Abstract
Spatial repellent (SR) products are envisioned to complement existing vector control methods through the continual release of volatile active ingredients (AI) providing: (i) protection against day-time and early-evening biting; (ii) protection in enclosed/semi-enclosed and peri-domestic spaces; (iii) various formulations to fit context-specific applications; and (iv) increased coverage over traditional control methods. SR product AIs also have demonstrated effect against insecticide-resistant vectors linked to malaria and Aedes-borne virus (ABV) transmission. Over the past two decades, key stakeholders, including World Health Organization (WHO) representatives, have met to discuss the role of SRs in reducing arthropod-borne diseases based on existing evidence. A key focus has been to establish a critical development path for SRs, including scientific, regulatory and social parameters that would constitute an outline for a SR target product profile, i.e. optimum product characteristics. The principal gap is the lack of epidemiological data demonstrating SR public health impact across a range of different ecological and epidemiological settings, to inform a WHO policy recommendation. Here we describe in brief trials that are designed to fulfill evidence needs for WHO assessment and initial projections of SR cost-effectiveness against malaria and dengue.
Keywords: Spatial repellents, WHO, Malaria, Dengue, Cost-effectiveness, Clinical trials, Social science
Graphical abstract
Highlights
-
•
75 years ago the potential value of spatial repellents (SRs) for vector-borne disease control was recognized.
-
•
SR protective efficacy has been demonstrated for human Aedes-borne virus infections and efficacy results for human malaria infections are promising.
-
•
Initial projections indicate that SRs have public health and economic benefits.
-
•
Limited data exists on (i) human behavior and SR use and (ii) when and where SRs are needed.
-
•
WHO requirements on SR epidemiological efficacy are partially satisfied.
1. Introduction
1.1. Context
In 2020, an estimated 241 million cases of malaria occurred worldwide (WHO, 2021). The global malaria deaths reached 627,000, an increase of 12% compared with 2019. Although malaria case incidence has fallen globally since 2010, the rate of decline in some regions has recently stalled or reversed (WHO, 2021). Mortality rates followed a similar pattern. Vector control has played a critical role in the global malaria response, including indoor residual spraying (IRS), and mass insecticide-treated net (ITN) distribution (D’Acremont et al., 2010; OʼMeara et al., 2010a, OʼMeara et al., 2010b; Bhatt et al., 2015). However, residual transmission remains where these approaches are ineffective for daytime, early evening, or outdoor biting mosquitoes and in conditions where their use is sub-optimal or infeasible or where mosquitoes have developed resistance to available insecticides (WHO, 2021).
Aedes aegypti, a day-biting mosquito, is a primary vector for Aedes-borne viruses (ABVs) including dengue, chikungunya, Zika, and urban yellow fever. ABVs can cause explosive epidemics, where high disease incidence and public fear combine to overwhelm health systems (Wilder-Smith et al., 2017). Dengue is the world’s most important human arthropod-borne viral infection worldwide (Brady et al., 2012; Bhatt et al., 2013) threatening almost 4 billion people in 128 countries with 5.2 million dengue cases reported to WHO in 2019. ABV control has traditionally focused on larval source reduction (including larvicides) and predominantly outdoor and limited indoor thermal/ULV space sprays, the latter restricted to public health emergencies. But, these methods require strict technical controls and access to households (Ritchie et al., 2021). Control of immature stages of Ae. aegypti is difficult to achieve due to its close adaptation to humans, especially in urban environments; broad distribution of eggs across a variety of artificial containers; desiccation resistant eggs; and constraints on intervention coverage that are often associated with inadequate resources. For these reasons, there is increasing recognition that ABV vector control programmes focused solely on larviciding and lacking indoor adulticiding interventions are insufficient for suppression of Aedes-borne diseases (Morrison et al., 2008; Achee et al., 2015).
New vector-control tools are thus essential to achieving current malaria eradication and ABV control goals. The World Health Organization’s (WHO’s) 2017 Global Vector Control Response framework (GVCR) (WHO/UNICEF, 2017) supports research to develop such tools, including spatial repellents (SRs). With the potential to deter or kill anopheline and aedine mosquitoes, SR products could represent an integrated disease control approach, simultaneously addressing malaria and ABVs.
1.2. Spatial repellent intervention class
WHO defines an intervention class in vector control as a group of products that share a common entomological effect by which it reduces pathogen transmission and thus reduces infection and/or disease in humans (WHO, 2020). Products in the SR intervention class are designed to release volatile chemicals that disperse in air and can be placed inside or around houses (Supplementary file S1). Currently, all SR products are pyrethroid-based but with a mode of action not intended just to kill. The volatile chemicals introduced into the air repel mosquitoes from entering the treated space, and/or disrupt their ability to detect or bite humans, and may impact their survival and reproductive behavior (Achee et al., 2012; WHO, 2013b). There are thousands of registered SR products already in the market, which are adopted and used by consumers to primarily prevent nuisance biting. These range from relatively simple inexpensive mosquito coils to more sophisticated higher-priced liquid vaporizers. However, due to insufficient evidence to support public health value (demonstrated impact on reduced clinical burden and/or fewer infections; WHO, 2022), WHO has not recommended any SR for public-sector use.
Temporary shelters where ITNs or IRS for malaria control would be impractical represent one potential public-sector application of SRs. SRs may also overcome a key limitation of ABV control in urban areas: the need for daytime access to homes. Since SRs employ a different mechanism of action for eliciting vector behavior effects (non-contact deterrence) than ITNs or IRS (contact-based mortality), they may also reduce the selection pressure that leads to insecticide resistance (Norris & Coats, 2017). SR pyrethroid AIs have demonstrated entomological efficacy against insecticide-resistant malaria and ABV mosquito vector species (Horstmann & Sonneck, 2016); however, this does not mean pyrethroid SRs are not affected by other resistance mechanisms, including Vgsc mutations (Nolden et al., 2021). Whether they act differently to reduce selection pressure is debatable and likely to depend on the resistance mechanisms present in the target insects.
1.3. Contribution to generating evidence for global policy recommendation
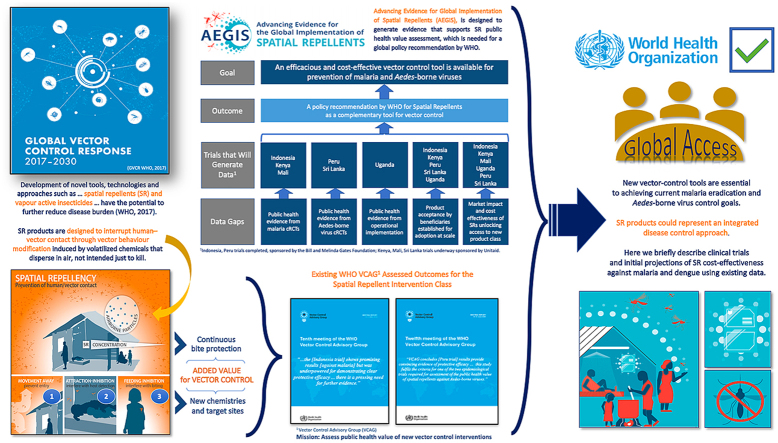
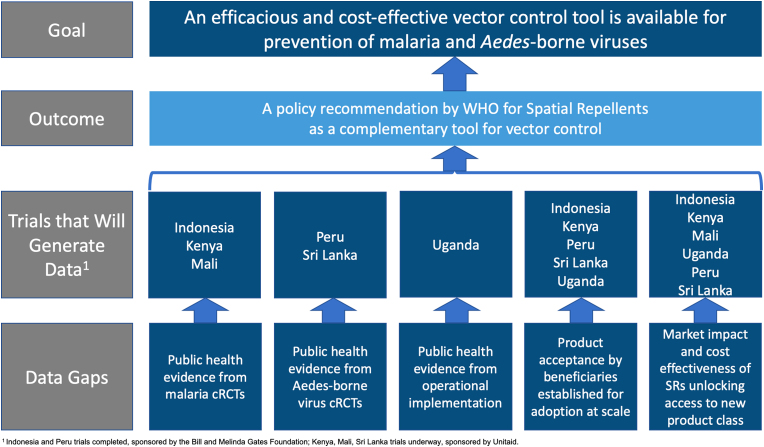
A WHO policy recommendation for a new vector control product must be based on rigorous evidence of public health value to ensure the intervention class represents efficacious and cost-effective beneficial tools. With this in mind, outputs from large-scale clinical trials are required to be linked to data on impact, feasibility and safety (WHO/UNICEF, 2017). Among ongoing laboratory, semi-field and small-scale spatial repellent studies from a broad range of academic, industry, and governmental stakeholders, a recently launched multi-country programme, Advancing Evidence for Global Implementation of Spatial Repellents (AEGIS), will contribute evidence regarding SR products for inclusion of SRs into public health programmes. The AEGIS programme was designed as a framework to generate data in support of a VCAG (Vector Control Advisory Group) endorsement for policy recommendation by WHO for SRs as a complement to existing vector control strategies (Fig. 1). AEGIS comprises three cluster-randomized, controlled trials (cRCTs) and one operational research (OR) study. All cRCTs will integrate epidemiological and entomological measures (WHO, 2013a).
Fig. 1.
Overview of spatial repellent data gaps, evidence generation, outcome and goal. 1 Indonesia and Peru trials completed, sponsored by the Bill and Melinda Gates Foundation; Kenya, Mali, Sri Lanka trials underway sponsored by Unitaid.
The malaria cRCTs are designed to evaluate the added benefit of SR protection against new human malaria infections in areas with high ITN coverage. The first malaria cRCT in Kenya will quantify protective efficacy in an East Africa setting with pyrethroid resistant malaria vectors and will quantify community/diversion effect to unprotected hosts (Ochomo et al., 2022). The second malaria cRCT in Mali is designed to measure SR protective efficacy in a West African setting with insecticide-resistant vector populations (Van Hulle et al., 2022). The ABV cRCT will be conducted in Sri Lanka and is designed to evaluate SR protective efficacy against human ABV infections, primarily dengue, and community effect in an Asian setting (AEGIS, 2022). The OR study is stage-gated (commencement based on positive Kenya cRCT interim results), planned to be conducted in Uganda and designed to evaluate SR effectiveness against malaria infections in rural African environments linked to displaced persons where challenges exist for effective deployment of ITNs and/or IRS. The basis of the OR study is to assess the feasibility of SR interventions to identify challenges in deployment at-scale for malaria control. Key outputs will include (i) quantifying the effectiveness and equivalence of impact through different distribution channels for donor market introduction and (ii) the development of WHO guidelines for operational implementation to support country-level adoption. Issues of implementation cost will be explored by evaluating efficacy in relation to coverage using varied intervention delivery systems (voucher, community health workers).
Social science endpoints will be gathered alongside the trials to better understand factors at the individual, household, community, and national levels that could influence the success of SRs. This includes capturing perceptions of the SR product and its performance over time, measuring activity patterns and time spent under the protection of the SR product, documenting availability and use of other mosquito control products, identifying opportunities to optimize distribution pathways, and developing key social and behavior change considerations.
The project will also measure cost of intervention implementation in relation to manufacturing, efficacy and coverage to model projections of SR cost-effectiveness to incentivize procurers. A key indicator for estimating public health impact will be additional infections averted and lives saved; while a key indicator for estimating economic impact will be treatment costs avoided and projected cost per person protected. The AEGIS project has calculated initial projected public health and economic impact of SRs for malaria and dengue based on available human case and vector control intervention costing data presented below. These projections will be improved through new data such as these generated by the AEGIS trials. Fig. 2 outlines key upcoming opportunities for SR global policy advancement.
Fig. 2.
Key upcoming opportunities for spatial repellent (SR) global policy advancement.
2. Modeling details
2.1. Projections of spatial repellent public health impact: Malaria
The goal of this analysis was to make projections of the potential public health impact of SRs with a given set of characteristics deployed for malaria prevention in Africa. We consider two potential SR coverage levels while accounting for the existence of other interventions including long-lasting insecticide nets (LLINs) and indoor residual spraying (IRS). In addition, we considered low and high estimates for the effectiveness of SR impacts based on existing estimates of SR impacts on mosquito behavior and mortality. Epidemiological dynamics were modeled using the Vector Control Optimization Model (VCOM) (Kiware et al., 2017). Using estimates regarding the impact of SRs, LLINs, and IRS on the ecology of the three main malaria vector species in sub-Saharan Africa (Anopheles gambiae, An. funestus and An. arabiensis), we calculated the expected global reduction in malaria cases and deaths based on detailed maps of the baseline annual incidence in 2015 from the Malaria Atlas Project (MAP). Results from Africa were then extrapolated globally based on recent estimates of the global malaria burden.
The epidemiological model is based around a set of differential equations that describe human infection through a simplistic susceptible-infectious-susceptible model structure, which leads to an equilibrium prevalence of infection in humans. Vector population dynamics are represented by equations that account for sequential events in the mosquito life-cycle: sequential blood-feeding, resting, sugar-feeding, oviposition site-seeking, emergence, mating, sugar-feeding, host-seeking, and encounters with humans indoors and outdoors and with livestock. Importantly, the model features baseline parameterizations that mimic the distinct behavior and ecology of the three dominant vector species in sub-Saharan Africa: An. gambiae, An. funestus, and An. arabiensis.
Different vector control interventions affect different aspects of vector population dynamics and malaria transmission in VCOM. Indoor spatial repellents (classified as housing modifications in VCOM) can reduce malaria transmission by (i) repelling a portion of blood-seeking Anopheles before they enter a treated dwelling, (ii) increasing the mosquito mortality rate in treated dwellings, and (iii) reducing the probability of a successful feeding among the surviving mosquitoes. The exact impacts of SRs on these different components of the malaria transmission are not yet known, and will be species-specific. To address this uncertainty, we modeled the impact of SRs using two sets of estimates: a low-effectiveness scenario with parameter values derived from several field trials of different SR products, and a high-effectiveness scenario that used the default parameter values for SRs in the VCOM model. The default VCOM parameter values assumed that indoor SRs would be as effective at reducing successful feeding of surviving mosquitoes as LLINs, hence they likely represent an upper bound on the potential impact of SRs on certain components of the transmission cycle.
First, SRs are assumed to repel a certain proportion of blood-seeking mosquitoes, lengthening the gonotrophic cycle and lowering the egg-laying rate. In field trials in Belize, transfluthrin cloth strips repelled 64.5% of Anopheles mosquitoes before entering experimental huts (Wagman et al., 2015). VCOM also includes parameters for increased mosquito mortality in dwellings with spatial repellents and decreased blood-feeding success in the presence of spatial repellents. We assumed that mosquito mortality prior to feeding in dwellings with spatial repellents was 38% (ten Bosch et al., 2018a). The probability that a mosquito would successfully feed in the presence of spatial repellents was 35%, based on experimental estimates for An. gambiae in the presence of transfluthrin coils (Ogoma et al., 2014). These parameter estimates are preliminary and in some cases are extrapolated from different products (SR coils vs longer-lasting SR sheets) and different Anopheles species. To examine how changes to these parameter estimates would affect our impact assessment, we also simulated the impact of spatial repellents using the default VCOM values for mosquito mortality and successful feeding in the presence of indoor spatial repellents. These values are less conservative than the literature-derived estimates and provide an upper bound on the impact assessment. The default probability of mortality in the presence of indoor spatial repellents is 41% for An. gambiae and An. funestus, and 13% for An. arabiensis. The default feeding success rate was 3% for An. gambiae and An. funestus, and 39% for An. arabiensis. The VCOM model also includes the potential effects from outdoor use of spatial repellents, but we assume that there will be no outdoor use in our impact assessment.
For a given parameterization, we simulated the model under default settings and extracted the entomological inoculation rate (EIR) at the end of the simulation as the primary output of interest. Under two different scenarios A and B, this yielded two different quantities: EIRA and EIRB. The ratio of these quantities is equal to the ratio of forces of infection, enabling a projection of incidence under scenario B based on incidence under scenario A and the ratio of EIRs under these scenarios, which is defined mathematically as
We obtained estimates of baseline annual incidence in 2015 from the Malaria Atlas Project (MAP; http://map.ox.ac.uk). Substituting location-specific values of annual incidence from MAP for IncidenceA and taking the ratio of EIRs under a given scenario, we used this equation to obtain an estimate of location-specific annual incidence in response to interventions that differ from baseline.
To account for variability in the ratio of EIRs due to differences in vector ecology, we computed the ratio of EIRs under parameterizations tailored to each of the three aforementioned vector species. We then computed the overall location-specific ratio of EIRs as the sum of the three species-specific EIRs weighted by the relative abundance of the three species at a location, as estimated by Sinka et al. (2016).
VCOM takes in a number of arguments to result in a projection of EIR following intervention, including the EIR in the absence of any intervention and the coverages of all interventions deployed in a given area. Because MAP-derived estimates of EIR incorporate effects of interventions, we had to back-calculate estimates of EIR in the absence of any intervention before we could project EIR under scenarios including SR. To do so, we first used VCOM to calculate EIR under intervention given (i) EIR in the absence of intervention ranging 1–1000, (ii) LLIN coverage ranging 0–100%, and (iii) IRS coverage ranging 0–80%. Next, for each 5 km × 5 km grid cell, we looked up the value of EIR in the absence of any intervention that corresponded with values of EIR under intervention, LLIN coverage, and IRS coverage for that grid cell according to MAP estimates. Then, we used VCOM to calculate EIR in each grid cell under assumed coverages of LLIN, IRS, and SR.
A spatial layer for EIR under baseline intervention coverages was only available for 2010, whereas our analysis involved use of more recent estimates for other variables from 2015. Thus, we derived estimates of EIR under baseline intervention coverages during 2015 based on estimates of incidence during 2015. To do so, we used EIR and incidence layers from 2010 to estimate site-specific scalars C that relate EIR and incidence according to the relationship,
These scalars reflect the product of (i) the probability that a blood meal by an infectious mosquito on a susceptible human results in infection of the human, and (ii) the proportion of parasite inoculations that result in clinically apparent infections. This yields a solution
of C in terms of EIR and incidence. We then applied the relationship
to yield an estimate of EIR for 2015.
SR impact projections were estimated for a five-year period from 2024 to 2028. We assume that EIR in the absence of any interventions in those years is equal to our estimate of EIR in the absence of any interventions from 2015. Projections of incidence and deaths in those years depend on assumptions about coverages of LLINs, IRS, and SR. With regard to LLIN and IRS coverage, we assume that they remain at 2015 levels over the duration of our projections. This amounts to a spatial average of 47.5% LLIN coverage and 7.5% IRS coverage in areas at risk for malaria. With regard to SR coverage, we consider the following scenarios:
-
1.
Equivalent to slow phase of LLIN rollout (2001–2005). This relatively conservative scenario assumes that rollout of SRs in each of the projected years 2024–2028 will proceed according to the same annual increases in household coverage as LLIN rollout in 2001–2005 starting with coverage at 0.4% in 2024. By 2028, this would result in approximately 5% household coverage on average.
-
2.
Equivalent to fast phase of LLIN rollout (2005–2009). This relatively aggressive scenario assumes that rollout of SRs in each of the projected years 2024–2028 will proceed according to the same annual increases in household coverage as LLIN rollout in 2005–2009. By 2028, this would result in approximately 22% household coverage on average.
Both the slow and fast rollouts were assumed to occur in a spatially homogeneous manner, with uniform SR coverage levels based on the mean LLIN coverage levels from 2001–2005 (or 2005–2009).
Applying calculations in a location-specific manner across MAP grid cells allowed us to capture an important nonlinearity associated with incidence reduction, wherein incidence is reduced more slowly at high per capita incidence at baseline in response to a given proportional reduction in EIR due to interventions than it is at low baseline per capita incidence. This nonlinearity arises from the saturating relationship between increasingly high rates of infection and the probability that a person will become infected, which cannot exceed 1.
After calculating location-specific incidence projections, we summed them to obtain a global estimate of incidence under a given intervention scenario. Location-specific projections of clinical cases and cases averted were obtained by multiplying the location-specific incidence by the local population size derived from the gridded 2015 WorldPop dataset (www.worldpop.org.uk). Location-specific estimates of malaria-associated deaths were calculated by multiplying local incidence by a country-specific case fatality ratio (CFR). The country-specific CFRs were calculated from the ratio of malaria-attributable deaths to clinical malaria cases in 2015 estimated for each country (Wang et al., 2016). Our spatially explicit calculations could only be performed for Africa due to the availability of MAP data, so we assumed that Africa-wide totals represent 88% of global totals. Numerical results presented in the tables should be interpreted as global totals that have been corrected in this way.
2.2. Projections of spatial repellent public health impact: Dengue
The goal of this analysis was to make crude projections of the potential public health and economic impact of SRs with a given set of characteristics deployed at a given coverage level under a realistic scenario about coverage levels of additional interventions. A number of aspects of dengue epidemiology and control are simpler than is the case for malaria, due to the fact that it only has one predominant vector (Ae. aegypti) and that there are no existing standards of care that need to be incorporated into our analysis. Various forms of vector control do take place for dengue control and prevention, but their impacts are thought to be variable and generally modest.
We used a model of the proportional reduction in dengue virus (DENV) force of infection attributable to spatial repellents deployed in a community at a given coverage of houses. This model accounts for multiple effects of a spatial repellent on mosquito behavior and life history, including repellency (reduced entry into a treated space), expellency (increased exiting from a treated space), excitatory effects on biting, and multiple sources of mortality. These sources of mortality include a probability of immediate knockdown upon entry into a treated space, augmentation of a mosquito’s background rate of mortality due to residual effects following exposure to the product, and elevated mortality associated with delays in blood-feeding and additional movement required to seek a blood meal in an untreated space.
Parameterization of this model was also performed based on a series of laboratory and experimental hut studies on Aedes aegypti using transfluthrin (ten Bosch et al., 2018a). Statistical analysis of those data yielded the following results: (i) exposure to transfluthrin increases time until a mosquito becomes fully blood-fed and increases propensity for partial biting; (ii) exposure to transfluthrin results in both acute and delayed mortality; and (iii) exposure to transfluthrin results in reduced entry into and exit out of treated spaces. The numerical effects measured in these experiments were introduced into the model, and other parameters largely canceled out in calculating a relative force of infection (i.e. force of infection in the presence of the spatial repellent divided by force of infection in its absence). Sensitivity of the relative force of infection to values of parameters that did not cancel out in the formula for relative force of infection was explored, and we used average values from that analysis in the projections described here.
Using the parameterization described, we based our projections on relative force of infection as the primary output of interest. Under two different scenarios (with and without spatial repellent), this yielded two different quantities: FOISR and FOIbaseline. This enables a projection of incidence under the scenario including spatial repellents based on incidence under a baseline scenario and the ratio of FOIs under these scenarios, which is defined mathematically as
The SR scenario should be interpreted as a situation with a spatial repellent with properties similar to that analyzed by ten Bosch et al. (2019) at a given coverage among houses in a community.
We obtained estimates of baseline annual incidence of 58.4 million clinically apparent cases globally in 2013 from Stanaway et al. (2016). These cases derive from an estimated population at risk of 3.9 billion people (Brady et al., 2012). After applying these numbers together with projections of relative force of infection in the presence of spatial repellents, we adopted default assumptions of Flasche et al. (2016) about the proportion of clinically apparent cases that result in hospitalization and the proportion that result in death. The latter was assumed to be approximated by a constant 0.0078. The former depends on whether an individual is experiencing a primary (0.111), secondary (0.209), or post-secondary infection (0.052). Our assumptions about annual incidence and population at risk imply a value of FOIbaseline = 0.015, which implies that the distribution of infections across these three categories is approximately one-third each (ten Bosch et al., 2018b). Thus, we assume that a proportion 0.124 of clinically apparent cases result in hospitalization.
Our projections pertain to each of the five years following the project, i.e. 2024–2028. We assume that FOIbaseline in the absence of intervention in those years is equal to our estimate of FOIbaseline based on estimates by Stanaway et al. (2016) for 2010. One factor that our projections depend on is our assumption about the relationship between coverage and relative force of infection. We considered a conservative scenario and a more optimistic scenario after ten Bosch et al. (2019). Another factor that our projections depend on is our assumption about coverage over time. Because there are currently no standard interventions for dengue control and prevention to inform this assumption, we based our projections on coverage estimates for long-lasting insecticidal nets (LLINs) for malaria (Bhatt et al., 2015). Specifically, we considered two alternative scenarios about coverage over time, one more conservative and one more optimistic:
-
1.
Equivalent to slow phase of LLIN rollout (2000–2004). This relatively conservative scenario assumes that rollout of SRs in each of the projected years 2024–2028 will proceed according to the same annual increases in household coverage as LLIN rollout in 2001–2005. By 2028, this would result in approximately 5% household coverage on average.
-
2.
Equivalent to fast phase of LLIN rollout (2005–2009). This relatively aggressive scenario assumes that rollout of SRs in each of the projected years 2024–2028 will proceed according to the same annual increases in household coverage as LLIN rollout in 2005–2009. By 2028, this would result in approximately 22% household coverage on average.
3. Results and discussion
3.1. Projected public health impact of SR introduction for malaria
Between 2000 and 2015, Plasmodium falciparum infection prevalence in malaria-endemic areas of Africa fell by 50% and the incidence of clinical disease fell by 40%, equivalent to an estimated 663 million cases averted in the past 15 years (Bhatt et al., 2015). The projected impact of SRs on number of cases averted across all ages for the AEGIS cRCTs (malaria and dengue) and OR study (emergency) settings are shown in Table 1. These values vary due to contextual differences in transmission dynamics (mosquito behavior, existing vector control interventions, probability of clinical disease). Estimates of ITN coverage used in projections of SR impact in emergency settings were high (ranging 71–80%). SRs will have a larger impact when ITN coverage is lower (as is the case in most malaria transmission settings) or zero (as is essentially the case for dengue). A summary of how uncertainties and assumptions in our model might alter the public health impact of SRs for both malaria and dengue (as well as the economic impacts discussed in the following sections) is presented in Fig. 3.
Table 1.
Spatial repellent estimated impact per cases averted for malaria, in emergency settings and dengue (severe and non-severe) compared to other vector control interventions (2024–2028).
| Malaria | Emergency | Dengue | |
|---|---|---|---|
| Cases averteda | 22,012,000 | 855,000 | 71,115,000 |
Rounded to nearest 10,000.
Fig. 3.
Limitations and uncertainties involved in estimating the public health and economic impacts of spatial repellents (SRs).
The goal of this analysis was to make projections of the potential public health impact of SRs with a given set of characteristics deployed for malaria prevention in Africa at a given coverage level under a realistic scenario about coverage levels of additional interventions. Results from Africa were extrapolated globally. We assumed that ITN and IRS coverage remain at 2015 levels over the duration of our projections, which corresponds to a spatial average of 47.5% ITN coverage and 7.5% IRS coverage in areas at risk for malaria. For SR coverage, we considered low and high coverage estimates equivalent to the slow and fast phases of ITN rollout (2001–2005 and 2005–2009). The conservative scenario corresponds to an increase in coverage from 0.4% in 2024 to 5.2% in 2028, and the more aggressive scenario would reach 22% by 2028. In addition, we considered low and high estimates for the effectiveness of SR impacts based on a range of studies.
3.2. Projected economic impact of SR introduction for malaria
A systematic review of cost and cost-effectiveness studies for various malaria control interventions found that the median cost of protecting one person for one year was $2.20 ($0.88–9.54) with ITNs and $6.70 ($2.22–12.85) with IRS (White et al., 2011). Our estimated cost of $12.60 to protect one household for one year with SRs falls within the range of costs for ITNs when compared on a per person basis ($2.52 or $3.15 assuming four or five individuals per household respectively) and is lower than the average per person cost of IRS treatment. The per person annual cost of ITN protection was considered to be highly cost-effective in many scenarios as the median financial cost of diagnosing a malaria case in the examined studies was $4.32 ($0.34–9.34) and the median financial costs of treating uncomplicated and severe malaria cases were $5.84 ($2.36–23.65) and $30.26 ($15.64–137.87). Recent studies have shown that ITNs are typically the most cost-effective intervention (relative to IRS, seasonal malaria chemotherapy (SMC), and the RTS,S malaria vaccine), but that additional interventions are often required to meet malaria reduction goals in high transmission settings (Korenromp et al., 2016). The relative cost-effectiveness of the other interventions became increasingly competitive as ITN coverage levels reached 50–60%, suggesting that the proper combination of interventions can act synergistically.
The cost savings associated with the use of SRs was estimated by calculating the monetary savings from cases averted versus the production and operational costs of the SR intervention being evaluated in the AEGIS trials. Costs averted were estimated by calculating location-specific savings based on the expected cost of treating both uncomplicated and severe malaria cases. Estimated costs of treating uncomplicated and severe clinical malaria cases were $5.84 and $30.26 (Galactionova et al., 2017). Costs for treatment included costs of medication, diagnosis with rapid diagnostic tests (RDT), non-medical and service-related expenditures by health facilities, travel and consumable costs, and hospitalization costs for severe cases. Global estimates of costs averted were obtained by summing location-specific cost savings projections and applying a correction to obtain global projections based on Africa-wide projections.
Costs associated with a given level of SR coverage were estimated by multiplying the number of people protected by the expected per person annual costs. We assumed that product costs for each individual SR would be $0.30 and that approximately 5 units would be needed to protect a household each month. Assuming a 6-month long malaria transmission season, an average of 30 units would be required annually to protect each household for an annual per person product cost of $1.80 assuming five people per house. Operational costs were assumed to contribute an additional 40% above the product costs based on a study of ITN programmes in five African countries that showed insecticide and nets represented 71% of the total cost of these programmes (Yukich et al., 2007).
Projections of both the global cost savings and costs savings in emergency settings under the low-coverage, high-effectiveness scenario are presented in Table 2. These numbers represent the difference between scenarios including and excluding SRs, and all impacts can therefore be attributed to SR rather than LLIN and IRS, the impact of which is reflected in the baseline scenario. Similarly, effects of drug-based and all other interventions are included implicitly in the baseline scenario and are assumed to carry on at 2015 levels. The net savings presented here represent a lower bound to expected savings because only treatment costs were included. When other costs such as death and DALYs are included, the cumulative net savings from 2024 to 2028 increases from $160.8 million to $9.67 billion under the low-coverage, high-effectiveness scenario.
Table 2.
Global projections of net savings based on treatment costs (in millions of USD) averted for malaria cases (severe and non-severe) when SRs are applied at the low coverage, high effectiveness scenario. Ranges represent results using low versus high coverage levels and low versus high estimates for the effect of spatial repellents on Anopheles spp. mortality and feeding success.
| Setting | Total SR costs | Treatment costs averted | Cumulative net savings |
|---|---|---|---|
| Global | $198.6 (198.6–1264.9) | $239.6 (30.4–1505.7) | $40.9 (−1060.7 to 240.9) |
| Emergency | $9.5 (9.5–43.2) | $9.3 (4.9–59.1) | $-0.2 (−28.8 to –0.2) |
Abbreviation: SR, spatial repellent.
3.3. Projected public health impact of SR introduction for dengue
Models of vector control impact and cost-effectiveness for dengue tend to be restricted to representative settings rather than truly global projections. Due to the fact that dengue tends to occur in epidemics that vary considerably across years, this is reasonable. We used a model of the proportional reduction in DENV transmission attributable to SRs deployed in a community at a given household coverage rate. The full range of impact projections we made included two scenarios about product effectiveness (Table 3). In the high-effectiveness scenario, we included estimates of all lethal and non-lethal effects of SRs on mosquitoes. In the low-effectiveness scenario, we included only non-lethal effects of SRs, which is a conservative assumption that could apply in contexts with high levels of resistance to the AI. We focus here on the high-effectiveness scenario, scenario, because during the initial assessment period (2024–2028) the lethal effects of SRs are likely to contribute significantly to their impact as resistance is not expected to develop rapidly (Norris & Coats, 2017).
Table 3.
Projections of total cases averted (2024–2028) for dengue (severe and non-severe) across all ages under a low coverage and high effectiveness scenario.
Rounded to nearest 10,000.
Rounded to nearest hundred.
We obtained estimates of baseline annual incidence of 58.4 million clinically apparent cases and 11,302 deaths globally in 2013. These cases derive from an estimated population at risk of 3.9 billion people. Because there are currently no standard interventions for dengue control and prevention to inform this assumption, we based our projections on coverage estimates for ITNs for malaria. Specifically, we considered a scenario equivalent to the slow phase of ITN rollout (2000–2004). This relatively conservative scenario assumes that rollout of SRs in each of the projected years 2024–2028 will proceed according to the same annual increases in household coverage as ITN rollout in 2001–2005. By 2028, this would result in approximately 5% household coverage on average.
3.4. Projected economic impact of SR introduction for dengue
The projected value for money using SR in a dengue setting is greater than for use of SR against malaria because of the underlying probability of preventing a large number of non-severe cases and the absence of a good vector control standard like ITNs in the case of malaria. Global projections of treatment costs (in millions USD) averted for dengue cases (severe and non-severe) when SRs are applied can be found in Table 4. The cost savings derived from use of SRs for dengue was estimated by calculating the monetary savings from dengue cases averted versus the cost of SRs. Following assumptions used in a recent estimate of the global economic burden of dengue (Shepard et al., 2016), our treatment cost projections are based on an assumed cost of $91 for direct treatment of a symptomatic dengue case, which averages over costs of direct treatment specific to hospitalized, ambulatory, and self-treated cases.
Table 4.
Global projections of net savings based on treatment costs (in millions of USD) averted for dengue cases (severe and non-severe) when SRs are applied at assumed coverage and effectiveness (2024–2028).
| Coverage | Effectiveness | Total SR costs | Treatment costs averteda | Cumulative net savingsa |
|---|---|---|---|---|
| Low | High | $980 | $3700 | $2700 |
Abbreviation: SR, spatial repellent.
Rounded to nearest hundred.
3.5. Looking ahead: Current limitations in impact assessments, updated public health impact, cost-effectiveness and market data
We acknowledge many limitations of the approach taken for projections of public health impact and cost-effectiveness and consider this analysis a work in progress that will mature over the course of the evidence generation. These estimates display a great deal of sensitivity to assumptions about effectiveness in particular. That is not surprising, given a number of simplifications of the VCOM modeling framework used for malaria and poor information to date on which to base estimates of effectiveness. Estimates of effectiveness should sharpen considerably over the course of this project, benefiting from the combination of rigorous field trials and model-based analyses that will directly connect empirical data to these impact projections. For context, we note that the magnitude of cases averted projected under the high coverage, high-effectiveness scenario is comparable to recent estimates of IRS impact. Thus, even our most optimistic projections should be considered to fall within a plausible range of outcomes.
Data availability from large-scale clinical trials will greatly reduce the uncertainty of the previously generated SR health impact estimates and help refine public health impact and cost-effectiveness estimates. They will also inform the potential for spatial repellent product introduction into different disease transmission contexts and thus clarify the expected market for the intervention class.
3.6. Advancing towards an SR global health policy: Closing remarks
The potential value of SR for vector-borne disease control was first recognized 75 years ago, with key research since then to support this claim. Epidemiological trials have demonstrated a metofluthrin-based (Syafruddin et al., 2014), and transfluthrin-based (Syafruddin et al., 2020) SR product in reducing malaria incidence in Indonesia, added impact of a transfluthrin-based SR product in reducing malaria incidence in China when combined with ITNs (Hill et al., 2014), and a transfluthrin-based SR product in reducing ABV incidence in Peru (Morrison et al., 2022).
In 2017, the WHO VCAG recommended additional clinical trials to evaluate SRs against malaria in Africa and against ABVs in Asia (WHO, 2017). There are also limited data in the scientific literature regarding factors that influence human use of SR products, and large-scale, public health procurers’ perceptions about when and where SR products are needed to supplement or serve as an alternative to more commonly used vector control strategies (i.e. ITNs and IRS for malaria; larvicides for ABV). Initial projections of public health and economic impact of SR introduction indicate beneficial cost-effectiveness, but more data are needed. These knowledge gaps are meant to be addressed by clinical trials underway in Kenya, Mali, and Sri Lanka as part of the AEGIS programme to inform a WHO SR policy recommendation, have national disease control programmes adopt an SR policy, and incentivize SR product research and development.
4. Conclusion
Recent outputs from WHO-endorsed clinical trials in Indonesia and Peru have moved the needle for reaching the required evidence-base for demonstrating spatial repellent efficacy but remain insufficient for VCAG public health value assessment; namely, two epidemiological trials per disease evaluation with conclusive, positive epidemiological outcomes from different ecological settings. The WHO VCAG requirements on epidemiological impact remain partially satisfied.
Funding
The AEGIS project is made possible thanks to Unitaid funding and support. Unitaid is a global health agency engaged in finding innovative solutions to prevent, diagnose, and treat diseases more quickly, effectively, and for affordable prices, in low- and middle-income countries. Unitaid’s work includes funding initiatives to address major diseases such as HIV/AIDS, malaria, and tuberculosis, as well as HIV co-infections and co-morbidities such as cervical cancer and hepatitis C, and cross-cutting areas, such as fever management. Unitaid is now applying its expertise to address challenges in advancing new therapies and diagnostics for the COVID-19 pandemic, serving as a key member of the Access to COVID Tools Accelerator. Unitaid is hosted by the World Health Organization.
Ethical approval
Not applicable.
CRediT author statement
The AEGIS program was conceived and designed by JPG and NLA. The Kenya cRCT was designed by JPG, NLA, EOO, and JEG. The Mali cRCT was designed by JPG, NLA, IS, and SVH. The Sri Lanka cRCT was designed by JPG, NLA, HAT, ACM, TWS and RCR. The Uganda cRCT was conceived and designed by JPG, NLA, and SVH. Social science studies in Kenya and Sri Lanka were conceived and designed by SAH and AM. Projection modeling was conducted by TAP and SMM. Statistical analyses plans for Kenya, Mali, and Uganda trials were designed by FL. The statistical analysis plan for the Sri Lanka cRCT was designed by RCR. The first draft of the manuscript was written by NLA, TAP, SMM, and JPG, with revision following comments from all the authors. All authors read and approved the final manuscript.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views, decisions or policies of the US Centers for Disease Control and Prevention.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank peer-reviewers for their constructive critiques on early versions of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crpvbd.2022.100107.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Achee N.L., Bangs M.J., Farlow R., Killeen G.F., Lindsay S., Logan J.G., et al. Spatial repellents: from discovery and development to evidence-based validation. Malar. J. 2012;11:164. doi: 10.1186/1475-2875-11-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achee N.L., Gould F., Perkins T.A., Reiner R.C., Jr., Morrison A.C., Ritchie S.A., et al. A critical assessment of vector control for dengue prevention. PLoS Negl. Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AEGIS . 2022. Spatial repellents for Aedes-borne virus control in Sri Lanka (AEGIS ABV). ClinicalTrials.gov identifier: NCT05452447.https://www.clinicaltrials.gov/ct2/show/ NCT05452447https://clinicaltrials.gov/ct2/show/NCT05452447?term=spatial+repellents&cond=Dengue&cntry=LK&draw=2&rank=1 (Accessed 11 July 2022) [Google Scholar]
- Bhatt S.W., Gething P., Brady O., Messina J., Farlow A., Moyes C. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S.W., Weiss F.D.J., Cameron E., Bisanzio D., Mappin B., Dalrympe U., et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady O.J., Gething P.W., Bhatt S., Messina J.P., Brownstein J.S., Hoen A.G., et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl. Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Acremont V., Lengeler C., Genton B. Reduction in the proportion of fevers associated with Plasmodium falciparum parasitaemia in Africa: A systematic review. Malar. J. 2010;9:240. doi: 10.1186/1475-2875-9-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flasche S., Jit M., Rodriguez-Barraquer I., Coudeville L., Recker M., Koelle K., et al. The long-term safety, public health impact, and cost-effectiveness of routine vaccination with a recombinant, live-attenuated dengue vaccine (Dengvaxia): A model comparison study. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1002181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galactionova K., Tediosi F., Camponovo F., Smith T.A., Gething P.W., Penny M.A. Country specific predictions of the cost-effectiveness of malaria vaccine RTS, S/AS01 in endemic Africa. Vaccine. 2017;35:53–60. doi: 10.1016/j.vaccine.2016.11.042. [DOI] [PubMed] [Google Scholar]
- Hill N., Zhou H.N., Wang P., Guo X., Carneiro I., Moore S.J. A household randomized, controlled trial of the efficacy of 0.03% transfluthrin coils alone and in combination with long-lasting insecticidal nets on the incidence of Plasmodium falciparum and Plasmodium vivax malaria in Western Yunnan Province, China. Malar. J. 2014;13:208. doi: 10.1186/1475-2875-13-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstmann S., Sonneck R. Contact bioassays with phenoxybenzyl and tetrafluorobenzyl pyrethroids against target-site and metabolic resistant mosquitoes. PLoS One. 2016;11 doi: 10.1371/journal.pone.0149738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiware S.S., Chitnis N., Tatarsky A., Wu S., Castellanos H.M.S., Gosling R., et al. Attacking the mosquito on multiple fronts: insights from the vector control optimization model (VCOM) for malaria elimination. PLoS One. 2017;12 doi: 10.1371/journal.pone.0187680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenromp E., Mahiané G., Hamilton M., Pretorius C., Cibulskis R., Lauer J., et al. Malaria intervention scale-up in Africa: Effectiveness predictions for health programme planning tools, based on dynamic transmission modelling. Malar. J. 2016;15:417. doi: 10.1186/s12936-016-1461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison A.C., Zielinski-Gutierrez E., Scott T.W., Rosenberg R. Defining the challenges and proposing new solutions for Aedes aegypti-borne disease prevention. PLoS Med. 2008;5:e68. doi: 10.1371/journal.pmed.0050068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison A.C., Reiner R.C., Jr., Elson W.H., Astete H., Guevara C., Del Aguila C., et al. Efficacy of a spatial repellent for control of Aedes-borne virus transmission: A cluster-randomized trial in Iquitos, Peru. Proc. Natl. Acad. Sci. USA. 2022;119 doi: 10.1073/pnas.2118283119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolden M., Brockmann A., Ebbinghaus-Kintscher U., Brueggen K.U., Horstmann S., Paine M.J.I., Nauen R. Towards understanding transfluthrin efficacy in a pyrethroid-resistant strain of the malaria vector Anopheles funestus with special reference to cytochrome P450-mediated detoxification. Curr. Res. Parasitol. Vector Borne Dis. 2021;1 doi: 10.1016/j.crpvbd.2021.100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris E.J., Coats J.R. Current and future repellent technologies: the potential of spatial repellents and their place in mosquito-borne disease control. Int. J. Environ. Res. Publ. Health. 2017;14:124. doi: 10.3390/ijerph14020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochomo E.O., Gimnig J.E., Bhattarai A., Samuels A.M., Kariuki S., Okello G., et al. Evaluation of the protective efficacy of a spatial repellent to reduce malaria incidence in children in western Kenya compared to placebo: study protocol for a cluster-randomized double-blinded control trial (the AEGIS program) Trials. 2022;23:260. doi: 10.1186/s13063-022-06196-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogoma S.B., Ngonyani H., Simfukwe E.T., Mseka A., Moore J., Maia M.F., Moore S.J., Lorenz L.M. The mode of action of spatial repellents and their impact on vectorial capacity of Anopheles gambiaesensu stricto. PLoS One. 2014;9 doi: 10.1371/journal.pone.0110433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OʼMeara W.P., Mangeni J.N., Steketee R., Greenwood B. Changes in the burden of malaria in sub-Saharan Africa. Lancet Infect. Dis. 2010;10:545–555. doi: 10.1016/S1473-3099(10)70096-7. [DOI] [PubMed] [Google Scholar]
- OʼMeara W.P., Mangeni J.N., Steketee R., Greenwood B. Changes in the burden of malaria in sub-Saharan Africa. Lancet Infect. Dis. 2010;10:545–555. doi: 10.1016/S1473-3099(10)70096-7. [DOI] [PubMed] [Google Scholar]
- Ritchie S.A., Devine G.J., Vazquez-Prokopec A., Lenhart A.E., Manrique-Saide P., Scott T.W. In: Innovative Strategies for Vector Control. Koenraadt C.M.J., Takken W., editors. Wageningen Academic Publishers; Wageningen, the Netherlands: 2021. Insecticide-based approaches for dengue vector control; pp. 59–89. [Google Scholar]
- Shepard D.S., Undurraga E.A., Halasa Y.A., Stanaway J.D. The global economic burden of dengue: A systematic analysis. Lancet Infect. Dis. 2016;16:935–941. doi: 10.1016/S1473-3099(16)00146-8. [DOI] [PubMed] [Google Scholar]
- Sinka M.E., Golding N., Massey N.C., Wiebe A., Huang Z., Hay S.I., Moyes C.L. Modelling the relative abundance of the primary African vectors of malaria before and after the implementation of indoor, insecticide-based vector control. Malar. J. 2016;15:142. doi: 10.1186/s12936-016-1187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanaway J.D., Shepard D.S., Undurraga E.A., Halasa Y.A., Coffeng L.E., Brady O.J., et al. The global burden of dengue: An analysis from the Global Burden of Disease Study 2013. Lancet Infect. Dis. 2016;16:712–723. doi: 10.1016/S1473-3099(16)00026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syafruddin D., Bangs M.J., Sidik D., Elyazar I., Asih P.B., Chan K., et al. Impact of a spatial repellent on malaria incidence in two villages in Sumba, Indonesia. Am. J. Trop. Med. Hyg. 2014;91:1079. doi: 10.4269/ajtmh.13-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syafruddin D., Asih P.B., Rozi I.E., Permana D.H., Hidayati A.P., Syahrani L. Efficacy of a spatial repellent for control of malaria in Indonesia: A cluster-randomized controlled trial. Am. J. Trop. Med. Hyg. 2020;103:344. doi: 10.4269/ajtmh.19-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Bosch Q.A., Castro-Llanos F., Manda H., Morrison A.C., Grieco J.P., Achee N.L., Perkins T.A. Model-based analysis of experimental data from interconnected, row-configured huts elucidates multifaceted effects of a volatile chemical on Aedes aegypti mosquitoes. Parasites Vectors. 2018;11:365. doi: 10.1186/s13071-018-2919-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Bosch Q.A., Clapham H.E., Lambrechts L., Duoung V., Buchy P., Althouse B.M., et al. Contributions from the silent majority dominate dengue virus transmission. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1006965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Bosch Q.A., Wagman J.M., Castro-Llanos F., Achee N.L., Grieco J.P., Perkins T.A. Community-level impacts of spatial repellents for control of diseases vectored by Aedes aegypti mosquitoes. PLoS Comput. Biol. 2019;16 doi: 10.1371/journal.pcbi.1008190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hulle S., Sagara I., Mbodji M., Nana G.I., Coulibaly M., Dicko A., et al. Evaluation of the protective efficacy of a spatial repellent to reduce malaria incidence in children in Mali compared to placebo: Study protocol for a cluster-randomized double-blinded control trial (the AEGIS program) Trials. 2022;23:259. doi: 10.1186/s13063-022-06197-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagman J.M., Grieco J.P., Bautista K., Polanco J., Briceño I., King R., Achee N.L. The field evaluation of a push-pull system to control malaria vectors in Northern Belize, Central America. Malar. J. 2015;14:184. doi: 10.1186/s12936-015-0692-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Naghavi M., Allen C., Barber R.M., Bhutta Z.A., Carter A., et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M.T., Conteh L., Cibulskis R., Ghani A.C. Costs and cost-effectiveness of malaria control interventions - a systematic review. Malar. J. 2011;10:337. doi: 10.1186/1475-2875-10-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . World Health Organization; Geneva: 2013. How to design vector control efficacy trials: Guidance on phase III vector control field trial design provided by the Vector Control Advisory Group. [Google Scholar]
- WHO . World Health Organization; Geneva: 2013. Guidelines for efficacy testing of spatial repellents.http://www.who.int/iris/handle/10665/78142 [Google Scholar]
- WHO . World Health Organization; Geneva: 2017. Seventh Meeting of the Vector Control Advisory Group, Geneva, Switzerland, 24–26 October 2017.http://www.who.int/neglected_diseases/vector_ecology/resources/WHO_HTM_NTD_VEM_2017.11/en/ [Google Scholar]
- WHO, 2020. Overview of intervention classes and prototype/products under Vector Control Advisory Group (VCAG) review for assessment of public health value. World Health Organization, Geneva. https://apps.who.int/iris/bitstream/handle/10665/274451/WHO-CDS-VCAG-2018.03-eng.pdf (Accessed 1 December 2022).
- WHO . World Health Organization; Geneva: 2021. World Malaria Report 2021.https://www.who.int/publications/i/item/9789240040496 [Google Scholar]
- WHO, 2022. Terms of reference of the Vector Control Advisory Group (VCAG). World Health Organization, Geneva Assessed https://www.who.int/publications/m/item/terms-of-reference-of-the-vector-control-advisory-group. (Accessed 16 November 2022).
- WHO/UNICEF . 2017. Global Vector Control Response 2017–2030. [Google Scholar]
- Wilder-Smith A., Gubler D.J., Weaver S.C., Monath T.P., Heymann D.L., Scott T.W. Epidemic arboviral diseases: Priorities for research and public health. Lancet Infect. Dis. 2017;17:e101–e106. doi: 10.1016/S1473-3099(16)30518-7. [DOI] [PubMed] [Google Scholar]
- Yukich, J., Tediosi, F., Lengeler, C., 2007. Operations, costs and cost-effectiveness of five insecticide-treated net programs (Eritrea, Malawi, Tanzania, Togo, Senegal) and two indoor residual spraying programs (Kwa-Zulu-Natal, Mozambique). USAID Report, 12 July 2007 Swiss Tropical Institute, Basel, Switzerland, 130 pp. https://www.researchgate.net/publication/242467217_Operations_Costs_and_Cost-Effectiveness_of_Five_Insecticide_Treated_Net_Programs_Eritrea_Malawi_Tanzania_Togo_Senegal_and_Two_Indoor_Residual_Spraying_Programs_Kwa-Zulu-Natal_Mozambique. (Accessed 16 November 2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.