Abstract
Introduction
Single-agent monoclonal antibody therapy against programmed death-ligand 1 (PD-L1) has modest effects in malignant pleural mesothelioma. Radiation therapy can enhance the antitumor effects of immunotherapy. Nevertheless, the safety of combining anti-PD-L1 therapy with stereotactic body radiation therapy (SBRT) is unknown. We present the results of a phase 1 trial to evaluate the safety of the anti-PD-L1 antibody avelumab plus SBRT in patients with malignant pleural mesothelioma.
Methods
This was a single-arm, investigator-initiated trial in patients who progressed on prior chemotherapy. Avelumab was delivered every other week, and SBRT was delivered to one lesion in three to five fractions (minimum of 30 Gy) followed by continuation of avelumab up to 24 months or until disease progression. The primary end point of the study was safety on the basis of grade 3+ nonhematologic adverse events (AEs) within 3 months of SBRT.
Results
Thirteen assessable patients received a median of seven cycles (range: 2–26 cycles) of avelumab. There were 27 grade 1, 17 grade 2, four grade 3, and no grade 4 or 5 avelumab-related AEs. The most common were infusion-related allergic reactions (n = 6), anorexia or weight loss (n = 6), fatigue (n = 6), thyroid disorders (n = 5), diarrhea (n = 3), and myalgia or arthralgias (n = 3). There were 10 grade 1, four grade 2, one grade 3, and no grade 4 or 5 SBRT-related AEs. The most common were diarrhea (n = 3), chest pain/myalgia (n = 2), fatigue (n = 2), cough (n = 2), dyspnea (n = 2), and nausea/vomiting (n = 2).
Conclusions
Combination avelumab plus SBRT seems tolerable on the basis of the prespecified toxicity end points of the first stage of this Simon two-stage design phase 1 study.
Keywords: Malignant pleural mesothelioma, Stereotactic body radiation therapy, Avelumab, Safety, Phase I Study
Introduction
Malignant pleural mesothelioma (MPM) remains an almost universally lethal diagnosis. There is currently no standard of care for second-line therapy after initial progression. A randomized trial in the United Kingdom revealed that single-agent nivolumab had improved median overall survival (OS) compared with placebo.1 Nevertheless, single-agent checkpoint inhibition alone seems to have only modest effects in mesothelioma.2, 1, 3
Radiation therapy (RT) is a highly effective local treatment modality for MPM that can be used in the neoadjuvant, adjuvant, or palliative settings.4, 5, 6, 7 Biologically, ionizing radiation can increase antigen exposure, enhance the T-cell receptor repertoire in tumor-infiltrating lymphocytes, and increase the secretion of proinflammatory cytokines,8, 9, 10, 11, 12, 13 thus providing a rationale for postulated synergy with immune checkpoint inhibitors. The ideal radiation dose to achieve optimal immunomodulatory effects seems to be in the range of 6 to 10 Gy per fraction.4,14, 15, 16 Checkpoint inhibition after thoracic chemo-RT has become the standard of care for inoperable stage III NSCLC.17 The phase 2 nonrandomized KEYNOTE-799 study was the first to reveal the safety and promising response rates of concurrent chemo-RT with pembrolizumab in patients with stage III NSCLC.18 Furthermore, concurrent thoracic RT and checkpoint inhibition is the subject of ongoing studies for NSCLC (e.g., PACIFIC-2; clinicaltrials.gov: NCT03519971; NRG LU-004; clinicaltrials.gov: NCT03801902) but has not been tested in MPM. We therefore designed this phase 1 study to evaluate the safety of combining avelumab, an antiprogrammed death-ligand 1 (anti–PD-L1) IgG1 monoclonal antibody, with thoracic stereotactic body RT (SBRT) using moderately high hypofractionated doses.
Materials and Methods
Fifteen patients were enrolled after written informed consent on this institutional review board-approved phase 1 study (NCT03399552) between January 2018 and September 2019. Key inclusion criteria were pathologically confirmed MPM (any histologic subtype), no plan for surgical resection, at least one prior line of chemotherapy, at least one targetable lesion appropriate for SBRT and one nontarget lesion for observation, age more than or equal to 18 years, Karnofsky performance status greater than or equal to 70%, and adequate hematologic, renal, and hepatic function. Exclusion criteria were prior RT precluding SBRT, current immunosuppressive medications at greater than 10 mg/d prednisone equivalent, active autoimmune disease, known hypersensitivity to avelumab, rapid widespread disease progression on prior immunotherapy, prior investigational or other systemic therapy within 4 weeks from study treatment, residual grade greater than or equal to 1 adverse events (AEs) from prior systemic therapy, concomitant use of other systemic therapies, pregnancy, active infection, serious unstable medical illness, and continuous oxygen use.
Treatment Plan
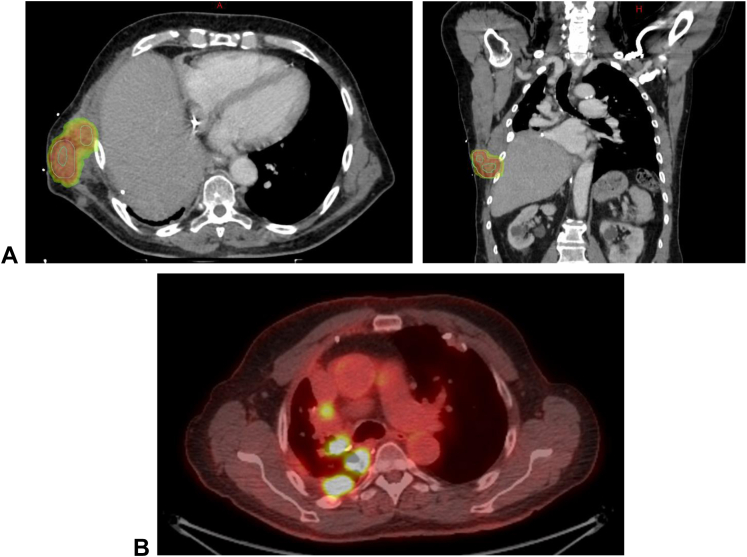
For each patient, one targetable lesion that required palliation was selected for SBRT, and at least one nontarget lesion was observed to assess effects of avelumab alone (Fig. 1). Common criteria to select the target lesion for SBRT included pleural lesions invading the chest wall causing pain, invading the spine or costovertebral joint causing pain, and putting the patient at risk of cord compression in case of further disease progression, and impending or present perihilar airway or vascular compromise. Biopsy of both lesions was done at baseline and after two cycles of avelumab and SBRT.
Figure 1.
Illustration of SBRT target and nontarget lesions. (A) Lesion targeted by SBRT with corresponding dose cloud of the radiation treatment plan. (B) Lesions not targeted by SBRT and used as a control to evaluate the effect of avelumab alone or potential synergy of SBRT and avelumab outside the radiation field. SBRT, stereotactic body radiation therapy.
Immunotherapy
Patients received avelumab 10 mg/kg every other week at the discretion of the treating medical oncologist. Standard supportive medications were administered. After two priming doses of avelumab, patients underwent SBRT and continued avelumab thereafter for up to 24 months or disease progression. Treatment beyond disease progression was permissible at the discretion of the treating medical oncologist.
SBRT Technique
Patients were immobilized in supine position with their arms raised in a customized α-cradle. Gross tumor volume was delineated on the basis of computed tomography (CT) scan at simulation and positron emission tomography scan within 6 weeks from treatment start. A four-dimensional CT was performed to account for respiratory motion and create an internal target volume. The clinical target volume was defined as the internal target volume plus a 2 mm margin. A planning target volume was generated using a 5 mm margin. SBRT was delivered in three to five fractions (minimum total prescription dose of 30 Gy) with 6 mV photons using the volumetric arc technique. Tissue inhomogeneity correction was used. The goal was to deliver the prescription dose to greater than or equal to 95% of the planning target volume while respecting normal tissue constraints (Supplementary Table 1). All SBRT treatments were planned in Eclipse and delivered on Varian linear accelerators (Varian Medical Systems).
Follow-Up
Patients were evaluated 1 month after RT and subsequently every 2 months. CT scans were performed at baseline and every other month after RT completion.
Biomarker Analyses
Exploratory studies on proinflammatory cytokines (interferon gamma, tumor necrosis factor-α, interleukin (IL)-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13) in peripheral blood samples obtained at baseline, 2 weeks post-SBRT, and 2 months post-SBRT were performed. Cytokine levels were measured using the MSD V-PLEX Proinflammatory Panel 1 (Meso Scale Diagnostics, LLC).
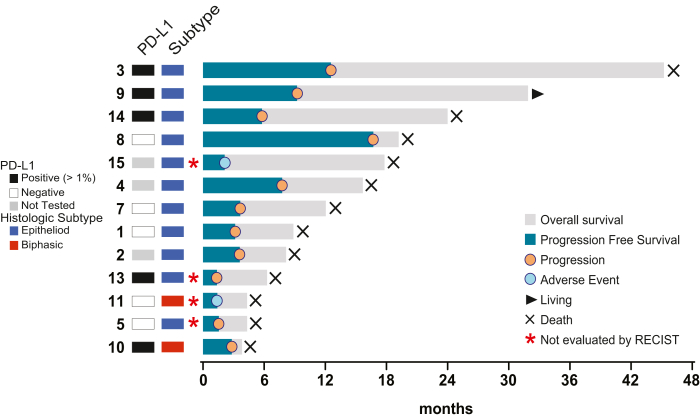
Next-generation sequencing was performed using the Memorial Sloan Kettering—Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT), which is a Food and Drug Administration–approved assay that detects genetic alterations involving 341 (version 1), 410 (version 2), or 468 (version 3) cancer-associated genes.19,20 Patients were selected and consented for tumor genomic testing to identify genetic alterations that are readily actionable or candidates for clinical trial as part of routine clinical care. Tissue samples analyzed by MSK-IMPACT were collected and sequenced before enrollment. The genes selected had greater than a 5% driver alteration frequency. The OncoPrint (Fig. 2) was made using cBioPortal (https://www.cbioportal.org/).
Figure 2.
Swimmer’s plot of patient outcomes. Overall survival, progression-free survival, and current status are indicated as found in the figure legend. PD-L1, programmed death-ligand 1; RECIST, Response Evaluation Criteria in Solid Tumors.
Immunohistochemistry for PD-L1 (Cell Signaling Technology, Danvers, MA; clone E1L3N) was performed on patient tissue. Appropriate control tissue was used for the PD-L1 antibody. PD-L1 was interpreted as a percentage of at least partial membranous expression in the tumor cells.
Study End Points and Statistical Considerations
The primary objective was to determine the safety of avelumab plus SBRT on the basis of grade greater than or equal to 3 radiation pneumonitis (i.e., hospitalization or requiring oxygen) following the National Cancer Institute Common Terminology Criteria for Toxicity and Adverse Event version 4.0 reporting. Toxicities were considered acute if they occurred within 3 months after SBRT. Patients were assessable for the primary end point if SBRT was completed and two or more doses of avelumab were administered.
A two-stage design was used with 13 patients enrolled in the first stage. If three or more patients experienced grade greater than or equal to 3 AEs, the trial would be stopped and declared unsafe. Otherwise, enrollment would be extended to 27 patients. This stopping rule had a power of 80% to declare the true AE rate of 0.1 or lower safe and a type I error rate (α) of 5% declaring the true AE rate of 0.3 or higher unsafe.
Secondary end points included the overall response rate of the irradiated and predetermined nonirradiated lesions, progression-free survival (PFS), and OS. All imaging studies were reviewed by a reference radiologist (S.H.) according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.
Results
Patient Characteristics
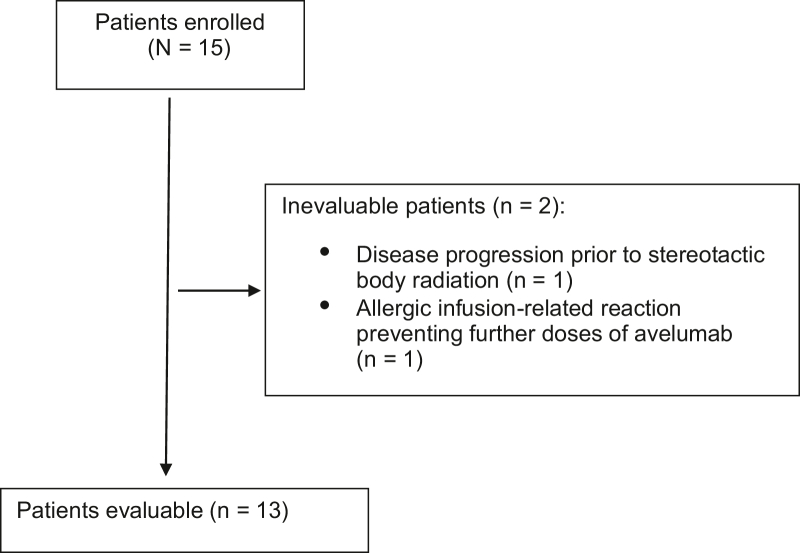
Of the 15 patients enrolled in the study, two were not assessable for the safety or efficacy end point (Fig. 3). One patient had disease progression before SBRT and therefore did not reach the first time point for evaluation. Another patient had a grade 3 allergic reaction with the first infusion of avelumab, could not safely receive additional doses of avelumab, and therefore came off study. The characteristics of the 13 assessable patients are found in Table 1. Median follow-up was 8.9 months (range: 4.3–32.7 mo). Median age at enrollment was 71 (range: 51–82) years, and the median Karnofsky performance status was 90% (range: 80%–90%). Ten patients were enrolled for second-line therapy, two for third-line therapy, and one for fourth-line therapy. None had received prior immunotherapy. All patients had biopsy-proven recurrence or disease progression after prior therapy. Nine patients had epithelioid, two had biphasic, and two had sarcomatoid histologic subtypes. Six patients had previous RT but were still deemed amenable to SBRT. In addition, 11 patients had next-generation sequencing of their tumor tissue performed, and the median tumor mutation burden (TMB) was 1.8 (range: 0–6.1 mutations per megabase [mt/Mb]). Twelve patients had PD-L1 immunohistochemistry of their tumor tissue performed, and the median tumoral PD-L1 expression was 0% (range: 0%–80%). One patient's tumor had a PD-L1 expression of 80%, another patient's tumor had a PD-L1 expression of 30%, two patients had a PD-L1 expression of 1%, and the remaining eight patient's tumors were PD-L1 negative.
Figure 3.
CONSORT diagram.
Table 1.
Patient, Disease, and Treatment Characteristics
| Patient Characteristics (N = 13) | n (%) |
|---|---|
| Median age, y (range) | 71 (51–82) |
| Median KPS (range) | 90 (80–90) |
| Sex | |
| Male | 11 (85) |
| Female | 2 (15) |
| Smoking status | |
| Current or former | 8 (62) |
| Never | 5 (38) |
| Asbestos exposurea | |
| Yes | 6 (46) |
| No | 4 (31) |
| Unknown | 3 (23) |
| Histologic subtype | |
| Epithelioid | 9 (69) |
| Biphasic | 2 (15.5) |
| Sarcomatoid | 2 (15.5) |
| Stage at diagnosis | |
| I–IIIA | 9 (69) |
| IIIB–IV | 4 (31) |
| Prior systemic therapy lines | |
| 1 | 10 (77) |
| 2 | 2 (15) |
| ≥3 | 1 (8) |
| Prior radiation | |
| Yes | 6 (46) |
| No | 7 (54) |
| Median TMB (n = 11) | |
| Mutations per megabase (range) | 1.8 (0–6.1) |
| Median PD-L1 expression (n = 12) (range) | 0% (0–80%) |
KPS, Karnofsky performance status; PD-L1, programmed death-ligand 1; TMB, tumor mutation burden.
Self-reported classic occupational exposure.
Treatment Characteristics
Patients received a median of seven (range: 2–26) cycles of avelumab for a median duration of 3.5 months. The median SBRT dose was 30 Gy in three fractions (range: 24–35 Gy in three to five fractions).
Safety of Avelumab and SBRT
The stage 1 primary safety end point was met with limited efficacy observed; therefore, the study did not continue to stage 2. Early stopping rules as defined in the biostatistics section were not met. Treatment-related AEs that were possibly, probably, or definitely treatment related are listed in Table 2 (avelumab-related AEs) and 3 (SBRT-related AEs). Two of 13 patients experienced a total of four grade 3 nonhematologic treatment-related AEs. These included an infusion-related allergic reaction, chest wall pain, myalgia, and anorexia. There were no avelumab-related grade 4 or 5 AEs observed.
Table 2.
Treatment-Related Adverse Events Possibly, Probably, or Definitely Attributed to Avelumab
| Events | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Total |
|---|---|---|---|---|---|---|
| Allergic infusion reaction | 0 | 5 | 1 | 0 | 0 | 6 |
| Anorexia or weight loss | 3 | 2 | 1 | 0 | 0 | 6 |
| Fatigue | 4 | 2 | 0 | 0 | 0 | 6 |
| Diarrhea | 2 | 1 | 0 | 0 | 0 | 3 |
| Myalgia or arthralgiaa | 1 | 1 | 1 | 0 | 0 | 3 |
| Hypothyroidisma | 1 | 2 | 0 | 0 | 0 | 3 |
| Hyperthyroidisma | 2 | 0 | 0 | 0 | 0 | 2 |
| Noncardiac chest pain | 1 | 0 | 1 | 0 | 0 | 2 |
| Dyspnea | 1 | 1 | 0 | 0 | 0 | 2 |
| Mucositis | 1 | 1 | 0 | 0 | 0 | 2 |
| Pruritus/rash | 2 | 0 | 0 | 0 | 0 | 2 |
| Fever/chills | 2 | 0 | 0 | 0 | 0 | 2 |
| Blood bilirubin increased | 0 | 1 | 0 | 0 | 0 | 1 |
| Dehydration | 0 | 1 | 0 | 0 | 0 | 1 |
| Palmar-plantar erythrodysesthesia syndrome | 1 | 0 | 0 | 0 | 0 | 1 |
| Dysgeusia | 1 | 0 | 0 | 0 | 0 | 1 |
| Headache | 1 | 0 | 0 | 0 | 0 | 1 |
| Eye redness or scleral erythema | 1 | 0 | 0 | 0 | 0 | 1 |
| Gastroesophageal reflux | 1 | 0 | 0 | 0 | 0 | 1 |
| Vomiting | 1 | 0 | 0 | 0 | 0 | 1 |
| Cough | 1 | 0 | 0 | 0 | 0 | 1 |
Note: Toxicities were graded using the CTCAE, version 4.0.
CTCAE, Common Terminology Criteria for Adverse Events.
Immune-related adverse event.
Avelumab-related grade 2+ AEs (Table 2) were most often infusion-related allergic reactions (n = 6), followed by anorexia or dehydration in three patients and fatigue or hypothyroidism in two patients each.
SBRT-related grade 2+ AEs (Table 3) included one patient with grade 3 noncardiac chest pain, likely representing a pain flare after SBRT, which was the only grade 3 SBRT-related toxicity. Three patients experienced grade 2 AEs that were possibly SBRT related, including cough in one, diarrhea and myalgia in another, and fatigue and dyspnea in a third patient. There were no SBRT-related grade 4 or 5 AEs observed.
Table 3.
Treatment-Related Adverse Events Possibly, Probably, or Definitely Attributed to SBRT
| Events | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Total |
|---|---|---|---|---|---|---|
| Diarrhea | 2 | 1 | 0 | 0 | 0 | 3 |
| Noncardiac chest pain or myalgia | 1 | 0 | 1 | 0 | 0 | 2 |
| Fatigue | 1 | 1 | 0 | 0 | 0 | 2 |
| Cough | 1 | 1 | 0 | 0 | 0 | 2 |
| Dyspnea | 1 | 1 | 0 | 0 | 0 | 2 |
| Nausea or vomiting | 2 | 0 | 0 | 0 | 0 | 2 |
| Fever | 1 | 0 | 0 | 0 | 0 | 1 |
| Gastroesophageal reflux disease | 1 | 0 | 0 | 0 | 0 | 1 |
Note: Toxicities were graded using the CTCAE, version 4.0.
CTCAE, Common Terminology Criteria for Adverse Events.
Response of Irradiated and Nonirradiated Lesions
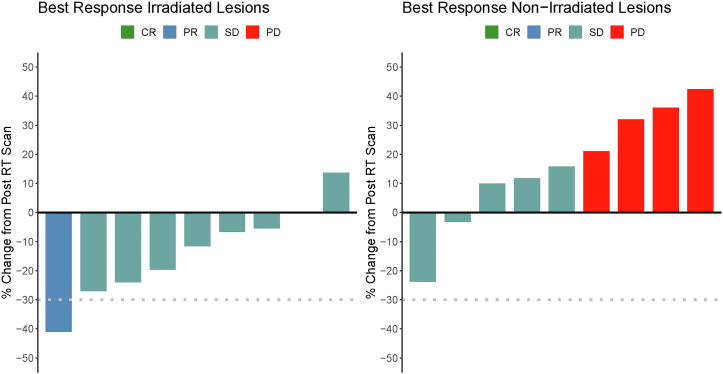
Nine of 13 assessable patients underwent the first CT scan 2 months post-SBRT for RECIST assessment; two patients had clinical progression before the RECIST assessment time point; two other patients had grade 2 and 3 infusion-related allergic reactions and were taken off protocol before the RECIST assessment. Of the remaining nine patients who were available for RECIST assessment, the irradiated lesion had a partial response in one patient and stable disease in eight patients (Fig. 4A). Seven of nine irradiated lesions demonstrated some shrinkage (Fig. 4A). All irradiated lesions were locally controlled at the end of follow-up.
Figure 4.
Response assessment of target lesions. To characterize the effects of avelumab alone and in combination with RT, only one lesion was irradiated per patient. Subsequently, both irradiated and nonirradiated lesions were monitored. (A) Response of irradiated lesion. (B) Response of nonirradiated lesion. Coloration denotes CR, PR, SD, and PD as indicated in the figure legends. CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; RT, radiation therapy.
The nonirradiated lesion demonstrated stable disease in five patients and disease progression in four patients, with no partial or complete responses (Fig. 4B). In seven of nine patients, the nonirradiated lesion had an increase compared to baseline at the time of SBRT.
PFS, OS, and Patterns of Recurrence
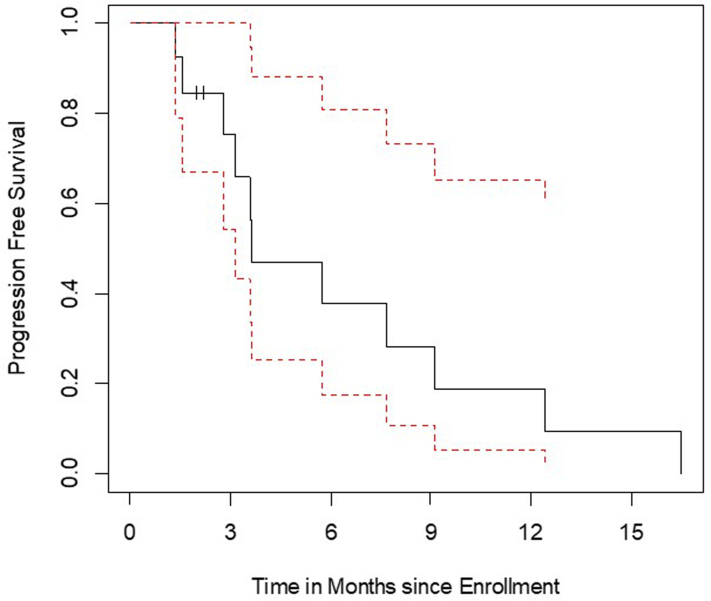
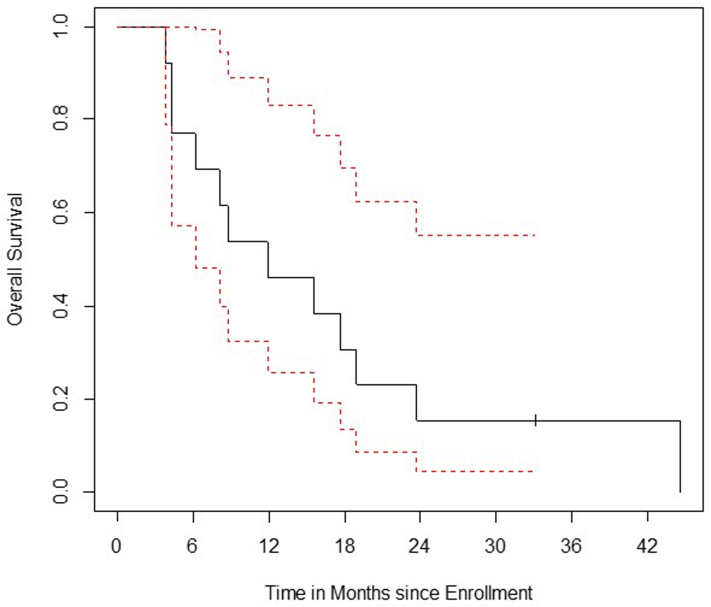
The median PFS and OS in all assessable patients were 3.7 months and 12.1 months, respectively (Supplementary Fig. 1A and B). All but one patient were deceased at the time of this analysis. There were two outliers with progression at 12.6 and 16.7 months and seven patients with survival more than 12 (range: 12.1–45.2) months (Fig. 2).
There was no clear correlation of clinical response with PD-L1 expression, though most patients (n = 8) had 0% PD-L1 expression. One patient with 30% tumoral PD-L1 expression had a PFS of 9.2 months, and one patient with 80% tumoral PD-L1 expression had a PFS of 1.4 months. Similarly, there was no clear association between TMB and PFS. The patient with the highest TMB (6.1 mt/Mb) had a PFS of 2.8 months, and a patient with a TMB of 3.5 mt/Mb had a PFS of 9.2 months. The two outliers with the longest PFS had 0% PD-L1 expression and no TMB analysis available.
Exploratory Analyses of Proinflammatory Cytokines in the Peripheral Blood
We did not observe any significant correlation of the examined proinflammatory cytokines in the peripheral blood with clinical response, including baseline levels, change from baseline to 2 weeks post-SBRT, or 2 months post-SBRT (data not shown; p>0.5). The most common mutations detected by MSK-IMPACT were truncating BAP mutations in three patients and deep deletions of CDKN2A and CDKN2B in five patients.
Discussion
There is currently no Food and Drug Administration–approved second-line therapy for patients with MPM. This study represents the first prospective trial combining anti–PD-L1 therapy with SBRT as second-line therapy in patients with MPM. We reported that SBRT and avelumab can be safely combined. We did not observe synergistic or excess toxicity per pre-defined AEs; only two patients experienced grade 3+ toxicity. In fact, the toxicities seemed additive and independently either related to avelumab (mostly infusion-related allergic reactions) or SBRT (mostly chest wall pain, possibly related to RT or the underlying tumor).
This also represents the first prospective study on the use of SBRT for MPM. Although others have studied the use of palliative RT with palliative or slightly hypofractionated doses (SYSTEMS-1 and -2),6, 7 we used moderately higher doses with a median biologically effective dose of 60 Gy (range: 43–60 Gy; α/β = 10), given in three to five fractions. These doses have a higher chance for extended local control, and indeed we observed that all nine patients who were assessable at the first imaging time point maintained local control of the irradiated lesion. Because many of these pleural targets were naturally located in or near the chest wall or the mediastinal pleura and perihilar zone and the goal was to obtain palliation and immune modulation in synergy with avelumab, we did not use ablative SBRT doses of biologically effective dose greater than 100 Gy, which has been reported to be optimal for peripherally located parenchymal early stage NSCLCs or lung metastases of various histologies.21, 22, 23 With these doses, we did not observe any unexpected AEs not previously known to occur with SBRT in general. We also did not observe an increased risk of SBRT-related AEs when combining it with avelumab relative to existing literature on AEs from SBRT alone.
Unfortunately, we did not observe a synergistic oncologic effect of the combination of avelumab and SBRT. We did not observe any abscopal effects either in this patient population. The overall response rate to avelumab was in line with previously reported single-agent anti–PD-L1 studies, but not superior in combination with SBRT. One possible reason may be that PD-L1 expression was relatively low with eight of 12 tumors having 0% PD-L1 expression. Although this may be affected by sampling error and small tissue specimens, it would align with the lack of response in most patients.
Last, we did not observe changes in proinflammatory cytokines in the peripheral blood correlating with response. We attempted to compare the outliers of best and worst responders but did not detect a pattern corresponding with clinical responses. These analyses may be limited by the small sample size of our study.
Our study has some limitations. Although there was no signal of excess toxicity and the combination of avelumab and SBRT seemed safe, the sample size is small as the study was stopped after completion of accrual to the first planned stage of this phase 1 study. Thus, a larger study would be needed for a more robust toxicity assessment. Similarly, the small sample size limited us in assessing the oncologic effects of avelumab plus SBRT. Although the effects seemed additive rather than synergistic, a larger study may detect a subset of patients who may particularly benefit from this combination therapy. Furthermore, correlative analyses were limited by only one patient having responded to avelumab. Analysis of a larger cohort may allow us to detect differences in the baseline immune phenotype or response of immune biomarkers to the combination therapy that would allow selection of patients with a greater response to therapy. Nevertheless, even the ability to find safety and tolerability of this regimen with additive effects of avelumab and SBRT is worthwhile and assuring.
In conclusion, SBRT in combination with avelumab is feasible and tolerable with an acceptable rate of AEs. This study provides the first prospective data on the use of SBRT for MPM and on combining thoracic SBRT with checkpoint inhibition for MPM. Although the effect seems additive rather than synergistic, we found that SBRT is highly effective as a local treatment option for patients with MPM. Further research needs to be done to optimize the combination with immunotherapy and maximize the immunologic effects.
CRediT Authorship Contribution Statement
Andreas Rimner: Conceptualization, Methodology, Formal analysis, Investigation, Enrolling patients, Writing—original draft, Writing—review and editing, Visualization, Supervision, Project administration.
Prasad S. Adusumilli: Methodology, Investigation, Enrolling patients, Writing—review and editing.
Michael D. Offin: Investigation, Writing—review and editing.
Stephen B. Solomon: Methodology, Investigation, Writing—review and editing.
Etay Ziv: Investigation, Writing—review and editing.
Sara A. Hayes: Methodology, Investigation, Writing—review and editing.
Michelle S. Ginsberg: Methodology, Investigation, Writing—review and editing.
Jennifer L. Sauter: Formal analysis, Investigation, Writing—review and editing, Visualization.
Daphna Y. Gelblum: Enrolling patients, Investigation, Writing—review and editing.
Annemarie F. Shepherd: Enrolling patients, Investigation, Writing—review and editing.
David M. Guttmann: Enrolling patients, Investigation, Writing—review and editing.
Jordan E. Eichholz: Visualization, Formal analysis, Writing—review and editing.
Zhigang Zhang: Conceptualization, Methodology, Formal analysis, Writing—review and editing.
Erika Ritter: Formal analysis, Investigation.
Phillip Wong: Methodology, Formal analysis, Investigation, Writing—review and editing.
Afsheen N. Iqbal: Enrolling patients, Investigation, Writing—review and editing.
Robert M. Daly: Enrolling patients, Investigation, Writing—review and editing.
Azadeh Namakydoust: Enrolling patients, Investigation, Writing—review and editing.
Henry Li: Project administration, Formal analysis, Writing—review and editing.
Megan McCune: Project administration, Formal analysis, Writing—review and editing.
Emily H. Gelb: Project administration, Formal analysis, Writing—review and editing.
Neil K. Taunk: Conceptualization, Methodology, Writing—review and editing.
Donata von Reibnitz: Conceptualization, Methodology, Writing—review and editing.
Neelam Tyagi: Methodology, Investigation, Writing—review and editing, Visualization.
Ellen D. Yorke: Methodology, Investigation, Writing—review and editing, Visualization.
Valerie W. Rusch: Enrolling patients, Investigation, Writing—review and editing.
Marjorie G. Zauderer: Conceptualization, Methodology, Enrolling patients, Investigation, Writing—review and editing, Supervision, Project administration.
Acknowledgments
Funding and avelumab were provided by Pfizer, as part of an alliance between Pfizer and the health care business of Merck KGaA, Darmstadt, Germany (CrossRef Funder identification: 10.13039/100009945). Pfizer and the health care business of Merck KGaA, Darmstadt, Germany (CrossRef Funder identification: 10.13039/100009945) reviewed this manuscript for medical accuracy only before journal submission. The authors are fully responsible for the content of this manuscript, and the views and opinions described in the publication reflect solely those of the authors. This work was partially supported by a National Cancer Institute Cancer Center Support Grant to Memorial Sloan Kettering Cancer Center (P30 CA008748). Dr. Adusumilli’s laboratory work is supported by grants from the National Institutes of Health (P30 CA008748, R01 CA236615, R01 CA235667, and U01 CA214195), the U.S. Department of Defense (CA170630, CA180889, and CA200437), the Batishwa Fellowship, the Comedy vs Cancer Award, the Cycle for Survival, the DallePezze Foundation, the Derfner Foundation, the Esophageal Cancer Education Fund, the Geoffrey Beene Foundation, the Memorial Sloan Kettering Technology Development Fund, the Miner Fund for Mesothelioma Research, the Mr. William H. Goodwin and Alice Goodwin, the Commonwealth Foundation for Cancer Research, and the Experimental Therapeutics Center of Memorial Sloan Kettering Cancer Center. Dr. Adusumilli’s laboratory receives research support from ATARA Biotherapeutics.
Footnotes
Disclosure: Dr. Rimner has consulted regarding oncology drug development with AstraZeneca, Merck, Boehringer Ingelheim, and Cybrexa; has received honorarium from MoreHealth and ResearchToPractice; has served on a scientific advisory board of Merck; and has received grants from Varian Medical Systems, Boehringer Ingelheim, Pfizer, AstraZeneca, and Merck. Dr. Adusumilli is a scientific advisory board member and consultant for ATARA Biotherapeutics, Bayer, Carisma Therapeutics, Imugene, ImmPACT Bio, Johnson & Johnson, and Outpace Bio; declares having patents, royalties, and intellectual property on mesothelin-targeted CAR and other T-cell therapies, which have been licensed to ATARA Biotherapeutics, issued patent method for detection of cancer cells using virus, and pending patent applications on PD-1–dominant negative receptor, wireless pulse oximetry device, and on an ex vivo malignant pleural effusion culture system. Memorial Sloan Kettering Cancer Center (MSK) has licensed intellectual property related to mesothelin-targeted CARs and T-cell therapies to ATARA Biotherapeutics and has associated financial interests. Dr. Offin has consulted regarding oncology drug development with Novartis, Jazz, and PharmaMar; and has received honorarium from Targeted Oncology, OncLive, and the American Society for Radiation Oncology. Dr. Wong reports receiving consultation fees from Leap Therapeutics. Dr. Rusch reports receiving grants from the National Institutes of Health (NIH)/National Cancer Institute, during the conduct of the study; grants from Genelux, Inc.; grants from Genentech, other from DaVinci Surgery; nonfinancial support from Bristol Myers Squibb; and personal fees from the NIH/Coordinating Center For Clinical Trials, outside the submitted work. Dr. Shepherd reports having stock or stock options in Doximity and ArcellX. Dr. Sauter reports having stock or stock options in Chemed Corporation, Merck & Co., Inc., Pfizer, Inc., and Thermo Fisher Scientific. Dr. Taunk reports receiving consulting fees from Boston Scientific; having advisory board participation at Boston Scientific, Point BioPharma, and Varian Medical Systems; and having research support from Varian Medical Systems. In the past 3 years, Dr. Zauderer has received consulting fees from Ikena, Takeda, GlaxoSmithKline, Aldeyra Therapeutics, and Novocure and honoraria for CME content from PER, Medscape, Research to Practice, Medical Learning Institute, and OncLive. Memorial Sloan Kettering receives research funding from the Department of Defense, the NIH, PreCog, GlaxoSmithKline, Epizyme, Polaris, Sellas Life Sciences, Bristol Myers Squibb, Millennium/Takeda, Curis, and ATARA for research conducted by Dr. Zauderer. Dr. Zauderer serves as Chair of the Board of Directors of the Mesothelioma Applied Research Foundation, uncompensated. The remaining authors declare no conflict of interest.
Cite this article as: Rimner A, Adusumilli PS, Offin MD, et al. A phase 1 safety study of avelumab plus stereotactic body radiation therapy in malignant pleural mesothelioma. JTO Clin Res Rep. 2023;4:100440.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2022.100440.
Supplementary Data
Supplementary Figure.
Supplementary Figure.
References
- 1.Fennell D.A., Ewings S., Ottensmeier C., et al. Nivolumab versus placebo in patients with relapsed malignant mesothelioma (CONFIRM): a multicentre, double-blind, randomised, phase 3 trial. Lancet Oncol. 2021;22:1530–1540. doi: 10.1016/S1470-2045(21)00471-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alley E.W., Lopez J., Santoro A., et al. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol. 2017;18:623–630. doi: 10.1016/S1470-2045(17)30169-9. [DOI] [PubMed] [Google Scholar]
- 3.Hassan R., Thomas A., Nemunaitis J.J., et al. Efficacy and safety of avelumab treatment in patients with advanced unresectable mesothelioma: phase 1b results from the JAVELIN Solid Tumor Trial. JAMA Oncol. 2019;5:351–357. doi: 10.1001/jamaoncol.2018.5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho B.C.J., Donahoe L., Bradbury P.A., et al. Surgery for malignant pleural mesothelioma after radiotherapy (SMART): final results from a single-centre, phase 2 trial. Lancet Oncol. 2021;22:190–197. doi: 10.1016/S1470-2045(20)30606-9. [DOI] [PubMed] [Google Scholar]
- 5.Rimner A., Zauderer M.G., Gomez D.R., et al. Phase II study of hemithoracic intensity-modulated pleural radiation therapy (IMPRINT) as part of lung-sparing multimodality therapy in patients with malignant pleural mesothelioma. J Clin Oncol. 2016;34:2761–2768. doi: 10.1200/JCO.2016.67.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacLeod N., Chalmers A., O’Rourke N., et al. Is radiotherapy useful for treating pain in mesothelioma?: a phase II trial. J Thorac Oncol. 2015;10:944–950. doi: 10.1097/JTO.0000000000000499. [DOI] [PubMed] [Google Scholar]
- 7.Ashton M., O’Rourke N., Macleod N., et al. SYSTEMS-2: a randomised phase II study of radiotherapy dose escalation for pain control in malignant pleural mesothelioma. Clin Transl Radiat Oncol. 2018;8:45–49. doi: 10.1016/j.ctro.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reits E.A., Hodge J.W., Herberts C.A., et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Twyman-Saint Victor C., Rech A.J., Maity A., et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curti B., Crittenden M., Seung S.K., et al. Randomized phase II study of stereotactic body radiotherapy and interleukin-2 versus interleukin-2 in patients with metastatic melanoma. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2020-000773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seung S.K., Curti B.D., Crittenden M., et al. Phase 1 study of stereotactic body radiotherapy and interleukin-2--tumor and immunological responses. Sci Transl Med. 2012;4:137ra74. doi: 10.1126/scitranslmed.3003649. [DOI] [PubMed] [Google Scholar]
- 12.Postow M.A., Callahan M.K., Barker C.A., et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharabi A.B., Lim M., DeWeese T.L., Drake C.G. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015;16:e498–e509. doi: 10.1016/S1470-2045(15)00007-8. [DOI] [PubMed] [Google Scholar]
- 14.de Perrot M., Feld R., Leighl N.B., et al. Accelerated hemithoracic radiation followed by extrapleural pneumonectomy for malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 2016;151:468–473. doi: 10.1016/j.jtcvs.2015.09.129. [DOI] [PubMed] [Google Scholar]
- 15.Demaria S., Guha C., Schoenfeld J., et al. Radiation dose and fraction in immunotherapy: one-size regimen does not fit all settings, so how does one choose? J Immunother Cancer. 2021;9 doi: 10.1136/jitc-2020-002038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marciscano A.E., Haimovitz-Friedman A., Lee P., et al. Immunomodulatory effects of stereotactic body radiation therapy: preclinical insights and clinical opportunities. Int J Radiat Oncol Biol Phys. 2021;110:35–52. doi: 10.1016/j.ijrobp.2019.02.046. [DOI] [PubMed] [Google Scholar]
- 17.Spigel DR, Faivre-Finn C, Gray JE, et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. J Clin Oncol. 2022;40(12):1301-1311. [DOI] [PMC free article] [PubMed]
- 18.Jabbour S.K., Lee K.H., Frost N., Breder V., Kowalski D.M., Pollock T., et al. Pembrolizumab plus concurrent chemoradiation therapy in patients with unresectable, locally advanced, stage III non-small cell lung cancer: the phase 2 KEYNOTE-799 nonrandomized trial. JAMA Oncol. 2021;7:1–9. doi: 10.1001/jamaoncol.2021.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng D.T., Mitchell T.N., Zehir A., et al. Memorial Sloan Kettering-integrated mutation profiling of actionable cancer targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17:251–264. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zehir A., Benayed R., Shah R.H., et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23:703–713. doi: 10.1038/nm.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto T., Niibe Y., Yamada K., et al. Significant reduction of oncologic pulmonary death by local control for pulmonary oligometastases treated with stereotactic body radiotherapy. Radiother Oncol. 2020;147:86–91. doi: 10.1016/j.radonc.2020.03.019. [DOI] [PubMed] [Google Scholar]
- 22.von Reibnitz D., Shaikh F., Wu A.J., et al. Stereotactic body radiation therapy (SBRT) improves local control and overall survival compared to conventionally fractionated radiation for stage I non-small cell lung cancer (NSCLC) Acta Oncol. 2018;57:1567–1573. doi: 10.1080/0284186X.2018.1481292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spratt D.E., Wu A.J., Adeseye V., et al. Recurrence patterns and second primary lung cancers after stereotactic body radiation therapy for early-stage non-small-cell lung cancer: implications for surveillance. Clin Lung Cancer. 2016;17:177–183.e2. doi: 10.1016/j.cllc.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.