 and interleukin-8 signaling inhibits cell viability, colony-forming activity, and cell migration in human triple-negative breast cancer and pancreatic cancer cells. Anticancer Res (2018) 38(11):6271–9. doi: 10.21873/anticanres.12983 [DOI] [PubMed] [Google Scholar]
- 174. Varney ML, Singh S, Li A, Mayer-Ezell R, Bond R, Singh RK. Small molecule antagonists for CXCR2 and CXCR1 inhibit human colon cancer liver metastases. Cancer Lett (2011) 300(2):180–8. doi: 10.1016/j.canlet.2010.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Ning Y, Labonte MJ, Zhang W, Bohanes PO, Gerger A, Yang D, et al. The CXCR2 antagonist, SCH-527123, shows antitumor activity and sensitizes cells to oxaliplatin in preclinical colon cancer models. Mol Cancer Ther (2012) 11(6):1353–64. doi: 10.1158/1535-7163.MCT-11-0915 [DOI] [PubMed] [Google Scholar]
- 176. Pedersen F, Waschki B, Marwitz S, Goldmann T, Kirsten A, Malmgren A, et al. Neutrophil extracellular trap formation is regulated by CXCR2 in COPD neutrophils. Eur Respir J (2018) 51(4). doi: 10.1183/13993003.00970-2017 [DOI] [PubMed] [Google Scholar]
- 177. Jurcevic S, Humfrey C, Uddin M, Warrington S, Larsson B, Keen C. The effect of a selective CXCR2 antagonist (AZD5069) on human blood neutrophil count and innate immune functions. Br J Clin Pharmacol (2015) 80(6):1324–36. doi: 10.1111/bcp.12724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Perez-Guijarro E, Day CP, Merlino G, Zaidi MR. Genetically engineered mouse models of melanoma. Cancer (2017) 123(S11):2089–103. doi: 10.1002/cncr.30684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Greene S, Robbins Y, Mydlarz WK, Huynh AP, Schmitt NC, Friedman J, et al. Inhibition of MDSC trafficking with SX-682, a CXCR1/2 inhibitor, enhances NK-cell immunotherapy in head and neck cancer models. Clin Cancer Res (2020) 26(6):1420–31. doi: 10.1158/1078-0432.CCR-19-2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Pawlick RL, Wink J, Pepper AR, Bruni A, Abualhassen N, Rafiei Y, et al. Reparixin, a CXCR1/2 inhibitor in islet allotransplantation. Islets (2016) 8(5):115–24. doi: 10.1080/19382014.2016.1199303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Ruffini PA. The CXCL8-CXCR1/2 axis as a therapeutic target in breast cancer stem-like cells. Front Oncol (2019) 9:40. doi: 10.3389/fonc.2019.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M, et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest (2010) 120(2):485–97. doi: 10.1172/JCI39397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Liotti F, De Pizzol M, Allegretti M, Prevete N, Melillo RM. Multiple anti-tumor effects of reparixin on thyroid cancer. Oncotarget (2017) 8(22):35946–61. doi: 10.18632/oncotarget.16412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Chen L, Fan J, Chen H, Meng Z, Chen Z, Wang P, et al. The IL-8/CXCR1 axis is associated with cancer stem cell-like properties and correlates with clinical prognosis in human pancreatic cancer cases. Sci Rep (2014) 4:5911. doi: 10.1038/srep05911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Miller BE, Mistry S, Smart K, Connolly P, Carpenter DC, Cooray H, et al. The pharmacokinetics and pharmacodynamics of danirixin (GSK1325756)–a selective CXCR2 antagonist –in healthy adult subjects. BMC Pharmacol Toxicol (2015) 16:18. doi: 10.1186/s40360-015-0017-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Nie G, Cao X, Mao Y, Lv Z, Lv M, Wang Y, et al. Tumor-associated macrophages-mediated CXCL8 infiltration enhances breast cancer metastasis: Suppression by danirixin. Int Immunopharmacol (2021) 95:107153. doi: 10.1016/j.intimp.2020.107153 [DOI] [PubMed] [Google Scholar]
- 187. Samuel P, Mulcahy LA, Furlong F, McCarthy HO, Brooks SA, Fabbri M, et al. Cisplatin induces the release of extracellular vesicles from ovarian cancer cells that can induce invasiveness and drug resistance in bystander cells. Philos Trans R Soc Lond B Biol Sci (2018) 373(1737). doi: 10.1098/rstb.2017.0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Cao YL, Zhuang T, Xing BH, Li N, Li Q. Exosomal DNMT1 mediates cisplatin resistance in ovarian cancer. Cell Biochem Funct (2017) 35(6):296–303. doi: 10.1002/cbf.3276 [DOI] [PubMed] [Google Scholar]
- 189. Matsumoto A, Takahashi Y, Nishikawa M, Sano K, Morishita M, Charoenviriyakul C, et al. Accelerated growth of B16BL6 tumor in mice through efficient uptake of their own exosomes by B16BL6 cells. Cancer Sci (2017) 108(9):1803–10. doi: 10.1111/cas.13310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Panigrahi GK, Praharaj PP, Peak TC, Long J, Singh R, Rhim JS, et al. Hypoxia-induced exosome secretion promotes survival of African-American and Caucasian prostate cancer cells. Sci Rep (2018) 8(1):3853. doi: 10.1038/s41598-018-22068-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Koch R, Aung T, Vogel D, Chapuy B, Wenzel D, Becker S, et al. Nuclear trapping through inhibition of exosomal export by indomethacin increases cytostatic efficacy of doxorubicin and pixantrone. Clin Cancer Res (2016) 22(2):395–404. doi: 10.1158/1078-0432.CCR-15-0577 [DOI] [PubMed] [Google Scholar]
- 192. Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest (2010) 120(2):457–71. doi: 10.1172/JCI40483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Wang X, Qiao D, Chen L, Xu M, Chen S, Huang L, et al. Chemotherapeutic drugs stimulate the release and recycling of extracellular vesicles to assist cancer cells in developing an urgent chemoresistance. Mol Cancer (2019) 18(1):182. doi: 10.1186/s12943-019-1114-z [DOI] [PMC free article] [PubMed] [Google Scholar]