Abstract
Exploring the genetic basis of human infertility is currently under intensive investigation. However, only a handful of genes have been validated in animal models as disease-causing genes in infertile men. Thus, to better understand the genetic basis of human spermatogenesis and bridge the knowledge gap between humans and other animal species, we construct the FertilityOnline, a database integrating the literature-curated functional genes during spermatogenesis into an existing spermatogenic database, SpermatogenesisOnline 1.0. Additional features, including the functional annotation and genetic variants of human genes, are also incorporated into FertilityOnline. By searching this database, users can browse the functional genes involved in spermatogenesis and instantly narrow down the number of candidates of genetic mutations underlying male infertility in a user-friendly web interface. Clinical application of this database was exampled by the identification of novel causative mutations in synaptonemal complex central element protein 1 (SYCE1) and stromal antigen 3 (STAG3) in azoospermic men. In conclusion, FertilityOnline is not only an integrated resource for spermatogenic genes but also a useful tool facilitating the exploration of the genetic basis of male infertility. FertilityOnline can be freely accessed at http://mcg.ustc.edu.cn/bsc/spermgenes2.0/index.html.
Keywords: FertilityOnline, Infertility, Database, Functional gene, Mutation
Introduction
Human infertility affects 10%–15% of couples at reproductive age, half of which are attributed to the male partner [1], [2]. Spermatogenesis is a delicate, prolonged cell differentiation process that involves the self-renewal of spermatogonial stem cells (SSCs), meiosis, and postmeiotic development [3], [4], [5]. Disruption of any step during this period will result in reduced fertility or complete infertility. For example, a defective proliferation of SSCs often leads to Sertoli cell-only syndrome (SCOS), and genetic interference in spermatocytes can cause spermatocyte development arrest (SDA) [1], [6], [7], [8]. Approximately 25%–50% of the cases of male infertility have been estimated to result from genetic abnormalities [8], [9]. A survey of the literature revealed that at least 2000 genes are involved in the process of spermatogenesis [10]. However, to date, only a small number of genetic mutations in men have been validated as the causes of human subfertility/infertility in animal models [11], [12], [13], [14].
With the advent of next-generation sequencing (NGS), a multitude of high-throughput methods such as whole-exome sequencing (WES) and whole-genome sequencing (WGS) have been adopted to search for pathogenic mutations in infertile patients [11], [12]. These approaches commonly generate tens of thousands of genetic mutations, which obstacles the identification of causative mutations underlying male infertility. To solve this problem, we constructed the FertilityOnline database. FertilityOnline integrates the functional spermatogenic genes reported in the literature into the only existing functional spermatogenic database, SpermatogenesisOnline 1.0 [15]. Apart from the basic annotations for manually curated genes (gene information, protein functional domains, pathways, orthologs, and paralogs), new features, such as functional annotation, gene expression data in different tissues and different testicular cell types, and genetic variants of human genes, have been incorporated in FertilityOnline. With gene or variant annotations in hand, users can filter the annotation list to prioritize the candidate genes associated with male infertility and perform an in-depth analysis to refine the number of candidates in a user-friendly web interface. Thus, FertilityOnline not only serves as an integrated database for the functional annotation of genes associated with spermatogenesis but also provides a straight pipeline for the identification of human disease-causing mutations for male infertility.
Implementation
FertilityOnline is implemented with PHP (a popular general-purpose scripting language for web development; https://www.php.net/), Bootstrap (an open-source front-end framework; https://getbootstrap.com/), and JQuery (a JavaScript library for web development; https://jquery.com/). MySQL (an open-source database management system; https://www.mysql.com/) is used to store all the data. The backend of the analysis module is supplied by Python (https://www.python.org/). FertilityOnline is hosted on a Dell 730 server using Linux-Apache-MySQL-PHP (LAMP) architecture. The server is equipped with two 12-core Intel processors (2.2 GHz each) and 128 GB Random Access Memory (RAM).
Database content and usage
Features and data statistics of FertilityOnline
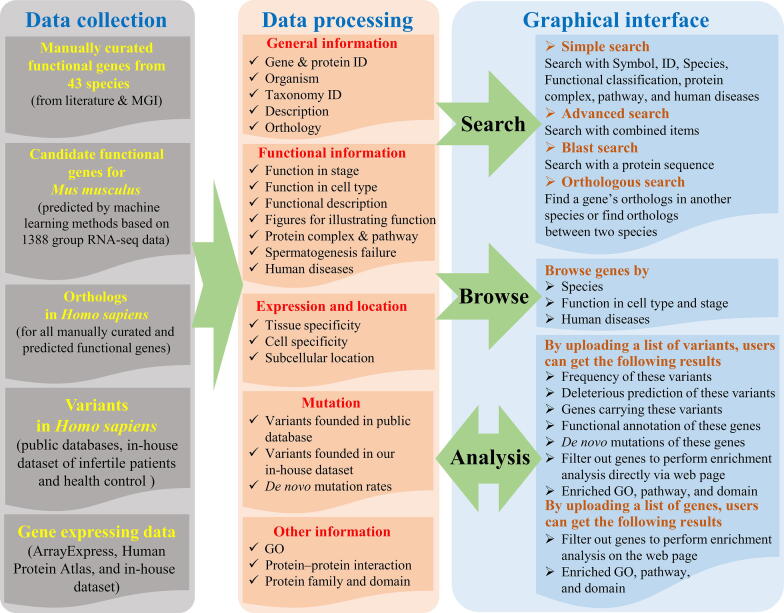
FertilityOnline is a comprehensive and systematic collection of functional annotations of spermatogenesis-related genes from the published literature. Information, such as gene expression, gene mutations, and homologs of spermatogenesis-related genes, is also integrated into this web resource (Table S1). Users can access all the information in FertilityOnline through the bowse and search page. Besides, the analysis page was also developed to facilitate batch retrieval gene and variant annotations for users (Figure 1).
Figure 1.
The overall structure of FertilityOnline
FertilityOnline is an integrated database that incorporates information on manually curated functional genes of spermatogenesis. Through data collection, the general and functional information, gene expression and location, and mutation information in human orthology are processed, and then graphical interfaces such as search, browsing, and analysis modules are visualized. MGI, mouse genome informatics; GO, Gene Ontology.
One of the goals of FertilityOnline is to provide an integrated resource that allows users to easily access information about spermatogenic genes and their mutations. To achieve this goal, we collected all the spermatogenic genes reported in the literature by employing a series of keywords to query in PubMed (see Method). Approximately 48,000 research articles published before July 1, 2019 were collected. Among these articles, 4736 records satisfying the criterion that the functions of genes in spermatogenesis have been validated by the experiment were sorted out. In total, 1610 unique spermatogenic genes with experimental validation from 43 species were curated in our database. The functional genes currently reported in spermatogenesis are mainly derived from mice, which account for 61.59% of curated genes, followed by humans (15.82%) and rats (10.07%). All other species together comprise the remaining (Table S2).
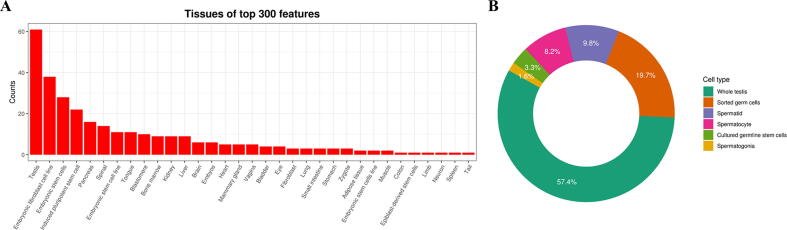
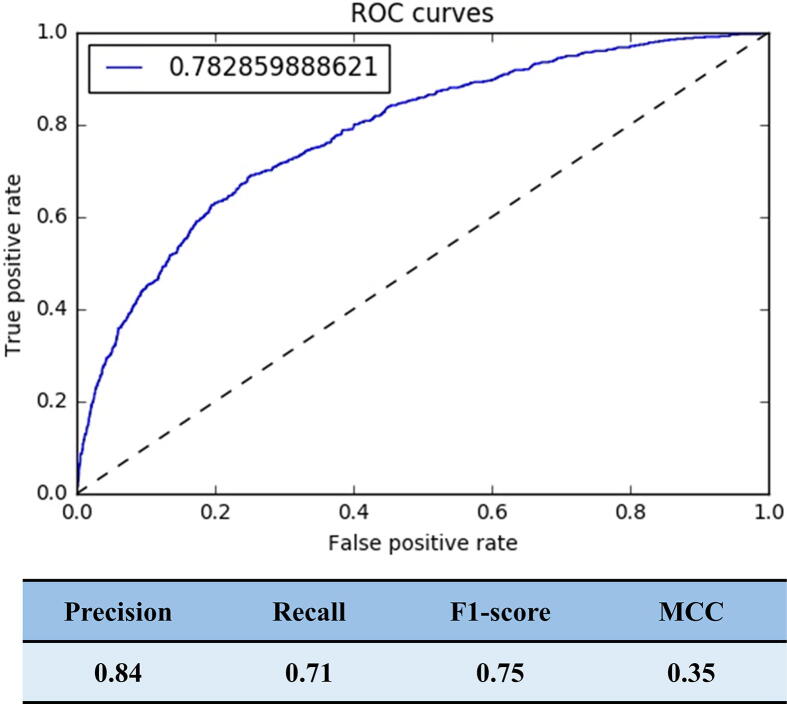
To further expand the utilization of FertilityOnline, a support vector machine (SVM) classifier was constructed to infer candidate functional spermatogenic genes. To build the training dataset, 654 functional genes reported in mice were collected as positive records, 3784 genes without any reproductive phenotype in knockout mice recorded in the Mouse Genome Informatics (MGI, http://www.informatics.jax.org/) database were labeled as negative records. Then 2627 RNA sequencing (RNA-seq) datasets from ArrayExpress were used as features (Table S3). We selected the top 300 most important features for constructing the SVM model (Figure S1; File S1). The area under the curve (AUC) of the receiver operating characteristic (ROC) curve of the trained SVM model was 0.78, representing that the model had a good ability to classify functional and non-functional genes (Figure S2). Ultimately, 3625 genes with probability values greater than 0.7 were sorted out as candidates.
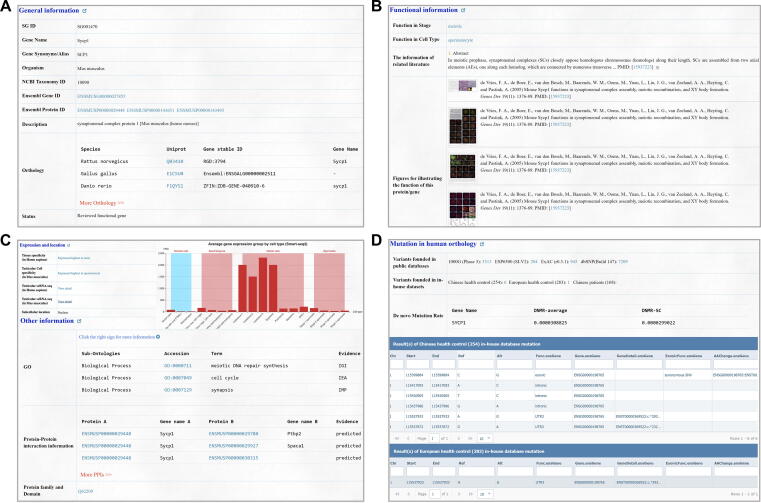
In addition to the general information such as gene/protein ID, taxonomy ID, general description, and orthology (Figure 2A), FertilityOnline provides high-quality functional information from literature for the spermatogenic genes. We classified genes based on their functions in developmental stages during spermatogenesis as well as in corresponding testicular cell types. Consequently, most of the reported genes were found during meiotic and postmeiotic stages (Table S4), corresponding to spermatocytes and spermatids, respectively (Table S5). Additionally, figures collected from references that support the functional classification are also displayed on the web. Moreover, we provided a manual annotation for the gene functions, signaling pathways, and their associated protein complexes (Figure 2B). Other information that implicates their functions in spermatogenesis, such as information about the reported function, gene expression, protein localization, structure, and protein–protein interactions, are included in FertilityOnline. This information provides additional references for users to select candidate genes for experimental validation (Figure 2C).
Figure 2.
Information integrated into FertilityOnline
Screenshots showing information about Sycp1 as an example. A. The general information of Sycp1, such as gene/protein ID, NCBI taxonomy ID, general description, and orthology. B. Functional information of Sycp1, including functional stage and cell types, related literature, and figures. C. Gene expression and protein location for Sycp1. D. Mutation information in its human orthology SYCP1. In particular, variant counts in different public databases and our in-house data are provided. The statistics of the de novo mutation rate of SYCP1 are also shown. Sycp1, synaptonemal complex protein 1.
To facilitate the screening of pathogenic mutations related to spermatogenesis disorder, FertilityOnline integrates a range of genetic databases, including 1000 Genomes Project, ESP6500, ExAC, and dbSNP [16], [17], [18], [19]. Users can acquire the counts of variants among different databases and retrieve the detailed variant information for each gene. Besides, the de novo mutation rate is an important parameter for assessing the pathogenicity of a gene [20], [21]. Therefore, we provided statistics of the de novo mutation rate for each gene in FertilityOnline. Users can access this information in the mutation section on the page (Figure 2D).
Search and browse spermatogenic genes
Our database provides a feature-rich visual interface for users to browse the genes related to spermatogenesis. Here are the functional modules of the web page:
Search
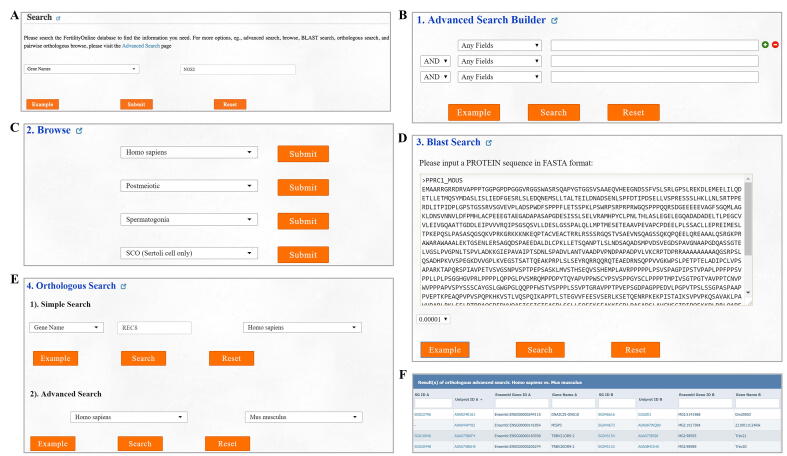
Users can search by a specific term, such as the gene/protein name, species, protein complexes, signaling pathways, functional classification, and disease characteristics, to determine the gene of interest (Figure S3A).
Advanced search
Users can refine their search results by combining multiple search terms (Figure S3B).
Browse
Users can browse all genes that are associated with a certain functional stage, cell type, or disease (Figure S3C).
BLAST search
By uploading a protein sequence in FASTA format, identical or homologous proteins present in FertilityOnline can be mapped (Figure S3D).
Homologous search
Users can input a gene name and species to obtain homologous genes in other species. Moreover, they can also select two species and obtain all homologous genes (Figure S3E and F).
Batch retrieval annotations for genes and mutations
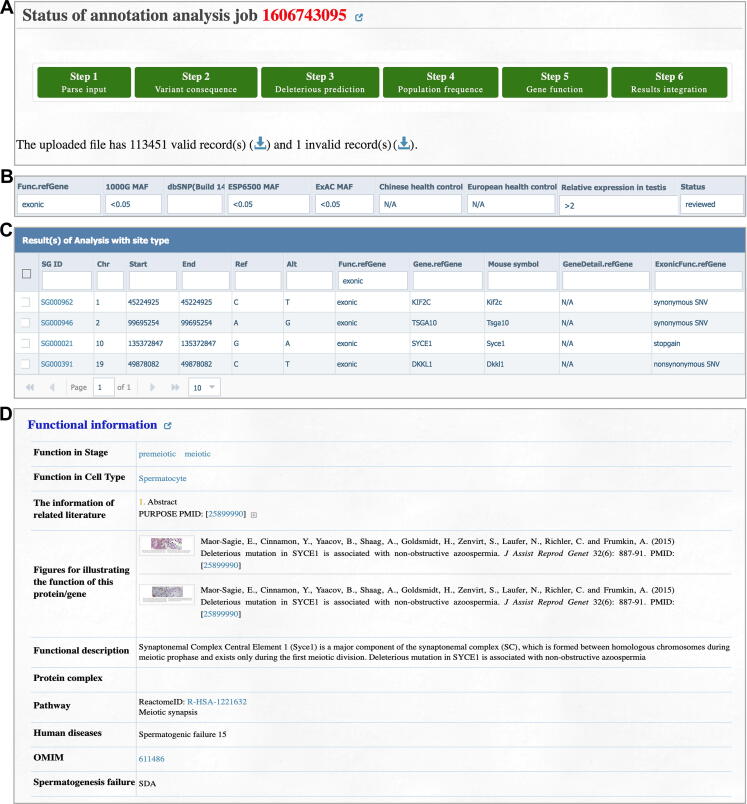
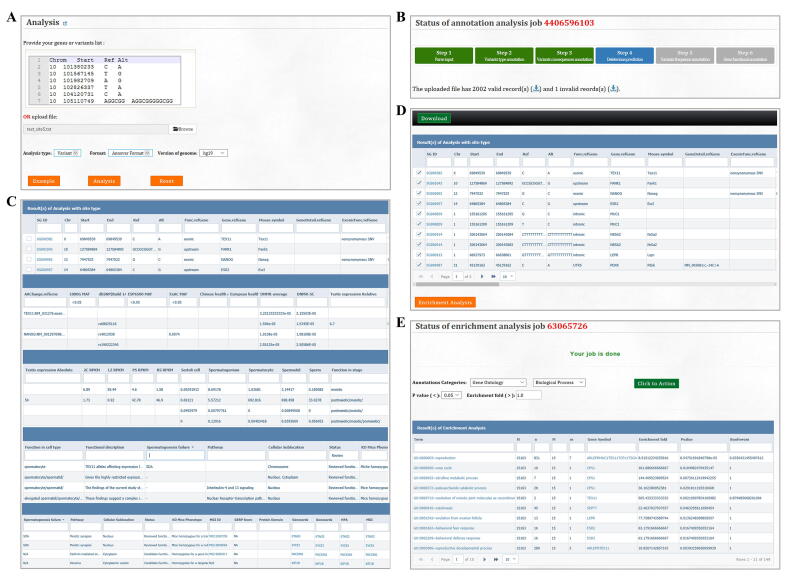
The major aim of FertilityOnline is to provide references to facilitate the screening of disease causal mutations associated with spermatogenic failure. Thus, the analysis module is provided for users to batch retrieval annotations for the genes or mutations. After uploading the gene or mutation list, the analysis module annotates the list with all available information in FertilityOnline (Figure S4A). Notably, the uploaded data are temporarily stored on the server and will be deleted automatically after 30 days. The progress of the analysis will be displayed in real-time (Figure S4B). It takes about 5 min to annotate a typical variant call format (VCF) file from WES (containing 100,000–400,000 variants) (Table S6). Additionally, the queuing module can execute more jobs in parallel. Finally, the annotation results will be displayed on the “Results” page, and users can filter these results according to their need to identify candidate genes or mutations (Figure S4C). Moreover, users can perform enrichment analysis for selected genes (Figure S4D and E). To facilitates the use of FertilityOnline in the screening of disease causal mutations, we provide a step-by-step protocol (Figure S5).
Case study
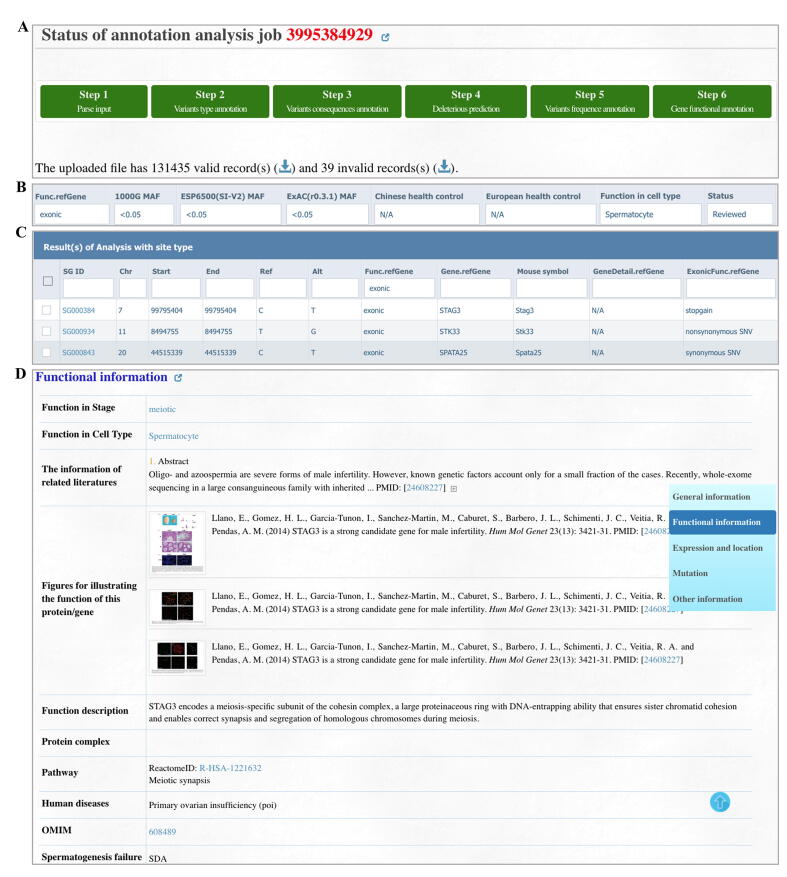
Herein, we provide two case studies to demonstrate how users can use FertilityOnline to screen potential pathogenic mutations. The patients P3793 and P2667 both displayed azoospermia without any other abnormality. First, we uploaded the mutations from patient P3793 obtained by WES in VCF format via the analysis module (Figure 3A). We set the following parameters in the filter box on the web page: 1) the mutation falls in the exons; 2) the minor allele frequency (MAF) in the 1000 Genomes Project, ESP6500, and ExAC is less than 0.05; 3) the mutation is not homozygous in any Chinese and Europeans with fertility history; 4) the expression level in the testes is more than twice than the expression level in other tissues; 5) the selection of the reviewed functional genes (Figure 3B). As a result, four mutations in four different genes were obtained (Figure 3C).
Figure 3.
A case study exhibits how FertilityOnline facilitates the discovery of disease causal mutations
A. Annotated mutations from a Chinese azoospermia patient P3793. B. The applied parameters in the filter box on the “Results” page. C. Filtered results displaying four mutations corresponding to four different genes. D. Functional information of the candidate gene SYCE1. SYCE1, synaptonemal complex central element protein 1.
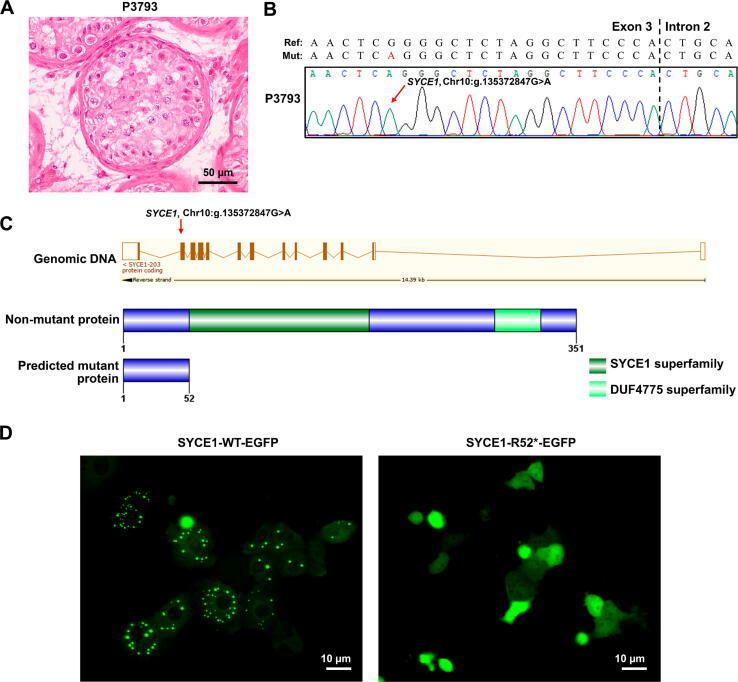
Among these genes, synaptonemal complex central element protein 1 (SYCE1), whose ortholog Syce1 has been reported to be crucial for mouse meiosis [22], [23], is consistent with the meiotic arrest phenotype observed in patient P3937 (Figure 3D, Figure 4A). Thus, the homozygous mutation in SYCE1 (g.135372847G>A, c.154C>T) likely causes the patient’s SDA phenotype. The SYCE1 mutation was further validated by Sanger sequencing (Figure 4B). This nonsense mutation generates a premature stop codon at amino acid residue 52 (p.R52*), and probably leads to a truncated SYCE1 protein (Figure 4C). SYCE1 has previously been shown to display aggregates when ectopically expressed in cultured mammalian cells [24]. We took advantage of this observation and examined whether the nonsense mutation of SYCE1 influences its protein localization in Vero cells. Remarkably, wild-type (WT) SYCE1 aggregated into multiple foci in transfected cells, whereas no focus was observed for mutant SYCE1 (Figure 4D). Thus, our results suggest that the nonsense mutation of SYCE1 abrogates the function of SYCE1 and is responsible for SDA in patient P3937.
Figure 4.
A novel nonsense mutation (c.154C>T, p.R52*) in SYCE1 was identified in the male-sterile patient P3793 by FertilityOnline
A. Representative image of testicular histology from patient P3793 displaying SDA. Scale bar, 50 µm. B. The chromatogram shows the Sanger sequencing result confirming the mutation (SYCE1, g.135372847G>A) in genomic DNA. The red arrow highlights the mutation site. C. The exonic map of SYCE1 is shown in the upper part of the Ensembl transcript (ENST00000368517). The red arrow on it represents the position of the novel identified mutation. The vertical boxes indicate exons, and the lines connecting these boxes indicate introns. The filled boxes represent the coding exons, and the unfilled, empty boxes represent the non-coding exons. The non-mutant protein sequence is represented in the lower part, having 351 amino acids with 2 domains. The predicted mutant protein length is 52 amino acids because of the nonsense mutation in exon 3. D. Mutational effect on the subcelluar localization of SYCE1. When overexpressed in Vero cells, WT SYCE1 has a punctate localization while mutant SYCE1 has diffuse localization. Protein expression was analyzed by immunofluorescence microscopy in Vero cells after 36 h of transfection. Scale bar, 10 µm. SYCE1, synaptonemal complex central element protein 1; SDA, spermatocyte development arrest; EGFP, enhanced green fluorescent protein; WT, wild-type.
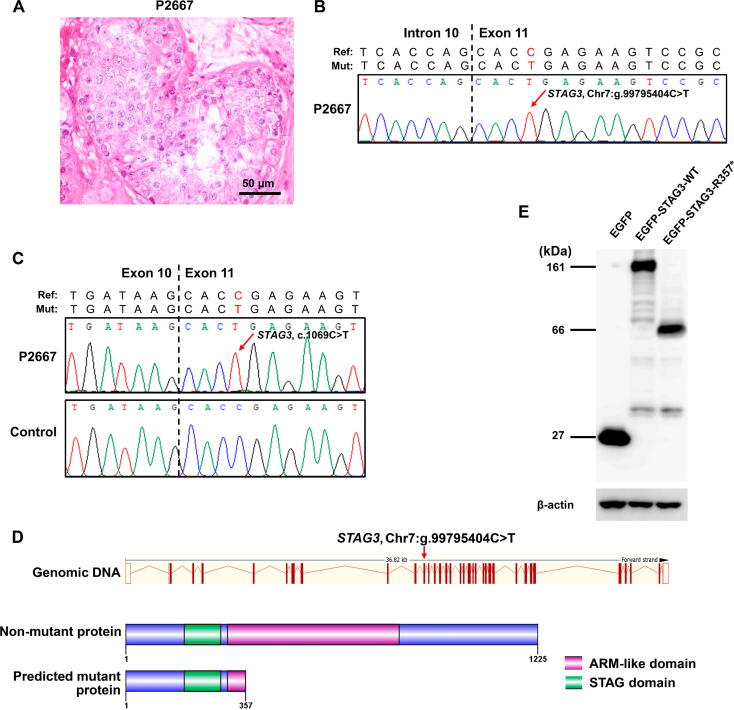
As another example, we uploaded all mutations from patient P2667 to FertilityOnline (Figure S6A). After obtaining the annotation results for variants and their carrier genes, we set parameters in the filter box on the web page (Figure S6B). We identified three mutations in three different genes after filtration (Figure S6C). Based on the SDA phenotype of patient P2667, we focused on a mutation in stromal antigen 3 (STAG3; g.99795404C>T, c.1069C>T), a gene encoding the component of the meiosis-specific cohesin complex necessary for meiosis (Figure 5A, Figure S6D) [25], [26]. The STAG3 mutation was further verified by Sanger sequencing at both the DNA and mRNA levels (Figure 5B and C). Likewise, this mutation introduces a premature stop codon at residue 357 (p.R357*) that possibly produces a C-terminal truncated protein (Figure 5D). To confirm this, we generated enhanced green fluorescent protein (EGFP)-tagged WT and mutant STAG3 (c.1069C>T). Then we transfected them into Vero cells. After that, Western blotting was performed on cell lysates. As expected, the mutant STAG3 indeed produced a truncated protein at 39 kDa, while the WT STAG3 showed a full-length protein at 134 kDa (Figure 5E). This result supported that the c.1069C>T mutation truncates the full-length STAG3 protein at the C-terminus, giving rise to meiotic arrest in patient P2667.
Figure 5.
A nonsense mutation (c.1069C>T,p.R357*) inSTAG3wasidentified inthemale-sterile patientP2667by FertilityOnline
A. Representative section of testicular histology of patient P2667 displaying SDA. Scale bar, 50 µm. B. The chromatogram shows the Sanger resequencing result confirming the mutation (STAG3, g.99795404C>T) in genomic DNA. The red arrow highlights the mutation site. C. Confirmation of the STAG3 (c.1069C>T) mutation at the mRNA level. D. Schematic representation of the exon map and the protein sequence of STAG3. The exonic map of STAG3 is shown in the upper part of the Ensembl transcript (ENST00000426455). The red arrow points out the position of the newly identified mutation. The WT protein sequence is represented in the lower part, having 1125 amino acids with 2 domains. The predicted mutant protein length is 357 amino acids, induced by the nonsense mutation in exon 11. E. Western blotting of EGFP-STAG3 using the protein lysate extracted from transfected Vero cells. The fusion protein EGFP-STAG3-R357* was ∼ 66 kDa corresponding to the predicted truncated STAG3 (∼ 39 kDa) fused to EGFP (∼ 27 kDa). β-actin was used as the internal control. STAG3, stromal antigen 3.
Method
Data collection
Manually curated functional genes
The following keywords were employed to search the PubMed database to collect functional spermatogenic gene information (published before July 1, 2019, in PubMed). For developmental stages, spermatogenesis, spermiogenesis, premeiotic, postmeiotic, and meiosis were employed to search the related literature. For testicular cell types, SSCs, spermatogonia, spermatocyte, spermatid, Sertoli cell, Leydig cell, and peritubular myoid cell were chosen as keywords. All collected references were manually curated, and only the genes with functional experimental validation were deemed functional genes associated with spermatogenesis. Moreover, figures and tables illustrating the functions of these genes were also collected.
Gene expression data
The gene expression data collected in this database can be divided into four parts: 1) RNA-seq data from Mus musculus which were downloaded from ArrayExpress (Table S3); 2) RNA-seq data from 37 human tissues (appendix, adrenal gland, adipose, bone marrow, colon, cerebral cortex, duodenum, esophagus, gallbladder, heart muscle, kidney, liver, lymph node, lung, ovary, prostate, placenta, pancreas, stomach, spleen, small intestine, skin, salivary gland, thyroid gland, testis, urinary bladder, and uterus) which were downloaded from Human Protein Atlas; 3) in-house RNA-seq data from five major mouse testicular cells (spermatogonium, spermatocyte, spermatid, sperm, and Sertoli cell); and 4) four sets of public single-cell RNA sequencing (scRNA-seq) data from human and mouse testes [27], [28], [29], [30] (Table S7).
Candidate functional genes in spermatogenesis (Mus musculus)
Because our curated functional genes associated with spermatogenesis were mainly from mice, we used mouse data to predict candidate functional genes by training an SVM classifier. The positive training dataset contained 653 manually curated genes that were reported to be functional during spermatogenesis. To construct the negative training dataset, we checked the phenotype data from MGI and selected 3783 genes in which mutation or deletion did not cause any abnormality in the reproductive system. The gene expression data (described in the “Gene expression data” section) were used as features to construct the model for predicting candidate functional genes in spermatogenesis. In total, a list of 300 most important features out of 2627 expression features was employed to train the SVM model (described in File S1). Among the predicted positive results, real positives were defined as true positives (TPs), while the others were defined as false positives (FPs). As described previously [15], the precision, recall, F1 score, and Matthews correlation coefficient (MCC) were adopted to evaluate the performance of our model. The equations are defined below:
Among the predicted negative results, real negatives were defined as true negatives (TNs), while others were defined as false negatives (FNs). Considering the small training dataset, we performed 4-fold cross-validations rather than 10-fold cross-validations, and the ROC curves were drawn with matplotlib packages.
Orthologous group information
Orthologous group information was downloaded from the InParanoid (version 8.0) and PANTHER (version 12.0) databases [31], [32]. Orthologous groups from these two databases were merged to avoid the loss of group members and redundancy.
Variants in Homo sapiens
In FertilityOnline, variants are classified into three categories: 1) variants present in public databases, including 1000G (Phase 3), ExAC (version r0.3.1), ESP6500 (ESP6500SI-V2), and dbSNP (build 147); 2) variants found in our in-house datasets, including Chinese health control (254 fertile men), European health control (283 fertile men), Chinese infertile patients (168 infertile men); 3) background de novo mutation rate obtained from previous reports [33].
Data processing
The collected data were processed to provide the following information for each gene. 1) General information, including gene and protein ID, source organism, taxonomic ID, description, and orthology. 2) Functional information, including the functional stage in which the gene is involved (premeiotic, meiotic, and postmeiotic), the cell type in which the gene is expressed (SSC, spermatogonium, spermatocyte, spermatid, Sertoli cell, Leydig cell, etc.), functional description, figures for illustration of function, protein complex and pathway, spermatogenesis disorder [SCO, SDA, and hypospermatogenesis (HSG)] and related human diseases. 3) Expression and localization, including the normalized value of gene expression in 37 human tissues and orthologous information in five types of mouse testicular cells. We also integrated four public scRNA-seq datasets covering germ and somatic cells. Moreover, the tissue with the highest expression was marked, and subcellular location information was also provided. 4) Mutation, providing the counts for variants of each gene found in public databases as well as in our in-house datasets. The de novo mutation rates were also provided. 5) Other annotations, including Gene Ontology (GO), protein–protein interaction, protein family, domain, etc.
WES and data analysis
WES was performed on genomic DNA (gDNA) isolated from the peripheral blood of nonobstructive azoospermia (NOA) patients using the QIAamp DNA Blood Mini Kit (Catalog No. 51206, Qiagen, Hilden, Germany) following the manufacturer’s instructions. An Agilent SureSelect Human All Exon v5 Kit (Catalog No. 5190-6208, Santa Clara, CA) was applied to capture the known exons and exon–intron boundary sequences. Sequencing was performed on a HiSeq 2000 platform, and raw reads (FASTQ format) were aligned to the human reference genome (GRCh37/hg19) using Burrows-Wheeler Aligner (BWA) software by applying default parameter settings [34]. The SAM file of each sample was converted to a BAM file by using SAMtools (http://samtools.sourceforge.net/) [35]. To remove PCR duplicates and to keep only properly paired reads, the Picard tool (http://broadinstitute.github.io/picard/) was used. The Genome Analysis Toolkit (GATK) (http://www.broadinstitute.org/gatk/) was used to further process the files, and then all BAM files were locally realigned by an indel realigner [36]. GATK’s Unified Genotyper was used on the processed BAM files to call both small insertions and deletions (InDels) and single-nucleotide variants (SNVs) within the captured coding exonic intervals.
Western blotting
Vero cells were transfected with EGFP-STAG3-WT or EGFP-STAG3-R357*. After 36 h of transfection, the cells were lysed, and proteins were separated on SDS-PAGE for Western blotting as described previously [37], [38].
Discussion
A large number of genes are implicated in the pathogenesis of human diseases, yet the genetic etiology underlying various diseases, e.g., male infertility, remains largely underdetermined [39]. The databases currently available lack depth and accuracy, which makes it difficult to obtain sufficient information to annotate the genes and their mutations. For example, more than two thousand genes that function across different developmental stages of spermatogenesis and in various testicular cell types are involved in the production of sperm [9], [10]. Perturbations at any substage during spermatogenesis may eventually lead to infertility; thus, the underlying causes of infertility are diverse. Without a detailed analysis of the specific phenotype of the abnormality, it is difficult to pinpoint the accurate causative gene and its mutation. Conventional gene annotation databases focus on providing broad-spectrum annotations, so it is not feasible to classify gene functions precisely based on developmental stages or cell types. Therefore, there is an urgent need for specialized databases for functional annotation in the field of reproductive biology. Here, the “Functional information” section provided by our database satisfies the aforementioned requirements. FertilityOnline provides not only detailed functional classification information but also additional information about genes and diseases. In particular, the phenotypes of genetically modified mice and their corresponding classification to the “spermatogenesis failure” of the patient can be examined. With this information, users could readily identify candidate variants based on the functional information of their carrier genes.
In recent years, WGS and WES have been used extensively to identify candidate pathogenic mutations in an unbiased manner [40], [41], but the number of mutations obtained by WES and WGS is very large. Therefore, integrated information on the expression, localization, and function of those genes that carry mutations will help greatly to screen candidate pathogenic mutations. In this regard, several online tools such as MARRVEL, VEP, and ANNOVAR have been developed for variant annotation [42], [43], [44]. Compared to existing tools, FertilityOnline provides more detailed information. First, FertilityOnline contains gene expression information across a panel of tissues and multiple types of cells in the testes. This set of information is particularly tailored for genes related to male infertility. For example, if the infertility of a patient is attributed to the meiotic arrest of spermatocytes, most likely, the genes with mutations are preferentially or highly expressed in spermatocytes, which allows us to reduce the number of candidate pathogenic genes and mutations for future validation. Second, we not only provide general information on gene orthologs across species but also collect the functional information on these orthologs published in the literature. Given that the functions of protein-coding genes are highly conserved and germ cells undergo similar developmental stages between model animals and humans, the information provided in our database will facilitate the screening of genes causing male infertility in humans.
Biologists often face the challenge of coping with high-throughput sequencing data. Our attempt to integrate the available databases with functional validation through animal models has provided reproductive biologists with a systematic module to quickly annotate a list of batch data on their own. In addition, a queuing mechanism is adopted to allow for the efficient analysis of uploaded tasks from users to ensure timely and stable annotation. For the analyzed results, a screening module is provided to allow users to reset parameters in the web interface directly to sort likely pathogenic mutations out of a large number of mutations. Furthermore, some hyperlinks are provided to help users directly access related databases quickly. For example, during the analyses of the cases presented above, the candidate pathogenic mutations were readily located in SYCE1 and STAG3. Notably, because we cannot acquire the testicular tissues of the patients to test the existence of mutant mRNAs directly, we cannot rule out the possibility of nonsense-mediated decay for the identified mutations. Instead, we validated the effects of the mutations in cell lines and found that both mutations affected the function of the protein. Therefore, our database provides an integrated and systematic platform that allows the batch annotation and screening of gene mutations causing spermatogenic disorders.
Taken together, our database is dedicated to providing a resource for integrating functional gene information regarding spermatogenesis. With this database, users can quickly access the functional information of spermatogenesis-associated genes or identify candidate disease-causing mutations related to spermatogenic disorders. In particular, this database provides a platform that facilitates the interpretation of the genetic causes of male infertility for diagnosis and research for clinicians as well as biologists.
Ethical statement
Written informed consents were obtained from the participating subjects, and all the human studies are approved by the institutional human ethics committee at the University of Science and Technology of China (USTC) with the approval number USTCEC20140003.
Data availability
FertilityOnline can be freely accessed at http://mcg.ustc.edu.cn/bsc/spermgenes2.0/index.html. The raw WES data reported in this study have been deposited in the Genome Sequence Archive [45] at the National Genomics Data Center, Beijing Institute of Genomics, Chinese Academy of Sciences / China National Center for Bioinformation (GSA: HRA000257), and are publicly accessible at https://ngdc.cncb.ac.cn/gsa.
CRediT author statement
Jianing Gao: Data curation, Methodology, Software, Writing - original draft. Huan Zhang: Resources, Data curation, Investigation. Xiaohua Jiang: Writing - original draft. Asim Ali: Data curation, Writing - original draft. Daren Zhao: Software. Jianqiang Bao: Writing - review & editing. Long Jiang: Investigation. Furhan Iqbal: Writing - review & editing. Qinghua Shi: Resources, Investigation, Funding acquisition. Yuanwei Zhang: Project administration, Conceptualization, Resources. All authors have read and approved the final manuscript.
Competing interests
The authors declared no competing interests.
Acknowledgments
This project was supported by the National Key R&D Program of China (Grant Nos. 2017YFC1001500, 2018YFC1003700, 2016YFC1000600, and 2018YFC1004700), the National Natural Science Foundation of China (Grant Nos. 31890780, 31630050, 31871514, 82071709, and 31771668), the Fundamental Research Funds for the Central Universities, China (Grant No. YD2070002006). We thank the USTC supercomputing center and the School of Life Science Bioinformatics Center for providing supercomputing resources for this project.
Handled by Sanghyuk Lee
Footnotes
Peer review under responsibility of Beijing Institute of Genomics, Chinese Academy of Sciences / China National Center for Bioinformation and Genetics Society of China.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gpb.2021.08.010.
Contributor Information
Xiaohua Jiang, Email: biojxh@mail.ustc.edu.cn.
Qinghua Shi, Email: qshi@ustc.edu.cn.
Yuanwei Zhang, Email: zyuanwei@ustc.edu.cn.
Supplementary material
The following are the Supplementary data to this article:
Supplementary figure 1.
Selected features for the SVM model. A. Histogram of tissues or cell lines from the top 300 features. B. Distribution of the testicular cell types of the top 300 features.
Supplementary figure 2.
The performance of the SVM model. The ROC curve showing the performance of the current dataset (AUC = 0.78). ROC, receiver operating characteristic; MCC, Matthews correlation coefficient.
Supplementary figure 3.
FertilityOnline provides a feature-rich visual interface for users to browse genes related to spermatogenesis. A. Users can initiate their search from the “Search” option for a query. B. The advanced search allows users to simultaneously input three terms as queries. C. Browse by species, the developmental stage during spermatogenesis, testicular cell types, or phenotype in humans. D. BLAST protein sequence search. E. Browse orthologs for a gene in all species and browse orthologs of all genes in two species. F. An example of pairwise orthologous browsing in humans and mice.
Supplementary figure 4.
FertilityOnline facilitates the discovery of variants causing male infertility. A. The analysis model in FertilityOnline is prepared for users to annotated genes or mutations through uploading a gene or mutation list. B. The real-time displayed progress bar when performing gene or mutation annotation. C. A screenshot of the annotation results. Users can sort out candidate genes or mutations by setting parameters in the filter boxes. D. Users can perform enrichment analysis on the same page by choosing genes of their interest through select boxes. E. The results page of enrichment analysis. The enriched items are categorized based on the functional categories, general annotation, gene ontology, disease, pathway, and protein domain.
Supplementary figure 5.
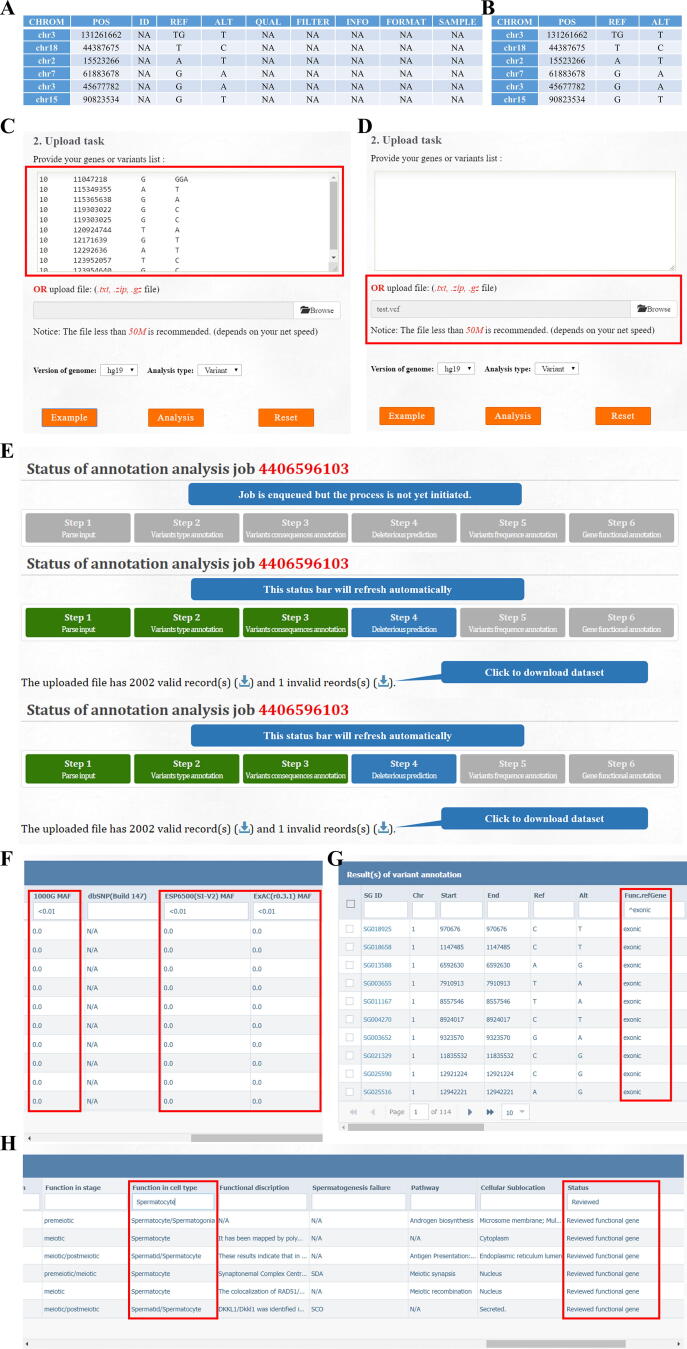
Step-by-step protocol of variant annotation and filtration. A step-by-step protocol for variant annotation and filtration using a VCF file containing 113,451 variants from the SYCE1 mutated SDA patient P3793. Preparing the input files. FertilityOnline accepts variants in two formats. A. VCF format suited for the output of regular GATK best practice. The NA columns mean the value of these columns is not necessary. B. Table-separated value (TSV) format, which is suitable for several mutation annotations of interest. Quick start. Users can paste the variants into the text form (C) or upload the variants in a file by setting the corresponding reference genome (D). E. A real-time displayed processing bar exhibits the analysis task status. Variants filtration. FertilityOnline provides a rich annotation of genes and mutations, including variant consequence, MAF in four public datasets and in-house fertile males, a summary of predicted deleterious effects from 13 software programs, and all gene information from FertilityOnline. We filtered variants to narrow down the candidates based on the following criteria: F. MAF < 0.01 in all public datasets (7778 variants left); G. Variants located in exonic regions (1339 variants left). H. Functional gene analysis by setting keywords ‘reviewed’ in the ‘status’ column and ‘spermatocyte’ as a function in the cell type column (6 variants left). Finally, the nonsense mutation on SYCE1 was the most relevant of these six mutations and had the highest probability of causing SDA in the patient. MAF, minor allele frequency.
Supplementary figure 6.
Example of how FertilityOnline facilitates gene and variant analysis. A. Annotated mutations from a Chinese azoospermia patient P2667. B. The applied parameters in the filter box on the results page. C. Three mutations in three different genes left after filtration. D. Functional information of the candidate gene STAG3. STAG3, stromal antigen 3.
Supplementary methods.
References
- 1.Wosnitzer M., Goldstein M., Hardy M.P. Review of azoospermia. Spermatogenesis. 2014;4:e28218. doi: 10.4161/spmg.28218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal A., Baskaran S., Parekh N., Cho C.L., Henkel R., Vij S., et al. Male infertility. Lancet. 2021;397:319–333. doi: 10.1016/S0140-6736(20)32667-2. [DOI] [PubMed] [Google Scholar]
- 3.Mäkelä J.A., Hobbs R.M. Molecular regulation of spermatogonial stem cell renewal and differentiation. Reproduction. 2019;158:R169–R187. doi: 10.1530/REP-18-0476. [DOI] [PubMed] [Google Scholar]
- 4.Wang L., Xu Z., Khawar M.B., Liu C., Li W. The histone codes for meiosis. Reproduction. 2017;154:R65–R79. doi: 10.1530/REP-17-0153. [DOI] [PubMed] [Google Scholar]
- 5.Griswold M.D. Spermatogenesis: the commitment to meiosis. Physiol Rev. 2016;96:1–17. doi: 10.1152/physrev.00013.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matzuk M.M., Lamb D.J. The biology of infertility: research advances and clinical challenges. Nat Med. 2008;14:1197–1213. doi: 10.1038/nm.f.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biswas L., Tyc K., El Yakoubi W., Morgan K., Xing J., Schindler K. Meiosis interrupted: the genetics of female infertility via meiotic failure. Reproduction. 2020;161:R13–R35. doi: 10.1530/REP-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zorrilla M., Yatsenko A.N. The genetics of infertility: current status of the field. Curr Genet Med Rep. 2013;1:247–260. doi: 10.1007/s40142-013-0027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krausz C., Riera-Escamilla A. Genetics of male infertility. Nat Rev Urol. 2018;15:369–384. doi: 10.1038/s41585-018-0003-3. [DOI] [PubMed] [Google Scholar]
- 10.Hochstenbach R., Hackstein J.H. The comparative genetics of human spermatogenesis: clues from flies and other model organisms. Results Probl Cell Differ. 2000;28:271–298. doi: 10.1007/978-3-540-48461-5_11. [DOI] [PubMed] [Google Scholar]
- 11.Krausz C., Escamilla A.R., Chianese C. Genetics of male infertility: from research to clinic. Reproduction. 2015;150:R159–R174. doi: 10.1530/REP-15-0261. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell M.J., Metzler-Guillemain C., Toure A., Coutton C., Arnoult C., Ray P.F. Single gene defects leading to sperm quantitative anomalies. Clin Genet. 2017;91:208–216. doi: 10.1111/cge.12900. [DOI] [PubMed] [Google Scholar]
- 13.Zhang B., Ma H., Khan T., Ma A., Li T., Zhang H., et al. A DNAH17 missense variant causes flagella destabilization and asthenozoospermia. J Exp Med. 2020;217:e20182365. doi: 10.1084/jem.20182365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin H., Ma H., Hussain S., Zhang H., Xie X., Jiang L., et al. A homozygous FANCM frameshift pathogenic variant causes male infertility. Genet Med. 2019;21:62–70. doi: 10.1038/s41436-018-0015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y., Zhong L., Xu B., Yang Y., Ban R., Zhu J., et al. SpermatogenesisOnline 1.0: a resource for spermatogenesis based on manual literature curation and genome-wide data mining. Nucleic Acids Res. 2013;41:D1055–D1062. doi: 10.1093/nar/gks1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu W., O’Connor T.D., Jun G., Kang H.M., Abecasis G., Leal S.M., et al. Analysis of 6,515 exomes reveals the recent origin of most human protein-coding variants. Nature. 2013;493:216–220. doi: 10.1038/nature11690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.1000 Genomes Project Consortium, Auton A., Brooks L.D., Durbin R.M., Garrison E.P., Kang H.M., et al. A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lek M., Karczewski K.J., Minikel E.V., Samocha K.E., Banks E., Fennell T., et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smigielski E.M., Sirotkin K., Ward M., Sherry S.T. dbSNP: a database of single nucleotide polymorphisms. Nucleic Acids Res. 2000;28:352–355. doi: 10.1093/nar/28.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Acuna-Hidalgo R., Veltman J.A., Hoischen A. New insights into the generation and role of de novo mutations in health and disease. Genome Biol. 2016;17:241. doi: 10.1186/s13059-016-1110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Awadalla P., Gauthier J., Myers R.A., Casals F., Hamdan F.F., Griffing A.R., et al. Direct measure of the de novo mutation rate in autism and schizophrenia cohorts. Am J Hum Genet. 2010;87:316–324. doi: 10.1016/j.ajhg.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamer G., Gell K., Kouznetsova A., Novak I., Benavente R., Hoog C. Characterization of a novel meiosis-specific protein within the central element of the synaptonemal complex. J Cell Sci. 2006;119:4025–4032. doi: 10.1242/jcs.03182. [DOI] [PubMed] [Google Scholar]
- 23.Bolcun-Filas E., Speed R., Taggart M., Grey C., de Massy B., Benavente R., et al. Mutation of the mouse Syce1 gene disrupts synapsis and suggests a link between synaptonemal complex structural components and DNA repair. PLoS Genet. 2009;5:e1000393. doi: 10.1371/journal.pgen.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernandez-Hernandez A., Masich S., Fukuda T., Kouznetsova A., Sandin S., Daneholt B., et al. The central element of the synaptonemal complex in mice is organized as a bilayered junction structure. J Cell Sci. 2016;129:2239–2249. doi: 10.1242/jcs.182477. [DOI] [PubMed] [Google Scholar]
- 25.Hopkins J., Hwang G., Jacob J., Sapp N., Bedigian R., Oka K., et al. Meiosis-specific cohesin component, Stag3 is essential for maintaining centromere chromatid cohesion, and required for DNA repair and synapsis between homologous chromosomes. PLoS Genet. 2014;10:e1004413. doi: 10.1371/journal.pgen.1004413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukuda T., Fukuda N., Agostinho A., Hernandez-Hernandez A., Kouznetsova A., Hoog C. STAG3-mediated stabilization of REC8 cohesin complexes promotes chromosome synapsis during meiosis. EMBO J. 2014;33:1243–1255. doi: 10.1002/embj.201387329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ernst C., Eling N., Martinez-Jimenez C.P., Marioni J.C., Odom D.T. Staged developmental mapping and X chromosome transcriptional dynamics during mouse spermatogenesis. Nat Commun. 2019;10:1251. doi: 10.1038/s41467-019-09182-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y., Zheng Y., Gao Y., Lin Z., Yang S., Wang T., et al. Single-cell RNA-seq uncovers dynamic processes and critical regulators in mouse spermatogenesis. Cell Res. 2018;28:879–896. doi: 10.1038/s41422-018-0074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo J., Grow E.J., Mlcochova H., Maher G.J., Lindskog C., Nie X., et al. The adult human testis transcriptional cell atlas. Cell Res. 2018;28:1141–1157. doi: 10.1038/s41422-018-0099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang M., Liu X., Chang G., Chen Y., An G., Yan L., et al. Single-cell RNA sequencing analysis reveals sequential cell fate transition during human spermatogenesis. Cell Stem Cell. 2018;23:599–614.e4. doi: 10.1016/j.stem.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Mi H., Muruganujan A., Thomas P.D. PANTHER in 2013: modeling the evolution of gene function, and other gene attributes, in the context of phylogenetic trees. Nucleic Acids Res. 2013;41:D377–D386. doi: 10.1093/nar/gks1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Brien K.P., Remm M., Sonnhammer E.L. Inparanoid: a comprehensive database of eukaryotic orthologs. Nucleic Acids Res. 2005;33:D476–D480. doi: 10.1093/nar/gki107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang Y.i., Li Z., Liu Z., Chen D., Wu W., Du Y., et al. mirDNMR: a gene-centered database of background de novo mutation rates in human. Nucleic Acids Res. 2017;45:D796–D803. doi: 10.1093/nar/gkw1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li H., Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKenna A., Hanna M., Banks E., Sivachenko A., Cibulskis K., Kernytsky A., et al. The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang X., Ma T., Zhang Y., Zhang H., Yin S., Zheng W., et al. Specific deletion of Cdh2 in Sertoli cells leads to altered meiotic progression and subfertility of mice. Biol Reprod. 2015;92:79. doi: 10.1095/biolreprod.114.126334. [DOI] [PubMed] [Google Scholar]
- 38.Gao Q., Khan R., Yu C., Alsheimer M., Jiang X., Ma H., et al. The testis-specific LINC component SUN3 is essential for sperm head shaping during mouse spermiogenesis. J Biol Chem. 2020;295:6289–6298. doi: 10.1074/jbc.RA119.012375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Price A.L., Spencer C.C.A., Donnelly P. Progress and promise in understanding the genetic basis of common diseases. Proc Biol Sci. 2015;282:20151684. doi: 10.1098/rspb.2015.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stranneheim H., Wedell A. Exome and genome sequencing: a revolution for the discovery and diagnosis of monogenic disorders. J Intern Med. 2016;279:3–15. doi: 10.1111/joim.12399. [DOI] [PubMed] [Google Scholar]
- 41.Yang Y., Muzny D.M., Reid J.G., Bainbridge M.N., Willis A., Ward P.A., et al. Clinical whole-exome sequencing for the diagnosis of mendelian disorders. N Engl J Med. 2013;369:1502–1511. doi: 10.1056/NEJMoa1306555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang J., Al-Ouran R., Hu Y., Kim S.Y., Wan Y.W., Wangler M.F., et al. MARRVEL: integration of human and model organism genetic resources to facilitate functional annotation of the human genome. Am J Hum Genet. 2017;100:843–853. doi: 10.1016/j.ajhg.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang K., Li M., Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McLaren W., Gil L., Hunt S.E., Riat H.S., Ritchie G.R.S., Thormann A., et al. The Ensembl Variant Effect Predictor. Genome Biol. 2016;17:122. doi: 10.1186/s13059-016-0974-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen T., Chen X., Zhang S., Zhu J., Tang B., Wang A., et al. The Genome Sequence Archive Family: toward explosive data growth and diverse data types. Genomics Proteomics Bioinformatics. 2021;19:578–583. doi: 10.1016/j.gpb.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary methods.
Data Availability Statement
FertilityOnline can be freely accessed at http://mcg.ustc.edu.cn/bsc/spermgenes2.0/index.html. The raw WES data reported in this study have been deposited in the Genome Sequence Archive [45] at the National Genomics Data Center, Beijing Institute of Genomics, Chinese Academy of Sciences / China National Center for Bioinformation (GSA: HRA000257), and are publicly accessible at https://ngdc.cncb.ac.cn/gsa.