Abstract
Background
Linezolid has been prioritized for treating multidrug-resistant tuberculosis (MDR TB), but toxicity limits its use. We report treatment outcomes for MDR TB patients in California who received standard-dose linezolid vs those who switched to low-dose.
Methods
We include culture-positive MDR TB cases treated with linezolid and receiving California MDR TB Service consultation during 2009–2016. Demographic, clinical, and laboratory data are analyzed using univariate analysis to compare patients who received linezolid of different dosing strategies. Analysis end points are linezolid treatment duration (measure of tolerability), treatment success (completion or cure), and adverse events (AEs).
Results
Sixty-nine of 194 (36%) MDR TB patients met inclusion criteria. While all patients began linezolid treatment at 600 mg daily, 39 (57%) continued at this dosage (standard-dose), and 30 (43%) switched to 300 mg daily (29%) or intermittent dosing (14%) (low dose). Patients on standard-dose linezolid were treated for 240 days, compared with 535 for those on low-dose (P < .0001). Sixty-three patients (91%) achieved treatment success, 2 (2.9%) died, 1 (1.5%) failed treatment, 1 (1.5%) stopped treatment due to side effects, and 2 (2.9%) were lost or moved. Treatment success was higher (P = .03) in the low-dose group. Sixty-two patients experienced ≥1 hematologic (71%) or neurologic (65%) AE. Those on low-dose linezolid experienced significantly (P = .03) fewer AEs per linezolid-month after switching (0.32 vs 0.10).
Conclusions
Patients who switched to low dose tolerated linezolid longer with better treatment outcomes and fewer recurring AEs.
Keywords: linezolid, low-dose, multidrug-resistant, treatment, tuberculosis
BACKGROUND
Tuberculosis (TB) is the leading infectious cause of death worldwide (excluding coronavirus disease 2019 [COVID-19]) [1]. Multidrug-resistant (MDR) and extensively drug-resistant (XDR) TB are particularly hard to treat because of few available drugs and related toxicity. Worldwide, MDR TB is associated with poor outcomes, with an estimated 56% of patients achieving treatment success [2].
World Health Organization (WHO) guidelines have prioritized drugs recommended for treating MDR TB [3]. Linezolid, an oxazolidinone first approved for the treatment of drug-resistant, gram-positive bacterial infections in 2000, has been shown to have good activity against drug-resistant strains of Mycobacterium tuberculosis complex (MTBC) in both in vitro and in vivo studies. Linezolid is now considered a Group A drug to be used for most patients and is a component of newer short-course regimens [4–8]. However, linezolid-related toxicity, primarily hematologic and neurologic, is frequently treatment-limiting.
Recent research suggests that linezolid-related toxicity can be mitigated by dose adjustment and intermittency [9–15]. We performed an analysis of MDR TB cases in California to describe the use of low-dose linezolid and related outcomes.
California has the highest number of both TB and MDR TB cases in the United States, reporting 23% (2091/9029) and 25% (32/128), respectively, in 2018 [16]. Consistent with Centers for Disease Control and Prevention recommendations, the California Department of Public Health MDR TB Service provides consultation for MDR TB management in California. Between 2002 and 2012, the proportion of patients receiving MDR TB Service consultation increased from 12% to 63% [17].
A report published in 2010 reported demographic and clinical characteristics, outcomes, and adverse events (AEs) of California MDR TB patients who received linezolid as part of an MDR TB regimen between 2003 and 2007 [18]. Linezolid was found to be well tolerated, with a low proportion of patients discontinuing treatment. In this report, we update these findings and compare standard-dose linezolid with reduced and intermittent dose. We aimed to determine the duration of linezolid treatment for each dosing strategy and investigate whether continuation of linezolid at a reduced dose allows for extended linezolid treatment.
METHODS
Study Population/Inclusion Criteria
Inclusion criteria were culture-positive MDR TB reported in California between January 1, 2009, and December 31, 2016, receipt of full MDR TB Service consultation (at least 1 initial consultation and 1 follow-up written consultation), and treatment with linezolid as part of an MDR TB regimen. During this period, the MDR TB Service aimed for at least 18 months of treatment with an individualized regimen after culture conversion to negative. Management of linezolid including the decision to stop or reduce linezolid dose or dose frequency was based on individualized provider decision, and no consistent clinical criteria were used to select between these regimens. All patients received treatment under directly observed therapy at least 5×/week.
MDR TB Service patient charts were abstracted and supplemented by the MDR TB Service database and the California TB Registry (data censored November 20, 2018). Data collected included demographic and clinical characteristics, treatment regimen, duration, and outcomes, and type, severity, and timing of AEs.
Definitions
See Supplementary Table 1 for drug-resistant (DR) TB and treatment outcome definitions. Low-dose linezolid was a daily (≥5×/week) dose of 300 mg or intermittent dose (600 mg administered <5×/week) of linezolid. The standard dose was 600 mg of linezolid daily (≥5×/week).
Linezolid tolerability was assessed by complete blood count (CBC) results and medical record review for symptoms of neuropathy. All symptoms and signs of peripheral neuropathy, optic neuropathy, or cytopenia (see detailed definition in Supplementary Table 2) occurring subsequent to linezolid initiation and within 1 month of linezolid stoppage were included if attributed to linezolid by a clinician or if linezolid was discontinued, held, or changed dose within 1 month after the event was first documented. Documentation of full resolution of cytopenia and/or symptoms relating to an AE within 1 month of linezolid treatment termination constituted resolution. We also assessed linezolid treatment duration as a measure of tolerability because adverse events often resulted in dosage change, not discontinuation. Linezolid treatment completion entailed continuation of linezolid until MDR TB treatment stop.
Analysis
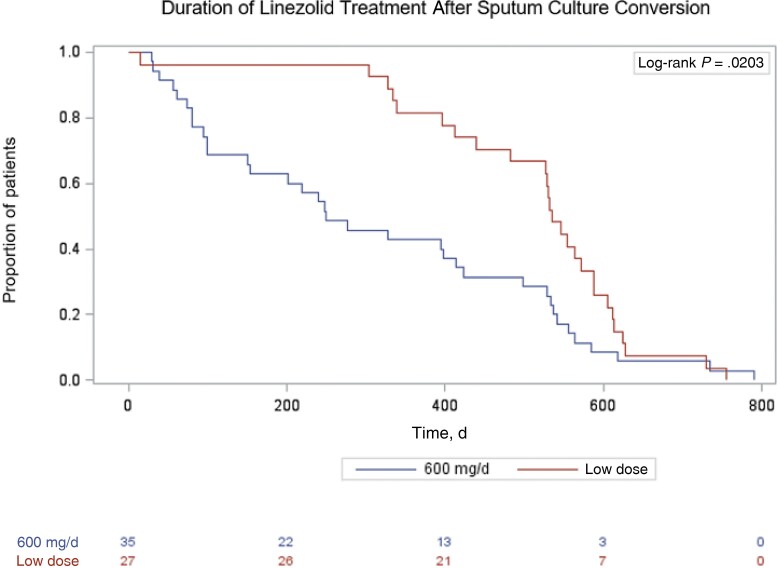
Patients switched to 300 mg daily and intermittent linezolid after initial treatment with the standard dose were individually analyzed and combined into 1 group, “low-dose,” for univariate analysis. We used the chi-square, Fisher exact, and Wilcoxon rank-sum tests to determine significant differences in demographic and clinical characteristics and treatment outcomes. We used the Cochran-Armitage test for trend for linezolid use, Kaplan-Meier curves to display probability of linezolid discontinuation, and the log-rank test to compare these curves. P values <.05 were considered statistically significant. Kaplan-Meier curves set day 0 as the date of culture conversion and the proportion of patients on linezolid to 1 at this date in all groups because both standard- and low-dose groups began treatment at the standard dosage (Figure 3). Analyses use SAS software, version 9.4 (SAS Institute, Cary, NC, USA).
Figure 3.
Kaplan-Meier curve of time from sputum culture conversion to linezolid discontinuation by linezolid dose among patients with MDR TB in California, 2009–2016. Abbreviation: MDR TB, multidrug-resistant tuberculosis.
Patient Consent
The analysis of routinely collected MDR Service data was reviewed and approved by the California Health and Human Service Agency's Committee for the Protection of Human Subjects. The analysis did not include factors necessitating patient consent.
RESULTS
Patient Characteristics
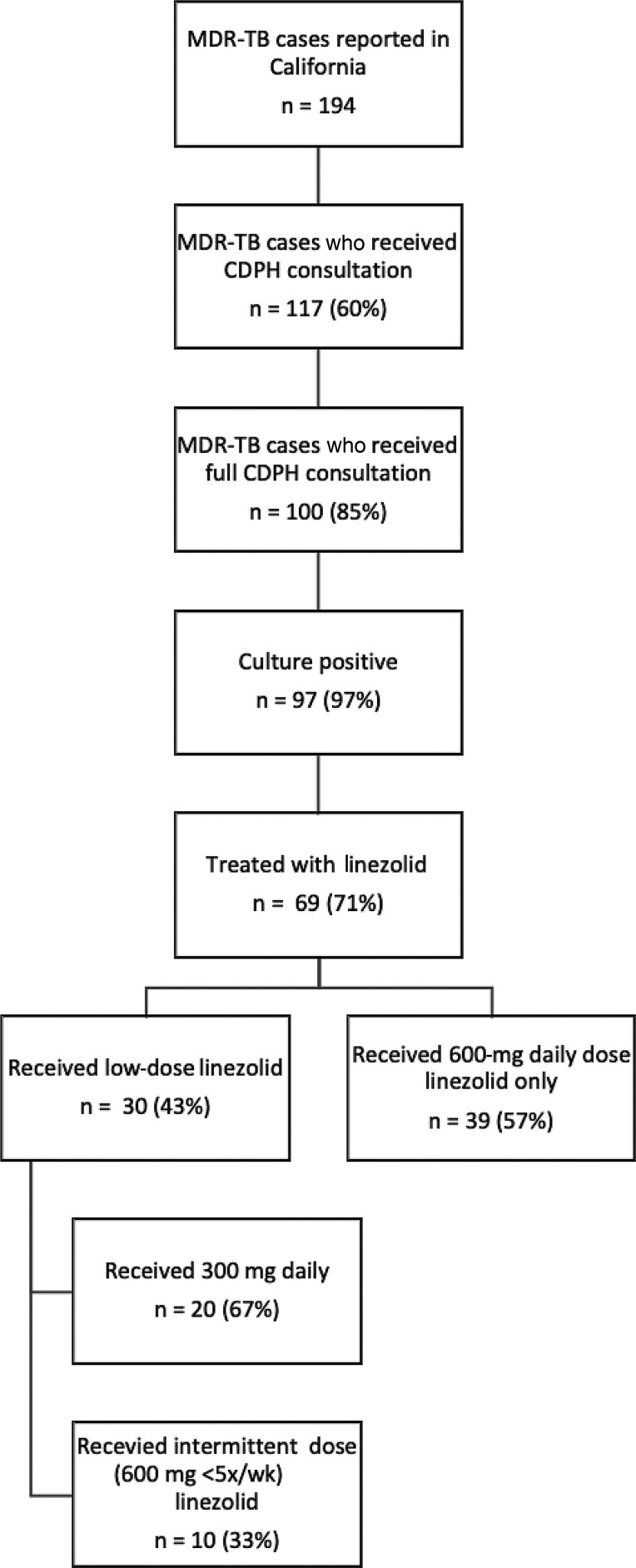
During 2009–2016, 194 MDR TB cases were reported in California; 117 (60%) patients received MDR TB Service consultation, either full (n = 100; 85%) or partial (n = 17; 15%) (Figure 1). Of those receiving full consultation, 97 (97%) were culture-positive; 14 (14%) had extrapulmonary disease, and 3 (3%) were clinically diagnosed. Sixty-nine of 97 (71%) patients were treated with a regimen including linezolid, and 30 (43%) of these 69 patients had linezolid dose (n = 20; 67%) or dose frequency (n = 10; 33%) reduced during treatment (Figure 1).
Figure 1.
Analysis population: patients with MDR TB treated with linezolid in California, 2009–2016. Abbreviations: CDPH, California Department of Public Health; MDR TB, multidrug-resistant tuberculosis.
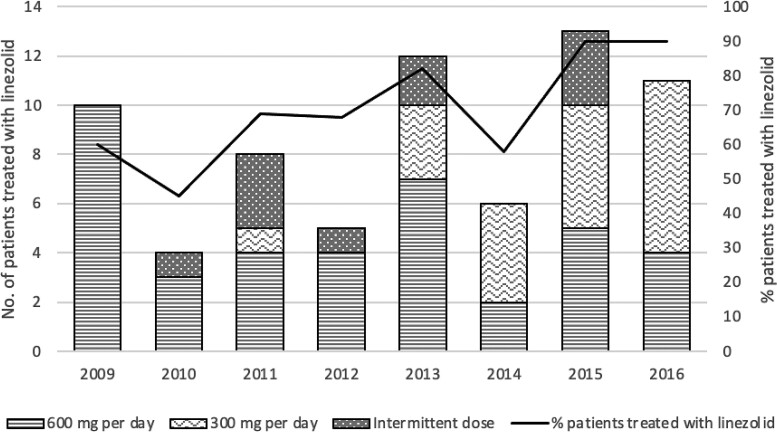
From 2009 to 2016, linezolid treatment among MDR cases increased from 30% to 38% (P = .016) (Figure 2). The proportion of linezolid-treated patients with modified dose or dose frequency also increased from 0 patients in 2009 to 7 of 12 (58%) patients in 2016.
Figure 2.
Linezolid treatment among patients with MDR TB who received California MDR TB Service consultation, 2009–2016. Abbreviation: MDR TB, multidrug-resistant tuberculosis.
Demographic Characteristics
The majority of patients treated with linezolid were non-US-born (87%) (Table 1), comparable to the proportion among California TB cases (83%) and California MDR TB cases (92%) in 2018 [19]. At MDR TB treatment initiation, the median age (range) was 37 (7–81) years.
Table 1.
Baseline Characteristics of Patients With MDR TB Treated With Linezolid by Linezolid Dose—California, 2009–2016
| Overall (n = 69) | Standard-Dose Linezolid (600 mg/d; n = 39) | Low-Dose Linezolid (Intermittent Dose and 300-mg Daily Dose Aggregate; n = 30) | |
|---|---|---|---|
| Age, median (range), y | 37 (7–81) | 36.5 (7–81) | 37 (19–77) |
| 0–15 y, No. (%) | 1 (1.4) | 1 (2.6) | 0 (0) |
| 15–50 y, No. (%) | 43 (62) | 25 (64) | 18 (60) |
| 50–75 y, No. (%) | 22 (32) | 11 (28) | 11 (37) |
| 75+ y, No. (%) | 3 (4.3) | 2 (5.1) | 1 (3.3) |
| Female, No. (%) | 32 (46) | 18 (46) | 14 (47) |
| Non-US-born, No. (%) | 60 (87) | 35 (90) | 25 (83) |
| Previous TB diagnosis, No. (%) | 20 (29) | 11 (28) | 9 (30) |
| Pulmonary only, No. (%) | 59 (86) | 33 (85) | 26 (87) |
| Extrapulmonary disease,a No. (%) | 10 (14) | 6 (15) | 4 (13) |
| Pre-extensively drug-resistant, No. (%) | 24 (35) | 14 (36) | 10 (33) |
| Fluoroquinolone-resistant, No. (%) | 20 (29) | 12 (31) | 8 (27) |
| Injectable-resistant, No. (%) | 4 (5.8) | 2 (5.1) | 2 (6.7) |
| Extensively drug-resistant, No. (%) | 5 (7.2) | 1 (2.6) | 4 (13) |
| Resistant to all 4 first-line drugs (RIF, INH, EMB, PZA), No. (%) | 21 (30) | 9 (23) | 12 (40) |
| Concurrent conditions | |||
| HIV+, No. (%) | 1 (1.4) | 0 (0) | 1 (3.3) |
| Diabetes mellitus, No. (%) | 17 (25) | 8 (21) | 9 (30) |
| Other immunosuppression, No. (%) | 7 (10) | 4 (10) | 3 (10) |
| Neurologic condition, No. (%) | 10 (14) | 6 (15) | 4 (13) |
| Visual deficit, No. (%) | 23 (33) | 11 (28) | 12 (40) |
| Cytopenia, No. (%) | 27 (39) | 16 (41) | 11 (37) |
| ≥1 other baseline concurrent conditions/risk factors, No. (%) | 49 (71) | 27 (69) | 22 (73) |
| Cavitary, No. (%) (of 63 pulmonary cases) | 25 (40) | 14 (39) | 11 (41) |
Abbreviations: EMB, ethambutol; INH, isoniazid; MDR, multidrug-resistant; PZA, pyrazinamide; RIF, rifampicin; TB, tuberculosis.
Four patients had both pulmonary and extrapulmonary TB.
Seventeen of 69 (25%) patients had concurrent diabetes mellitus (DM) (Table 1). Eight (12%) patients had documented immunosuppression, 1 of whom (1.4%) was HIV positive. Baseline neurologic and hematologic conditions were common: 10 (14%) neurologic conditions, 23 (33%) visual deficits, and 27 (39%) cytopenias.
TB Disease Characteristics
Of 69 MDR TB patients on linezolid, 59 (86%) had only pulmonary disease, 6 (9%) had only extrapulmonary disease, and 4 (6%) had both pulmonary and extrapulmonary involvement (Table 1).
Twenty (29%) patients had documented previous TB disease (Table 1). Of pulmonary cases, 25 (40%) showed cavitary changes noted on their initial chest radiograph or chest computed tomography (Table 1).
Treatment
The median number of TB drugs over the course of MDR TB treatment (range) was 9 (5–15), and all regimens included a fluoroquinolone and an injectable agent, unless resistance to these drug classes was documented (Table 2). Nearly all patients, 68/69 (99%), were given concurrent vitamin B6.
Table 2.
Treatment Characteristics and Outcomes Among Patients With MDR TB Treated With Linezolid by Dose—California, 2009–2016
| Overall (n = 69) | Standard-Dose Linezolid (600 mg/d; n = 39) | Low-Dose Linezolid (Intermittent Dose and 300-mg Daily Dose; n = 30) | |
|---|---|---|---|
| Total number of drugs over course of MDR TB treatment, median (range) | 9 (5–15) | 9 (5–15) | 9 (6–14) |
| Vitamin B6, No. (%) | 68 (99) | 38 (97) | 30 (100) |
| Vitamin B6 ≥200 mg daily, No. (%) | 7 (10) | 2 (5.1) | 5 (17) |
| Concurrent TB medications | |||
| ≥900 mg daily INH, No. (%) | 1 (1.4) | 1 (2.6) | 0 (0) |
| Moxifloxacin, No. (%) | 60 (87) | 35 (90) | 25 (83) |
| Levofloxacin, No. (%) | 28 (41) | 13 (33) | 15 (50) |
| Ethambutol, No. (%) | 50 (72) | 28 (72) | 22 (73) |
| Cycloserine, No. (%) | 47 (68) | 24 (62) | 23 (77) |
| Rifampin, No. (%) | 9 (13) | 8 (21) | 1 (3.3) |
| Days to culture conversion from MDR TB treatment start, median (range) | 41 (1–127) | 41.5 (5–127) | 39 (1–90) |
| aImaging improvement, No. (%) | 6/8 (75) | 3/4 (75) | 3/4 (75) |
| aWeight gain, No. (%) | 10/10 (100) | 6/6 (100) | 4/4 (100) |
| Treatment outcome | |||
| Cure, No. (%) | 48 (70) | 23 (59) | 25 (83) |
| Treatment complete, No. (%) | 15 (22) | 10 (26) | 5 (17) |
| Treatment failed, No. (%) | 1 (1.4) | 1 (2.5) | 0 (0) |
| Stopped treatment due to side effects, No. (%) | 1 (1.4) | 1 (2.5) | 0 (0) |
| Died, No. (%) | 2 (2.9) | 2 (5.1) | 0 (0) |
| Lost to follow-up, No. (%) | 2 (2.9) | 2 (5.1) | 0 (0) |
| Successful outcome, No. (%) | 63 (91) | 33 (85) | 30 (100) |
| On linezolid at end of treatment of those who completed treatment, No. (%) | 35/63 (56) | 13/33 (39) | 22/30 (73) |
| On linezolid at end of treatment of those who completed treatment AND had ≥1 adverse event, No. (%) | 32/58 (55) | 11/28 (39) | 21/30 (70) |
| ≥ 6 mo linezolid treatment completed, No. (%) | 53 (77) | 24 (62) | 29 (97) |
| Reason linezolid stopped | |||
| Treatment completion, No. (%) | 35 (51) | 13 (33) | 22 (73) |
| Adverse event, No. (%) | 26 (38) | 19 (49) | 7 (23) |
| Treatment end (failure), No. (%) | 1 (1.4) | 1 (2.6) | 0 (0) |
| Other, No. (%) | 7 (10) | 6 (15) | 1 (3.3) |
| Linezolid treatment duration, median (range), d | 413 (14–790) | 240 (14–790) | 535 (304–755) |
| MDR TB treatment duration, median (range), d | 591 (42–857) | 592 (42–857) | 589.5 (412–844) |
Abbreviations: INH, isoniazid; MDR, multidrug-resistant; TB, tuberculosis.
These statistics only include patients with extrapulmonary TB involvement. The denominator for imaging improvement is 8, the number of extrapulmonary patients with repeat imaging data.
The median (range) duration of linezolid therapy, excluding drug interruptions, was 413 (14–790) days (Table 2). For those treated only with standard-dose linezolid, the median duration (range) was shorter (P < .0001) at 240 (14–790) days than for those on low-dose, 535 (304–755) days. The median duration of MDR TB treatment overall (range) was 591 (42–857) days and was comparable among treatment subgroups: for standard-dose 592 days (42–857) days and for low-dose 589.5 (412–844) days.
Over the course of linezolid treatment, patients received concurrent TB medications as part of their drug regimen, some of which are associated with neurologic and/or hematologic adverse events. These medications included high-dose isoniazid (1, 1.4%), moxifloxacin (60, 87%), levofloxacin (28, 41%), ethambutol (50, 72%), cycloserine (47, 68%), and rifampin (9, 13%) (Table 2).
Treatment Outcomes
The median time to culture conversion (range) was 41 (1–127) days, excluding extrapulmonary cases and those with a documented culture conversion date before MDR TB treatment initiation (Table 2). Sixty-three (91%) patients achieved treatment success.
Patients treated with low-dose linezolid were more likely to have successful outcomes (P = .03) than those receiving the standard dose, with 30/30 (100%) and 33/39 (85%) cured or having completed treatment, respectively. No patients on low-dose linezolid failed treatment, were lost to follow-up, or died. Of those on standard-dose linezolid, 1 (2.5%) failed treatment, 1 (2.5%) stopped treatment due to side effects, 2 (5%) were lost to follow-up, and 2 (5%) died.
Of 10 patients with extrapulmonary disease, 8 (80%) had repeat imaging studies during MDR TB treatment and 6 of 8 (75%) showed signs of improvement. All patients with extrapulmonary TB gained weight between their first and last weight measurements. Overall, all 10 extrapulmonary cases had a successful outcome; 4 (40%) were cured, and 6 (60%) completed treatment.
Of all patients with successful outcomes (n = 63), 35 (56%) continued linezolid to MDR TB treatment completion. Those on low-dose linezolid were significantly (P = .001) more likely to continue linezolid therapy to treatment completion (22/30, 73%) than those on the standard dose (13/33, 39%). Furthermore, 53 (77%) patients on linezolid completed 6 months or more of therapy, and those in the low-dose group were significantly (P < .0001) more likely to do so.
Patients on intermittent linezolid therapy were initially treated with the standard dose for 75 (range, 14–425) days before continuing at a reduced dose frequency for 518 (range, 147–708) days (Table 3). Those on 300 mg of daily linezolid were initially treated at the standard dose for 146.5 (range, 2–472) days before continuing at a lower dose for 308 (range, 12–640) days. Those whose dose was reduced due to an AE were treated on standard-dose linezolid for shorter durations (115.5 [range, 2–472]) than those whose reason for dose reduction was prophylactic or other (135 [range, 14–360]). One patient (3%) treated with standard-dose linezolid stopped linezolid before culture conversion (Figure 3). Following culture conversion, those treated with low-dose linezolid stayed on linezolid for significantly longer than those who did not switch (P = .0006).
Table 3.
Timing and Reason for Changing to Low-Dose Linezolid Among Patients With MDR TB Treated With Linezolid by Dosing Regimen—California, 2009–2016
| Low-Dose Linezolid Overall (Intermittent Dose and 300-mg Daily Dose Aggregate; n = 30) | Intermittent Dose of Linezolid (n = 10) | 300 mg/d of Linezolid (n = 20) | |
|---|---|---|---|
| Time on 600 mg daily linezolid before reduction, median (range), d | 121 (2–472) | 75 (14–425) | 146.5 (2–472) |
| Time on 600 mg daily linezolid before reduction of those whose dose change was due to an adverse event, median (range), d | 115.5 (2–472) | 94 (14–425) | 149 (2–472) |
| Time on 600 mg daily linezolid before reduction of those whose dose change was NOT due to an adverse event, median (range), d | 135 (14–360) | 14 (14–14)a | 144 (51–360) |
| Time on low-dose linezolid, median (range), d | 330 (12–708) | 518 (147–708) | 308 (12–640) |
| Reason for dose change | |||
| Adverse event, No. (%) | 22 (73) | 9 (90) | 13 (65) |
| Prophylactic, No. (%) | 7 (23) | 0 (0) | 7 (35) |
| Other, No. (%) | 1 (3.3) | 1 (10) | 0 (0) |
Abbreviation: MDR TB, multidrug-resistant tuberculosis.
There was only 1 patient in the intermittent group whose dose change was not due to an adverse event.
The most common reason for linezolid discontinuation was treatment completion (35, 51%), followed by AE (26, 38%) (Table 2). Patients in the low-dose group were more likely (P = .001) to stop linezolid as a result of treatment completion and less likely to stop linezolid due to an AE (P = .03) than those in the standard-dose group (Table 2).
Drug Toxicity
Of all 69 patients treated with linezolid, 62 (90%) had documentation of at least 1 hematologic or neurologic AE over the course of MDR TB treatment (Table 4). There were 147 AEs (2.13 per person) (Table 5). Forty-nine (71%) patients experienced ≥1 hematologic AE with a total of 89 hematologic AEs (1.30 per person). Seven (10%) patients developed neutropenia, 34 (49%) anemia, and 21 (30%) thrombocytopenia. Forty-five (65%) patients experienced ≥ 1 neurologic AE, with a total of 58 neurologic AEs (0.84 per person). Thirty-two (46%) patients developed peripheral neuropathy, 21 (30%) optic neuropathy or visual changes, and 2 (3%) had other neurologic AEs.
Table 4.
Patients With MDR TB Treated With Linezolid With ≥1 Linezolid-Related Adverse Event—California, 2009–2016
| Overall (n = 69) | Standard-Dose Linezolid (600 mg/d; n = 39) | Low-Dose Linezolid (Intermittent Dose and 300-mg Daily Dose; n = 30) | Intermittent Dose of Linezolid (n = 10) | 300 mg/d of Linezolid (n = 20) | |
|---|---|---|---|---|---|
| Any adverse events, No. (%) | 62 (90) | 33 (85) | 29 (97) | 10 (100) | 19 (95) |
| Hematologic, No. (%) | 49 (71) | 25 (64) | 24 (80) | 9 (90) | 15 (75) |
| Leukopenia, No. (%) | 27 (39) | 17 (25) | 10 (33) | 2 (20) | 8 (40) |
| Neutropenia, No. (%) | 7 (10) | 4 (10) | 3 (10) | 1 (10) | 2 (10) |
| Anemia, No. (%) | 34 (49) | 16 (44) | 18 (60) | 7 (70) | 11 (55) |
| Thrombocytopenia, No. (%) | 21 (30) | 10 (26) | 11 (37) | 6 (60) | 5 (25) |
| Neurologic, No. (%) | 45 (65) | 26 (67) | 19 (63) | 7 (70) | 12 (60) |
| Symptoms or diagnosis of peripheral neuropathy, No. (%) | 32 (46) | 18 (46) | 14 (47) | 4 (40) | 10 (50) |
| Optic neuropathy or visual changes, No. (%) | 21 (30) | 10 (26) | 11 (37) | 5 (50) | 6 (30) |
| Other, No. (%) | 2 (2.9) | 1 (2.6) | 1 (3.3) | 0 (0) | 1 (5) |
| Adverse event rate per linezolid-month on 600 mg/d | 0.21 | .20 | 0.32a | 0.44 | 0.28 |
| Adverse event rate per linezolid-month on low dose | N/A | N/A | 0.10a | 0.09 | 0.11 |
Abbreviation: N/A, not available; MDR TB, multidrug-resistant tuberculosis.
P = .03.
Table 5.
Characteristics of Adverse Events Among Patients With MDR TB Treated With Linezolid—California, 2009–2016
| Adverse Event | Severity, No. (%) | Attributed to Linezolid, No. (%) | Days Before Occurrence, Median (Range) | Linezolid Held Due to AE, No. (%) | Linezolid Discontinued Due to AE, No. (%) | Linezolid Dose Reduced Due to AE, No. (%) | Medical Management, No. (%) | Documented Resolution, No. (%) |
|---|---|---|---|---|---|---|---|---|
| Hematologic (n = 89) | N/A | 30 (34) | 46 (9–755) | 31 (35) | 9 (10) | 16 (18) | 10 (11) | 62 (70) |
| Anemia (n = 34) | Mild: 18 (53); Moderate: 10 (29); Severe: 6 (18) | 17 (50) | 37.5 (9–755) | 12 (35) | 6 (18) | 8 (24) | 8 (24) | 15 (44) |
| Leukopenia (n = 37) | N/A | 1 (3.7) | 62 (13–366) | 8 (30) | 1 (3.7) | 2 (7.4) | 1 (3.7) | 23 (85) |
| Thrombocytopenia (n = 21) | Mild: 16 (76); Moderate: 3 (14); Severe: 2 (9.5) | 10 (48) | 30 (13–30) | 8 (38) | 2 (9.5) | 6 (29) | 0 (0) | 18 (86) |
| Neutropenia (n = 7) | Mild: 5 (71); Moderate: 2 (29); Severe: 0 (0) | 2 (29) | 94 (25–379) | 3 (43) | 0 (0) | 0 (0) | 1 (14) | 6 (86) |
| Neurologic (n = 61) | N/A | 27 (44) | 119.5 (0–820) | 24 (39) | 23 (38) | 12 (20) | 25 (41) | 22 (36) |
| Symptoms or diagnosis of peripheral neuropathy (n = 34) | N/A | 20 (59) | 119.5 (24–820) | 12 (35) | 15 (44) | 7 (21) | 19 (56) | 8 (24) |
| Vision changes or optic neuropathy (n = 25) | N/A | 6 (24) | 163 (0–668) | 11 (44) | 8 (32) | 5 (20) | 5 (20) | 12 (48) |
| Other (n = 2) | N/A | 1 (50) | 24 (10–38) | 1 (50) | 0 (0) | 0 (0) | 1 (50) | 2 (100) |
Abbreviations: AE, adverse event; N/A, not available; MDR TB, multidrug-resistant tuberculosis.
Among 89 hematologic adverse events, 18 (53%) anemia, 16 (76%) thrombocytopenia, and 5 (71%) neutropenia events were classified as mild (Table 5). Hematologic AEs occurred a median (range) of 46 (9–755) days after linezolid was started. Overall, 30/89 (34%) hematologic AEs were attributed to linezolid, and 31 (35%) led to linezolid being held, 16 (18%) to the dose being reduced, and 9 (10%) to discontinuation. Ten of 89 (11%) hematologic AEs received medical management (ie, vitamin B6 dose increase or erythropoietin initiation). A high percentage of hematologic AEs (62, 70%) showed full documented resolution at completion of treatment.
Neurologic AEs occurred at a median (range) of 124 (6–820) days on linezolid. Twenty-seven (47%) neurologic AEs were attributed to linezolid, and 24 (41%) led to linezolid being held, 12 (21%) to the dose being reduced, and 23 (40%) to discontinuation. Twenty-two (38%) neurologic AEs required medical management (ie, vitamin B6 dose increase, gabapentin initiation, or pregabalin initiation). Twenty-one (36%) neurologic AEs demonstrated full documented resolution at the end of treatment.
Overall, 69 of 147 (47%) AEs occurred in the low-dose group. Few AEs recurred following dose reduction, with 34 (81%) hematologic and 21 (78%) neurologic events occurring before dose reduction and only 8 (19%) and 5 (19%), respectively, occurring after reduction. Patients receiving low-dose linezolid experienced significantly more (P = .03) AEs per linezolid-month on 600 mg of linezolid (0.32) than they did after switching to low-dose (0.10) (Table 4).
DISCUSSION
In this retrospective analysis, we found that among patients with MDR TB receiving consultation from the California MDR TB service, linezolid use was associated with successful outcomes and that dose reduction seemed to mitigate the toxicity associated with the drug. Patients were able to tolerate linezolid for a longer duration with fewer AEs and better treatment outcomes when switched to a reduced dose or intermittent dosing.
Over the course of the study period, linezolid use for treatment of MDR TB increased significantly [9]. We expect this trend to continue in the United States and globally due to the reprioritization of linezolid for treatment of MDR TB, now a Group A drug per the WHO Drug-Resistant TB Guidelines, and the inclusion of linezolid in newer shorter regimens for MDR TB [3, 4, 8].
We found that patients taking linezolid as part of an MDR TB treatment regimen had good treatment outcomes overall, but those switched to low-dose linezolid were more likely to complete therapy, with 100% of patients who switched to low dose achieving treatment success compared with 85% of patients on the standard dose. Previous studies report high efficacy of linezolid at doses of ≥600 mg daily, but studies reporting efficacy of 300 mg daily and intermittent 600 mg dose linezolid are few [19–21]. Studies that do address the efficacy of reduced dosage suggest that linezolid maintains its efficacy, even at lower or intermittent doses [9–13, 15, 16]. Our analysis adds to the robustness of this finding.
Importantly, because both the standard- and low-dose groups began linezolid treatment on the standard dose, our findings do not suggest that treatment can be initiated at a low dose. Rather, we found that lowering the linezolid dose after an initial period of treatment with the standard dose was associated with good patient outcomes, leaving room for the possibility that an initial period of treatment at the standard dose is essential to the observed positive outcomes. The strategy of linezolid reduction after an initial period is consistent with the successful results reported in other trials including TB PRACTECAL, in which patients were treated with 600 mg of linezolid daily for an initial period of 16 weeks (in addition to bedaquiline and pretomanid), after which the linezolid dose was decreased to 300 mg daily or 600 mg 3 times per week for the final 8 weeks [22].
Furthermore, we found that patients treated with a reduced dose or dose frequency of linezolid were able to tolerate linezolid significantly longer than those treated solely with the standard dose. The current literature consistently finds that linezolid given at ≥600 mg daily is associated with toxicity despite its efficacy [4, 12, 14]. Debilitating cytopenias and neuropathies have been reported to cause early termination of the drug [4, 23, 24]. Existing studies examining low-dose linezolid treatment suggest that it is associated with fewer toxicities relative to the standard dose [9–14, 18]. Using linezolid treatment duration as a measure of tolerability, our findings support the hypothesis that dose reduction allows for greater tolerability.
Linezolid-related hematologic and neurologic AEs were common in our study population. It is possible that a contributor to this relatively high frequency of AEs was the high prevalence of comorbidities in our population. Cytopenias were slightly more common than neuropathies but were more likely to fully resolve by the end of treatment, a pattern consistent with previous findings [25]. Patients experienced far fewer AEs after switching to low-dose linezolid, suggesting that linezolid was better tolerated at a lower dose.
Pharmacokinetic and pharmacodynamic (PK/PD) studies of linezolid suggest that a dose of 300 mg daily likely retains the efficacy of higher doses while reducing toxicity [26–30]. One study utilizing a Monte Carlo simulation found that a linezolid dose of 300 mg daily achieves the optimal sterilizing effect in 87% of patients and, simultaneously, does not achieve the AUC0–24 associated with linezolid toxicity. On the other hand, 600 mg daily dosing achieves the optimal sterilizing effect in >99% of patients, but also achieves AUC0–24s associated with linezolid toxicity in <20% of patients [30]. Other PK/PD studies support this conclusion as well [15, 26–29]. For individual linezolid dosing, some experts recommend a dose that maintains trough linezolid levels below the mitochondrial toxicity level, 2 mcg/mL [29]. These studies emphasize linezolid's narrow therapeutic index. Because of individual differences in linezolid absorption, patients may require differing doses of linezolid to reach this concentration. For this reason, these studies recommend therapeutic drug monitoring (TDM), a tailored treatment approach that uses individual drug levels of linezolid and linezolid minimum inhibitory concentrations (MICs) of individual MTB isolates to recommend dosing. Patients in our analysis did not receive TDM, in part because data on using TDM in preventing linezolid toxicity emerged at the end of the study period [29, 31]. Additionally, TDM can be challenging to access in some settings in California. In settings where TDM is not available, it appears that low-dose linezolid—or, as seen in our population, linezolid dose or frequency reduction post–culture conversion—may be an acceptable option [29]. More recently, it has been proposed that monitoring hemoglobin levels at 4 weeks for a 10% decline from baseline is another indicator of toxicity in patients receiving linezolid for MDR TB [32].
Limitations
There are several limitations to our study, most of which are the result of the retrospective observational analytic design. First, because all patients started treatment with standard-dose linezolid and were switched to low-dose based on clinical recommendations, the 2 groups may have differed in ways not detected with data captured. However, the 2 groups were comparable on the characteristics most likely to predict success such as age, cavitary disease, and others. Where differences did exist, the low-dose group tended to have less favorable attributes. Second, we had insufficient numbers to make separate conclusions for the 2 low-dose strategies. Third, though a thorough review of patient consultation charts was conducted, there were gaps in documentation, especially during post-treatment follow-up. For this reason, we cannot provide complete data on the persistence of AEs or a comprehensive account of all potential comorbidities and concurrent medications. Fourth, onset and recovery dates for new symptoms may have reflected the dates these events were documented by consultants and thus may have lagged actual occurrence. Additionally, treatment practice shifted toward prophylactic dose reduction later in the study period. This could have led to differences within the treatment subgroups, complicating data interpretation.
CONCLUSIONS
Our study, reflecting real-world use of linezolid outside of a study setting, shows that the use of lower or intermittently dosed linezolid after an initial period at the standard dose may allow for treatment with linezolid for a longer duration with a greater likelihood of use until the end of treatment. Further, our results can inform clinical decision-making for MDR TB patients who have linezolid-related toxicity, which has been observed in recent trials investigating all-oral shorter treatment courses as well as in patients receiving longer, individualized treatment regimens for drug-resistant TB [4, 22, 33].
Additional results from randomized clinical trials may help evaluate the effect of lower- or intermittent-dose linezolid on tolerability and treatment outcomes. In MDR treatment programs, AE monitoring and documentation should be systematized for clinical as well as research purposes.
Supplementary Material
Acknowledgments
We thank all members of the California Department of Public Health Tuberculosis Control Branch who gave input and helped support us in completing this manuscript, including Jenna Feraud, Araxi Polony, Rebecca Wang, and Marya Husary.
Funding statement. There were no sources of funding for this analysis and manuscript.
CDPH required disclaimer. The findings and conclusions in this article are those of the authors and do not necessarily represent the views or opinions of the California Department of Public Health or the California Health and Human Services Agency.
Contributor Information
Anjeli Mase, Division of Hematology/Oncology, University of California, San Francisco, California, USA.
Phil Lowenthal, California Department of Public Health Tuberculosis Control Branch, Richmond, California, USA.
Lisa True, California Department of Public Health Tuberculosis Control Branch, Richmond, California, USA.
Leslie Henry, California Department of Public Health Tuberculosis Control Branch, Richmond, California, USA.
Pennan Barry, California Department of Public Health Tuberculosis Control Branch, Richmond, California, USA.
Jennifer Flood, California Department of Public Health Tuberculosis Control Branch, Richmond, California, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. World Health Organization . Global tuberculosis report 2016. 2016. Available at: https://apps.who.int/iris/bitstream/handle/10665/250441/9789241565394-eng.pdf? sequence=1. Accessed October 29, 2021.
- 2. World Health Organization . Global tuberculosis report 2019. 2019. Available at: https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf. Accessed October 29, 2021.
- 3. World Health Organization . WHO Consolidated Guidelines on Tuberculosis. Module 4: Treatment—Drug-Resistant Tuberculosis Treatment. World Health Organization; 2020. [PubMed] [Google Scholar]
- 4. Conradie F, Diacon AH, Ngubane N, et al. Treatment of highly drug-resistant pulmonary tuberculosis. N Engl J Med 2020; 382:893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cynamon MH, Klemens SP, Sharpe CA, Chase S. Activities of several novel oxazolidinones against Mycobacterium tuberculosis in a murine model. Antimicrob Agents Chemother 1999; 43:1189–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Migliori GB, Eker B, Richardson MD, et al. A retrospective TBNET assessment of linezolid safety, tolerability and efficacy in multidrug-resistant tuberculosis. Eur Respir J 2009; 34:387–93. [DOI] [PubMed] [Google Scholar]
- 7. Molicotti P, Ortu S, Bua A, et al. In vitro efficacy of linezolid on clinical strains of Mycobacterium tuberculosis and other mycobacteria. New Microbiol 2006; 29:275–80. [PubMed] [Google Scholar]
- 8. Nyang’wa B-T, Motta I, Kazounis E, Berry C. Early termination of randomisation into TB-PRACTECAL, a novel six months all-oral regimen drug resistant TB study. Paper presented at: 11th International AIDS Society Conference on HIV Science; May 19, 2021; Virtual. [Google Scholar]
- 9. Chang KC, Yew WW, Cheung SW, et al. Can intermittent dosing optimize prolonged linezolid treatment of difficult multidrug-resistant tuberculosis? Antimicrob Agents Chemother 2013; 57:3445–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Condos R, Hadgiangelis N, Leibert E, et al. Case series report of a linezolid-containing regimen for extensively drug-resistant tuberculosis. Chest 2008; 134:187–92. [DOI] [PubMed] [Google Scholar]
- 11. Koh WJ, Kwon OJ, Gwak H, et al. Daily 300 mg dose of linezolid for the treatment of intractable multidrug-resistant and extensively drug-resistant tuberculosis. J Antimicrob Chemother 2009; 64:388–91. [DOI] [PubMed] [Google Scholar]
- 12. Lee M, Lee J, Carroll MW, et al. Linezolid for treatment of chronic extensively drug-resistant tuberculosis. N Engl J Med 2012; 367:1508–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sotgiu G, Centis R, D'Ambrosio L, et al. Linezolid to treat extensively drug-resistant TB: retrospective data are confirmed by experimental evidence. Eur Respir J 2013; 42:288–90. [DOI] [PubMed] [Google Scholar]
- 14. Tang S, Yao L, Hao X, et al. Efficacy, safety and tolerability of linezolid for the treatment of XDR-TB: a study in China. Eur Respir J 2015; 45:161–70. [DOI] [PubMed] [Google Scholar]
- 15. Wasserman S, Denti P, Brust JCM, et al. Linezolid pharmacokinetics in South African patients with drug-resistant tuberculosis and a high prevalence of HIV coinfection. Antimicrob Agents Chemother 2019; 63:e02164-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World TB day—March 24, 2019. MMWR Morb Mortal Wkly Rep 2019; 68:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shah NS, Westenhouse J, Lowenthal P, et al. The California multidrug-resistant tuberculosis consult service: a partnership of state and local programs. Public Health Action 2018; 8:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schecter GF, Scott C, True L, Raftery A, Flood J, Mase S. Linezolid in the treatment of multidrug-resistant tuberculosis. Clin Infect Dis 2010; 50:49–55. [DOI] [PubMed] [Google Scholar]
- 19. Jeong BH, Jeon K, Park HY, et al. Outcomes of pulmonary MDR-TB: impacts of fluoroquinolone resistance and linezolid treatment. J Antimicrob Chemother 2015; 70:3127–33. [DOI] [PubMed] [Google Scholar]
- 20. Agyeman AA, Ofori-Asenso R. Efficacy and safety profile of linezolid in the treatment of multidrug-resistant (MDR) and extensively drug-resistant (XDR) tuberculosis: a systematic review and meta-analysis. Ann Clin Microbiol Antimicrob 2016; 15:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Singla R, Caminero JA, Jaiswal A, et al. Linezolid: an effective, safe and cheap drug for patients failing multidrug-resistant tuberculosis treatment in India. Eur Respir J 2012; 39:956–62. [DOI] [PubMed] [Google Scholar]
- 22. Berry C, du Cros P, Fielding K, et al. TB-PRACTECAL: study protocol for a randomised, controlled, open-label, phase II–III trial to evaluate the safety and efficacy of regimens containing bedaquiline and pretomanid for the treatment of adult patients with pulmonary multidrug-resistant tuberculosis. Trials 2022; 23:484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hanai Y, Matsuo K, Ogawa M, et al. A retrospective study of the risk factors for linezolid-induced thrombocytopenia and anemia. J Infect Chemother 2016; 22:536–42. [DOI] [PubMed] [Google Scholar]
- 24. Singh B, Cocker D, Ryan H, Sloan DJ. Linezolid for drug-resistant pulmonary tuberculosis. Cochrane Database Syst Rev 2019; 3:CD012836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spellberg B, Yoo T, Bayer AS. Reversal of linezolid-associated cytopenias, but not peripheral neuropathy, by administration of vitamin B6. J Antimicrob Chemother 2004; 54:832–5. [DOI] [PubMed] [Google Scholar]
- 26. Alffenaar JW, van Altena R, Harmelink IM, et al. Comparison of the pharmacokinetics of two dosage regimens of linezolid in multidrug-resistant and extensively drug-resistant tuberculosis patients. Clin Pharmacokinet 2010; 49:559–65. [DOI] [PubMed] [Google Scholar]
- 27. Barry P, Deck D, Farkas A. Pharmacokinetic modeling of linezolid dosing regimens for multidrug resistant tuberculosis. Paper presented at: IDWeek 2014; December 1, 2014; Philadelphia, PA. [Google Scholar]
- 28. Millard J, Pertinez H, Bonnett L, et al. Linezolid pharmacokinetics in MDR-TB: a systematic review, meta-analysis and Monte Carlo simulation. J Antimicrob Chemother 2018; 73:1755–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Song T, Lee M, Jeon HS, et al. Linezolid trough concentrations correlate with mitochondrial toxicity-related adverse events in the treatment of chronic extensively drug-resistant tuberculosis. EBioMedicine 2015; 2:1627–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Srivastava S, Magombedze G, Koeuth T, et al. Linezolid dose that maximizes sterilizing effect while minimizing toxicity and resistance emergence for tuberculosis. Antimicrob Agents Chemother 2017; 61:e00751-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brown AN, Drusano GL, Adams JR, et al. Preclinical evaluations to identify optimal linezolid regimens for tuberculosis therapy. mBio 2015; 6:e01741-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Imperial MZ, Nedelman JR, Conradie F, Savic RM. Proposed linezolid dosing strategies to minimize adverse events for treatment of extensively drug-resistant tuberculosis. Clin Infect Dis 2021; 74:1736–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Conradie F, Bagdasaryan TR, Borisov S, et al. Bedaquiline–pretomanid–linezolid regimens for drug-resistant tuberculosis. N Engl J Med 2022; 387:810–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.