Abstract
Indian mustard is an economic and highly important industrial oilseed crop. In this study, genetic diversity among 135 Indian mustard germplasm accessions was evaluated using 11 agro-morphological descriptors and 227 SSRs. Morphological characterization of Indian mustard germplasm accessions exhibited a broad range of variation for characters including biological yield (CV = 25.63%), seed yield (CV = 23.23% and 1000-seed weight (CV = 23.14%); whereas traits such as days to maturity (CV = 2.91%) showed lowest degree of variation. Out of 227 SSR markers evaluated, a total of 159 (70.04%) SSRs produced polymorphic products and 68 (29.96%) SSRs resulted into monomorphic amplicons. The polymorphic markers amplified 575 alleles and the number of alleles ranged from 2-7 with 3.61 average number of alleles per locus. SSR markers BRMS-030, Ra2-E11, Ra2-G05, Ni4-G10 and Ol10B11 generated the highest number of alleles (7). SSR marker Ra2-G05 was having the highest allele frequency (0.84), while BRMS-002 was having the lowest major allele frequency (0.33). Polymorphism information content (PIC) values ranged from 0.24-0.61 with an average value of 0.39 per primer pair. Unweighted pair group method with arithmetic mean (UPGMA) cluster analysis based on morphological traits grouped Indian mustard genotypes into three clusters, while two clusters were obtained based on SSR based clustering. Population structure analysis provided a better estimate of genetic diversity and divided all the genotypes into five subpopulations. Genetically diverse accessions identified may be used for hybridization in Indian mustard crop improvement programs in future.
Keywords: Brassica juncea, Genetic diversity, Agro-morphological traits, SSR markers, Population structure
Brassica juncea, Genetic diversity, Agro-morphological traits, SSR markers, Population structure.
1. Introduction
Indian mustard (Brassica juncea L. Czern. & Coss) is an economically important oilseed crop belonging to rapeseed-mustard (RM, oilseed Brassica) group and is being cultivated mainly in Indian subcontinent and in some parts of Canada, China, Australia and Russia. It is a predominantly self-pollinating crop with an extent of 8–12% cross-pollination. It has been developed in nature by crossing between B. nigra (BB, 2n = 16) and B. rapa (AA, 2n = 20) followed by subsequent chromosome duplication thousands of years ago (Redden et al., 2009). Indian mustard is the most dominating crop of RM group which is presently being cultivated over >85% of RM acreage in India. It accounts for 23.3% of the total acreage and 26.2% of the total oilseed production in India (Govt. of India, 2019). Besides its use as cooking oil, Indian mustard oil has found numerous applications in food, chemical, biofertilizer and paint industries. Its oil contains higher amount of erucic acid, which makes it quite useful in paint and varnishes industry. In addition, the presence of high erucic acid in its oil is the main reason for its use in biodiesel production in automobile industry (Premi et al., 2013). Mustard oil is an enriched source of various antioxidants and α-tocopherol, which provides health benefits to the consumers. Its oil has both culinary and therapeutic uses. It contains rich amount of monounsaturated and polyunsaturated fatty acids (MUFA and PUFA) and optimum ratio of ω-3 and ω-6 fatty acids, which is very much essential for curing cardiac disorders. Due to antimicrobial properties of mustard oil, it is also used in pickle industry for preservation purpose. Besides mustard oil, its seedmeal is an excellent source of feed and nutrition for chicken industry and India has now become world’s largest exporter of mustard seedmeal (Thakur et al., 2020). However, due to the exponential increase in population and consumers’ changing taste preferences towards mustard oil, it will become a challenging assignment to fulfil the growing edible oil demand in future. On the other hand, several biotic and abiotic stress constraints leading to considerable yield losses seriously hampers overall production of this crop. This necessitates the development of high yielding mustard varieties having resistance to both abiotic and biotic stresses so that they can grow in a diverse array of agroclimatic conditions.
Information about the genetic diversity patterns of various germplasm accessions and their genetic relationships is of paramount relevance in formulating breeding strategies and germplasm management programs. Utilization of genetically diverse germplasm lines in breeding programs can be used to harness allelic richness to create new gene combinations. Traditionally, various agro-morphological traits had been used to estimate genetic diversity among Indian mustard germplasms (Singh et al., 2018, 2020). Morphological characterization of germplasm has certain advantages including less cost involvement, less technically demanding and simplicity of characterization protocols. Estimates of genetic variability can be indirectly done using morphological traits, but these estimates do not provide the exact picture of genetic diversity as they work under the influence of environmental factors (Pandey et al., 2018) and developmental stages of the plant. Further, the lesser number of available morphological markers render them unfit for diversity characterization in crops. Whereas, molecular markers are not influenced by the environmental factors or any developmental stage of the plant. Nowadays, it has been suggested to evaluate genetic diversity using both agro-morphological traits and molecular markers to get more precise estimates of the genetic variability (Vinu et al., 2013; Thakur et al., 2017).
To date, several type of DNA-based marker systems were used for evaluation of genetic diversity in Indian mustard germplasm accessions including random amplified polymorphic DNA (RAPD) (Khan et al., 2008; Sharma et al., 2015), inter simple sequence repeat (ISSR) markers (Huangfu et al., 2009; Yadav and Rana, 2012), restriction fragment length polymorphism (RFLP) (Mir et al., 2015), amplified fragment length polymorphism (AFLP) (Srivastava et al., 2001; Qi et al., 2008) and simple sequence repeat (SSR) markers (Vinu et al., 2013; Pratap et al., 2015; Thakur et al., 2015; Sudan et al., 2016). Among these marker systems, SSR markers have proved highly useful in genetic diversity analysis in Indian mustard owing to their abundance in eukaryotic systems, ease of doing and scorability, high reproducibility, co-dominant and multi-allelic inheritance patterns and less cost involvement (Viera et al., 2016). With this background knowledge, the present research investigation was planned to evaluate genetic diversity in Indian mustard germplasm accessions using various agro-morphological characters and SSR markers.
2. Materials and methods
2.1. Plant material
A total of 135 Indian mustard germplasm accessions were used as plant material (Table 1). The pure, selfed seeds of germplasm accessions were obtained from the Germplasm Division of ICAR-DRMR, Bharatpur. All 135 germplasm accessions were grown in augmented block design in a 3 m length row with a row-to-row spacing of 45 cm along with plant-to-plant spacing of 10 cm for two consecutive years in the rabi season of 2017 and 2018, following standard package of practices. Two Indian mustard varieties viz. Giriraj and NRCHB 101 were kept as check genotypes. The composition of the soil was kept same for growing different genotypes of Indian mustard.
Table 1.
List of Brassica juncea germplasm accessions used in the present study.
| Code | Name/identity of the genotype | IC/EC No. | Source | Country of origin |
|---|---|---|---|---|
| 1 | B 123 | IC 494164 | Unknown | India |
| 2 | JBT 41/15 | IC 520747 | Jharkhand | India |
| 3 | 03–530 | IC 609262 | Unknown | Unknown |
| 4 | KLM 4 | IC 609278 | PAU, Ludhiana | India |
| 5 | BT 15 | IC 609452 | Unknown | Unknown |
| 6 | SNTM 53 | IC 411750 | Jammu and Kashmir | India |
| 7 | HUJM 05-01 | IC 511426 | BHU, Varanasi | India |
| 8 | JBT 41/60 | IC 520759 | Jharkhand | India |
| 9 | SN 99 | IC 609983 | Andhra Pradesh | India |
| 10 | EC 511481 | EC 511481 | Saskatchewan, Canada | Canada |
| 11 | BT 31 | IC 607046 | Unknown | Unknown |
| 12 | NE 19 | IC 609882 | Assam | India |
| 13 | HP 35 | IC 609380 | Himachal Pradesh | India |
| 14 | UP I-23 | IC 346131 | Uttar Pradesh | India |
| 15 | UP I-75 | IC 346183 | Uttar Pradesh | India |
| 16 | DU 10 | IC 609529 | Unknown | Unknown |
| 17 | SN 24 | IC 609927 | Andhra Pradesh | India |
| 18 | RH 08-2 | IC 511367 | CCSHAU, Hisar | India |
| 19 | SEL 4 | IC 511598 | Unknown | India |
| 20 | SEL 9 | IC 511602 | Unknown | India |
| 21 | VKG 29/80 | IC 447832 | Bihar | India |
| 22 | JBT 41/4 | IC 520766 | Jharkhand | India |
| 23 | JCR 914 | IC 427136 | Himachal Pradesh | India |
| 24 | PSR 11226 | IC 436220 | Telangana | India |
| 25 | SN 29 | IC 609935 | Andhra Pradesh | India |
| 26 | P 83 | IC 346769 | Punjab | India |
| 27 | SV 41708 | EC 182920 | Sweden | Sweden |
| 28 | B 267 | IC 494266 | Unknown | India |
| 29 | RESJ 564 | EC 399288 | Rothamsted | England |
| 30 | Pusa Bold x BJ-1058 | IC 609897 | Unknown | India |
| 31 | NBPG 29 | IC 609235 | Unknown | Unknown |
| 32 | HPLM 06-46 | IC 609255 | Unknown | Unknown |
| 33 | Parasmani 8 | IC 609293 | Shakti Vardhak Hybrid Seeds Pvt.Ltd., Hisar | India |
| 34 | BBM 06-02 | IC 511432 | RRS, IGKVV, Jagdalpur | India |
| 35 | RRN 615 | IC 511431 | ARS, SKRAU, Navgaon | India |
| 36 | RRN 605 | IC 511434 | ARS, SKRAU, Navgaon | India |
| 37 | HUJM 05-02 | IC 609321 | BHU, Varanasi | India |
| 38 | Kranti | IC 491638 | CSAUA & T, Kanpur | India |
| 39 | DMRS 186 | IC 266917 | Uttarakhand | India |
| 40 | UP II-76 | IC 345930 | Uttar Pradesh | India |
| 41 | UP II-6 | IC 345860 | Uttar Pradesh | India |
| 42 | P 4 | IC 346690 | Punjab | India |
| 43 | PR 2001-84 | IC 609324 | GBPUA&T, Pantnagar | India |
| 44 | HP 57 | IC 609363 | Himachal Pradesh | India |
| 45 | SKM 125 | IC 609378 | SDAU, SK Nagar | India |
| 46 | P 63 | IC 346749 | Punjab | India |
| 47 | HP 14 | IC 347670 | Himachal Pradesh | India |
| 48 | HP 30 | IC 609402 | Himachal Pradesh | India |
| 49 | UP II-62 | IC 345916 | Uttar Pradesh | India |
| 50 | UP II-18 | IC 345872 | Uttar Pradesh | India |
| 51 | P 37 | IC 346723 | Punjab | India |
| 52 | JGM 01-15 | IC 609490 | RVSKVV, Gwalior | India |
| 53 | RN 593 | IC 609494 | ARS, SKRAU, Navgaon | India |
| 54 | DU 32 | IC 609533 | Unknown | Unknown |
| 55 | VKG 13/75 | IC-267715 | Bihar | India |
| 56 | RGN 42 | IC 609543 | ARS, SKRAU, Sriganganagar | India |
| 57 | B/K/S 67 | IC 310767 | Haryana | India |
| 58 | B/K/S 135 | IC 310807 | Madhya Pradesh | India |
| 59 | B/K/S 42 | IC 310748 | Rajasthan | India |
| 60 | CS 1100-1-2-2-3 | IC 511389 | CSSRI, Karnal | India |
| 61 | CS 3000-1-1-1-2-4 | IC 511390 | CSSRI, Karnal | India |
| 62 | NDRS 2010-5-1 | IC 511393 | NDUA&T, Faizabad | India |
| 63 | CS 610-5-2-5P1 | IC 511455 | CSSRI, Karnal | India |
| 64 | NDR 05-02 | IC 511503 | NDUA&T, Faizabad | India |
| 65 | YS,SKJ 5 | IC 312514 | Haryana | India |
| 66 | SKJ/DPS 9 | IC 312524 | Haryana | India |
| 67 | JBT 41/32 | IC 520768 | Jharkhand | India |
| 68 | VKG 31/8 | IC 521376 | Jharkhand | India |
| 69 | EC 511436 | EC 511436 | Saskatoon, Canada | Canada |
| 70 | EC 511589 | EC 511589 | Saskatoon, Canada | Canada |
| 71 | PAB 9511 | IC 607054 | GBPUA&T, Pantnagar | India |
| 72 | EC 511682 | EC 511682 | Saskatoon, Canada | Canada |
| 73 | EC 511447 | EC 511447 | Saskatoon, Canada | Canada |
| 74 | EC 511684 | EC 511684 | Saskatoon, Canada | Canada |
| 75 | SKM 158 | IC 609336 | SDAU, SK Nagar | India |
| 76 | P 18 | IC 346704 | Punjab | India |
| 77 | Krishna Shorty | IC 609188 | Krishna Agril. Res. & Dev., Agra | India |
| 78 | RGN 13 | IC 296337 | ARS, SKRAU, Sriganganagar | India |
| 79 | PRB 2006-5 | IC 573439 | GBPUA&T, Pantnagar | India |
| 80 | Parasmani 3 | IC 609946 | DRMR, Bharatpur | India |
| 81 | MRN J 2001-12 | IC 511467 | ZARS, RVSKVV, Morena | India |
| 82 | PBR 2004-06 | IC 511487 | PAU, Ludhiana | India |
| 83 | CS 219-1 | IC 511537 | CSSRI, Karnal | India |
| 84 | RH 118 | IC 609894 | CCSHAU, Hisar | India |
| 85 | NRC 323-1 | IC 511525 | DRMR, Bharatpur | India |
| 86 | UP II-9 | IC 345863 | Uttar Pradesh | India |
| 87 | HP 10 | IC 609511 | Himachal Pradesh | India |
| 88 | UP II-75 | IC 345929 | Uttar Pradesh | India |
| 89 | SN 92 | IC 609923 | Andhra Pradesh | India |
| 90 | SN 57 | IC 609931 | Andhra Pradesh | India |
| 91 | SN 55 | IC 609933 | Andhra Pradesh | India |
| 92 | SN 34 | IC 609938 | Andhra Pradesh | India |
| 93 | SN 28 | IC 609941 | Andhra Pradesh | India |
| 94 | SKACV 09-62 | IC 571685 | Karnataka | India |
| 95 | UP I-21 | IC 346129 | Uttar Pradesh | India |
| 96 | NE 68 | IC 609916 | Nagaland | India |
| 97 | UP I-24 | IC 346132 | Uttar Pradesh | India |
| 98 | UP II-111 | IC 345965 | Uttar Pradesh | India |
| 99 | P 11 | IC 346697 | Punjab | India |
| 100 | UP II-25 | IC 345879 | Uttar Pradesh | India |
| 101 | UP II-49 | IC 345903 | Uttarakhand | India |
| 102 | UP II-4 | IC 345858 | Uttar Pradesh | India |
| 103 | UP I-62 | IC 346170 | Uttar Pradesh | India |
| 104 | PR 2006 | IC 511618 | GBPUA&T, Pantnagar | India |
| 105 | UP II-21 | IC 345875 | Uttar Pradesh | India |
| 106 | B/K/S 130 | IC 310802 | NBPGR, New Delhi | India |
| 107 | NRCHB 06-5912 | IC 609944 | DRMR, Bharatpur | India |
| 108 | JBT 42/RP-3/43 | IC 526318 | Bihar | India |
| 109 | IB 1521 | IC 493679 | Unknown | India |
| 110 | LET 3 | IC 511546 | IARI, New Delhi | India |
| 111 | Domo 1 | IC 609210 | Unknown | Unknown |
| 112 | OMK 15 | IC 609257 | SAREC, CSKHPKVV, Kangra | India |
| 113 | BT 13 | IC 609451 | Unknown | Unknown |
| 114 | DU 25 | IC 609532 | Unknown | Unknown |
| 115 | B/K/S 1 | IC 609565 | Unknown | Unknown |
| 116 | SEL 15 | IC 511608 | Unknown | India |
| 117 | VKG 21/208 | IC 342778 | Bihar | India |
| 118 | RCC 4 x Zem-2 | IC 609905 | Unknown | Unknown |
| 119 | HPLM 06-35 | IC 609250 | Unknown | Unknown |
| 120 | HPLM 06-36 | IC 609251 | Unknown | Unknown |
| 121 | Pusa Basant | IC 491533 | IARI, New Delhi | India |
| 122 | RK 07-2 | IC 609288 | CSAUA&T, Kanpur | India |
| 123 | RH 0305 | IC 575576 | CCSHAU, Hisar | India |
| 124 | DMRS 97 | IC 266828 | Uttarakhand | India |
| 125 | UP II-119 | IC 345973 | Uttar Pradesh | India |
| 126 | DU 15 | IC 609531 | Unknown | Unknown |
| 127 | B/K/S 19 | IC 310730 | Rajasthan | India |
| 128 | SEL 23 | IC 511483 | UAS, Bangalore | India |
| 129 | SEL 68 | IC 511485 | UAS, Bangalore | India |
| 130 | SEL 6 | IC 511600 | Unknown | India |
| 131 | SEL 14 | IC 511607 | Unknown | India |
| 132 | SKJ/DPS 10 | IC 312525 | NBPGR, New Delhi | India |
| 133 | YSR/SKJ 45 | IC 355345 | Uttar Pradesh | India |
| 134 | JCR 996 | IC 427206 | Himachal Pradesh | India |
| 135 | SN 66 | IC 609940 | Andhra Pradesh | India |
2.2. Morphological characterization
Observations of 11 agro-morphological traits were taken as per the DUS guidelines (Singh et al., 2006). Data was recorded on five randomly selected plants per genotype for various traits including days to flower initiation (DFI, 50%), days to maturity (DM), plant height (PH, cm), main shoot length (MSL, cm), siliqua length (SL, cm), siliquae on main shoot (SMS), seeds per siliqua (SS), 1000-seed weight (SW, g), seed yield (SY, Kg/ha), biological yield i.e. dry matter production excluding roots (BY, Kg/ha) and harvest index (HI, %).
2.3. Genomic-DNA isolation and purification
Genomic-DNA was isolated from the pooled leaf tissues collected from five randomly selected healthy plants per genotype and purified using the protocol of Thakur et al. (2013).
2.4. Genotyping
A set of 227 genomic-SSR markers covering all the linkage groups of Indian mustard as reported by Singh et al. (2021) were used in present study for genotyping. The mastermix was prepared in a 2.0 ml centrifuge tube in 25 μl volume by taking 50 ng genomic-DNA, 1XPCR buffer, 2.0 mM Mgcl2, 0.2 mM of each dNTP, 1.0 U Taq DNA polymerase (GCC Biotech, India) and 400 nM primers using Verity 96-w PCR machine. In thermal cycler, initial denaturation cycle comprised of 94 °C temperature for 5 min which was followed by 45 cycles at 94 °C for 30 s, 50–60 °C (depending on the annealing temperature of the SSR primers) for 30 s, 45 s of extension at 72 °C and in the last, primer extension step at 72 °C for 7 min. PCR amplicons containing 5 μl loading dye were resolved in a 3.5% Super Fine Resolution (SFR) agarose (Amresco, USA). Further, gel pictures were taken in a gel documentation unit (Syngene Gel Doc, UK).
2.5. Data analysis
Statistical analysis of mean values of each of agro-morphological trait was carried out using the Fit Model of SAS JMP9.2. DARwin 5 software (Perrier and Jacquemoud-Collet, 2006) was used to construct UPGMA-based dendrogram on the basis of similarity coefficients based on Euclidean distances.
SSR bands were scored based on allele size and allelic data was subjected to using Power Marker software v3.25 (Liu and Muse, 2005) for computation of major allele frequency, PIC value and gene diversity for individual SSR marker. UPGMA-dendrogram was developed to demonstrate the relationship among different accessions under investigation using MEGA version 5.03 (Tamanna and Khan, 2005).
2.6. Population structure analysis
Population structure was analyzed to determine number of subpopulations in the germplasm panel using STRUCTURE v. 2.3.4 software (Pritchard and Wen, 2003). Ten independent runs were carried out for each number of population (K) set from 1-10. Burn-in time and Markov Chain Monte Carlo (MCMC) replication number were both set to 100,000 for each run. The optimal value of K was determined by examining delK statistic and L(K) (Evanno et al., 2005) using Structure Harvester software (Earl and VonHoldt 2012). Analysis of molecular variance (AMOVA) and principal coordinate analysis (PCoA) was computed using software GenALex6.5 (Peakall and Smouse, 2012).
3. Results
3.1. Germplasm performance based on agro-morphological characters
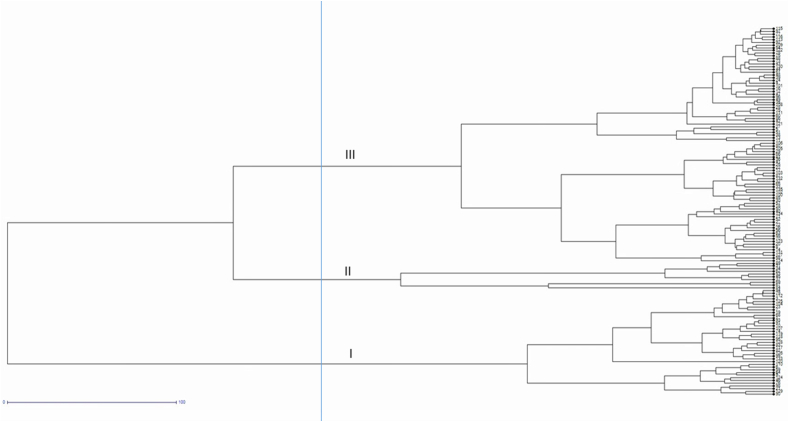
The genotype panel used in present study consisted of 135 B juncea germplasm lines, both exotic and indigenous as-well-as some advanced breeding lines also. For each of the traits evaluated under field conditions, their descriptive statistics including mean, standard deviation and coefficient of variation (CV) are described in Table 2. Morphological characterization of Indian mustard germplasm accessions exhibited a wide range of variation for traits including biological yield (CV = 25.63%), seed yield (CV = 23.23%) and 1000-seed weight (CV = 23.14%); whereas traits such as days to maturity (CV = 2.91%) showed lowest degree of variation (Table 2). In the present study, genotype KLM 4 was the earliest flowering line (33 days), while genotype P 63 was the late flowering genotype (77 days). The plant height varied from 148 cm to 268 cm. NDRS 2010-5-1 was the tallest with plant height of 268 cm and HP 35 had the shortest plant height (148 cm). Main shoot length and the number of siliqua it bears are the critical factors determining seed yield. In this study, main shoot length ranged from 45.5 cm (BT 15) to 115 cm (UP II-76) and the number of siliqua on main shoot varied from 77 (HP 35) to 130 (SEL 68). In this study, Indian mustard genotypes had seeds per siliqua in the range of 8–18. While 1000-seed weight ranged from 2.2 to 7.5 g. Further, for overall yield enhancement, such genotypes are required which bear more seeds per siliqua and have higher 1000-test seed weight. Four such genotypes identified in this study are RRN 605 (17 seeds/siliqua, 6.01 g seed weight), SKJ/DPS 9 (14.72 seeds/siliqua, 6.07 g seed weight), P 4 (14 seeds/siliqua, 6.61 g seed weight) and HP 10 (14.20 seeds/siliqua, 6.87 g seed weight). Overall, seed yield is the most important and critical trait of any crop. Seed yield showed extensive range between genotypes and varied from 800 kg/ha to 3088.9 kg/ha. Genotype YSR/SKJ 45 was the highest yielding genotype. The results of ANOVA inferred that there is enough genetic diversity among the Indian mustard germplasm lines for formulating breeding programs with the aim of improving seed size and grain yield. The UPGMA-dendrogram grouped all the 135 germplasm accessions into three main clusters (Figure 1) on the basis of agro-morphological traits. Cluster I consisted of 39 genotypes, cluster II with 10 genotypes and cluster III was comprised of 86 genotypes.
Table 2.
Range, mean and coefficient of variation for agro-morphological traits among 135 germplasm accessions of Indian mustard.
| Sr. No. | Trait | Range |
Mean | Standard deviation (SD) | Coefficient of variation (%) | |
|---|---|---|---|---|---|---|
| Minimum | Maximum | |||||
| 1 | Time of flowering initiation (IF, 50%) | 33.00 | 77.00 | 53.13 | 8.23 | 15.49 |
| 2 | Days to maturity (DM) | 123.00 | 143.00 | 130.31 | 3.78 | 2.91 |
| 3 | Plant height (PH, cm) | 148.00 | 268.00 | 205.4 | 22.16 | 10.79 |
| 4 | Main shoot length (MSL, cm) | 45.50 | 115.00 | 83.73 | 11.01 | 13.16 |
| 5 | Siliqua length (SL, cm) | 2.64 | 5.63 | 4.16 | 0.62 | 14.95 |
| 6 | Siliquae on main shoot (SMS) | 77 | 130 | 108.00 | 11.57 | 10.73 |
| 7 | Number of seeds per siliqua (SS) | 8 | 18 | 14.29 | 1.67 | 11.74 |
| 8 | 1000-seed weight (SW, g) | 2.20 | 7.5 | 4.60 | 1.06 | 23.14 |
| 9 | Seed yield (SY, Kg/Ha) | 800.00 | 3088.8 | 2198.9 | 509.85 | 23.23 |
| 10 | Biological Yield (BY, Kg/Ha) | 3955.55 | 30074 | 10901 | 2816.31 | 25.63 |
| 11 | Harvest index (HI, %age) | 9.93 | 27.57 | 20.4 | 2.94 | 14.43 |
Figure 1.
UPGMA dendrogram showing genetic relationship among 135 Indian mustard germplasm accessions based on 11 agro-morphological traits.
3.2. Allelic diversity
Of 227 SSR markers evaluated, a total of 159 (70.04%) SSRs generated polymorphic products, while 68 (29.96%) SSRs resulted into monomorphic amplicons. The results for allele number per SSR locus, major allele frequency, PIC value and gene diversity for polymorphic markers have been presented in Table 3. PCR amplification profile of SSR marker SA0306 is presented as Figure 2. The polymorphic markers amplified 575 alleles with allele number varying from 2-7 and 3.61 average number of alleles per locus. SSR markers BRMS-030, Ra2-E11, Ra2-G05, Ni4-G10 and Ol10B11 generated the highest number of alleles (7). Major allele frequency denotes the frequency of main key allele of a molecular marker and it varied from 33% to 84%. SSR marker Ra2-G05 was having the highest major allele frequency (84%), while BRMS-002 was having the lowest major allele frequency (33%) along with 62% as the average major allele frequency per SSR locus (Table 3). PIC values varied from 0.24-0.61 with an average value of 0.39 per SSR locus. Thus, cnu_m604a marker was the most efficient primer pair having the maximum PIC value (0.61). Three SSR markers viz. BRMS-002, BrgMS638 and BrgMS713 depicted the highest gene diversity value (0.67); while SSR marker Ra2-G05 resulted into the lowest gene diversity value (0.27) along with 0.47 as average gene diversity value per SSR marker. UPGMA-dendrogram divided all 135 accessions into two separate clusters (Figure 3). Cluster I comprised of only one germplasm line i.e. HPLM 06–36 (120), while cluster II consisted of two sub-clusters – IIa and IIb. Sub-cluster IIa again comprised of one genotype only i.e. EC 511684 (74), which is a Canadian genotype. These two germplasm accessions viz. HPLM 06–36 and EC 511684 were found to be the most divergent in the present study, hence may be deployed in breeding programs for creation of genetic variability. Rest of 133 germplasm accessions were grouped into sub-cluster IIb. PCoA was also performed to depict the genetic relationship among genotypes, which could better distinguish the genetically diverse accessions (Figure 4).
Table 3.
Genetic diversity parameters of 159 polymorphic SSR markers used in the present study.
| S. No. | Marker ID | Annealing temperature (°C) | Total amplified bands (TB) | Polymorphic bands (PB) | Percentage of polymorphic bands (PPB) | Major allele frequency | PIC value | Gene diversity |
|---|---|---|---|---|---|---|---|---|
| 1 | BRMS-002 | 51 | 3 | 1 | 33.33 | 0.33 | 0.59 | 0.67 |
| 2 | BRMS-003 | 55 | 3 | 1 | 33.33 | 0.34 | 0.59 | 0.66 |
| 3 | BRMS-005 | 50 | 3 | 1 | 33.33 | 0.63 | 0.37 | 0.47 |
| 4 | BRMS-006 | 49 | 3 | 1 | 33.33 | 0.34 | 0.59 | 0.66 |
| 5 | BRMS-007 | 53 | 4 | 4 | 100.00 | 0.56 | 0.49 | 0.56 |
| 6 | BRMS-011 | 50 | 6 | 6 | 100.00 | 0.70 | 0.40 | 0.45 |
| 7 | BRMS-015 | 56 | 3 | 1 | 33.33 | 0.52 | 0.46 | 0.54 |
| 8 | BRMS-017 | 56 | 4 | 4 | 100.00 | 0.66 | 0.38 | 0.45 |
| 9 | BRMS-027 | 50 | 5 | 5 | 100.00 | 0.65 | 0.44 | 0.49 |
| 10 | BRMS-029 | 55 | 2 | 1 | 50.00 | 0.50 | 0.38 | 0.50 |
| 11 | BRMS-030 | 57 | 7 | 7 | 100.00 | 0.74 | 0.37 | 0.41 |
| 12 | BRMS-033 | 53 | 4 | 4 | 100.00 | 0.66 | 0.40 | 0.46 |
| 13 | Ra1-F03 | 53 | 3 | 2 | 66.66 | 0.34 | 0.58 | 0.66 |
| 14 | Ra2-A01 | 53 | 4 | 4 | 100.00 | 0.69 | 0.36 | 0.43 |
| 15 | Ra2-A11 | 55 | 5 | 5 | 100.00 | 0.73 | 0.35 | 0.41 |
| 16 | Ra2-C09 | 55 | 4 | 1 | 25.00 | 0.51 | 0.54 | 0.61 |
| 17 | Ra2-D04 | 54 | 3 | 1 | 33.33 | 0.53 | 0.45 | 0.54 |
| 18 | Ra2-E04 | 54 | 4 | 4 | 100.00 | 0.69 | 0.37 | 0.44 |
| 19 | Ra2-E11 | 54 | 7 | 7 | 100.00 | 0.67 | 0.45 | 0.49 |
| 20 | Ra2-E12 | 56 | 3 | 1 | 33.33 | 0.35 | 0.58 | 0.65 |
| 21 | Ra2-F11 | 54 | 6 | 6 | 100.00 | 0.77 | 0.32 | 0.36 |
| 22 | Ra2-G05 | 56 | 7 | 7 | 100.00 | 0.84 | 0.24 | 0.27 |
| 23 | Ra2-G08 | 54 | 5 | 5 | 100.00 | 0.76 | 0.31 | 0.36 |
| 24 | Ra2-G09 | 55 | 2 | 2 | 100.00 | 0.53 | 0.35 | 0.47 |
| 25 | Ra2-C11 | 56 | 4 | 4 | 100.00 | 0.71 | 0.34 | 0.41 |
| 26 | Ra3-H09 | 56 | 3 | 3 | 100.00 | 0.64 | 0.36 | 0.46 |
| 27 | Ra3-H10 | 54 | 6 | 6 | 100.00 | 0.75 | 0.34 | 0.38 |
| 28 | BrgMS60 | 54 | 3 | 1 | 33.33 | 0.66 | 0.35 | 0.45 |
| 29 | BrgMS63 | 54 | 3 | 1 | 33.33 | 0.67 | 0.34 | 0.44 |
| 30 | BrgMS68 | 55 | 5 | 1 | 20.00 | 0.60 | 0.37 | 0.48 |
| 31 | BrgMS70 | 54 | 4 | 4 | 100.00 | 0.72 | 0.33 | 0.40 |
| 32 | BrgMS75 | 54 | 3 | 2 | 66.66 | 0.63 | 0.37 | 0.47 |
| 33 | BrgMS309 | 54 | 2 | 1 | 50.00 | 0.50 | 0.38 | 0.50 |
| 34 | BrgMS316 | 53 | 3 | 1 | 33.33 | 0.66 | 0.35 | 0.45 |
| 35 | BrgMS329 | 54 | 2 | 1 | 50.00 | 0.66 | 0.26 | 0.34 |
| 36 | BrgMS334 | 57 | 3 | 2 | 66.66 | 0.63 | 0.37 | 0.47 |
| 37 | BrgMS337 | 53 | 5 | 1 | 20.00 | 0.70 | 0.38 | 0.44 |
| 38 | BrgMS338 | 56 | 3 | 3 | 100.00 | 0.70 | 0.30 | 0.37 |
| 39 | BrgMS344 | 53 | 4 | 3 | 75.00 | 0.73 | 0.32 | 0.39 |
| 40 | BrgMS372 | 55 | 4 | 3 | 75.00 | 0.70 | 0.35 | 0.42 |
| 41 | BrgMS388 | 56 | 4 | 4 | 100.00 | 0.71 | 0.34 | 0.41 |
| 42 | BrgMS397 | 54 | 4 | 1 | 25.00 | 0.69 | 0.36 | 0.43 |
| 43 | BrgMS399 | 55 | 4 | 4 | 100.00 | 0.68 | 0.37 | 0.44 |
| 44 | BrgMS409 | 55 | 3 | 3 | 100.00 | 0.67 | 0.34 | 0.44 |
| 45 | BrgMS412 | 53 | 5 | 5 | 100.00 | 0.74 | 0.34 | 0.40 |
| 46 | BrgMS418 | 56 | 2 | 1 | 50.00 | 0.50 | 0.38 | 0.50 |
| 47 | BrgMS421 | 54 | 3 | 2 | 66.66 | 0.60 | 0.39 | 0.48 |
| 48 | BrgMS422 | 55 | 3 | 2 | 66.66 | 0.59 | 0.41 | 0.50 |
| 49 | BrgMS429 | 54 | 3 | 2 | 66.66 | 0.41 | 0.54 | 0.61 |
| 50 | BrgMS430 | 55 | 3 | 2 | 66.66 | 0.65 | 0.36 | 0.45 |
| 51 | BrgMS432 | 54 | 3 | 1 | 33.33 | 0.66 | 0.35 | 0.44 |
| 52 | BrgMS521 | 55 | 3 | 3 | 100.00 | 0.67 | 0.34 | 0.44 |
| 53 | BrgMS590 | 54 | 4 | 1 | 25.00 | 0.72 | 0.33 | 0.40 |
| 54 | BrgMS638 | 53 | 3 | 3 | 100.00 | 0.34 | 0.59 | 0.67 |
| 55 | BrgMS710 | 54 | 2 | 2 | 100.00 | 0.52 | 0.36 | 0.48 |
| 56 | BrgMS713 | 55 | 3 | 1 | 33.33 | 0.34 | 0.59 | 0.67 |
| 57 | BrgMS751 | 54 | 2 | 1 | 50.00 | 0.50 | 0.38 | 0.50 |
| 58 | BrgMS776 | 55 | 4 | 4 | 100.00 | 0.74 | 0.31 | 0.38 |
| 59 | BrgMS778 | 54 | 3 | 1 | 33.33 | 0.67 | 0.35 | 0.44 |
| 60 | BrgMS780 | 55 | 3 | 3 | 100.00 | 0.65 | 0.36 | 0.46 |
| 61 | BrgMS782 | 54 | 4 | 4 | 100.00 | 0.69 | 0.36 | 0.43 |
| 62 | BrgMS783 | 54 | 2 | 1 | 50.00 | 0.51 | 0.37 | 0.49 |
| 63 | BrgMS787 | 54 | 6 | 5 | 83.33 | 0.66 | 0.46 | 0.51 |
| 64 | BrgMS794 | 53 | 2 | 2 | 100.00 | 0.51 | 0.37 | 0.49 |
| 65 | BrgMS799 | 54 | 2 | 1 | 50.00 | 0.50 | 0.38 | 0.50 |
| 66 | BrgMS801 | 56 | 4 | 4 | 100.00 | 0.69 | 0.36 | 0.43 |
| 67 | BrgMS802 | 54 | 2 | 1 | 50.00 | 0.50 | 0.38 | 0.50 |
| 68 | BrgMS848 | 54 | 2 | 1 | 50.00 | 0.50 | 0.38 | 0.50 |
| 69 | BrgMS961 | 55 | 3 | 2 | 66.66 | 0.57 | 0.42 | 0.51 |
| 70 | BrgMS1237 | 55 | 4 | 3 | 75.00 | 0.74 | 0.32 | 0.39 |
| 71 | BrGMS2766 | 51 | 4 | 1 | 25.00 | 0.74 | 0.32 | 0.39 |
| 72 | BrGMS2767 | 54 | 3 | 1 | 33.33 | 0.61 | 0.38 | 0.48 |
| 73 | BrgMS4508 | 55 | 4 | 4 | 100.00 | 0.71 | 0.34 | 0.41 |
| 74 | BrgMS4533 | 55 | 3 | 1 | 33.33 | 0.65 | 0.35 | 0.45 |
| 75 | Ni2A01 | 55 | 2 | 2 | 100.00 | 0.54 | 0.34 | 0.46 |
| 76 | Ni2A02 | 55 | 2 | 1 | 50.00 | 0.50 | 0.38 | 0.50 |
| 77 | Ni2A07 | 55 | 3 | 1 | 33.33 | 0.67 | 0.34 | 0.43 |
| 78 | Ni2A08 | 50 | 6 | 1 | 16.66 | 0.71 | 0.40 | 0.44 |
| 79 | Ni2A12 | 54 | 3 | 1 | 33.33 | 0.62 | 0.38 | 0.47 |
| 80 | Ni2B01 | 52 | 3 | 3 | 100.00 | 0.66 | 0.35 | 0.45 |
| 81 | Ni2B02 | 54 | 4 | 4 | 100.00 | 0.51 | 0.54 | 0.61 |
| 82 | Ni2B03 | 55 | 5 | 5 | 100.00 | 0.61 | 0.48 | 0.54 |
| 83 | Ni2B07 | 55 | 4 | 4 | 100.00 | 0.63 | 0.43 | 0.49 |
| 84 | Ni2B08 | 53 | 3 | 3 | 100.00 | 0.66 | 0.35 | 0.45 |
| 85 | Ni2C09 | 52 | 5 | 5 | 100.00 | 0.78 | 0.29 | 0.34 |
| 86 | Ni2D03 | 48 | 4 | 4 | 100.00 | 0.76 | 0.29 | 0.36 |
| 87 | Ni2D10 | 52 | 4 | 1 | 25.00 | 0.73 | 0.32 | 0.39 |
| 88 | Ni2E05 | 58 | 6 | 6 | 100.00 | 0.75 | 0.34 | 0.38 |
| 89 | Ni2F11 | 52 | 3 | 1 | 33.33 | 0.64 | 0.36 | 0.46 |
| 90 | Ni4A06 | 54 | 2 | 1 | 50.00 | 0.51 | 0.37 | 0.49 |
| 91 | Ni4A09 | 53 | 4 | 4 | 100.00 | 0.75 | 0.31 | 0.38 |
| 92 | Ni4B04 | 51 | 3 | 2 | 66.66 | 0.68 | 0.34 | 0.43 |
| 93 | Ni4C02 | 56 | 3 | 3 | 100.00 | 0.60 | 0.40 | 0.49 |
| 94 | Ni4C06 | 55 | 3 | 3 | 100.00 | 0.55 | 0.43 | 0.52 |
| 95 | Ni4C09 | 53 | 5 | 1 | 20.00 | 0.53 | 0.56 | 0.62 |
| 96 | Ni4C11 | 54 | 4 | 4 | 100.00 | 0.61 | 0.44 | 0.51 |
| 97 | Ni4F09 | 54 | 5 | 1 | 20.00 | 0.74 | 0.33 | 0.38 |
| 98 | Ni4F11 | 55 | 5 | 1 | 20.00 | 0.78 | 0.29 | 0.34 |
| 99 | Ni4G06 | 50 | 4 | 1 | 25.00 | 0.71 | 0.34 | 0.41 |
| 100 | Ni4G10 | 55 | 7 | 7 | 100.00 | 0.77 | 0.33 | 0.37 |
| 101 | nia-m091a | 52 | 3 | 1 | 33.33 | 0.65 | 0.36 | 0.46 |
| 102 | nia-m066a | 53 | 3 | 1 | 33.33 | 0.64 | 0.36 | 0.46 |
| 103 | SJ3838 | 51 | 5 | 5 | 100.00 | 0.79 | 0.28 | 0.33 |
| 104 | SJ4933 | 51 | 5 | 5 | 100.00 | 0.80 | 0.27 | 0.32 |
| 105 | SJ6846 | 52 | 5 | 5 | 100.00 | 0.76 | 0.31 | 0.36 |
| 106 | SJ3302RI | 52 | 2 | 1 | 50.00 | 0.50 | 0.38 | 0.50 |
| 107 | SJ03104 | 51 | 2 | 1 | 50.00 | 0.50 | 0.37 | 0.50 |
| 108 | SJ3627R | 52 | 4 | 4 | 100.00 | 0.71 | 0.34 | 0.41 |
| 109 | SB1672 | 48 | 5 | 5 | 100.00 | 0.69 | 0.39 | 0.44 |
| 110 | SB1752 | 53 | 4 | 4 | 100.00 | 0.61 | 0.44 | 0.51 |
| 111 | SB2131 | 50 | 2 | 1 | 50.00 | 0.50 | 0.38 | 0.50 |
| 112 | SA0306 | 51 | 5 | 5 | 100.00 | 0.52 | 0.56 | 0.61 |
| 113 | SB0372 | 53 | 3 | 3 | 100.00 | 0.62 | 0.38 | 0.48 |
| 114 | SB1935A | 51 | 5 | 3 | 60.00 | 0.72 | 0.35 | 0.40 |
| 115 | SJ8033 | 51 | 4 | 4 | 100.00 | 0.68 | 0.38 | 0.45 |
| 116 | SB0202I | 51 | 2 | 2 | 100.00 | 0.50 | 0.38 | 0.50 |
| 117 | SB3140 | 52 | 6 | 6 | 100.00 | 0.63 | 0.48 | 0.52 |
| 118 | SJ6842 | 53 | 4 | 1 | 25.00 | 0.57 | 0.48 | 0.55 |
| 119 | SB2556 | 52 | 3 | 1 | 33.33 | 0.63 | 0.37 | 0.46 |
| 120 | SB3872 | 51 | 2 | 2 | 100.00 | 0.50 | 0.38 | 0.50 |
| 121 | SJ0338 | 52 | 3 | 3 | 100.00 | 0.63 | 0.37 | 0.47 |
| 122 | SJ1505 | 49 | 2 | 1 | 50.00 | 0.51 | 0.37 | 0.49 |
| 123 | SJ3640I | 50 | 3 | 3 | 100.00 | 0.59 | 0.40 | 0.49 |
| 124 | SJ39119I | 51 | 4 | 4 | 100.00 | 0.68 | 0.37 | 0.44 |
| 125 | SJ13133 | 51 | 4 | 3 | 75.00 | 0.62 | 0.42 | 0.49 |
| 126 | SJ1536 | 51 | 5 | 5 | 100.00 | 0.68 | 0.40 | 0.46 |
| 127 | SB1937 | 50 | 3 | 2 | 66.66 | 0.64 | 0.36 | 0.46 |
| 128 | SJ4633 | 47 | 2 | 2 | 100.00 | 0.50 | 0.38 | 0.50 |
| 129 | SB1728 | 52 | 3 | 3 | 100.00 | 0.62 | 0.38 | 0.47 |
| 130 | SJ34121 | 51 | 4 | 3 | 75.00 | 0.66 | 0.39 | 0.46 |
| 131 | SJ1668I | 50 | 2 | 1 | 50.00 | 0.50 | 0.38 | 0.50 |
| 132 | SB3751 | 51 | 2 | 1 | 50.00 | 0.50 | 0.37 | 0.50 |
| 133 | cnu_m587a | 52 | 3 | 3 | 100.00 | 0.65 | 0.35 | 0.45 |
| 134 | cnu_m593a | 52 | 2 | 1 | 50.00 | 0.50 | 0.38 | 0.50 |
| 135 | cnu_m596a | 53 | 4 | 1 | 25.00 | 0.72 | 0.33 | 0.40 |
| 136 | cnu_m597a | 54 | 4 | 4 | 100.00 | 0.72 | 0.33 | 0.40 |
| 137 | cnu_m600a | 51 | 4 | 1 | 25.00 | 0.75 | 0.30 | 0.37 |
| 138 | cnu_m602a | 56 | 3 | 1 | 33.33 | 0.65 | 0.35 | 0.45 |
| 139 | cnu_m604a | 53 | 6 | 1 | 16.66 | 0.51 | 0.61 | 0.66 |
| 140 | cnu_m611a | 55 | 2 | 2 | 100.00 | 0.51 | 0.37 | 0.49 |
| 141 | cnu_m626a | 54 | 5 | 5 | 100.00 | 0.73 | 0.34 | 0.40 |
| 142 | sORA43 | 55 | 4 | 1 | 25.00 | 0.70 | 0.35 | 0.42 |
| 143 | Ol09A01 | 54 | 5 | 5 | 100.00 | 0.79 | 0.28 | 0.33 |
| 144 | Ol10A11 | 55 | 3 | 1 | 33.33 | 0.34 | 0.59 | 0.66 |
| 145 | Ol10B01 | 54 | 4 | 1 | 25.00 | 0.45 | 0.58 | 0.64 |
| 146 | Ol10B11 | 52 | 7 | 7 | 100.00 | 0.79 | 0.31 | 0.34 |
| 147 | KBRH138G23 | 52 | 3 | 3 | 100.00 | 0.67 | 0.34 | 0.44 |
| 148 | KBRH139B23 | 52 | 3 | 3 | 100.00 | 0.66 | 0.35 | 0.45 |
| 149 | E018 | 55 | 3 | 3 | 100.00 | 0.66 | 0.35 | 0.45 |
| 150 | GOL2 | 56 | 3 | 2 | 66.66 | 0.38 | 0.56 | 0.63 |
| 151 | GOL3 | 55 | 3 | 3 | 100.00 | 0.34 | 0.59 | 0.66 |
| 152 | EJU1 | 53 | 2 | 1 | 50.00 | 0.50 | 0.38 | 0.50 |
| 153 | EJU3 | 52 | 4 | 1 | 25.00 | 0.73 | 0.32 | 0.39 |
| 154 | EJU5 | 55 | 3 | 1 | 33.33 | 0.57 | 0.41 | 0.51 |
| 155 | ENA2 | 55 | 5 | 5 | 100.00 | 0.60 | 0.50 | 0.56 |
| 156 | ENA20 | 54 | 4 | 4 | 100.00 | 0.72 | 0.33 | 0.40 |
| 157 | ENA28 | 54 | 2 | 1 | 50.00 | 0.51 | 0.37 | 0.49 |
| 158 | ENA9 | 54 | 3 | 1 | 33.33 | 0.45 | 0.50 | 0.59 |
| 159 | MB4 | 46 | 2 | 2 | 100.00 | 0.51 | 0.37 | 0.49 |
| Mean | 3.61 | 0.62 | 0.39 | 0.47 | ||||
Figure 2.
A representative gel picture depicting the amplification profile of SSR marker SA0306 in 135 Indian mustard germplasm accessions (M: 50 bp DNA ladder; Samples 1–135: Indian mustard germplasm accessions).
Figure 3.
UPGMA dendrogram showing genetic relationship among 135 Indian mustard germplasm accessions based on 159 SSR loci.
Figure 4.
Principal coordinate analysis (PCoA) of Indian mustard germplasm accessions on the basis of SSR markers.
3.3. Population structure analysis and AMOVA
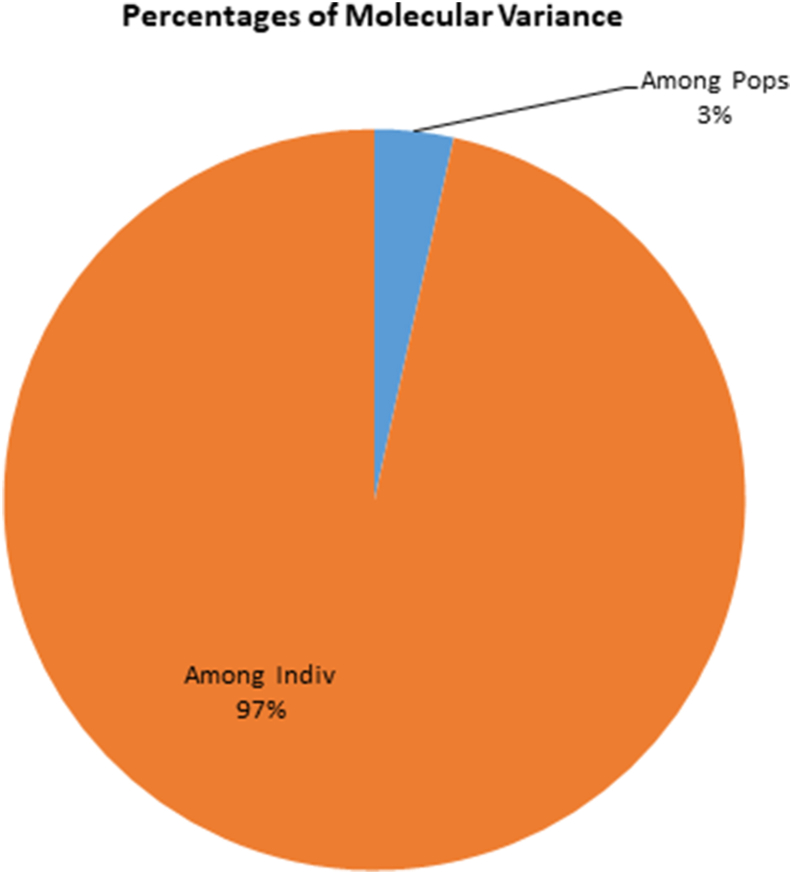
The log likelihood displayed by structure revealed the optimum K value as 5 (K = 5), which inferred that whole germplasm panel can be divided into five subpopulations (Figure 5). The summary statistics obtained from STRUCTURE software as depicted in color chart inferred varying degree of genetic intermixing between genotypes under study (Figure 6). AMOVA statistics of Indian mustard genotypes exhibited 3% variation among populations and 97% variation within individuals (Table 4, Figure 7).
Figure 5.
Population structure analysis using LnP(D) derived delta K for determining optimum number of subpopulations. The maximum of adhoc measure delta K determined by Structure Harvester was found to be K = 5, which inferred that the whole population can be divided into 5 subpopulations.
Figure 6.
Population structure of 135 Indian mustard germplasm accessions with K = 5 estimated using 159 SSR loci.
Table 4.
Summary of Analysis of variance (AMOVA).
| Source of variation | df | SS | MSS | Est. Var. | %age |
|---|---|---|---|---|---|
| Among populations | 4 | 683.039 | 170.760 | 1.572 | 3 |
| Among individuals | 130 | 11494.709 | 88.421 | 44.210 | 97 |
| Total | 134 | 12177.748 | 45.782 | 100 |
Figure 7.
Analysis of molecular variance of 135 Indian mustard accessions based on SSR markers.
4. Discussion
A high volume of genetic information pertaining to genetic diversity is required to formulate breeding strategies (Carvalho et al., 2019). Since Indian mustard germplasm has a narrow genetic base (Chauhan et al., 2011), so we used both agro-morphological traits and SSR marker systems to evaluate genetic diversity in a panel of Indian mustard germplasm collection. In the present investigation, we characterized 135 Indian mustard germplasm accessions using 11 agro-morphological traits. Various traits including biological yield, seed yield and 1000-seed weight expressed wide range of variation. Whereas, almost all the Indian mustard accessions exhibited a narrow range of variation for days to maturity. In a similar study, Singh et al. (2018) also reported least variability for days to maturity in Indian mustard varieties. In another study conducted to evaluate genetic variability in Indian mustard accessions, maximum variation for seed yield per plant followed by secondary branches per plant, primary branches per plant, 1000-seed weight and the number of siliqua on main shoot was recorded (Singh et al., 2020). In another attempt to decipher the genetic variation in 50 Indian mustard germplasm and breeding lines, maximum variability for biological yield followed by seed yield and minimum variability for days to maturity followed by days to flower initiation was reported (Singh et al., 2013). All the germplasm lines initiated flowering between 33-77 days interval. Lesser plant height is a desirable agronomic trait in Indian mustard as it eases various agronomic operations. Hence, genotypes HP 35 (148 cm), BT 31 (154 cm) and JCR 996 (158 cm) are having low plant height and may be used as donor for breeding for low plant height trait. Number of seeds per siliqua and 1000-seed weight are important yield component traits. A total of 14 germplasm lines were having 1000-seed weight above 6.0 g, out of which genotype JBT 41/32, UP II-21 and BT 13 were having 1000-seed weight above 7.0 g, inferring the utility of these genotypes for breeding for bold seededness trait. Overall, a high degree of genetic diversity was observed in Indian mustard germplasm accessions for agro-morphological traits, which is consistent with the previous reports (Vinu et al., 2013; Singh et al., 2018). UPGMA-based dendrogram grouped all the Indian mustard germplasm accessions into three major groups.
Genetic diversity estimation aids crop improvement programs by establishing genetic relationship in germplasm accessions, which assists in effective utilization of genetic resources. In the present investigation, 159 SSRs resulted into polymorphic amplicons, which were further considered for computation of various allelic diversity parameters. The number of alleles ranged from 2-7 with a mean value of 3.61 per SSR locus. A lesser average allele number (2.37) per SSR marker than the present study was reported in a similar study by Sudan et al. (2016), where 23 genotypes of Indian mustard were characterized using 16 SSRs. Thakur et al. (2015) also reported 2.17 average number of alleles per SSR marker when they characterized 12 popular varieties of B. juncea. Higher allele number reported in the present study can be credited to the large number of genotypes evaluated and higher number of SSRs used than earlier studies. PIC value describes the discriminatory potential of a molecular marker and it ranged from 0.24-0.61 with a mean value of 0.39 per SSR locus. A lesser average PIC value was reported in a similar study involving evaluation of genetic diversity in B. juncea genotypes for Alternaria blight tolerance using 25 SSRs (Pratap et al., 2015). Similarly, Singh et al. (2016) observed a lesser average PIC value (0.30) than the present study when they assessed the polymorphic potential of 134 genic-SSRs in six B. juncea genotypes. Lower PIC value in these studies might be due to the lesser diversity in the germplasm used. Botstein et al. (1980) developed a PIC value scale to estimate the level of genetic variability. According to them, the locus having PIC value > 0.5 was considered of high genetic diversity; with PIC value < 0.25 means of low genetic diversity and with PIC value in the range of 0.25–0.5, with intermediate diversity. In this study, an average PIC value of 0.39 per SSR locus was obtained, which inferred that the present data set consists of intermediate genetic diversity level. Further, it was also reported by another group of scientists (Akkaya and Buyukunal Bal, 2004) that markers having PIC values of 0.5 or above 0.5 are considered highly useful in differentiating the genotypes. In this study, out of 159 polymorphic SSRs, 16 markers recorded PIC values > 0.5, which can be considered as highly polymorphic and thus informative and useful for genetic diversity studies, linkage map construction and QTL mapping in Indian mustard. Gene diversity is the probability that two randomly chosen alleles are differing in the genotype (Choukan and Warburton, 2005). In the present study, gene diversity values were in the range of 0.27–0.67 with a mean value of 0.47 per SSR marker.
UPGMA-dendrogram grouped all 135 accessions into two separate clusters (Figure 3). It has been observed that even exotic accessions from Canada except for EC 511684, Sweden and England used in the present study showed grouping with the Indian genotypes. There was no grouping of genotypes observed on the basis of geographical origin. The most plausible explanation for that these exotic germplasm lines might have been imported from India earlier in various international collaborative programs or these genotypes might have involved Indian genotypes as parents somewhere. Further, the SSR-based grouping of Indian mustard genotypes did not show any coherence with the quantitative trait-based grouping. The reason for such a mismatch might be due to that molecular marker differentiation is affected by a number of factors including genetic drift, mutation and gene flow; whereas, the differentiation in agro-morphological traits is more dependent on environmental factors’ influence (Govindaraj et al., 2015). In a similar study, Singh et al. (2013) also obtained different clustering patterns of Indian mustard germplasm lines based on agro-morphological trait and RAPD marker data showing no coherence between genetic and phenotypic diversity. In another study, different clustering patterns were obtained in rice genotypes using both morphological and SSR markers showing no correspondence with each other (Sruthi et al., 2020). In the present study, PCoA could provide a better estimate of genetic diversity inherent in Indian mustard germplasm accessions than the morphological trait and SSR allele-based UPGMA. Indian mustard genotypes EC 511589 (70) from Canada and DMRS 186 (39) from Uttarakhand, India were the most genetically diverse accessions, followed by EC 511684 (74) from Canada and UP II-18 (50) from U.P., India. Two germplasm lines from India viz. RN 593 (53) from Navgaon, Rajasthan and BBM 06-02 (34) from Jagdalpur, Chhatisgarh formed a separate cluster. Similarly, two germplasm accessions from Bihar, India viz. VKG 29/80 (21) and VKG 13/75 (55) clustered together in a separate group (Figure 4).
It has been observed that the genetically diverse accessions identified in the present study using SSR allelic data viz. HPLM 06–36 (120) is having 127 siliquae on main shoot and thus may be a potential donor for this trait. Similarly, EC 511684 (74), an exotic accession from Canada is high yielding genotype (seed yield 2986 kg/ha). EC 51189 (70) from Canada and HPLM 06–36 (120) are also having a higher siliqua length (4.59 cm). Germplasm accessions viz. DMRS 186 (39) from India and EC 51189 (70) from Canada attained maturity in 126–127 days, hence can be used for breeding for early maturity. DMRS 186 (39) also exhibited the trait of bearing higher number of seeds per siliqua (16 seeds/siliqua), thus can be deployed for breeding for both early maturity and higher yield. Population structure analysis is useful for distinguishing the number of subpopulations based on the distribution of allele frequency among genotypes. It can further be utilized to identify pure genotypes from the admixtures in the gene banks and while formulating hybridization strategies (Chen et al., 2017). In the present study, five subpopulations were obtained based on STRUCTURE analysis and all the subpopulations exhibited significant number of admixture genotypes. Actually, B. juncea has a narrow genetic base as reported by Chauhan et al. (2011). It has been further reported that Indian mustard variety Varuna or its derivatives had been used as parent in the development of a number of Indian mustard varieties and breeding lines. Further, there might have occurred the exchange of genetic material over the time among various genotypes used in the present study resulting into admixture genotypes in structure analysis. AMOVA partitioned the genetic variation as 3% variation among populations and 97% variation within individuals.
5. Conclusion
In the present study, agro-morphological traits along with SSR markers were used for characterization of Indian mustard germplasm panel. The study inferred that Indian mustard germplasm expressed considerable diversity based on agro-morphological traits. However, the genetic diversity at molecular level was found relatively low. Finally, dissimilarities between agro-morphological and molecular markers-based clustering of genotypes inferred that geographic and genetic distances are not related in Indian mustard accessions. The results of cluster analysis may be utilized to formulate strategies to enhance genetic diversity by crossing genetically diverse parents in future. The second approach may involve crossing of high yielding genotypes that possess many random genetic differences, which may increase the number of transgressive segregants. This study inferred that both morphological and molecular marker systems are supplementing each other as they have identified separate list of genotypes as diverse ones. The genetically diverse material identified in the present study may be utilized for Indian mustard crop improvement programs in future.
Declarations
Author contribution statement
Lal Singh, M.Sc; Deepika Sharma, M.Sc; Nehanjali Parmar, Ph.D: Performed the experiments.
J Nanjundan, Ph.D.: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
K H Singh, Ph.D: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Rohit Jain, Ph.D: Analyzed and interpreted the data.
Ajay Kumar Thakur: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interests statement
The authors declare no competing interests.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are thankful to the Director, ICAR-Directorate of Rapeseed-Mustard Research, Bharatpur, Rajasthan for providing funding to carry out this research work. Authors also wish to express their gratitude to Dr. S.S. Banga, ex-National Professor, ICAR, PAU, Ludhiana, Punjab, India for providing B-genome specific SSR markers.
Footnotes
This article is a part of the "Genetic enhancement and advances in crop breeding" Special issue
References
- Akkaya M.S., Buyukunal Bal E.B. Assessment of genetic variation of bread wheat varieties using microsatellite markers. Euphytica. 2004;135:179–185. [Google Scholar]
- Botstein D., White R.L., Skolnick M., Davis R.W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am. J. Hum. Genet. 1980;32:314–331. [PMC free article] [PubMed] [Google Scholar]
- Carvalho Y.G.S., Vitorino L.C., de Souza U.J.B., Bessa L.A. Recent trends in research on the genetic diversity of plants: implications for conservation. Diversity. 2019;11 [Google Scholar]
- Chauhan J.S., Singh K.H., Singh V.V., Kumar S. Hundred years of rapeseed-mustard breeding in India: accomplishments and future strategies. Indian J. Agric. Sci. 2011;81(12):1093–1109. [Google Scholar]
- Chen H., Hong C., Liangliang H., Lixia W., Suhua W., Ming L.W., Cheng X. Genetic diversity and a population structure analysis of accessions in the Chinese cowpea [Vigna unguiculata (L.) Walp.] germplasm collection. Crop J. 2017;5(5):363–372. [Google Scholar]
- Choukan R., Warburton M.I. Use of SSR data to determine relationships among early maturing Iranian maize inbred lines. Maydica. 2005;50:163–170. [Google Scholar]
- Earl D.A., vonHoldt B.M. Structure harvester: a website and program for visualizing Structure output and implementing the Evanno method. Conserv. Genet. Resour. 2012;4:359–361. [Google Scholar]
- Evanno G., Regnaut S., Goudet J. Detecting the number of clusters of individuals using the software structure: a simulation study. Mol. Ecol. 2005;14:2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x. [DOI] [PubMed] [Google Scholar]
- Govindaraj M., Vetriventhan M., Srinivasan M. Importance of genetic diversity assessment in crop plants and its recent advances: an overview of its analytical perspectives. Genet. Res. Int. 2015;431487 doi: 10.1155/2015/431487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govt. of India . Directorate of Economics and Statistics, Department of Agriculture and Cooperation, Ministry of Agriculture, Govt. of India; New Delhi: 2019. Agricultural Statistics at a Glance.http://cands.daenet.nic.in/PDF [Google Scholar]
- Huangfu C., Song X., Qiang S.G. ISSR variation within and among wild Brassica juncea populations: implication for herbicide resistance evolution. Genet. Resour. Crop Evol. 2009;56:913–924. [Google Scholar]
- Khan M.A., Rabbani M.A., Muhammad M., Ajmal S.K., Malik M.A. Assessment of genetic variation within Indian mustard germplasm using RAPD markers. J. Integr. Plant Biol. 2008;50:385–392. doi: 10.1111/j.1744-7909.2007.00630.x. [DOI] [PubMed] [Google Scholar]
- Liu K., Muse S.V. PowerMarker: an integrated analysis environment for genetic marker analysis. Bioinformatics. 2005;21(9):2128–2129. doi: 10.1093/bioinformatics/bti282. [DOI] [PubMed] [Google Scholar]
- Mir J.I., Islam I., Kudesia R. Evaluation of genetic diversity in Brassica juncea (L.) using protein profiling and molecular marker (RFLP) Int. J. Plant Breed. Genet. 2015;9(2):77–85. [Google Scholar]
- Pandey S., Ansari W.A., Pandey M., Singh B. Genetic diversity of cucumber estimated by morpho-physiological and EST-SSR markers. Physiol. Mol. Biol. Plants. 2018;24(1):135–146. doi: 10.1007/s12298-017-0489-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peakall R., Smouse P.E. GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research–an update. Bioinformatics. 2012;28:2537–2539. doi: 10.1093/bioinformatics/bts460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier X., Jacquemoud-Collet J.P. 2006. DARwin Software.http://darwin.cirad.fr/darwin [Google Scholar]
- Pratap P., Thakur A.K., Meena P.D., Meena H.S., Sharma P., Singh D., Majumdar R. Genetic diversity assessment in Indian mustard (Brassica juncea L) for Alternaria blight tolerance using SSR markers. J. Oilseed Brassica. 2015;6(1):175–182. [Google Scholar]
- Premi O.P., Kandpal B.K., Rathore S.S., Shekhawat K., Chauhan J.S. Green manuring, mustard residue recycling and fertilizer application affects productivity and sustainability of Indian mustard (Brassica juncea L.) in Indian semi-arid tropics. Ind. Crop. Prod. 2013;41:423–429. [Google Scholar]
- Pritchard J.K., Wen W. Department of Human Genetics, University of Chicago; Chicago: 2003. Documentation for the Structure Software.http://pritch.bsd.uchicago.edu/software [Google Scholar]
- Qi X., Yang J., Zhang M. AFLP-based genetic diversity assessment among Chinese vegetable mustard (Brassica juncea (L.) Czern.) Genet. Resour. Crop Evol. 2008;55:705–711. [Google Scholar]
- Redden R., Vardy M., Edwards D., Raman H., Batley J. 16th Australian Research Assembly on Brassicas, Ballarat, Victoria. 2009. Genetic and morphological diversity in the Brassicas and wild relative.http://www.cropwildrelatives.org/fileadmin/www.cropwildrelaves Retrieved from. [Google Scholar]
- Sharma S., Kaushik S., Singhal A., Changwal G., Das A., Joshi B. Assessment of genetic variation and relationship among Brassica species by RAPD marker. Int. J. Curr. Res. Biosci. Plant Biol. 2015;2(6):35–42. [Google Scholar]
- Singh B.K., Mishra D.C., Yadav S., Ambawat S., Vaidya E., Tribhuvan K.U., Kumar A., Kumar S., Kumar S., Chaturvedi K.K., Rani R., Yadav P., Rai A., Rai P.K., Singh V.V., Singh D. Identification, characterization, validation and cross-species amplification of genic-SSRs in Indian mustard (Brassica juncea) J. Plant Biochem. Biotechnol. 2016;25(4):410–420. [Google Scholar]
- Singh K.H., Misra A.K., Kumar A. National Research Centre on Rapeseed Mustard; Bharatpur, Rajasthan, India: 2006. Draft Guidelines to Conduct the Tests for Distinctness, Uniformity and Stability in Rapeseed and Mustard (Brassica) [Google Scholar]
- Singh K.H., Shakya R., Nanjundan J., Thakur A.K., Singh K., Singh K.K. Genetic diversity assessment and characterization of Indian mustard (Brassicaceae) varieties using agro-morphological traits. Indian J. Plant Genet. Resour. 2018;31(1):44–50. [Google Scholar]
- Singh K.H., Shakya R., Thakur A.K., Chauhan D.K., Chauhan J.S. Assessment of genetic diversity in Indian mustard (Brassica juncea L Czern & Coss) using morphological traits and RAPD markers. Natl. Acad. Sci. Lett. 2013;36(4):419–427. [Google Scholar]
- Singh L., Nanjundan J., Singh K.H., Sharma D., Parmar N., Watts A., Jain R., Thakur A.K. Development of a set of SSR markers for characterization of Indian mustard germplasm and varieties. J. Plant Biochem. Biotechnol. 2021 [Google Scholar]
- Singh V.K., Avtar R., Mahavir Kumari N., Manjeet Kumar R., Rathore V. Assessment of genetic relationship among diverse Indian mustard (Brassica juncea L.) genotypes using XLSTAT. Electron. J. Plant Breed. 2020;11(2):674–680. [Google Scholar]
- Srivastava A., Gupta V., Pental D., Pradhan A.K. AFLP based genetic diversity assessment amongst agronomically important natural and some newly synthesized lines of Brassica juncea. Theor. Appl. Genet. 2001;102:193–199. [Google Scholar]
- Sruthi, et al. Evaluation of genetic diversity of parental lines for development of heterotic groups in hybrid rice (Oryza sativa L.) J. Plant Biochem. Biotechnol. 2020;29(2):236–252. [Google Scholar]
- Sudan J., Khajuria P., Gupta S.K., Singh R. Analysis of molecular diversity in Indian and Eexotic genotypes of Brassica juncea using SSR markers. Indian J Genet. 2016;76(3):361–364. [Google Scholar]
- Tamanna A., Khan A.U. Mapping and analysis of simple sequence repeats in the Arabidopsis thaliana genome. Bioinformation. 2005;1:64–68. doi: 10.6026/97320630001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur A.K., Parmar N., Singh K.H., Nanjundan J. Current achievements and future prospects of genetic engineering in Indian mustard (Brassica juncea L. Czern & Coss.) Planta. 2020;252:56. doi: 10.1007/s00425-020-03461-8. [DOI] [PubMed] [Google Scholar]
- Thakur A.K., Singh B.K., Verma V., Chauhan J.S. Direct organogenesis in Brassica juncea var. NRCDR-2 and analysis of genetic uniformity using RAPD markers. Natl. Acad. Sci. Lett. 2013;36:403–409. [Google Scholar]
- Thakur A.K., Singh K.H., Singh L., Nanjundan J., Khan Y.J., Singh D. Patterns of subspecies genetic diversity among oilseed Brassica rapa as revealed by agro-morphological traits and SSR markers. J. Plant Biochem. Biotechnol. 2017;26(3):282–292. [Google Scholar]
- Thakur A.K., Singh K.H., Singh L., Nanjundan J., Rana M.K., Singh D. Transferability of SSR markers of Brassica species to some popular varieties of Brassica juncea. Proc. Natl. Acad. Sci. India B Biol. Sci. 2015;85(4):1001–1010. [Google Scholar]
- Vieira M.L., Santini L., Diniz A.L., Munhoz Cde F. Microsatellite markers: what they mean and why they are so useful. Genet. Mol. Biol. 2016;39:312–328. doi: 10.1590/1678-4685-GMB-2016-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinu V., Singh N., Vasodev S., Yadav D.K., Kumar S., Naresh S., Bhat S.R., Prabhu K.V. Assessment of genetic diversity in Brassica juncea (Brassicaceae) genotypes using phenotypic differences and SSR markers. Rev. Biol. Trop. 2013;61(4):1919–1934. [PubMed] [Google Scholar]
- Yadav M., Rana J.S. ISSR markers assisted studies on genetic diversity in Brassica juncea. Int. J. Pharma Bio Sci. 2012;3(2):402. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.