Abstract
Environments prone to naturally non-biodegradable metal pollution have remained hot areas of research. Here, concentrations of Cu, Cr, Cd and Pb in 90 bovine tissue (kidney, liver and muscle) samples collected from Gondar City abattoir and Nara-Awudarda in Central Gondar Zone, Ethiopia, were investigated. The samples were dry-ashed at 550 °C for 3.45 h, mineralized with 2 mL conc. HNO3, and analysed using flame atomic absorption spectrometer. Pattern recognition methods, viz. principal component analysis (PCA) and partial least squares – discriminant analysis (PLS-DA), were employed to investigate heavy metal profiles in bovine tissues and classify them based on their geographic source. Results showed that the mean concentrations of the metals (mg/kg) extended in the range: 1.5–11.5 (Cu), 0.42–1.5 (Pb), BDL–1.05 (Cr) and BDL–0.41 (Cd). The concentrations of Cu and Cd, except Cd in muscle samples from Gondar City abattoir, were below the permissible limits while Pb and Cr, except for Pb in kidney and Cr in all samples at Gondar City abattoir, were above the permissible limits. Hence, it calls for enforceable food safety monitoring practices. PCA visualizes grouping of the studied bovine tissues based on the contents of analyzed metals where liver contained highest concentrations of the studied metals, except for Cd, which was highest in kidney. PLS-DA model clearly classified the tissue samples into two clusters depending on their geographic source. In general, the accumulation burden of heavy metals exhibited tissue-specific and sample source differences, probably due to varied organ functions and environmental conditions.
Keywords: Bovine tissues, Heavy metal, Partial least squares – discriminant analysis, Principal component analysis
Bovine tissues, Heavy metal, Partial least squares – discriminant analysis, Principal component analysis.
1. Introduction
Meat constitutes an important part of the human diet, and is mainly composed of protein, fat and some essential elements as well as vitamins (Akan et al., 2010; Kurnaz and Filazi 2011; Shaheen et al., 2016). Heavy metals may also occur as inherent components of meat (Iwegbue et al., 2008), in which their accumulations may influence the quality of meat. Majority of these metals are toxic to living organisms, thereby affecting biochemical processes and even those considered essential can be toxic if present in excess (Yabe et al., 2012; Nkansah and Ansah 2014).
Meat contains very small amounts of these metals whose concentrations can substantially increase due to natural and anthropogenic factors (Aljerf and Aljurf 2020). In this respect, animal feedstuffs, drinking water, and rearing of livestock in proximity to polluted environment are the main routes of exposure (Nwude et al., 2010; Oladipo and Okareh 2015) by which they are transferred to animals, and then to humans through diet (Alkmim Filho et al., 2014; Hashemi 2018). In addition, contamination of meat and meat products by toxic metals during processing, and dirty slaughterhouses have been reported (Brito et al., 1990; Milam et al., 2015).
In recent years, the risk of heavy metal contamination in meat is of great concern for both food safety and human health because of their toxicity, bioaccumulation and biomagnifications in the food chain (Demirezen and Uruç 2006; Santhi et al., 2008; Oladipo and Okareh 2015). Hence, study of heavy metals in different cattle organs has gained increased attention due to variations related to factors such as breed, the age of the animal, feeding practices and geographical conditions, of which environmental conditions and type of pasture mainly determine their levels in meat (Demirezen and Uruç 2006). Despite the difficulty of entirely avoiding contamination of animal feed by toxic metals given their ubiquitous occurrence in the environment, the contamination needs to be minimized in order to reduce both direct and indirect effects on animal and human health, respectively (SCAN 2003). Thus, monitoring the levels of heavy metals in meat that forms significant proportion of our diet is of great importance in view of their potential health risk.
In Ethiopia, meat is a common source of protein which is widely consumed by the population with an annual per capita meat consumption of 9 kg (Avery 2004; Kasa and Guyo 2015). In spite of the considerable production and consumption of meat in the country, there are very limited data on contamination of meat and edible organs by heavy metals ascribed to low food safety monitoring practices. So far, only a few studies have investigated trace metal residue concentrations in different bovine tissues collected from Selale highland in the central (Khalili et al., 1993), and Jimma zone in the south-western region of the country (Dermauw et al., 2014). However, the levels of metal residues in bovine muscle, liver and kidney samples from the north-west of the country, particularly in the Central Gondar Zone, have yet to be examined.
In the Central Gondar Zone, which is the focus of our study, cattle production is one of the main agricultural activities in rural areas where cattle are freely ranging and given locally grown green fodder. In contrast, spent grain and other feed supplements are the major feedstuffs in most towns and cities owing to cattle fattening activities. In Chilga County, part of the same zonal administration, there is a coal deposit in a small village called, Nara-Awudarda, where coal fly ash is believed to contaminate the surrounding environment since the local people use coal for cooking purpose. Apparently, cattle reared in the area may be highly exposed to potentially toxic metal contaminants via ingestion of polluted soil, vegetation and drinking water. For instance, previous studies conducted in this village reported appreciable amounts of trace metals (e.g. Cr, Cd and Pb) in the soil, water and cow's milk samples (Birhanu et al., 2014; Lijalem et al., 2015; Akele et al., 2017), suggesting high mineral enrichment near the coal deposit. Consequently, the present study was motivated to assess the risk associated with consumption of domestic meat in the Central Gondar Zone where there is an increasing demand of ensuring food safety.
Therefore, the objectives of this study are (i) to determine the concentrations of essential (Cu and Cr) and toxic (Cd and Pb) heavy metal residues in bovine tissue (muscle, liver and kidney) samples collected from Gondar City abattoir and Nara-Awudarda village in Chilga County; (ii) to evaluate the fitness of the studied muscle and organ tissues by comparing metal levels against their respective maximum residual limits; and (iii) to classify the three tissue samples based on their type and farm origin employing principal component analysis (PCA) and partial least square–discriminant analysis (PLS–DA).
2. Materials and methods
2.1. Description of the study area
Ethiopia has the largest livestock population in Africa (Kasa and Guyo 2015) that contributes 12–16% of the total Gross Domestic Product (GDP). Amhara Regional State accounts for 25% cattle production of the country where livestock contributes 33% of the region's GDP. In this regard, the highland areas in the former North Gondar Zone (recently divided into three zonal administrations: namely, North-, Central- and West-Gondar) contribute 54% to the total legal export volume, out of which Gondar city and its suburb together with Chilga County cover 22 %, clearly indicating the potential of the study area for livestock production (Mulugeta et al., 2007).
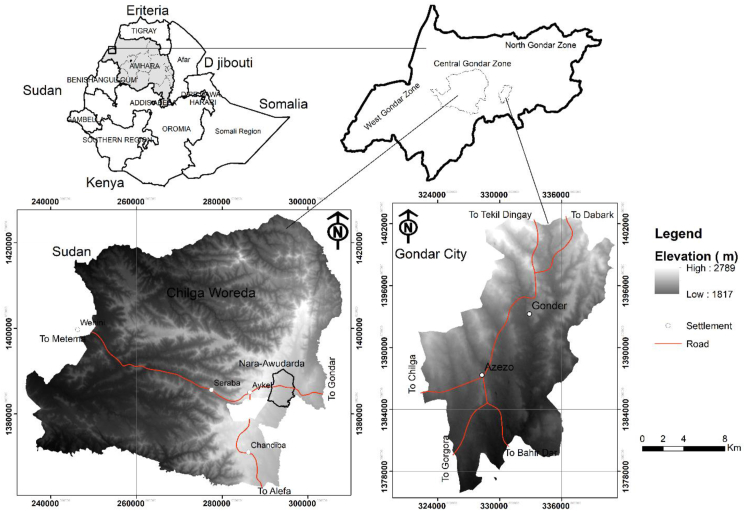
Nara-Awudarda is a small village in Chilga County, situated ca. 43 km west of Gondar city. It is an active geological area with a bed-rock, comprising lime-stone and coal deposits (Figure 1). Cattle in this village are free ranging in the vicinity of a coal deposit, and commonly consume locally produced feeds as major portions of their feed. In Gondar city, however, beef cattle fattening cooperatives have grown drastically; and they often feed their cattle with spent grain and concentrated feed. Cattle watering from polluted rivers, crossing the city, have also become common practice, which calls for a comprehensive assessment of meat safety for human consumption.
Figure 1.
Location map of the study area, Gondar City and Chilga County, in Central Gondar Zone, Ethiopia.
2.2. Sample collection
For this study, ninety different bovine tissue samples were collected from 30 cattle in both study sites. Briefly, 45 fresh bovine muscle, liver and kidney samples, about 50 g each, from 15 cattle were collected at Gondar City abattoir. Cattle slaughtered at this abattoir came from different counties in North-, Central- and West-Gondar Zones. Similarly, the remaining 45 samples were obtained from free grazing cattle reared, for at least 4 years, near the coal deposit in Nara-Awudarda village. The age of the cattle ranged 5–8 years. All the samples were packed in contaminant-free polyethylene bags, properly labeled with permanent marker, and immediately brought to the laboratory with an icebox. In the laboratory, a representative sample, for each bovine tissue and sampling site, was prepared by removing gross fat, connective tissue and major blood vessels, and, after homogenization, kept frozen at −20 °C until analysis.
2.3. Reagents and standards
All chemicals were of analytical reagent grade, and double distilled water was used throughout our analysis. HNO3 (70% Spectrosol; BDH, England) was used for the digestion of the sample matrix; standard stock solutions containing 1000 mg/L (BUCK SCIENTIFIC GRAPHIC™) of the respective metals in 2% (w/w) HNO3 were used for the calibration and recovery test. Plastic bags were soaked in 20% (w/w) HNO3 for 24 h, and rinsed with distilled water before sample collection. Glassware was also soaked in 10% (w/w) HNO3 for 24 h, rinsed with distilled water and dried in a dust-free environment prior to use.
2.4. Digestion of meat and offal tissue samples
All bovine tissue samples were subject to dry-ashing, followed by acid digestion with conc. HNO3 (70%) until a clear, colourless solution was obtained. First, the frozen samples were defrosted to ambient temperature, and 5 g of each sample (muscle, liver and kidney) was transferred into a porcelain crucible, and oven-dried (Gallenkamp, UK) at 85 °C for 24 h. The samples were then placed in a muffle-furnace (Stuart Scientific, UK), and reduced to ash at 550 °C for 3.45 h. The white ash was transferred into a beaker containing 2 mL of conc. HNO3, and heated on a hot plate at 50 °C for a couple of minutes to ensure entire dissolution. After cooling, distilled water (15 mL) was added, and the mineralized solution was, subsequently, filtered with Whatman no. 42 filter paper into 25 mL volumetric flask; finally, it was made up to the mark with distilled water. Digestion was performed in triplicate for each bulk sample and a reagent blank under the same conditions. The digested samples were kept in the refrigerator (Hitachi, Japan) at 4 °C until analysis by FAAS (Buck Scientific, 210VGP, USA), equipped with a deuterium background corrector and air-acetylene flame.
2.5. Method performance and validation
The limit of detection (LOD) and quantification (LOQ) of the applied analytical method were estimated based on the numerical values of calibration curves regression parameters, following the ICH–Q2 guideline on method validation (ICH-Q2 2005). According to which, LOD = 3.3 × σ/S and LOQ = 10 × σ/S, where (σ) and (S) refer to the standard deviation of the intercept of calibration curve, and the slope of the calibration line at levels approaching the limits, respectively. The other validation parameters, viz. repeatability and intermediate precision, are expressed as relative standard deviation (RSD) for a series of replicate measurements of a given test sample in a single analytical cycle, and for all the results obtained in all the three-bovine tissue (kidney, liver and muscle) samples analysed, respectively. Reagent blanks and bovine tissue samples spiked with known amount of each analyte (at a concentration corresponding to the average measured value for each metal) were analysed with test samples for recovery estimate purposes. Heavy metal concentrations are reported as mg/kg wet weight.
2.6. Data analysis
Concentrations were expressed as mean ± standard deviation. One-way analysis of variance (ANOVA) with a post hoc test (Tukey's HSD test) was used, after checking the normal distribution and homogeneity of variance, to test significant differences in the mean levels of metals in both study sites. The level of significance throughout the study was set at p < 0.05. Different pattern recognition methods were applied on the data to explore associations among the different bovine tissue samples and their sources. First a preliminary investigation based on principal component analysis (PCA) (Destefanis et al., 2000) was applied to visualize different bovine tissues trends and evaluate the discriminatory characteristics of the determined metals. PCA analysis was performed on a data set that consisted of a matrix in which the rows and the columns represented the individual tissue samples and concentrations of metals, respectively. In addition, partial least squares–discriminant analysis (PLS-DA) (Ballabio and Consonni 2013) was applied to build a classification model useful for the prediction of the geographical source of the tissues based on their metals concentrations. To build the PLS-DA model, the same data set used in the PCA was used, but in this case, tissue samples from both study sites were represented in the rows of the matrix while the concentrations of metals for the three studied tissues were arranged in the columns. The tissue samples from each source were equally split into two data sets using the Kennard-Stone algorithm (Kennard and Stone 1969) to be used in the PLS-DA training and external validation steps. Two thirds of the total tissue samples (10 from Gondar + 10 from Nara-Awudarda) were used in the training and the remaining (5 from Gondar + 5 from Nara-Awudarda) in the external validation of the PLS-DA model. In order to overcome the influence of the different scales of the variables, the original variables were auto-scaled by subtracting the mean value of the variable and dividing the result by the standard deviation before analysis with PCA and PLS-DA. Samples with concentrations below the method detection limits were assigned a value of half of the limit of detection values for statistical analysis. Chemometric data analysis was performed in the MATLAB environment version R2019a (MathWorks Inc., Natick, MA) using the PLS Toolbox package version 8.5 (Eigenvector Research Inc., Manson, WA). The confidence interval used throughout the study was set at 95%.
3. Results and discussion
3.1. Method performance and method validation
Instrument and method performance parameters were anticipated for precision, accuracy, linearity, detection limits and LOQ. The method detection limit (mg/kg) for each metal under investigation was slightly higher than the corresponding instrument detection limit. Analysis of heavy metals revealed small differences among the triplicates, with relative standard deviations, RSD < 10%. A recovery test of the total analytical procedure conducted for each metal in kidney, liver and muscle samples gave acceptable results, with recovery rates ranging from 85.6–105% (Cd); 93–106% (Cr); 99–111% (Cu); and 91–100% (Pb).
3.2. Concentrations of metals in bovine tissue (kidney, liver and muscle) samples
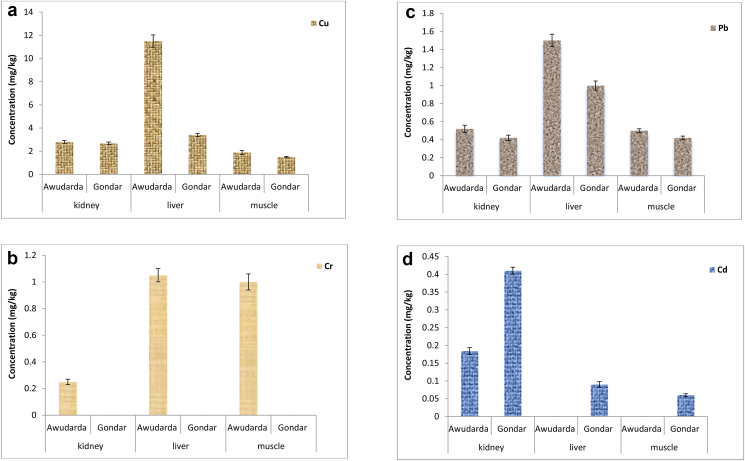
The present study was conducted with the aim of providing baseline data on current levels of Cu, Cr, Pb and Cd analysed in kidney, liver and muscle tissue samples (mg/kg) of bovines slaughtered at Gondar City abattoir and Nara-Awudarda village in the Central Gondar Zone. The overall mean concentrations of the four metals ranged from: 1.5–11.5 for Cu; BDL–1.05 for Cr; 0.42–1.5 for Pb; and BDL–0.41 for Cd (cf. Figure 2a, b, c and d). Of these metals, the highest mean concentration obtained was for Cu (11.50 ± 0.53 mg/kg in liver samples) and the lowest for Cd (0.060 ± 0.004 mg/kg in muscle samples) on both study sites. In general, liver contained highest concentrations of the studied metals compared to kidney and muscle (Figure 2a, b, c and d), except for Cd, for which concentrations were highest in kidney (p < 0.05).
Figure 2.
a–g Contents ± standard deviations of heavy metals in bovine kidney, liver and muscle tissue samples (mg/kg wet weight; n = 3) collected from two study sites: Nara-Awudarda in Chilga County and Gondar City abattoir, Central Gondar Zone, Ethiopia. 2a for Cu (MRLs: 20 mg/kg for muscle); 2b for Cr (MRLs: 1 mg/kg for kidney and muscle, and 0.5 mg/kg for liver); 2c for Pb (MRLs: 0.1 mg/kg for muscle and 0.5 mg/kg for liver and kidney); and 2d for Cd (MRLs: 0.05 mg/kg for muscle and 0.5 mg/kg for liver and 1 mg/kg for kidney).
Copper was the most abundant of all the metals studied, clearly indicating their expected roles as major components of animal nutrients, that are required for the activities of several metalloenzymes (Kurnaz and Filazi 2011; Abd EI-Salam et al., 2013). It occurred in all the three bovine tissue samples with concentrations ranging from 1.50 ± 0.06 (in muscle) to 11.5 ± 0.53 mg/kg (in liver) which are all below the permissible limit of 20 mg kg−1 for meat (Akan et al., 2010). The detection of lower Cu levels in virtually all the samples analysed, except in those liver samples from Nara-Awudarda village, is indicative of Cu deficiency in the study area (Dermauw et al., 2014). Similar studies also reported higher Cu accumulation in the liver than other tissues of cattle (Abou-Arab 2001; Vukasinovic et al., 2007; López-Alonso et al., 2016; Hashemi 2018). The mean Cu concentration in the liver sample from Nara-Awudarda was significantly higher (p < 0.05) than that found in the samples collected at Gondar City abattoir (Figure 2a). This is in agreement with high mineral enrichment in Chilga, particularly at our study site (Birhanu et al., 2014; Akele et al., 2017). The copper levels observed in this study were, in general, comparable to those detected in other studies (Akan et al., 2010; Dermauw et al., 2014; Hashemi 2018). But, they were lower than their respective levels reported elsewhere (Abou-Arab 2001; Sedki et al., 2003; Mariam et al., 2004; Alonso et al., 2004; Waegeneers et al., 2009; Yabe et al., 2012; López-Alonso et al., 2016). Despite its importance as a constituent of various enzymes and role in bone formation, skeletal mineralization and maintaining the integrity of the connective tissues (Akan et al., 2010), very high intake of this metal can result in deleterious health effects, such as liver and kidney damage (Brito et al., 1990). The lethal dose of Cu for humans is 100 mg kg−1, and the maximum observed value was approximately nine orders of magnitude below the lethal dose, revealing absence of potential risk of Cu poisoning for human consumers (Vos et al., 1987).
Chromium was detected only in those meat tissue samples from Nara-Awudarda (cf. Figure 2b), with the highest concentration of Cr (1.05 ± 0.05 mg/kg) found in the liver samples. Contrary to this, the levels of Cr content in all the three bovine tissue samples collected from Gondar City abattoir were all below the minimum detection limit (0.06 mg/kg). Here, higher concentrations of Cr that exceeded their corresponding permissible limits (i.e., 0.5 mg/kg for liver and 1.0 mg/kg for muscle) were observed in liver and muscle tissue samples from Nara-Awudarda (USDA 2006), reflecting contamination of the soil probably ascribed to metal leaching from coal deposits (Sahoo et al., 2016). Cr is apparently an essential nutrient that potentiates insulin action and thus influences carbohydrate, lipid, and protein metabolism (Hua et al., 2012). However, excess amounts of Cr may cause irritation, itching and flushing and other adverse health effects (Duran et al., 2009; Akan et al., 2010; Iwegbue 2011; Tchounwou et al., 2012; Abd EI-Salam et al., 2013; Shaheen et al., 2016). The levels of Cr reported in this study are, in most instances, comparable to mean levels reported elsewhere (Demirezen and Uruç 2006; Santhi et al., 2008; Akan et al., 2010; Iwegbue 2011; Yabe et al., 2012).
With respect to Pb, the highest concentration (1.50 ± 0.07 mg/kg) was found in liver from Nara-Awudarda, whereas the lowest (0.42 ± 0.03 mg/kg) in kidney and muscle from Gondar City abattoir, all above the tolerable limits (0.5 mg/kg for kidney and liver; and 0.1 mg/kg for muscle tissues), except for kidney samples collected from Gondar City abattoir. As can be seen in Figure 2c, highest concentrations of Pb were obtained in liver samples collected from both study sites. Significant differences (P < 0.05) in the Pb concentrations were noted between liver and other tissue samples, in agreement with similar studies (Koréneková et al., 2002; Miranda et al., 2006; Akan et al., 2010). The sources of Pb contamination of livestock come from the water they drink and food they eat (Aljerf et al., 2018). Almost all the samples analysed from both study sites generally contain Pb in high doses (above the tolerable limit), probably due to metal leaching from the coal deposit in the vicinity of Nara-Awudarda and high metal contents in cattle feedstuffs during cattle fattening practices in Gondar city. Vehicle emissions could also contribute to elevated levels of Pb in those cattle grazing on pasture close to an international highway, crossing Nara-Awudarda village to Sudan (Akele et al., 2017). Consumption of high amount of Pb above the threshold level can induce reduced cognitive development and intellectual performance in children and increase blood pressure and cardiovascular disease in adults (National Research Council, 1993; EFSA 2010). The levels of Pb reported in this study are similar to the values reported for India (Mariam et al., 2004), but are higher than those found in Nigeria (Ihedioha and Okoye 2012), Spain (Miranda et al., 2006), and Kenya (Oyaro et al., 2007).
The highest mean level of Cd was observed in the kidney samples from Gondar City abattoir at 0.41 ± 0.01 mg/kg while Cd contents in muscle and liver tissues from Nara-Awudarda were below the method detection limit (BDL). This clearly shows that the concentrations of Cd did not surpass the maximum residue limits of 1.00 and 0.50 mg/kg for kidney and liver, respectively (USDA 2006). However, only muscle sample from Gondar City abattoir, with mean value of 0.06 mg/kg, slightly exceeded its corresponding permissible limit (0.05 mg/kg), indicating a potential health risk to meat consumers in the city. This is most likely due to mineral-rich supplements fed to dairy cattle (Bohstedt 1942). Small-scale workshops, garages and municipal sources (batteries, fertilizers, oil from garage wastewater etc.) might also be the sources of Cd pollution (Wuana and Okieimen 2011).
Cadmium exists at low concentrations in all soils and phosphate fertilizers and is toxic to virtually every system in the animal body (Akan et al., 2010). It is almost absent in the human body at birth, but accumulates with age, especially in the kidneys in comparison to other organs like liver (Abou-Arab 2001; Waegeneers et al., 2009; Canty et al., 2014). Its accumulation occurs in the body, thereby interacting with several minerals (e.g., Zn and Cu) due to their chemical similarities and competition for binding site (McLaughlin et al., 1999). Hence, it may induce kidney dysfunction, skeletal damage and reproductive deficiencies (Solidum et al., 2013). The highest level of cadmium in the kidney samples reveals long-term exposure and appears to depend mainly on the binding of Cd to sulfhydryl groups in metallothionein protein in the kidney (Klaassen et al., 2009). The concentrations of Cd in these samples were higher than the levels reported in Kenya and Slovakia (Koréneková et al., 2002; Oyaro et al., 2007), but lower than the levels reported in Korea and India (Mariam et al., 2004; Kim et al., 2016).
The results of correlations showed strong, moderate and weak relationships between the levels of the four metals. A strong, but negative, correlation was observed between Cd–Cr (r = –0.988). A weak negative correlation (r = –0.32) was also observed between Cd–Cu, suggesting their complex antagonistic interactions in animals, probably ascribed to the displacement of Cu from the kidney and liver by Cd in competition for metallothionein binding sites (Alonso et al., 2000). In contrast, the strongest Pb–Cu (r = 0.994) and moderately significant associations exist between the levels of Cr–Pb (r = 0.53) and Cr–Cu (r = 0.44) in the tissue samples analysed. The positive Cr–Cu association could be linked to the physiological functions of both metals, including being cofactors of many enzymes (Nordberg et al., 2007; Tchounwou et al., 2012).
3.3. Multivariate Exploratory analysis by PCA
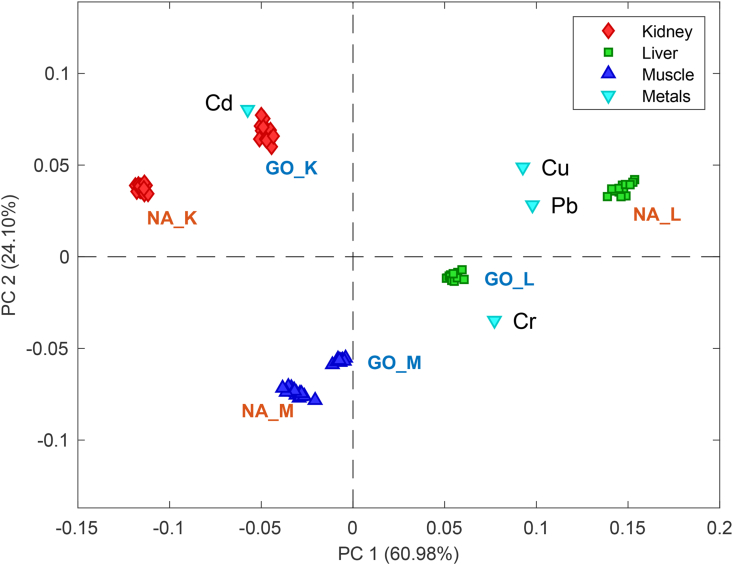
In the present study, the data was analyzed with PCA as unsupervised pattern recognition method to characterize the collected data. PCA compresses the original information (Cu, Cr, Pb and Cd concentrations) into new axes, or principal components (PCs), which represents the orthogonal directions of the most important variability in the original data (Destefanis et al., 2000). Therefore, PCA allows the extraction of the most important information hidden in the multivariate dataset and represents them in a graphical plot (Figure 3). Here, the PCA model was built with three PCs, as indicated by cross-validation, capturing a total variance of 98.77% of the original data. The information of the first two PCs are shown in the bi-plot (Figure 3), which depicts the relationship between the PCA scores related to the tissue samples in red (kidney), green (liver) and blue (muscle) markers; and the loadings, related to the different metal variables studied, in cyan marker. As illustrated in Figure 3, PCA appears to be an effective procedure for data analysis of bovine tissue samples based on their metal contents and sample origin, through the reduction in dimensionality, which permits visual interpretation of the data represented by two-dimensional PCA bi-plot. As can be seen from loadings of variables along the first two PCs in Figure 3, all the studied metals, but Cd, are placed in the positive side of the first PC. The loadings plot also indicates strong direct correlation between Cu and Pb; however, inverse correlation was observed between Cd and Cr in the analyzed data. The pairs of metals Cu and Pb were found to be poorly correlated with Cd and Cr as indicated in the loading plot.
Figure 3.
Bi-plot (scores and loadings) of the first two principal components from PCA model built using the metal concentrations from bovine tissues samples, kidney (K), liver (L) and muscle (M), collected in Nara-Awudarda (NA) and Gondar (GO) regions.
Examination of score plot of the tissue samples in the PCA bi-plot defined by the first two PCs reveals six main groups (Figure 3). Each cluster represents a group of 15 samples and are labeled according to the type of bovine tissues (K: kidney, L: liver and M: muscle) and sampling site (GO: Gondar and NA: Nara-Awudarda). Liver samples, especially from Nara-Awudarda (NA_L), are grouped closer to the variables Cu and Pb in the positive sides of the two PCs and characterized by high contents of Cu and Pb, which present the highest concentrations in all samples. In contrast, the clusters of kidney samples from both sources (GO_K and NA_K) are placed towards the negative side of PC1 and close to the variable Cd, indicating higher levels of Cd and lower levels of Cr than other tissues, particularly those kidney samples from Gondar (GO_K) are closer to Cd variable in the bi-plot. As for liver samples (NA_L), muscle samples from Nara-Awudarda (NA_M) also show relatively higher levels of Cr, but lower levels of Cd. In general, Gondar samples are grouped towards the center of the bi-plot. This is explained by the overall lower metal concentration present in Gondar samples, except for Cd, when comparing the same type of tissues to Nara-Awudarda samples.
The present study revealed that different concentrations of the four metals were detected in the three cattle tissues collected at both study sites; and the prevailing high levels of variability, which occurred in the metals profile across the bovine tissues, is a function of varied organ functions. Liver samples generally contained higher concentrations of metals, especially Pb and Cu, than kidney and muscle samples since it is always responsible for detoxification, leading to the highest level of exposure to the contaminants (Klaassen et al., 2009). Moreover, substantial amounts of Cr were detected in both liver and muscle tissues which is in agreement with other studies (Sedki et al., 2003; Hashemi 2018). On the other hand, kidney contained the highest levels of Cd, probably due to increased levels of a small, cysteine-rich, metal-binding protein–metallothionein (MT)–that plays an important role in tissue Cd sequestration (Klaassen et al., 2009). Cd–MT complex is mainly formed in the liver in response to Cd exposure, released into the blood stream from damaged hepatocytes, then filtered by the kidney and finally taken up into proximal tubule cells (Sabolić et al., 2010). It is responsible for the long biological half-life of Cd in the body (Klaassen et al., 2009). In contrast to our finding, others reported higher concentration of cadmium in liver than in muscle and kidney samples (Nriagu et al., 2009; Adetunji et al., 2014).
3.4. Classification of bovine tissue sample source by PLS-DA
The supervised pattern recognition model was built using PLS-DA method, which is based on PLS regression. Because there were two classes in this study, a PLS2 method was used (Ballabio and Consonni 2013). Each class (sample source) was represented by a dummy variable which resulted in a Y matrix to build the PLS2 model. The aim is to predict the class membership from the X variables (bovine tissues metals concentrations) describing the sample. During the development of the PLS-DA model, variables were auto-scaled and a full cross-validation was used to define the size of the model and evaluate the quality of the calibration model. The external validation test set was applied to evaluate the predictability of the model for the classification of new bovine tissue samples.
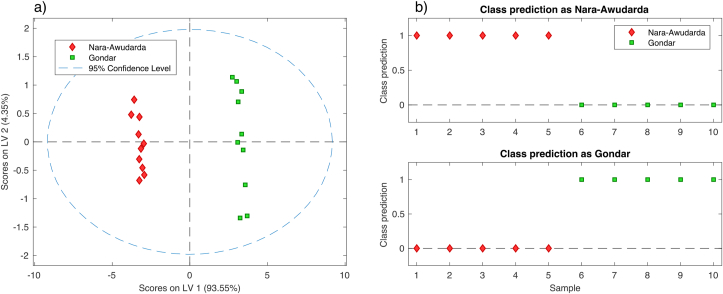
The first two PLS latent variables (LVs) were used in this model as indicated by cross-validation. They explained 97.9 % of the total variance of the calibration data (20 samples, 10 from each class). The score plot of the first two LVs is shown in Figure 4a. It clearly shows that calibration samples are grouped in two clusters. The cluster in the left side of the score plot represents bovine tissue samples from Nara-Awudarda, in red diamonds, whereas the opposite side shows tissue samples collected from Gondar, in green squares. Gondar samples are more dispersed in the score plot than samples from Nara-Awudarda, which is explained by the fact that cattle slaughtered at Gondar city abattoir came from a more spread area than those Nara-Awudarda cattle. As can be seen from the two non-overlapping clusters in Figure 4a, the recognition ability to distinguish between Nara-Awudarda and Gondar samples indicates a satisfactory calibration model.
Figure 4.
(a) Score plot of the first two LVs from PLS-DA calibration model; (b) Class membership prediction plot for Gondar class in the top plot and as Nara-Awudarda in the bottom plot.
To assess the classification quality of the final PLS-DA model, known data from an external validation test set were predicted. The external validation procedure involved feeding the regression model with observed X values, i.e. metal concentrations for the different tissues, from the new tissue samples and predicts the Y values, i.e. predicted class membership. External validation of the PLS-DA model was performed with the remaining 5 tissue samples (15 tissue samples) from each class. The class prediction has shown 100% classification efficiency for both classes as shown in the test set class prediction plot in Figure 4b.
A multivariate analysis with PLS-DA for classification has shown that the levels of metal contents corresponding to the two bovine sample sources are significantly different. These preliminary results demonstrate that profiling of metal contents in the different bovine tissues combined with multivariate analysis models is promising for the characterization and classification of cattle sources. Further studies are necessary to understand whether this methodology is suitable to classify bovine tissues from other regions.
4. Conclusion
The investigated four metals were detected at different concentration ranges in all bovine tissue samples from both study sites. Heavy metal levels, in most tissue samples, exceeded their respective maximum residual limits set by FAO/WHO, which necessitates food safety monitoring practices in the region. Application of PCA clearly grouped the studied tissue samples based on their contents of analyzed metals. Clear distributions in the accumulation of metals among the tissues were also another interesting feature to observe. The liver is the target organ for bioaccumulation of virtually all metals, while the kidney, in which cadmium levels have been highest, appears to be the organ of preference for cadmium accumulation. PLS-DA model has shown 100% classification efficiency in differentiating the samples based on their geographic source. In general, the present study demonstrates the importance of chemometric methods of analysis in profiling metal contents in different bovine tissues to maintain sound authentication of bovine tissues based on their origin, thereby ensuring the increasing demand of food safety in the region. As it is a preliminary study, further research is required to ascertain the suitability of the developed models in classifying similar bovine tissue samples from other regions.
Declarations
Author contribution statement
Mulugeta Legesse Akele; Setegn Kassawmar Desalegn: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Tilahun Belayneh Asfaw; Atnafu Guadie Assefa; Addis Kokeb Alemu: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools; Wrote the paper.
Rodrigo Rocha de Oliveira: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest's statement
The authors declare no competing interests.
Acknowledgements
The authors would like to thank Mr. Zerihun Dawit, lecturer at the Department of Geology, University of Gondar, Ethiopia, for his assistance with GIS. Special thanks go to Dr. Solomon Abraha, former Administrative Vice President, University of Gondar, for the logistic support during the fieldwork. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Abd Ei-Salam N.M., Ahmad S., Basir A., et al. Distribution of heavy metals in the liver, kidney, heart, pancreas and meat of cow, buffalo, goat, sheep and chicken from Kohat Market Pakistan. Life Sci. J. 2013;10:937–940. [Google Scholar]
- Abou-Arab A.A.K. Heavy metal contents in Egyptian meat and the role of detergent washing on their levels. Food Chem. Toxicol. 2001;39:593–599. doi: 10.1016/s0278-6915(00)00176-9. [DOI] [PubMed] [Google Scholar]
- Adetunji V.O., Famakin I.O., Chen J. Lead and cadmium levels in cattle muscle and edible tissues collected from a slaughter slab in Nigeria. Food Addit. Contam., Part B. 2014 doi: 10.1080/19393210.2013.848942. [DOI] [PubMed] [Google Scholar]
- Akan J.C., Abdulrahman F.I., Sodipo O.A., Chiroma Y.A. Distribution of heavy metals in the liver, kidney and meat of beef, mutton, caprine and chicken from Kasuwan Shanu Market in Maiduguri Metropolis, Borno State, Nigeria. Res. J. Appl. Sci. Eng. Technol. 2010;2:743–748. [Google Scholar]
- Akele M.L., Abebe D.Z., Alemu A.K., et al. Analysis of trace metal concentrations in raw cow’s milk from three dairy farms in North Gondar, Ethiopia: chemometric approach. Environ. Monit. Assess. 2017;189:499. doi: 10.1007/s10661-017-6203-0. [DOI] [PubMed] [Google Scholar]
- Aljerf L., Aljurf M. Improvements in the ecological and nutritional aspects of down’s syndrome. Preprints. 2020 [Google Scholar]
- Aljerf L., AlMasri N., Prince U. Statistical relationship between milk constituents used in breeding programs during lactation: French case study. Madridge J. Case Rep. Stud. 2018;2:90–93. [Google Scholar]
- Alkmim Filho J.F., Germano A., Dibai W.L.S., et al. Heavy metals investigation in bovine tissues in Brazil. Food Sci. Technol. 2014;34:110–115. [Google Scholar]
- Alonso M.L., Benedito J.L., Miranda M., et al. Arsenic, cadmium, lead, copper and zinc in cattle from Galicia, NW Spain. Sci. Total Environ. 2000;246:237–248. doi: 10.1016/s0048-9697(99)00461-1. [DOI] [PubMed] [Google Scholar]
- Alonso M.L., Montaña F.P., Miranda M., et al. Interactions between toxic (As, Cd, Hg and Pb) and nutritional essential (Ca, Co, Cr, Cu, Fe, Mn, Mo, Ni, Se, Zn) elements in the tissues of cattle from NW Spain. Biometals. 2004;17:389–397. doi: 10.1023/b:biom.0000029434.89679.a2. [DOI] [PubMed] [Google Scholar]
- Avery A. International Livestock Research Institute; Addis Ababa, Ethiopia: 2004. Red Meat and Poultry Production and Consumption in Ethiopia and Distribution in Addis Ababa. [Google Scholar]
- Ballabio D., Consonni V. Classification tools in chemistry. Part 1: linear models. PLS-DA. Anal. Methods. 2013;5:3790. [Google Scholar]
- Birhanu W.T., Chaueby A.K., Teklemariam T.T., et al. Elemental analysis of soil around aykle in Chilga district North Gondar, Ethiopia using the technique of instrumental neutron activation analysis (INAA) Int. J. Adv. Sci. Tech. Res. 2014;6:455–468. [Google Scholar]
- Bohstedt G. Minerals in dairy cattle nutrition: a review. J. Dairy Sci. 1942;25:441–458. [Google Scholar]
- Brito G., Díaz C., Galindo L., et al. Levels of metals in canned meat products: intermetallic correlations. Bull. Environ. Contam. Toxicol. 1990;44:309–316. doi: 10.1007/BF01700152. [DOI] [PubMed] [Google Scholar]
- Canty M.J., Scanlon A., Collins D.M., et al. Cadmium and other heavy metal concentrations in bovine kidneys in the Republic of Ireland. Sci. Total Environ. 2014;485–486:223–231. doi: 10.1016/j.scitotenv.2014.03.065. [DOI] [PubMed] [Google Scholar]
- Demirezen D., Uruç K. Comparative study of trace elements in certain fish, meat and meat products. Meat Sci. 2006;74:255–260. doi: 10.1016/j.meatsci.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Dermauw V., Lopéz Alonso M., Duchateau L., et al. Trace element distribution in selected edible tissues of zebu (Bos indicus) cattle slaughtered at Jimma, SW Ethiopia. PLoS One. 2014;9 doi: 10.1371/journal.pone.0085300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destefanis G., Barge M.T., Brugiapaglia A., Tassone S. The use of principal component analysis (PCA) to characterize beef. Meat Sci. 2000;56:255–259. doi: 10.1016/s0309-1740(00)00050-4. [DOI] [PubMed] [Google Scholar]
- Duran A., Tuzen M., Soylak M. Trace metal contents in chewing gums and candies marketed in Turkey. Environ. Monit. Assess. 2009;149:283–289. doi: 10.1007/s10661-008-0202-0. [DOI] [PubMed] [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain (CONTAM) Scientific opinion on lead in food. EFSA J. 2010;8:1570. [Google Scholar]
- Hashemi M. Heavy metal concentrations in bovine tissues (muscle, liver and kidney) and their relationship with heavy metal contents in consumed feed. Ecotoxicol. Environ. Saf. 2018;154:263–267. doi: 10.1016/j.ecoenv.2018.02.058. [DOI] [PubMed] [Google Scholar]
- Hua Y., Clark S., Ren J., Sreejayan N. Molecular mechanisms of chromium in alleviating insulin resistance. J. Nutr. Biochem. 2012;23:313–319. doi: 10.1016/j.jnutbio.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICH . 2005. Validation of Analytical Procedures Q2(R1): Methodology. International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use.http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf 2005 November. Available from: [Google Scholar]
- Ihedioha J.N., Okoye C.O.B. Cadmium and lead levels in muscle and edible offal of cow reared in Nigeria. Bull. Environ. Contam. Toxicol. 2012;88:422–427. doi: 10.1007/s00128-011-0509-3. [DOI] [PubMed] [Google Scholar]
- Iwegbue C.M.A. Trace metal contents in some brands of canned beef in Nigeria. Toxicol. Environ. Chem. 2011;93:1368–1374. [Google Scholar]
- Iwegbue C.M.A., Nwozo S.O., Ossai E.K., Nwajei G.E. Heavy metal composition of some imported canned fruit drinks in Nigeria. Am. J. Food Technol. 2008;3:220–223. [Google Scholar]
- Kasa T., Guyo S. A review on compositional constituents , quality characteristics and trace elements in beef , sheep and goat meat in Ethiopia A review on compositional constituents , quality characteristics and trace elements in beef , sheep and goat meat in Ethiopia. Food Sci. Qual. Manag. 2015;46:3–8. [Google Scholar]
- Kennard R.W., Stone L.A. Computer aided design of experiments. Technometrics. 1969;11:137. [Google Scholar]
- Khalili M., Lindgren E., Varvikko T. A survey of mineral status of soil, feeds and cattle in the Selale Ethiopian highlands. II. Trace elements. Trop. Anim. Health Prod. 1993;25:193–201. doi: 10.1007/BF02250867. [DOI] [PubMed] [Google Scholar]
- Kim D.-G., Kim M., Shin J.Y., Son S.-W. Cadmium and lead in animal tissue (muscle, liver and kidney), cow milk and dairy products in Korea. Food Addit. Contam. B. 2016;9:33–37. doi: 10.1080/19393210.2015.1114032. [DOI] [PubMed] [Google Scholar]
- Klaassen C.D., Liu J., Diwan B.A. Metallothionein protection of cadmium toxicity. Toxicol. Appl. Pharmacol. 2009;238:215–220. doi: 10.1016/j.taap.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koréneková B., Skalická M., Nad’ P. Concentration of some heavy metals in cattle reared in the vicinity of a metallurgic industry. Vet. Arh. 2002;72:259–267. [Google Scholar]
- Kurnaz E., Filazi A. Determination of metal levels in. Fresenius Environ. Bull. 2011;20:2896–2901. [Google Scholar]
- Lijalem T., Jeevan A., Bekele T., Berhe A. Determination of some essential and toxic metals in low grade coal and drinking water in Chilga, Amhara region, Ethiopia. 2015;17:412–422. [Google Scholar]
- López-Alonso M., Miranda M., Benedito J.L., et al. Essential and toxic trace element concentrations in different commercial veal cuts in Spain. Meat Sci. 2016;121:47–52. doi: 10.1016/j.meatsci.2016.05.013. [DOI] [PubMed] [Google Scholar]
- Mariam I., Iqbal S., Nagra S.A. Distribution of some trace and macrominerals in beef , mutton and poultry. Int. J. Agric. Biol. 2004;6:816–820. [Google Scholar]
- McLaughlin M., Parker D., Clarke J. Metals and micronutrients – food safety issues. Field Crop. Res. 1999;60:143–163. [Google Scholar]
- Milam C., Dimas B., Jang A., Eneche J. Determination of some heavy metals in vital organs of cows and bulls at Jimeta abattoir, Yola, Adamawa state, Nigeria. Am. Chem. Sci. J. 2015;8:1–7. [Google Scholar]
- Miranda M., Alonso M.L., Benedito J.L. Copper, zinc, iron, and manganese accumulation in cattle from Asturias (northern Spain) Biol. Trace Elem. Res. 2006;109:135–144. doi: 10.1385/BTER:109:2:135. [DOI] [PubMed] [Google Scholar]
- Mulugeta E., Gebremedhin B., Hoekstra D., Jabbar M., Yehun I., Ayehu G., Ejigu M. Improving Productivity and Market Success of Ethiopian Farmers Project (IPMS). IPMS Working Paper 4. ILRI; Nairobi (Kenya): 2007. Analysis of the Ethio-Sudan cross-border cattle trade: the case of Amhara Regional State; p. 41. [Google Scholar]
- National Research Council . National Academies Press; Washington, D.C: 1993. Measuring Lead Exposure in Infants, Children, and Other Sensitive Populations. [PubMed] [Google Scholar]
- Nkansah M.A., Ansah J.K. Determination of Cd, Hg, as, Cr and Pb levels in meat from the Kumasi central abattoir. Int. J. Sci. Res. Publ. 2014;4:1–4. [Google Scholar]
- Nordberg G.F., Fowler B.A., Nordberg M., Friberg L.T., editors. Handbook on the Toxicology of Metals. third ed. Elsevier; 2007. [Google Scholar]
- Nriagu J., Boughanen M., Linder A., et al. Levels of As, Cd, Pb, Cu, Se and Zn in bovine kidneys and livers in Jamaica. Ecotoxicol. Environ. Saf. 2009;72:564–571. doi: 10.1016/j.ecoenv.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Nwude D.O., Okoye P.A.C., Jo Babayemi. Heavy metal levels in animal muscle tissue: a case study of Nigerian raised cattle. Res. J. Appl. Sci. 2010;5:146–150. [Google Scholar]
- Oladipo T.A., Okareh O.T. Determination of heavy metals in selected tissues and organs of slaughtered cattle from Akinyele central abattoir, Ibadan, Nigeria. J. Biol. Agric. Healthcare. 2015;5:25–30. [Google Scholar]
- Oyaro N., Juddy O., Murago E.N.M., Gitonga E. The contents of Pb, Cu, Zn and Cd in meat in Nairobi, Kenya. J. Food Agric. Environ. 2007;5:119–121. [Google Scholar]
- Sabolić I., Breljak D., Škarica M., Herak-Kramberger C.M. Role of metallothionein in cadmium traffic and toxicity in kidneys and other mammalian organs. Biometals. 2010;23:897–926. doi: 10.1007/s10534-010-9351-z. [DOI] [PubMed] [Google Scholar]
- Sahoo P.K., Equeenuddin S.M., Powell M.A. Trace elements in soils around coal mines: current scenario, impact and available techniques for management. Curr Pollut Reports. 2016;2:1–14. [Google Scholar]
- Santhi D., Balakrishnan V.B., Kalaikannan A., Radhakrishnan K T. Presence of heavy metals in pork products in Chennai (India) Am. J. Food Technol. 2008;3:192–199. [Google Scholar]
- SCAN, 2003. Opinion of the Scientific Committee on Animal Nutrition (SCAN) on Undesirable Substances in Feed, Adopted on 20 February 2003. https://ec.europa.eu/food/sites/food/files/safety/docs/animal-feed_additives_rules_scan-old_report_out126_bis.pdf. (Accessed 20 September 2020).
- Sedki A., Lekouch N., Gamon S., Pineau A. Toxic and essential trace metals in muscle, liver and kidney of bovines from a polluted area of Morocco. Sci. Total Environ. 2003;317:201–205. doi: 10.1016/S0048-9697(03)00050-0. [DOI] [PubMed] [Google Scholar]
- Shaheen N., Ahmed M.K., Islam M.S., et al. Health risk assessment of trace elements via dietary intake of ‘non-piscine protein source’ foodstuffs (meat, milk and egg) in Bangladesh. Environ. Sci. Pollut. Res. 2016;23:7794–7806. doi: 10.1007/s11356-015-6013-2. [DOI] [PubMed] [Google Scholar]
- Solidum J.M., Vera MJD De, Abdulla A.-R.D.C., et al. Quantitative analysis of lead, cadmium and chromium found in selected fish marketed in metro Manila, Philippines. Int. J. Environ. Sustain Dev. 2013;4:207–211. [Google Scholar]
- Tchounwou P.B., Yedjou C.G., Patlolla A.K., Sutton D.J. Molecular, clinical and environmental toxicology volume 3: environmental toxicology. Mol. Clin. Environ. Toxicol. 2012;101:133–164. [Google Scholar]
- USDA . Peoples Republic of China; 2006. FAIRS Product Specific Maximum Levels of Contaminants in Foods. [Google Scholar]
- Vos G., Hovens J.P.C., Delft W.V. Arsenic, cadmium, lead and mercury in meat, livers and kidneys of cattle slaughtered in The Netherlands during 1980–1985. Food Addit. Contam. 1987;4:73–88. doi: 10.1080/02652038709373617. [DOI] [PubMed] [Google Scholar]
- Vukasinovic M., Kaljevic V., Sekler M., et al. The effect of copper and zinc concentrations in feed and water on their distribution in beef cattle tissues. Biotechnol. Anim. Husb. 2007;23:35–48. [Google Scholar]
- Waegeneers N., Pizzolon J.C., Hoenig M., De Temmerman L. Accumulation of trace elements in cattle from rural and industrial areas in Belgium. Food Addit. Contam., Part A. 2009;26:326–332. doi: 10.1080/02652030802429096. [DOI] [PubMed] [Google Scholar]
- Wuana R.A., Okieimen F.E. Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol. 2011;1–20 2011. [Google Scholar]
- Yabe J., Nakayama S.M.M., Ikenaka Y., et al. Accumulation of metals in the liver and kidneys of cattle from agricultural areas in Lusaka, Zambia. J. Vet. Med. Sci. 2012;74:1345–1347. doi: 10.1292/jvms.12-0142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.