Abstract
Background
Data characterizing risk factors and long-term outcome studies on human immunodeficiency virus (HIV)-associated pulmonary hypertension (PH) in Africa are lacking.
Methods
The Pan African Pulmonary Hypertension Cohort, a multinational registry of 254 consecutive patients diagnosed with PH (97% of African descent) from 9 centers in 4 African countries was implemented. We compared baseline characteristics and 3-year survival of an HIV-infected cohort newly diagnosed with PH (PH/HIV+) to an HIV-uninfected cohort with PH (PH/HIV−).
Results
One hundred thirty-four participants with PH completed follow up (47 PH/HIV+ and 87 PH/HIV−; age median, 36 versus 44 years; P = .0004). Cardiovascular risk factors and comorbidities were similar except for previous tuberculosis (62% versus 18%, P < .0001). Six-minute walk distance (6MWD) <300 meters was common in PH/HIV− (P = .0030), but PH/HIV+ had higher heart (P = .0160) and respiratory (P = .0374) rates. Thirty-six percent of PH/HIV+ and 15% of PH/HIV− presented with pulmonary arterial hypertension (PAH) (P = .0084), whereas 36% of PH/HIV+ and 72% of PH/HIV− exhibited PH due to left heart disease (PHLHD) (P = .0009). Pulmonary hypertension due to lung diseases and hypoxia (PHLD) was frequent in PH/HIV+ (36% versus 15%) but did not reach statistical significance. Human immunodeficiency virus-associated PAH tended to have a poorer survival rate compared with PHLHD/PHLD in HIV-infected patients.
Conclusions
The PH/HIV + patients were younger and commonly had previous tuberculosis compared to PH/HIV− patients. Despite a better 6MWD at presentation, they had more signs and symptoms of early onset heart failure and a worse survival rate. Early echocardiography assessment should be performed in HIV-infected patients with history of tuberculosis who present with signs and symptoms of heart failure or posttuberculosis lung disease.
Keywords: Africa, AIDS, HIV, pulmonary hypertension, tuberculosis
The HIV cohort within the Pan African Pulmonary Hypertension Cohort is particularly relevant given the colliding epidemics of HIV/tuberculosis in Africa. Due to immunosuppression, young people living with HIV/AIDS contract tuberculosis several times, increasing their risk of post-tuberculosis lung diseases and HIV-associated pulmonary hypertension in particular.
Pulmonary hypertension (PH) is a life-threatening disease characterized by elevated pulmonary arterial pressures and pulmonary vascular resistance leading to right ventricular failure and premature death [1]. Human immunodeficiency virus (HIV) infection, chronic systemic inflammation, and antiretroviral therapy (ART)-associated side effects may affect the development and progression of cardiovascular diseases [2, 3]. The prevalence of HIV-associated pulmonary arterial hypertension (HIV-PAH) is estimated to be approximately 0.5% in people with HIV (PWH) [4] and 1000-fold higher compared to the general population [5, 6]. Before large-scaled rollout of effective ART, the prevalence of HIV-PAH was underestimated, because opportunistic infections were limiting life expectancy of PWH [2].
Patients with HIV-PAH suffer increased mortality compared to PWH without PAH [7]. Half of the deaths reported in patients with HIV-PAH are due to cardiopulmonary complications, for example, right ventricular failure or sudden cardiac death [4, 7]. Human immunodeficiency virus-PAH can develop at any stage irrespective of the degree of immunosuppression [5] and results in remodeling and stiffening of the pulmonary artery. Moreover, it remains controversial whether the prevalence of HIV-related PH has remained unchanged since the advent of ART [8]. Human immunodeficiency virus infection also confers an increased risk for a variety of infectious lung diseases, including bacterial pneumonia, tuberculosis (TB), and Pneumocystis pneumonia [9]. The impact of HIV on TB has been devastating, with approximately 10% of global cases attributable to HIV [8]. Indeed, the incidence of TB dramatically increased worldwide due to HIV [8]. Human immunodeficiency virus-infected patients are at great risk of TB due to the associated immunosuppression. The mechanism of developing PH after successfully treated pulmonary TB is thought to result from residual pulmonary structural damage such as fibrosis and persistent cavitation and pulmonary function abnormalities leading to gas exchange abnormalities and chronic hypoxia [10].
Human immunodeficiency virus infection has also been recognized as a prothrombotic condition and is associated with a 2- to 10-fold increased risk of venous thrombosis in comparison with a general population of the same age [11]. There are several intersecting mechanisms associated with HIV infection and ART that are emerging, which may lead to vasculopathy and hypercoagulability [11]. These hypercoagulable states predispose the patient to severe thrombosis and potentially life-threatening thromboembolic events [4]. Furthermore, the estimated prevalence of PH among HIV-infected people in Africa varies from 5% to 13% [4]. It is notably high compared to those in developed countries [12, 13]. Approximately 25 million PWH reside in sub-Saharan Africa (SSA) [13], and between 1.2 and 3.2 million are at risk of developing PAH [4]. In addition to the prevalence of HIV in Africa, poverty and homelessness, migration, armed conflicts and wars, rapid urbanization and household air pollution, lack of public health infrastructure, and limited access to medical care especially during COVID-19 lockdowns contribute to the increase of pulmonary TB incidence.
The Pan African Pulmonary Hypertension Cohort (PAPUCO) study is a multinational registry study established in 2011 in response to the paucity of information on the prevalence of PH in Africa; a continent considered the epicenter of the global TB/HIV epidemic, which are both risk factors of PH [12]. To the best of our knowledge, PAPUCO is the only PH registry in Africa to date, and the main findings of the entire cohort have previously been published [4, 7, 12, 14]. This subanalysis focuses on HIV-associated PH and risk factors such as TB that are highly prevalent in this population and compares HIV-infected and HIV-uninfected patients with PH.
METHODS
The broad design, aims, and specific methods of the PAPUCO registry, the largest contemporary cohort study of PH in Africa, have been described in greater detail previously (ClinicalTrials.gov NCT02265887) [14].
Patient Consent Statement
The PAPUCO protocol was approved by the Human Research Ethics Committee of the University of Cape Town (HRED REF 241/2011) and by all local ethics committees of participating centers. The PAPUCO study conforms to ethical norms, standards, and principles of the Declaration of Helsinki. Patient’s written consent was obtained from all participants (minors via their guardians/parents) before study enrollment, before any study-related procedures and HIV testing was performed, according to national guidelines, with consent.
Study Design and Setting
The PAPUCO research group was established in 2011 with the aim of establishing a prospective registry cohort study of de novo PH cases representative of the wider African population. Consequently, 9 specialist care referral centers in Cameroon, Mozambique, Nigeria, and South Africa contributed to the registry. Each site recruited consecutive patients on the following basis: (1) newly diagnosed with PH based on standardized clinical and echocardiography criteria; (2) the capacity to return for 6-month follow-up if alive; (3) aged ≥8 years (except for pediatric centers in Mozambique, Cameroon, and Nigeria, data presented elsewhere); and (4) provision of written informed consent to participate. Center eligibility included the following: (1) availability of echocardiography and training in assessing right heart function; (2) experience in diagnosing PH according to World Health Organization (WHO) classification; (3) experience in clinical management of patients with right heart failure (RHF); and (4) resources to review patients at 6-month follow up and at 3-year follow up for the HIV cohort (PH/HIV+).
Study Profiling and Outcome Data
All study data were collected on electronic case report forms (web-based platform) and stored on a dedicated secure central database. At least 2 investigators reviewed cases for completion and data validation. As described previously, once definitive assessment and treatment had been applied, the following data were documented for each participant (1) sociodemographic and ethnic profile; (2) antecedent risk profile (including environmental exposures and cardiovascular risk factors); (3) medical history including HIV test results, previous CD4 counts, and HIV viral loads; (4) relevant clinical findings; (5) prescribed treatment and management; (6) all major cardiovascular diagnoses according to International Classification of Diseases, Tenth Revision (ICD-10) coding; and (7) multimorbidity data with up to 5 non-cardiovascular diagnoses according to ICD-10 coding [15]. Clinical assessment included symptoms scoring, a full clinical examination, a physical, and clinical status. Functional tests included WHO Functional Class (FC), 6-minute walk test/distance (6MWT/D), and Karnofsky Performance Score. Technical procedures included echocardiography, chest x-ray, and 12-lead electrocardiogram (ECG). Patient outcomes, including hospitalization and death during planned 36-month follow up, were also prospectively collected. A verbal autopsy was performed to record survival at the end of the study. Three-year survival data were available for 68% (32 of 47) of patients in the PH/HIV+ group.
Case Definition
A diagnostic algorithm to diagnose PH in resource-constraint settings without access to right heart catheterization of the European Society of Cardiology (ESC) and European Respiratory Society (ERS) was adopted [14], pretested, and data were compared between centers. On this basis, PH was diagnosed by specialist cardiologists and defined as documented elevated right ventricular systolic pressure >35 mm Hg on transthoracic echocardiography in the absence of pulmonary stenosis and acute RHF; accompanied by dyspnoea, fatigue, peripheral edema, and other cardiovascular symptoms, and ECG and chest x-ray changes in keeping with PH. Additional investigations such as chest computed tomography, ventilation/perfusion scans, or right heart catheterizations were performed at the discretion of the treating physician if available [12]. Patients were classified into 5 PH subgroups using the updated WHO classification of pulmonary hypertension [1, 2]. Group 1, pulmonary arterial hypertension; group 2, pulmonary hypertension due to left heart disease (PHLHD); group 3, pulmonary hypertension due to lung diseases and/or hypoxemia (PHLD); group 4, pulmonary hypertension due to pulmonary artery obstructions (often also referred to as chronic thromboembolic pulmonary hypertension [CTEPH]); and group 5, miscellaneous. The PAPUCO review committee (F.T., A.D., K.S., and A.O.M.) reviewed all clinical data and confirmed the WHO classification according to the most likely classification. None of the study participants were added to 2 subgroups.
Statistical Analysis
The data were analyzed using GraphPad Prism version 7.03 for Windows (GraphPad Software, La Jolla California). Continuous data are presented as medians plus interquartile ranges (IQR), and categorical data are presented as numbers (%). For group comparisons, we used (1) χ2 analysis with calculation of odds ratios and 95% confidence interval ([CI] where appropriate) for discrete variables and (2) Student t test and analysis of variance (ANOVA) for normally distributed continuous variables. Normal distribution was assessed using D’Agostino-Pearson normality test. For multiple group comparison, we used one-way ANOVA and χ2 where appropriate. Mortality data were used to generate Kaplan-Meier survival curves. Significance was accepted at the 2-sided level of less than .05.
RESULTS
Study Cohort
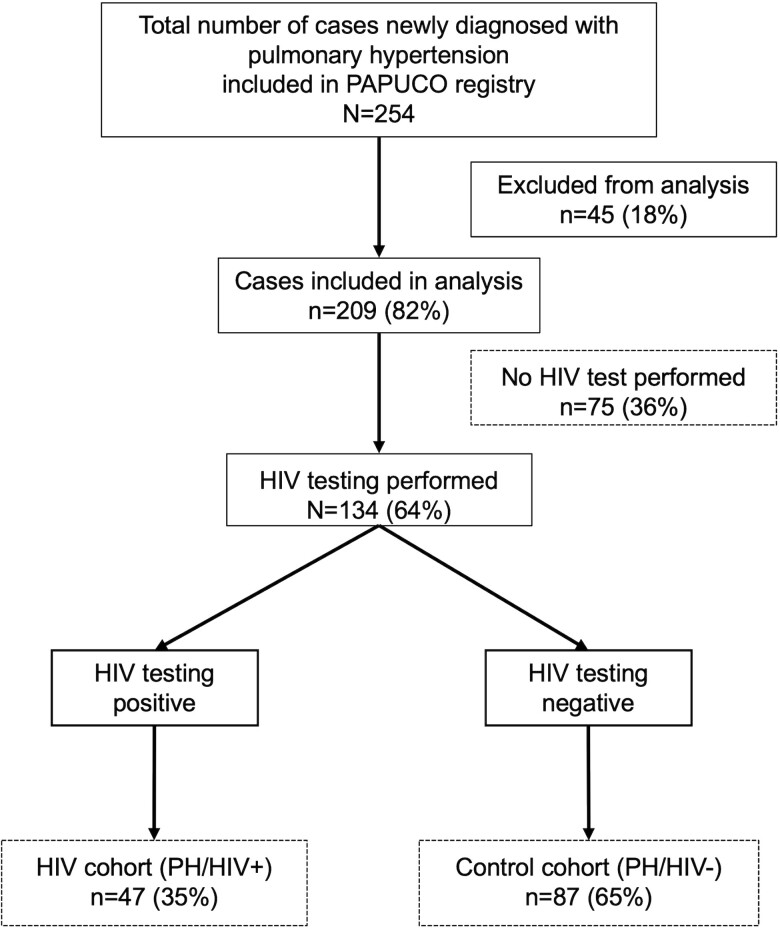
In total, 254 patients with PH were recruited into the PAPUCO study (Figure 1). Of these, 45 patients (18%) were excluded from analysis due to incomplete data or being minors (<18 years of age). Of the remaining 209 patients, 134 (64%) PH patients were tested for HIV. At the time of presentation, 47 (35%) patients tested positive for HIV and comprise the HIV cohort (PH/HIV+), and 87 (65%) patients tested negative for HIV and comprise the control cohort (PH/HIV−).
Figure 1.
Consort diagram of the Pan Africa Pulmonary hypertension Cohort (PAPUCO) study. HIV, human immunodeficiency virus; PH, pulmonary hypertension; PH/HIV+, HIV-associated pulmonary hypertension cohort; PH/HIV−, control cohort of pulmonary hypertension in HIV-uninfected patients.
Sociodemographic and Risk Profile
Table 1 summarizes the sociodemographic characteristics and risk factor profile of the PH cohort stratified by HIV status. The median age of the PH/HIV+ was 36 years (range, 23–62 years) compared to 44 years of the PH/HIV− (range, 19–91 years; P = .0004). The PH/HIV+ were more likely to be living in temporary shelters such as shacks compared to the PH/HIV− (40% versus 28%, P = .0215). No differences were observed in noncommunicable comorbidities and cardiovascular risk factors. Chronic liver disease was present in 6% of the PH/HIV+, all with positive hepatitis B surface antigen. No case of chronic liver disease was reported in the PH/HIV−. The number of previous or concurrent TB was significantly higher in the PH/HIV+ (n = 29, 62%) compared to the PH/HIV− (n = 16, 18%, P < .0001). However, no difference was observed in the number of previous TB episodes between the 2 cohorts. The mean number of TB episodes was generally high per patient (1.44 ± 0.09).
Table 1.
Sociodemographic and Risk Factor Profile of the 134 Patients With Pulmonary Hypertension Stratified by HIV Status
| Variables | All (n = 134) | PH/HIV+ (n = 47) | PH/HIV− (n = 87) | P Value |
|---|---|---|---|---|
| Sociodemographic Characteristics | ||||
| Median age in years (range) | 39 (19 to 91) | 36 (23 to 62) | 44 (19 to 91) | .0004 |
| Female | 79 (59) | 33 (70) | 46 (53) | .15 |
| African descent | 128 (95) | 47 (100) | 81 (93) | .18 |
| Education | ||||
| Never went to school or completed only primary school | 74 (55) | 29 (62) | 45 (52) | .54 |
| Income <30 USD per month | 43 (32) | 13 (28) | 30 (34) | .57 |
| Housing Conditions | ||||
| Temporary shelter (eg, shacks) | 35 (26) | 19 (40) | 16 (18) | .0215 |
| Traditional hut | 4 (3) | 2 (4) | 2 (2) | .82 |
| Risk Factor Profile | ||||
| Cardiovascular Risk Factors | ||||
| Family history for CVD | 45 (33) | 15 (32) | 30 (34) | .96 |
| Hypercholesterolemia | 5 (4) | 1 (2) | 4 (5) | .77 |
| Hypertension | 45 (33) | 14 (30) | 31 (36) | .79 |
| Diabetes | 10 (7) | 2 (4) | 8 (9) | .58 |
| Comorbidities | ||||
| Hemolytic anaemia | 0 | 0 | 0 | - |
| Rheumatic disease | 6 (4) | 1 (2) | 5 (6) | .63 |
| Chronic liver disease | 3 (<1) | 3 (6) | 0 | .0584 |
| Chronic lung disease | 19 (14) | 10 (21) | 9 (10) | .22 |
| Previous DVT/PE | 6 (<1) | 4 (11) | 2 (<1) | .25 |
| Chronic Infectious Diseases | ||||
| Previous or concurrent tuberculosis | 45 (34) | 29 (62) | 16 (18) | <.0001 |
| Concurrent tuberculosis | 10 (7) | 6 (13) | 4 (5) | .23 |
| TB episodes (mean ± SEM) | 1.44 ± 0.09 | 1.55 ± 0.12 | 1.25 ± 0.11 | .08 |
| Exposure to Smoke and Recreational Drugs | ||||
| Indoor cooking/heating without chimney | 35 (26) | 12 (26) | 23 (26) | .99 |
| History of smoking | 23 (17) | 10 (21) | 13 (15) | .65 |
| Alcohol abuse | 26 (19) | 11 (23) | 15 (17) | .69 |
| Recreational drug use | 3 (2) | 1 (2) | 2 (2) | .99 |
Abbreviations: CVD, cardiovascular disease; DVT, deep vein thrombosis; HIV, human immunodeficiency virus; PE, pulmonary embolism; PH, pulmonary hypertension; SEM, standard error of the mean; TB, tuberculosis; USD, US dollar.
NOTES: Data are number (percentage) or otherwise specified; P values based on t test, χ2, or Fisher's exact test where appropriated.
Clinical Findings and Signs of Heart Failure
Table 2 summarizes the clinical profile of the PH cohort stratified by HIV status. Symptoms at presentation were similar in both cohorts, but almost one third of the PH/HIV+ experienced angina-type chest pain (28% versus 1%, P = .0098). No difference was found with regards to New York Heart Association functional class or Karnofsky performance score. The median 6MWD was 264 meters (IQR, 120–360). The 6MWD below 300 meters was common in PH/HIV− (36% versus 1%, P = .0030) but achieved in most of PH/HIV+ (300 m versus 235 m). Heart rate in PH/HIV+ was 96/minute (IQR, 80–106) compared to 87/minute (IQR, 72–96, P = .01609), and respiration rate in PH/HIV+ was 28/minute (IQR, 20–34) compared to 24/minute (IQR, 20–28, P = .0374). On electrocardiogram, atrial fibrillation was rare in PH/HIV+ (1% versus 22%, .0282), but P-pulmonale was common (38% versus 14%, P = .0051) when comparing to PH/HIV−. No difference was found on chest radiographic imaging. On echocardiography, right ventricular (RV) function as assessed by tricuspid annular plane systolic excursion (TAPSE) was similar between HIV+ and HIV− patients. However, combined right atrial and ventricular hypertrophy showed a trend to significant difference (higher in HIV+).
Table 2.
Clinical Findings of the 134 Patients With Pulmonary Hypertension Stratified by HIV Status
| Clinical observation | All (n = 134) | PH/HIV+ (n = 47) | PH/HIV− (n = 87) | P Value |
|---|---|---|---|---|
| Symptoms at Presentation | ||||
| Dyspnoea | 122 (91) | 41 (87) | 81 (93) | .53 |
| Cyanosis | 16 (12) | 5 (11) | 11 (13) | .94 |
| Cough | 78 (58) | 28 (60) | 50 (57) | .97 |
| Fatigue | 116 (87) | 38 (81) | 78 (90) | .36 |
| Dizziness | 47 (35) | 17 (36) | 30 (35) | .98 |
| Syncope | 12 (1) | 7 (15) | 5 (1) | .21 |
| Palpitations | 96 (72) | 36 (77) | 60 (69) | .65 |
| Chest pain | 20 (15) | 13 (28) | 7 (1) | .0098 |
| NYHA Functional Class (n = 134) | ||||
| NYHA FC I/II | 49 (37) | 13 (28) | 36 (41) | .29 |
| NYHA FC III | 54 (40) | 22 (47) | 32 (37) | .51 |
| NYHA FC IV | 30 (22) | 12 (25) | 18 (21) | .81 |
| Karnofsky performance score* | 70 (50–80) | 70 (50–80) | 70 (50–80) | .89 |
| 6MWT distance* | 264 (120–360) | 300 (120–405) | 235 (114–346) | .18 |
| 6MWT <300m | 35 (26) | 4 (1) | 31 (36) | .003 |
| BMI* | 22.5 (19.9–27.9) | 22.8 (19.8–28.4) | 22.4 (19.8–27.1) | .83 |
| Heart rate at rest (beats/min)* | 90 (75–98) | 96 (80–106) | 87 (72–96) | .0160 |
| Systolic BP (mmHg)* | 118 (109–130) | 118 (111–131) | 120 (109–130) | .80 |
| Diastolic BP (mmHg)* | 77 (70–85) | 80 (70–85) | 76 (67–85) | .31 |
| Respiratory rate at rest (breaths/min)* | 24 (20–30) | 28 (20–34) | 24 (20–28) | .0374 |
| Pulse oximetry at rest (%)* | 97 (92–98) | 97 (93–99) | 97 (92–98) | .54 |
| Cardiac Auscultation | ||||
| Systolic murmur | 34 (25) | 7 (15) | 27 (31) | .12 |
| Loud P2 | 55 (41) | 27 (57) | 28 (32) | .0179 |
| Electrocardiogram | ||||
| Sinus rhythm | 57 (42) | 19 (40) | 38 (44) | .94 |
| Sinus tachycardia | 23 (17) | 9 (19) | 14 (16) | .90 |
| Atrial fibrillation | 21 (16) | 2 (1) | 19 (22) | .0282 |
| P-pulmonale | 30 (22) | 18 (38) | 12 (14) | .0051 |
| Right ventricular strain pattern | 25 (19) | 13 (28) | 12 (14) | .15 |
| Left ventricular strain pattern | 19 (14) | 8 (1) | 11 (13) | .79 |
| Chest X-Ray | ||||
| Cardiomegaly | 69 (52) | 20 (43) | 49 (56) | .31 |
| Prominent pulmonary arteries | 31 (23) | 11 (23) | 20 (23) | .99 |
| Echocardiography | ||||
| Median LVEF (%)* | 50 (37–66) | 51 (40–65) | 50 (36–66) | .67 |
| Median RVSP (mmHg)* | 56 (47–68) | 56 (47–67) | 56 (47–68) | .70 |
| Median TAPSE (mm)* | 15 (11–19) | 13 (10–16) | 15 (12–22) | .60 |
| Right atrial hypertrophy | 112 (84) | 42 (89) | 70 (80) | .41 |
| Right ventricular hypertrophy | 108 (81) | 41 (87) | 67 (77) | .37 |
| Combined right atrial and right ventricular hypertrophy | 101 (75) | 41 (87) | 60 (60) | .06 |
| TAPSE <15mm | 50 (37) | 22 (47) | 28 (32) | .25 |
| Signs of RHF at baseline | ||||
| Raised JVP | 98 (73) | 38 (81) | 60 (69) | .33 |
| Peripheral edema | 83 (62) | 30 (64) | 53 (61) | .95 |
| Raised JVP or peripheral oedema | 68 (51) | 26 (55) | 42 (48) | .74 |
| Diagnosis of RHF at baseline | 41 (31) | 19 (40) | 22 (25) | .19 |
Abbreviations: BMI, body mass index; BP, blood pressure; FC, functional class; HIV, human immunodeficiency virus; JVP, jugular venous pressure; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; 6MWT, 6-minute walk test; RHF, right heart failure; RVSP, right ventricular systolic pressure; TAPSE, tricuspid annular plane systolic excursion.
NOTES: Data are number (percentage) except for variables with asterisk (*) indicated where they are median (interquartile range); P values based on t test, χ2, or Fisher's exact test where appropriated.
The overall prevalence of signs of heart failure at baseline was >50% based on raised jugular venous pressure and peripheral edema, but only 31% were diagnosed with heart failure by the treating physician at this time.
Prevalence of Pulmonary Hypertension According to Subgroup Classification
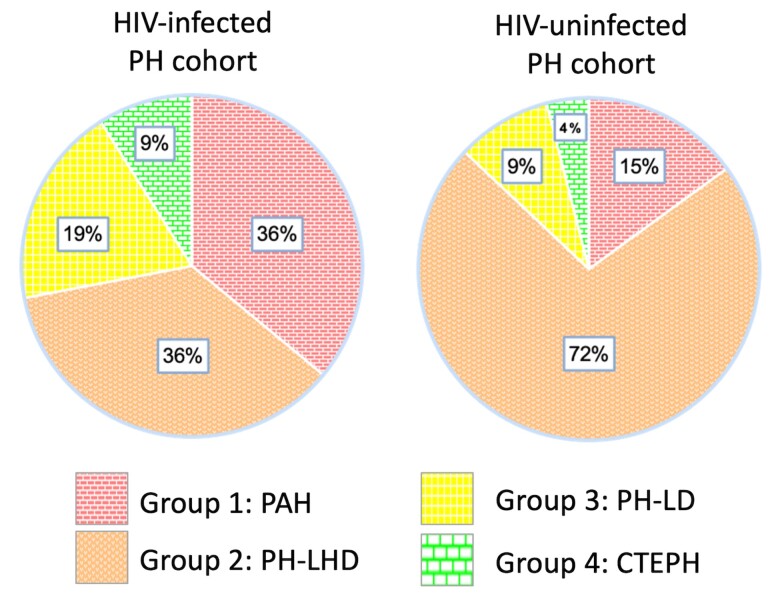
Figure 2 displays the prevalence of PH subgroups among PH/HIV+ and PH/HIV− patients in the PAPUCO study. Overall, 36% of patients in PH/HIV+ were classified group 1 PAH compared to 15% in PH/HIV− (P = .0084). In contrast, 36% of patients in PH/HIV+ were classified as group 2 PHLHD compared to 72% in PH/HIV− (P = .0009). Group 3 PHLD was more common in PH/HIV+ with no statistical significance (19% in PH/HIV+ versus 9% in PH/HIV−, P = .1102) but attributed to previous TB in PH/HIV+ (100%) and PH/HIV− (67%). Group 4 PH due to pulmonary artery obstruction, that is, chronic thromboembolism was rare in both cohorts, and none of the patients were classified group 5 PH with unclear and/or multifactorial mechanism.
Figure 2.
Prevalence of pulmonary hypertension (PH) according to the updated World Health Organization (WHO) classification in the human immunodeficiency virus (HIV)-infected PH cohort (n = 47) and in the HIV-uninfected PH cohort (n = 87) enrolled in the Pan Africa Pulmonary hypertension Cohort (PAPUCO) study. Group 1: PAH, pulmonary arterial hypertension; Group 2: PH-LHD, pulmonary hypertension due to left heart disease; Group 3: PH-LD, pulmonary hypertension due to lung disease and/or hypoxia; Group 4: pulmonary hypertension due to pulmonary artery obstructions (chronic thromboembolic pulmonary hypertension [CTEPH]).
Survival Estimates Among Human Immunodeficiency Virus-Infected Patients With Pulmonary Hypertension by Subgroup Classification
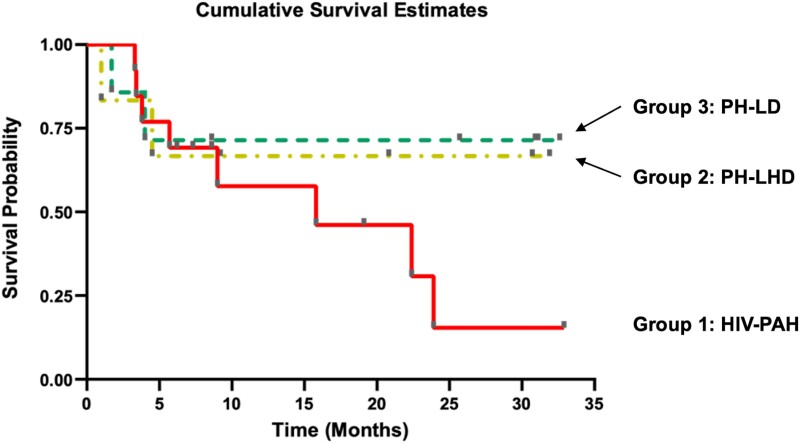
Figure 3 shows 3-year survival rate among HIV-infected patients by PH subgroups in the PAPUCO study. Overall, HIV-infected patients with PH exhibited a relatively high mortality at 3-year follow up. However, compare to HIV-infected patients classified as PHLHD or PHLD, those classified as PAH tended to a very poor survival rate especially after the second year of follow up.
Figure 3.
Kaplan-Meier plot showing cumulative survival estimates/time to death in months by pulmonary hypertension (PH) subtype of the human immunodeficiency virus (HIV)-infected cohort. Group 1: HIV-PAH, HIV-associated pulmonary arterial hypertension; Group 2: PH-LHD, pulmonary hypertension due to left heart disease; Group 3: PH-LD, pulmonary hypertension due to lung disease and/or hypoxia.
DISCUSSION
This study describes the baseline characteristics, analyses, and long-term outcome among Black African HIV-infected versus HIV-uninfected patients with PH included in the PAPUCO registry. The PAPUCO represents the largest prospective longitudinal study on patients diagnosed with PH in SSA, and the main results have been previously published [12, 16, 17]. It provides important insights into the clinical profile and treatment of PH from a uniquely African context [12]. Furthermore, it looked at PH and its multitude of etiologies prevalent in the region including HIV-associated PH, PH due to left heart diseases, PH due to respiratory diseases and/or hypoxemia, and the contribution of the TB epidemic to this condition; CTEPH, which may be due to a hypercoagulable state in patients coinfected with HIV and Mycobacterium tuberculosis.
Four main characteristics may define the profile of our HIV-infected patients who presented with PH. First, the age at presentation is younger in HIV-infected patients as previously described [18]. Second, most HIV-infected patients exhibited PAH (36%). Third, PHLD and chronic embolism were also higher in HIV-infected compared to HIV-uninfected patients. This is in line with reports on PH in HIV-infected people in the era of ART in France [19]. Before ART, the prevalence of PAH was 0.5% (95% CI, .10%–.90%) [20]. This indicates that ART did not reduce the prevalence of HIV-PAH. Numerous studies have concluded that the impact of antiretroviral drugs has been minimal, suggesting that the virus may influence vascular cells in the microenvironment where distribution of antiretroviral drugs is uneven [7]. The mechanism of HIV causing PAH therefore remains unclear. However, 3 main mechanisms suggested are as follows: HIV viral proteins found in the pulmonary vascular endothelium induce vascular oxidative stress, smooth myocyte proliferation and migration, and endothelial injury; cytokines due to the presence of HIV also induce dysregulation of endothelial wall and increase the genetic predisposition due to HIV [21, 22]. Fourth, HIV-infected patients with PAH exhibit high mortality rates [7], which was confirmed in our cohort study. The survival estimates of HIV-PAH are drastically reduced compared to the other groups of PH.
Pulmonary hypertension due to lung disease was higher in HIV-infected patients compared to the HIV-uninfected patients. Phenotypically, our data outline a high median respiratory rate in HIV-infected patients compared to HIV-uninfected patients, which might indicate the presence of lung disease in the HIV-infected population. In our cohort, the mean of previous TB episodes per patient was relatively high. Pulmonary TB is one of the most prominent lung infections affecting HIV-infected individuals [10]. In 2015, there were an estimated 10.4 million incident TB cases globally. Of these, approximately 11% occurred in HIV-infected individuals and more than 50% of those are living in the WHO African Region, mainly in southern Africa [23]. Considering the outlined prevalence, incidence, and history of TB in HIV-infected patients, PHLD must be considered highly prevalent in SSA, thus underreported, and an important contributor to the overall burden of PH in the African population. Mechanistically, TB commonly causes chronic structural lung disease such as fibrocavitation and bronchiectasis and pulmonary function abnormalities leading to gas exchange abnormalities and chronic hypoxia with consecutive PH [10]. Equally, there was a similar RV function as assessed by TAPSE between HIV-infected patients with PH and those without HIV-infection with PH. Among PWH, it had been shown that exposure to ART was associated with an increase in estimated PAP, whereas being unexposed to ART was associated with a decrease in RV systolic activity. Thus, lower respiratory tract infection leads to decreased RV systolic activity in ART-naive people [24]. Furthermore, combined right atrial and ventricular hypertrophy showed a trend to significant difference (higher in HIV-infected). Right ventricular dysfunction in HIV-infected people can be distinct from left ventricular/global cardiomyopathy or PH and warrants further investigation [25].
In addition, the data obtained in this study indicate that the prevalence of CTEPH was high in HIV-infected patients compared to HIV-uninfected patients. Human immunodeficiency virus infection has been recognized as a prothrombotic condition and is associated with a 2- to 10-fold increased risk of venous thromboembolism (VTE) in comparison with the general population of same age [11]. There are several intersecting mechanisms associated with HIV infection and antiviral therapy that are emerging, which may lead to vasculopathy and hypercoagulability in HIV-infected patients [23]. Furthermore, the risk appears to increase in the context of HIV/TB coinfection. In a retrospective study (n = 852, median age 45 years) in Kimberly, South Africa, more than half (n = 443, 52.0%) of patients with confirmed deep venous thrombosis (DVT) over a 5-year period were HIV-infected (n = 333, 39.1%), and HIV infection was the most prevalent cause of DVT (52.0%), followed by TB (12.4%) [26]. Among inpatients with HIV/TB coinfection, the septic state is high with an estimated risk of Mycobacterium tuberculosis blood stream infection of 45% (95% CI, 38–52) [27]. Infection-induced thrombosis is normally mediated by inflammation (also known as thromboinflammation), which causes platelet activation and endothelial damage, resulting in fibrin deposition and thrombus formation [28]. Specifically, inflammation of the lower respiratory tract causes fibrin secretion, which is characteristic of diseases such as pneumonia and acute respiratory distress syndrome, as well as the generation of pulmonary and systemic thrombin [29]. Such mechanism (plus alteration of pulmonary blood flow in response to the parenchymal process, disrupting Virchow's triad and classic DVT to pulmonary embolism transition) has been largely evoked with coronavirus disease 2019 pneumonia [30, 31]. Similarly, DVT during TB has been associated with an increase in plasma fibrinogen, impaired fibrinolysis, and a reduction in antithrombin III [26, 32]. This hypercoagulable condition might be exacerbated by hospitalization-related immobility and the use of anti-tuberculosis medications, which can potentially predispose to an elevated risk of VTE among PLHIV [32, 33].
One limitation of our cohort study is that most of the participants with HIV-associated PH (n = 30) were from clinics in Khayelitsha, South Africa. Khayelitsha is a densely populated, low-income, periurban township with one of the highest TB/HIV prevalence rates in the world. Echocardiography was the most appropriate strategy for our limited resource settings, but we cannot completely rule out the possibility of over diagnosis. Another important limitation of our study is that the cause of death has not been systematically assessed, and we report on overall morality knowing that PWH and people without HIV may die at different rates with PWH at risk of dying from opportunist infections. To our knowledge, this study is the first to compare the risk of PH in HIV-infected individuals in the Africa context. Notwithstanding these shortcomings, we learned that time is of essence in managing PH in the SSA context. Acknowledging the atypical phenotype of PH in this region—PWH who are younger than traditionally described [34] and have a relatively long 6MWD—our patients experienced delay in access to care, especially cardiac and tertiary [35] care; efforts need to be undertaken to substantially improve the quality of care of patients with PH in SSA [36]. The ECG and echocardiography are valuable diagnostic procedures for HIV-infected patients who are at high risk of heart and pulmonary disease. Reflecting on the high burden of TB-HIV coinfection in SSA, we advocate for further debates with regards to the implementation of ECG and echocardiography screening among at-risk HIV patients, which includes patients with previous episodes of TB [3, 16].
CONCLUSIONS
People with HIV in Africa are vulnerable to PH; this is associated with high prevalence rates of risk factors such as TB in the region. In this population, HIV-infected patients with PAH exhibit high mortality rates compared to other subtypes of PH. Monitoring for clinical signs and symptoms for PH in people with HIV is required. It shall be underlined that assessment of cardiac disease is part of standard HIV care. Additional research to better understand the complex pathophysiology and management of HIV-associated PH in the context of TB in the African setting is urgently needed.
Acknowledgments
We thank the Pan Africa Pulmonary Hypertension Cohort (PAPUCO) study teams at all African sites for their help with collecting medical reports and retention of study patients.
Author contributions. All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Financial support. Financial support was provided by unconditional research grants from the Pulmonary Vascular Research Institute (Grant Number 422348) and Bayer Healthcare Berlin (Grant Number 411278). The Hatter Institute for Cardiovascular Research in Africa of the University of Cape Town provided institutional support.
The PAPUCO Study Team: Lori Blauwet, Okechukwu S. Ogah, Amam Mbakwem, Patience Udo, Kemi Tibazarwa, Albertino Damasceno, Ashley K. Keates, Simon Stewart, Ahmed S. Ibrahim, and Rosie Burton.
Contributor Information
Friedrich Thienemann, General Medicine & Global Health, Cape Heart Institute, Faculty of Health Science, University of Cape Town, Cape Town, South Africa; Department of Medicine, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa; Department of Internal Medicine, University Hospital Zurich, University of Zurich, Zurich, Switzerland.
Patrick D M C Katoto, General Medicine & Global Health, Cape Heart Institute, Faculty of Health Science, University of Cape Town, Cape Town, South Africa; Department of Medicine and Center for Infectious Diseases, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa; Department of Medicine, Division of Respiratory Medicine and Prof. Lurhuma Biomedical Research Laboratory, Mycobacterium Unit, Catholic University of Bukavu, Bukavu, Democratic Republic of Congo.
Feriel Azibani, General Medicine & Global Health, Cape Heart Institute, Faculty of Health Science, University of Cape Town, Cape Town, South Africa; INSERM U942, Paris, France.
Vitaris Kodogo, General Medicine & Global Health, Cape Heart Institute, Faculty of Health Science, University of Cape Town, Cape Town, South Africa.
Sandra L Mukasa, General Medicine & Global Health, Cape Heart Institute, Faculty of Health Science, University of Cape Town, Cape Town, South Africa; Department of Medicine, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa.
Mahmoud U Sani, Department of Medicine, Bayero University Kano and Aminu Kano Teaching Hospital, Kano, Nigeria.
Kamilu M Karaye, Department of Medicine, Bayero University Kano and Aminu Kano Teaching Hospital, Kano, Nigeria.
Irina Mbanze, Faculty of Medicine, Universidade Eduardo Mondlane, Maputo, Mozambique.
Ana O Mocumbi, Faculty of Medicine, Universidade Eduardo Mondlane, Maputo, Mozambique; Instituto Nacional de Saúde, Maputo, Mozambique.
Anastase Dzudie, Department of Internal Medicine, Douala General Hospital, Cameroon.
Karen Sliwa, General Medicine & Global Health, Cape Heart Institute, Faculty of Health Science, University of Cape Town, Cape Town, South Africa; Department of Medicine, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa.
References
- 1. Janda S, Quon BS, Swiston J. HIV and pulmonary arterial hypertension: a systematic review. HIV Med 2010; 11:620–34. [DOI] [PubMed] [Google Scholar]
- 2. Schwarze-Zander C, Pabst S, Hammerstingl C, et al. Pulmonary hypertension in HIV infection: a prospective echocardiographic study. HIV Med 2015; 16:578–82. [DOI] [PubMed] [Google Scholar]
- 3. Thienemann F, Ntusi NAB, Battegay E, Mueller BU, Cheetham M. Multimorbidity and cardiovascular disease: a perspective on low- and middle-income countries. Cardiovasc Diagn Ther 2019; 10:376–85.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bigna JJR, Sime PSD, Koulla-Shiro S. HIV related pulmonary arterial hypertension: epidemiology in Africa, physiopathology, and role of antiretroviral treatment. AIDS Res Ther 2015; 12:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. ten Freyhaus H, Vogel D, Lehmann C, et al. Echocardiographic screening for pulmonary arterial hypertension in HIV-positive patients. Infection 2014; 42:737–41. [DOI] [PubMed] [Google Scholar]
- 6. Dellegrottaglie S, García-Alvarez A, Guarini P, Perrone-Filardi P, Fuster V, Sanz J. Prevalence and severity of ventricular dysfunction in patients with HIV-related pulmonary arterial hypertension. Heart Lung 2014; 43:256–61. [DOI] [PubMed] [Google Scholar]
- 7. Almodovar S, Cicalini S, Petrosillo N, Flores SC. Pulmonary hypertension associated with HIV infection: pulmonary vascular disease: the global perspective. Chest 2010; 137:6S–12S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bauer AL, Hogue IB, Marino S, Kirschner DE. The effects of HIV-1 infection on latent tuberculosis. Math Model Nat Phenom 2008; 3:229–66. [Google Scholar]
- 9. Almodovar S, Hsue PY, Morelli J, Huang L, Flores SC. Pathogenesis of HIV-associated pulmonary hypertension. Proc Am Thorac Soc 2011; 8:308–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahmed AEH, Ibrahim AS, Elshafie SM. Pulmonary hypertension in patients with treated pulmonary tuberculosis: analysis of 14 consecutive cases. Clin Med Insights Circ Respir Pulm Med 2011; 5:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sule AA, Pandit N, Handa P, et al. Risk of venous thromboembolism in patients infected with HIV: a cohort study. Int J Angiol 2013; 22:95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thienemann F, Dzudie A, Mocumbi AO, et al. The causes, treatment, and outcome of pulmonary hypertension in Africa: insights from the pan African pulmonary hypertension cohort (PAPUCO) registry. Int J Cardiol 2016; 221:205–11. [DOI] [PubMed] [Google Scholar]
- 13. HIV.gov. The Global HIV/AIDS Epidemic . Global Statistics. Available at: https://www.hiv.gov/hiv-basics/overview/data-and-trends/global-statistics. Accessed 22 December 2022.
- 14. Thienemann F, Dzudie A, Mocumbi AO, et al. Rationale and design of the pan African pulmonary hypertension cohort (PAPUCO) study: implementing a contemporary registry on pulmonary hypertension in Africa. BMJ Open 2014; 4:e005950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS) endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46:903–75. [DOI] [PubMed] [Google Scholar]
- 16. Balieva I, Dzudie A, Thienemann F, et al. Prevalence and predictive value of electrocardiographic abnormalities in pulmonary hypertension: evidence from the pan-African pulmonary hypertension cohort (PAPUCO) study. Cardiovasc J Afr 2017; 28:370–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dzudie A, Dzekem BS, Tchoumi CT, et al. Pulmonary hypertension as seen in a rural area in sub-Saharan Africa: high prevalence, late clinical presentation and a high short-term mortality rate during follow up. Cardiovasc J Afr 2018; 29:208–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Petitpretz P, Brenot F, Azarian R, et al. Pulmonary hypertension in patients with human immunodeficiency virus infection. Comparison with primary pulmonary hypertension. Circulation 1994; 89:2722–7. [DOI] [PubMed] [Google Scholar]
- 19. Sitbon O, Lascoux-Combe C, Delfraissy JF, et al. Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med 2008; 177:108–13. [DOI] [PubMed] [Google Scholar]
- 20. Speich R, Jenni R, Opravil M, Pfab M, Russi EW. Primary pulmonary hypertension in HIV infection. Chest 1991; 100:1268–71. [DOI] [PubMed] [Google Scholar]
- 21. Thienemann F, Sliwa K, Rockstroh JK. HIV and the heart: the impact of antiretroviral therapy: a global perspective. Eur Heart J 2013; 34:3538–46. [DOI] [PubMed] [Google Scholar]
- 22. Jarrett H, Barnett C. HIV-associated pulmonary hypertension. Curr Opin HIV AIDS 2017; 12:566–71. [DOI] [PubMed] [Google Scholar]
- 23. World Health Organization (WHO) . Global tuberculosis report 2017. Available at: https://www.who.int/publications/i/item/9789241565516. Accessed 22 December 2022.
- 24. Idris NS, Uiterwaal CSPM, Burgner DP, Grobbee DE, Kurniati N, Cheung MMH. Effects of HIV infection on pulmonary artery pressure in children. Glob Heart 2019; 14:367–72. [DOI] [PubMed] [Google Scholar]
- 25. Simon MA, Lacomis CD, George MP, et al. Isolated right ventricular dysfunction in patients with human immunodeficiency virus. J Card Fail 2014; 20:414–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mampuya FK, Steinberg WJ, Raubenheimer JE. Risk factors and HIV infection among patients diagnosed with deep vein thrombosis at a regional/tertiary hospital in Kimberley, South Africa. S Afr Fam Pract 2018; 60:107–13. [Google Scholar]
- 27. Barr DA, Lewis JM, Feasey N, et al. Mycobacterium tuberculosis bloodstream infection prevalence, diagnosis, and mortality risk in seriously ill adults with HIV: a systematic review and meta-analysis of individual patient data. Lancet Infect Dis 2020; 20:742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Beristain-Covarrubias N, Perez-Toledo M, Thomas MR, Henderson IR, Watson SP, Cunningham AF. Understanding infection-induced thrombosis: lessons learned from animal models. Front Immunol 2019; 10:2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ha H, Kim KH, Park JH, et al. Thromboembolism in Mycobacterium tuberculosis infection: analysis and literature review. Infect Chemother 2019; 51:142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Price LC, McCabe C, Garfield B, Wort SJ. Thrombosis and COVID-19 pneumonia: the clot thickens! Eur Respir J 2020; 56:2001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huertas A, Montani D, Savale L, et al. Endothelial cell dysfunction: a major player in SARS-CoV-2 infection (COVID-19)? Eur Respir J 2020; 56:2001634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Robson SC, White NW, Aronson I, Woollgar R, Goodman H, Jacobs P. Acute-phase response and the hypercoagulable state in pulmonary tuberculosis. Br J Haematol 1996; 93:943–9. [DOI] [PubMed] [Google Scholar]
- 33. Hodkinson KE, Mahlangu JN. Deep-vein thrombosis in the era of high HIV and tuberculosis prevalence: a prospective review of its diagnosis and treatment in a quaternary centre. S Afr Med J 2017; 107:859–63. [DOI] [PubMed] [Google Scholar]
- 34. Hoeper MM, Humbert M, Souza R, et al. A global view of pulmonary hypertension. Lancet Respir Med 2016; 4:306–22. [DOI] [PubMed] [Google Scholar]
- 35. Dzudie A, Kengne AP, Thienemann F, Sliwa K. Predictors of hospitalisations for heart failure and mortality in patients with pulmonary hypertension associated with left heart disease: a systematic review. BMJ Open 2014; 4(7):e004843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dzudie A, Dzekem BS, Ojji DB, et al. Pulmonary hypertension in low- and middle-income countries with focus on sub-Saharan Africa. Cardiovasc Diagn Ther 2020; 10:316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]