Abstract
Introduction
In the REFLECT trial, lenvatinib was found to be noninferior compared to sorafenib in terms of overall survival. Here, we analyze the effects of lenvatinib in the real-life experience of several centers across the world and identify clinical factors that could be significantly associated with survival outcomes.
Methods
The study population was derived from retrospectively collected data of HCC patients treated with lenvatinib. The overall cohort included western and eastern populations from 23 center in five countries.
Results
We included 1,325 patients with HCC and treated with lenvatinib in our analysis. Median OS was 16.1 months. Overall response rate was 38.5%. Multivariate analysis for OS highlighted that HBsAg positive, NLR >3, and AST >38 were independently associated with poor prognosis in all models. Conversely, NAFLD/NASH-related etiology was independently associated with good prognosis. Median progression-free survival was 6.3 months. Multivariate analysis for progression-free survival revealed that NAFLD/NASH, BCLC, NLR, and AST were independent prognostic factors for progression-free survival. A proportion of 75.2% of patients suffered from at least one adverse effect during the study period. Multivariate analysis exhibited the appearance of decreased appetite grade ≥2 versus grade 0–1 as an independent prognostic factor for worse progression-free survival. 924 patients of 1,325 progressed during lenvatinib (69.7%), and 827 of them had a follow-up over 2 months from the beginning of second-line treatment. From first-line therapy, the longest median OS was obtained with the sequence lenvatinib and immunotherapy (47.0 months), followed by TACE (24.7 months), ramucirumab (21.2 months), sorafenib (15.7 months), regorafenib (12.7 months), and best supportive care (10.8 months).
Conclusions
Our study confirms in a large and global population of patients with advanced HCC, not candidates for locoregional treatment the OS reported in the registration study and a high response rate with lenvatinib.
Keywords: Hepatocellular carcinoma, Lenvatinib, ALBI, Neutrophils to lymphocyte ratio, Nonalcoholic steatohepatitis, Nonalcoholic fatty liver disease, Tyrosine kinase inhibitors, Outcome, Second line
Introduction
Hepatocellular carcinoma (HCC), the most frequent primary liver malignancy, ranks as the sixth cancer worldwide in terms of incidence [1, 2]. HCC commonly develops in the context of advanced liver disease, e.g., chronic viral infections (hepatitis B or C), alcohol abuse or nonalcoholic fatty liver disease (NAFLD), and nonalcoholic steatohepatitis (NASH) [3, 4].
The efficacy of sorafenib in the treatment of advanced HCC has been demonstrated in two randomized phase 3 trials, the SHARP study [5] and the Asia-Pacific study [6]. In both trials, sorafenib achieved a significant improvement in overall survival (OS) and time to progression. In absolute terms, the median survival prolongation was approximately 3 months in the SHARP study, and 2 months in the Asian study, with a hazard ratio (HR) of 0.69 and 0.68, respectively. Based on these results, sorafenib was approved by the EMA and FDA for the treatment of HCC in October 2007.
After sorafenib approval, several phase 3 trials have failed to demonstrate improved outcomes over sorafenib in the first-line setting [7, 8, 9, 10, 11]. Recently, REFLECT trial meets its primary endpoint [12]. Treatment with lenvatinib resulted in an OS of 13.6 months compared to 12.3 months in the sorafenib arm. Lenvatinib showed a statistically significant improvement compared to sorafenib for all secondary efficacy endpoints as determined by investigator tumor assessments based on modified Response Evaluation Criteria in Solid Tumors. The median time to progression was 8.9 months with lenvatinib and 3.7 months with sorafenib (HR: 0.63; p < 0.00001). In addition, lenvatinib demonstrated a significantly higher overall response rate compared with sorafenib (24% vs. 9%; odds ratio: 3.13; p < 0.00001) [12].
Two phase 3 trials of programmed cell death 1 (PD-1) inhibitors nivolumab (KEYNOTE-240) and pembrolizumab (CheckMate 459) did not reach their primary endpoints, in first line and in second line, respectively [12, 13], although pembrolizumab has been demonstrated superior to best supportive care as second-line therapy in Asian patients (KEYNOTE-394) [14]. IMbrave150 trial demonstrated a statistically significant improvement in OS and PFS with atezolizumab and bevacizumab compared to sorafenib [15]. Recently, the phase III trial HIMALAYA trial demonstrated a statistically significant improvement in OS with the combination of the anticytotoxic T-lymphocyte antigen 4 (CTLA-4) tremelimumab plus the anti-PD-L1 durvalumab (STRIDE regiment) compared to sorafenib [16].
Recently, Pfister et al. [17] demonstrated that the increase of hepatic CD8+PD1+ T cells induced by immunotherapy impairs immune surveillance and could trigger hepatocarcinogenesis in mouse model of NASH. Furthermore, Pfister and collaborators performed a meta-analysis of three large immunotherapy studies using aggregate data and not on individual patient data (CheckMate 459, IMbrave150, and KEYNOTE-240) and analyzed the survival results based on etiology (viral vs. nonviral). Notably, in the subset of patients with nonviral HCC, they did not show an improved survival with immunotherapy. Conversely, survival was improved in HBV- and HCV-related HCC patients treated with immunotherapy. However, it should be mentioned that this meta-analysis was performed on published data and included nonhomogeneous trials, run in first- and second-line setting, with single agents and a combination, the nonviral etiology included both alcohol and NASH, and not only NASH; therefore, the results can be considered only hypothesis generating. Recently, data from HIMALAYA trial highlighted the superiority of immunotherapy in nonviral patients [16]. Based on the different results achieved in different trials, no conclusion regarding etiology and treatment outcome can be drawn.
Moving from the scenario of randomized clinical trials to a real-life setting, this study aims to analyze the efficacy of lenvatinib in the real-life experience of several centers across the world. Beyond that, we identified clinical factors that could be significantly associated with survival outcomes.
Patients and Methods
Patients and Treatment
The study population derived from retrospectively collected data of patients treated with lenvatinib as first line for advanced-stage HCC (Barcelona Clinic Liver Cancer [BCLC]-C) or for intermediate HCC (BCLC-B) was deemed not eligible for surgical or locoregional therapies. The overall cohort included western and eastern populations from 23 centers in 5 countries (Italy, Japan, China, Germany, and Republic of Korea) treated between August 2010 and February 2021.
The primary outcome of the study was OS, defined as the time from initiation of lenvatinib treatment to the date of death or the patient's last follow-up. Follow-up ended in June 2021.
Lenvatinib was administered once daily orally as described in the REFLECT trial [18]. Patients received 12 mg if baseline bodyweight was ≥60 kg or 8 mg if baseline bodyweight was <60 kg.
Treatment interruptions and dose reductions were allowed to manage adverse events (AEs). AEs were recorded and graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.03 [19]. All hematologic blood tests and patients' characteristics were carried out at baseline the day before the start of the treatment.
The study was approved by the Ethics Committee (number DSAN854-A-OS/5 of Ethical committee of San Raffaele Hospital), complied with the provisions of the Good Clinical Practice guidelines and the Declaration of Helsinki and local laws, and fulfilled the Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to processing of personal data.
Statistical Analysis
The aim of this analysis was to examine the association between baseline characteristics and OS in patients with HCC treated with lenvatinib. Association between categorical variables was assessed using Fisher's exact test, when appropriate. In case of unknown cut-off in literature, X-tile 3.6.1 software (Yale University, New Haven, CT, USA) was used to determine the cut-off value for baseline levels.
OS was defined as the time interval between the time from the start date of treatment to the date of death or last follow-up visit. OS was estimated by the Kaplan-Meier method, and curves were compared by the log-rank test. The multivariate analyses were conducted using three models. Model 1 included all basal positive analysis that we found in univariate analysis plus BCLC and Child-Pugh (CP) stage. In the other two models, we split the BCLC and CP in their components.
The significance level of 5% was used to determine statistical significance. MedCalc package (MedCalc® version 16.8.4) was used for all statistical analysis.
Results
Patients
Between August 2010 and February 2021, we included 1,325 patients with HCC treated with lenvatinib in our analysis. The study sample included 1,040 males (78.5%) and 285 females (21.5%) with a median age at diagnosis of 72 years (range 25–97). 1,173 patients (88.5%) had CP class-A. 749 patients (56.5%) had BCLC-C disease stage. 31.1% of patients had an α-fetoprotein (AFP) level >400 ng/mL. The most common underlying etiology was hepatitis infection from C virus (37.2%) followed by other etiology (23.1%), hepatitis B (20.9), and NAFLD/NASH (18.8%). NAFLD/NASH population was defined as follows: we used European Association for the Study of the Liver, European Association for the Study of Diabetes, and European Association for the Study of Obesity Clinical Practice Guidelines to define NAFLD-related HCC. NASH-related HCC was defined based on the presence of these parameters: Presence of steatosis in >5% of hepatocytes according to histological analysis; patients without a history of alcohol abuse (30 g for men and 20 g for women) per day; HBV and HCV negative. Other characteristics were reported in Table 1.
Table 1.
Basal characteristics of patients; univariate and multivariate analysis for OS
| Patients, % | Univariate analysis | Multivariate, model 1 | p value | Multivariate, model 2 | p value | Multivariate, model 3 | p value | ||
|---|---|---|---|---|---|---|---|---|---|
| Age | |||||||||
| <70 >70 |
41.4 58.6 |
1 0.90 (0.76–1.08) |
0.2601 | ||||||
| Gender | |||||||||
| Male Female |
78.5 21.5 |
1 1.04 (0.84–1.28) |
0.7129 | ||||||
| ECOG PS | |||||||||
| 0 >0 |
82.7 17.3 |
1
1.47 (1.15–1.88) |
0.0019 | 1 1.25 (0.80–1.94) |
0.3189 | 1 1.32 (0.85–2.04) |
0.2083 | ||
| HCV | |||||||||
| No Yes |
62.8 37.2 |
1 0.98 (0.82–1.16) |
0.7984 | ||||||
| HBV | |||||||||
| No Yes |
79.1 20.9 |
1
1.26 (1.03–1.56) |
0.0275 |
1
1.56 (1.13–2.17) |
0.0071 |
1
1.77 (1.27–2.48) |
0.0008 |
1
1.61 (1.15–2.25) |
0.0049 |
| NAFLD/NASH | |||||||||
| No Yes |
81.2 18.8 |
1
0.71 (0.58–0.87) |
0.0012 |
1
0.58 (0.33–0.98) |
0.0438 |
1
0.55 (0.34–0.88) |
0.0137 |
1
0.61 (0.38–0.99) |
0.0489 |
| Other etiology | |||||||||
| No Yes |
76.9 23.1 |
1 1.15 (0.93–1.41) |
0.1759 | ||||||
| AFP | |||||||||
| <400 >400 |
68.9 31.1 |
1
1.74 (1.43–2.11) |
<0.0001 | 1 1.29 (0.95–1.78) |
0.1025 | 1 1.26 (0.93–1.73) |
0.1388 | 1 1.35 (0.95–1.91) |
0.0545 |
| ALBI grade | |||||||||
| 1 2 |
89.4 10.6 |
1
2.81 (2.04–3.86) |
<0.0001 | 1 1.05 (0.61–1.79) |
0.8615 | ||||
| BCLC | |||||||||
| B C |
43.5 56.5 |
1
1.65 (1.39–1.95) |
<0.0001 |
1
1.64 (1.19–2.27) |
0.0027 | ||||
| Macrovascular invasion | |||||||||
| No Yes |
79.8 20.2 |
1
1.68 (1.34–2.11) |
<0.0001 | 1 1.02 (0.72–1.43) |
0.9199 | 1 1.03 (0.73–1.46) |
0.8668 | ||
| CP | |||||||||
| A B |
88.5 11.5 |
1
2.33 (1.72–3.15) |
<0.0001 | 1 0.97 (0.57–1.68) |
0.9302 | ||||
| NLR | |||||||||
| <3 >3 |
63.4 36.6 |
1
1.80 (1.46–2.22) |
<0.0001 |
1
1.95 (1.46–2.60) |
<0.0001 |
1
1.93 (1.44–2.59) |
<0.0001 |
1
2.01 (1.50–2.69) |
<0.0001 |
| ALT | |||||||||
| <32 >32 |
51.5 48.5 |
1
1.54 (1.30–1.82) |
<0.0001 | 1.01 (0.73–1.40) | 0.9263 | 1 1.02 (0.74–1.41) |
0.9162 | 1 1.03 (0.74–1.42) |
0.8630 |
| AST | |||||||||
| <38 >38 |
45.2 54.8 |
1
1.70 (1.44–2.02) |
<0.0001 |
1
1.52 (1.08–2.13) |
0.0167 |
1
1.41 (1.03–1.95) |
0.0339 |
1
1.55 (1.09–2.20) |
0.0147 |
| Bilirubin | |||||||||
| <NV >NV |
78.7 21.3 |
1
1.81 (1.44–2.28) |
<0.0001 |
1
1.43 (1.03–1.97) |
0.0319 | ||||
| Albumin | |||||||||
| <35 >35 |
29.6 70.4 |
1
0.44 (0.36–0.53) |
<0.0001 |
1
0.54 (0.38–0.77) |
0.0007 | ||||
| Creatinine | |||||||||
| <0.67 >0.67 |
22.3 77.7 |
1 1.03 (0.84–1.26) |
0.7955 | ||||||
| Alkaline phosphatase | |||||||||
| >200 <200 |
37.7 62.3 |
1
0.67 (0.50–0.91) |
0.01 | 1 0.98 (0.70–1.39) |
0.9364 | 1 0.77 (0.54–1.09) |
0.1450 | 1 0.86 (0.61–1.21) |
0.4001 |
TACE, transarterial chemoembolization; BCLC, Barcelona Clinic Liver Cancer; ECOG PS, Eastern Cooperative Oncologic Group Performance Status; AFP, alpha-fetoprotein; CV, continuous variables; AST, aspartate aminotransferase; ALT, alanine aminotransferase; NLR, neutrophils to lymphocyte ratio.
At the time of analysis (August 2021), 781 (59%) patients were still alive (415 on treatment) and 544 (41%) had died. Median follow-up was 13.8 months (95% CI: 13.2–14.9).
Efficacy and Prognostic Factor
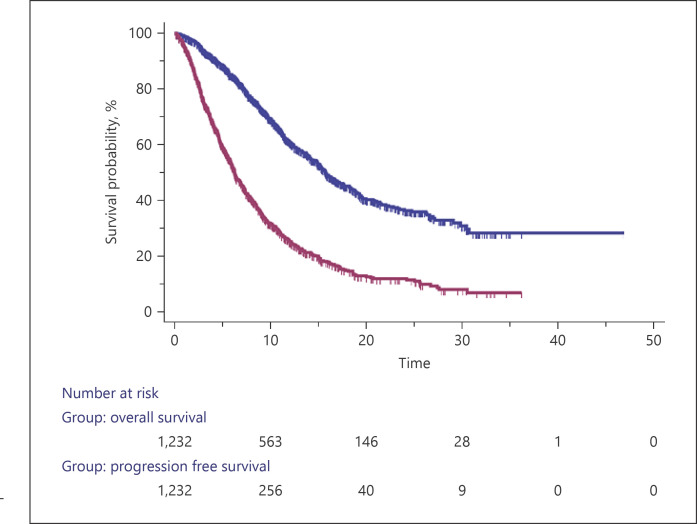
The median OS was 16.1 months (95% CI: 15.2–51.6) (Fig. 1), and 70.1% of the patients were alive after 1 year. Considering basal characteristics, the univariate analysis identified ECOG > 0 (vs. 0; HR 1.47; p = 0.0019), HBsAg positive (vs. negative; HR 1.26; p = 0.0275), AFP > 400 (vs. ≤400; HR 1.74; p < 0.0001), ALBI grade 2 (vs. 1; HR 2.81; p < 0.0001), BCLC-C (vs. B; HR 1.65; p < 0.0001), portal vein invasion positive (vs. negative; HR 1.68; p < 0.0001), CP B (vs. A; HR 2.33; p < 0.0001), neutrophils to lymphocyte ratio (NLR) > 3 (vs. <3; HR 1.80; p < 0.0001), alanine aminotransferase (ALT) >32 (vs. <32; HR 1.54; p < 0.0001), aspartate aminotransferase (AST) > 38 (vs. <38; HR 1.70; p < 0.0001), bilirubin > NV (vs. <NV; HR 1.81; p < 0.0001) as potential prognostic factors for poorer OS. Conversely, the univariate analysis identified NAFLD/NASH-related etiology yes (vs. no; HR 0.71; p = 0.0012) and phosphatases alkaline <200 (vs. >200; HR 0.67; p = 0.01) as potential prognostic factors for better OS (Table 1).
Fig. 1.
Kaplan-Meier curve for OS and progression-free survival.
To test all these variables in a multivariate model without incurring in the bias of multicollinearity, we constructed three models: model 1 consisted of HBV, NAFLD/NASH, AFP, BCLC, CP class, NLR, ALT, and AST; model 2 consisted of ECOG, HBV, NAFLD/NASH, AFP, ALBI grade, portal vein invasion, NLR, ALT, and AST; model 3 consisted of ECOG, HBV, NAFLD/NASH, AFP, portal vein invasion, NLR, ALT, AST, bilirubin, and albumin.
These models showed that HBsAg positive (Fig. 2a), NLR > 3 (Fig. 2b), and AST > 38 (Fig. 2c) were independently associated with poorer prognosis in all models. Conversely, NAFLD/NASH-related etiology (Fig. 2d) was independently associated with a good prognosis. BCLC-C was associated with poorer prognosis (Fig. 2e). The ALBI grade was not associated with prognostic impact, but when we analyzed bilirubin (Fig. 2f) and albumin (Fig. 2g) separately, both presented a prognostic impact (Table 1).
Fig. 2.
Kaplan-Meier curve for OS for HBsAg (a), NLR 3 (b), AST (c), NASH-related etiology (d), BCLC stage (e), bilirubin (f), and albumin (g).
Best response data were available in 1,298 patients. Sixty-eight patients (5.2%) showed a complete response, 433 patients (33.3%) a partial response, 542 patients (41.7%) a stable disease, and 255 patients (19.8%) a progressive disease. So, the disease control rate was 80.2%.
Progression-Free Survival and Clinical Outcome
Our date showed a median progression-free survival of 6.3 months (95% CI: 6.0–46.5) (Fig. 1). For basal characteristics, the univariate analysis identified ECOG > 0 (vs. 0; HR 1.25; p = 0.0209), HBsAg positive (vs. negative; HR 1.25; p = 0.0061), AFP > 400 (vs. 400; HR 1.28; p = 0.001), ALBI grade 2 (vs. 1; HR 1.67; p = 0.0001), BCLC-C (vs. B; HR 1.36; p < 0.0001), portal vein invasion yes (vs. no; HR 1.30; p = 0.0029), CP B (vs. A; HR 1.37; p = 0.0075), NLR > 3 (vs. <3; HR 1.24; p = 0.0077), ALT > 32 (vs. <32; HR 1.19; p = 0.0107), AST > 38 (vs. <38; HR 1.27; p = 0.0004), bilirubin > NV (vs. <NV; HR 1.26; p = 0.0083) as potential prognostic factors for poorer progression-free survival.
Conversely, the univariate analysis identified NAFLD/NASH-related etiology yes (vs. no; HR 0.83; p = 0.0237) and albumin > NV (vs. <NV; HR 0.78; p = 0.0013) as potential prognostic factors for good progression-free survival (Table 2). After adjusting for positive clinical covariates used in model 1, the multivariate analysis highlighted NAFLD/NASH yes (vs. no; HR 0.87, 95% CI: 0.75–0.93, p = 0.0090) (online suppl. Fig. 1A; for all online suppl. material, see www.karger.com/doi/10.1159/000525145), BCLC-C (vs. BCLC-B; HR 1.33, 95% CI: 1.14–1.55, p = 0.0002) (online suppl. Fig. 1B), NLR > 3 (vs. <3 HR 1.16, 95% CI: 1.01–1.36, p = 0.0482) (online suppl. Fig. 1C), and AST > 38 (vs. <38; HR 1.21, 95% CI: 1.01–1.45, p = 0.0365) (online suppl. Fig. 1D) as independent prognostic factors for progression-free survival (Table 2).
Table 2.
Univariate and multivariate analysis for progression-free survival
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Age | ||||
| <70 >70 |
1 0.93 (0.81–1.06) |
0.2867 | ||
| Gender | ||||
| Male | 1 | 0.4646 | ||
| Female | 0.94 (0.80–1.10) | |||
| ECOG PS | ||||
| 0 >0 |
1
1.25 (1.03–1.51) |
0.0209 | ||
| HCV | ||||
| No Yes |
1 0.99 (0.87–1.14) |
0.9465 | ||
| HBV | ||||
| No Yes |
1
1.25 (1.07–1.47) |
0.0061 | 1 1.05 (0.87–1.15) |
0.2940 |
| NAFLD/NASH | ||||
| No Yes |
1
0.83 (0.71–0.97) |
0.0237 |
1
0.87 (0.75–0.93) |
0.0090 |
| Other etiology | ||||
| No Yes |
1 0.97 (0.83–1.13) |
0.7188 | ||
| AFP | ||||
| <400 >400 |
1
1.28 (1.10–1.48) |
0.001 | 1 1.11 (0.95–1.31) |
0.1968 |
| ALBI grade |
1
1.67 (1.29–2.15) |
|||
| 1 2 |
0.0001 | |||
| BCLC | ||||
| B C |
1
1.36 (1.20–1.55) |
<0.0001 |
1
1.33 (1.14–1.55) |
0.0002 |
| Portal vein invasion | ||||
| No Yes |
1
1.30 (1.09–1.55) |
0.0029 | ||
| CP | ||||
| A B |
1
1.37 (1.09–1.72) |
0.0075 | 1 1.17 (0.91–1.50) |
0.2052 |
| NLR | ||||
| <3 >3 |
1
1.24 (1.06–1.44) |
0.0077 |
1
1.16 (1.01–1.36) |
0.0482 |
| ALT | ||||
| <32 >32 |
1
1.19 (1.04–1.35) |
0.0107 | 1 0.98 (0.82–1.17) |
0.8008 |
| AST | ||||
| <38 >38 |
1
1.27 (1.11–1.45) |
0.0004 |
1
1.21 (1.01–1.45) |
0.0365 |
| Bilirubin | ||||
| <NV >NV |
1
1.26 (1.06–1.49) |
0.0083 | ||
| Albumin | ||||
| <35 >35 |
1
0.78 (0.67–0.90) |
0.0013 | ||
| Creatinine | ||||
| <0.67 | 1 | 0.2549 | ||
| >0.67 | 1.10 (0.93–1.28) | |||
| Alkaline phosphatase | ||||
| >200 <200 |
1 0.92 (0.76–1.12) |
0.4329 |
BCLC, Barcelona Clinic Liver Cancer; ECOG PS, Eastern Cooperative Oncologic Group Performance Status; AFP, alpha fetoprotein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; NLR, neutrophils to lymphocyte ratio.
Safety and Clinical Outcome
A proportion of 75.2% of patients presented at least one adverse effect during the study period. The most common events were other toxicity (46.0%), fatigue (32.5%), and decreased appetite (31.9%). 29.2% of the events were grade (G) 3 (Table 3). No drug-related death was reported.
Table 3.
Treatment-emergent AEs
| Grade 0, n (%) | Grade 1, n (%) | Grade 2, n (%) | Grade 3–4, n (%) | |
|---|---|---|---|---|
| HFSR | 1,023 (79.4) | 146 (9.6) | 127 (8.4) | 29 (2.6) |
| Diarrhea | 1,056 (75.2) | 142 (12.0) | 99 (10.7) | 28 (2.0) |
| Hypertension | 905 (63.0) | 108 (8.3) | 232 (21.0) | 80 (7.8) |
| Fatigue | 895 (61.6) | 185 (15.2) | 180 (15.8) | 65 (7.4) |
| Decreased appetite | 903 (65.5) | 174 (13.9) | 179 (15.5) | 69 (5.1) |
| Proteinuria | 1,001 (75.2) | 126 (9.6) | 101 (6.9) | 97 (8.3) |
| Hypothyroidism | 946 (67.0) | 124 (11.2) | 243 (20.8) | 12 (1.0) |
| Other | 715 (52.3) | 400 (29.7) | 110 (10.2) | 100 (7.8) |
HFSR, hand-foot skin reaction.
We explored possible AEs associated with differences in OS. At univariate analysis, a difference in OS was detected for decreased appetite yes versus no (HR 1.24; p = 0.0193); decreased appetite G ≥ 2 versus G 0–1 (HR 1.49; p = 0.0004); diarrhea yes versus no (HR 0.78; p = 0.0196); diarrhea G ≥ 2 versus G 0–1 (HR 0.72; p = 0.0152); hand-foot skin reaction (HFSR) yes versus no (HR 0.72; p = 0.0007); HFSR G ≥ 2 versus G 0–1 (HR 0.74; p = 0.0141); arterial hypertension yes versus no (HR 0.75; p = 0.0014); arterial hypertension G ≥ 2 versus G 0–1 (HR 0.69; p = 0.0001); hypothyroidism yes versus no (HR 0.68; p < 0.0001); hypothyroidism G ≥ 2 versus G 0–1 (HR 0.77; p = 0.0135); proteinuria yes versus no (HR 0.68; p = 0.0001); proteinuria G ≥ 2 versus G 0–1 (HR 0.72; p = 0.0044) (Table 4).
Table 4.
Univariate and multivariate analysis for treatment-emergent AEs
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Decreased appetite | ||||
| No | 1 | 0.0193 | ||
| Yes | 1.24 (1.03–1.49) | |||
| Decreased appetite | ||||
| Grade <2 Grade ≥2 |
1
1.49 (1.19–1.87) |
0.0004 | 1.74 (1.55–2.14) | 0.0072 |
| Diarrhea | ||||
| No Yes |
1
0.78 (0.64–0.96) |
0.0196 | 1 0.9597 0.99 (0.63–1.55) |
0.9597 |
| Diarrhea | ||||
| Grade <2 | 1 | 0.0152 | ||
| Grade ≥2 | 0.72 (0.55–0.94) | |||
| Fatigue | ||||
| No | 1 | 0.1749 | ||
| Yes | 0.88 (0.74–1.06) | |||
| Fatigue | ||||
| Grade <2 | 1 | 0.8554 | ||
| Grade ≥2 | 0.98 (0.79–1.21) | |||
| HFSR | ||||
| No Yes |
1
0.72 (0.60–0.87) |
0.0007 | 0.93 (0.67–1.28) | 0.6612 |
| HFSR | ||||
| Grade <2 | 1 | 0.0141 | ||
| Grade ≥2 | 0.74 (0.58–0.94) | |||
| Hypertension | ||||
| No | 1 | 0.0014 | ||
| Yes | 0.75 (0.62–0.89) | |||
| Hypertension | ||||
| Grade <2 Grade ≥2 |
1
0.69 (0.56–0.83) |
0.0001 | 0.2159 0.89 (0.77–1.35) |
0.2159 |
| Hypothyroidism | ||||
| No Yes |
1
0.68 (0.57–0.81) |
<0.0001 | ||
| Hypothyroidism | ||||
| Grade <2 Grade ≥2 |
1
0.77 (0.63–0.95) |
0.0135 | 0.2159 0.89 (0.77–1.35) |
0.2159 |
| Proteinuria | ||||
| No Yes |
1
0.68 (0.57–0.83) |
0.0001 | 0.95 (0.67–1.34) | 0.7897 |
| Proteinuria | ||||
| Grade <2 | 1 | 0.0044 | ||
| Grade ≥2 | 0.72 (0.57–0.90) | |||
| Other toxicity | ||||
| No | 1 | 0.1266 | ||
| Yes | 1.14 (0.96–1.35) | |||
| Other toxicity | ||||
| Grade <2 | 1 | 0.8938 | ||
| Grade ≥2 | 0.98 (0.79–1.23) |
After adjusting for positive clinical covariates used in model 1, the multivariate analysis highlighted the appearance of decreased appetite G ≥ 2 versus G 0–1 as an independent prognostic factor for OS (HR 1.74, 95% CI: 1.55–2.14, p = 0.0072) (Table 4; online suppl. Fig. 2).
Post-Lenvatinib Treatment
924 patients of 1,325 progressed during lenvatinib (69.7%) and 827 of them had a follow-up over 2 months from the beginning of second-line treatment. The analyses were performed on this population. Three hundred ninety-one (47.3%) patients received best supportive care, 174 received sorafenib (21.0%), 160 received transarterial chemoembolization (TACE) (19.3%), 60 patients received immunotherapy (7.2%), 28 patients ramucirumab (3.4%), and 14 patients regorafenib (1.7%). We performed analysis in term of OS from the first line with lenvatinib.
From first-line therapy, the longest median OS was obtained with the sequence lenvatinib and immunotherapy (47.0 months), followed by TACE (24.7 months), ramucirumab (21.2 months), sorafenib (15.7 months), regorafenib (12.7 months), and best supportive care (10.8 months). From second-line therapy, the longest median OS was obtained by the sequence lenvatinib and immunotherapy (17.2 months), followed by ramucirumab (10.3 months), TACE (8.7 months), sorafenib (8.6 months), regorafenib (5.0 months), and no treatment (4.5 months). Patients treated with immunotherapy had a higher response rate compared to other treatments (p < 0.0001).
Following adjustment, the choice of treatment in second line with known prognostic variables in second line (AFP, BCLC, CP, and median PFS in first line), multivariate analysis highlighted that immunotherapy was an independent prognostic factor for better OS (HR = 0.65; 95% CI: 0.45–0.97; p = 0.0394). Regorafenib and placebo were independent prognostic factors for worse OS (HR = 3.62; 95% CI: 1.40–9.36; p = 0.008; HR = 2.58; 95% CI: 1.23–3.14; p = 0.0014; respectively).
Discussion
Moving from the scenario of randomized clinical trial to a real-life setting, we confirmed the efficacy of lenvatinib showing high median OS in patients like in the registration study [12]. Overall, 70% of patients were still alive at 1 year. Several studies in real life have previously confirmed the results of the REFLECT trial [20, 21], but the strength of our data is in the very high number and geography heterogeneity of the patients enrolled. Furthermore, lenvatinib confirmed the impressively high response and disease control rates (about 40% and 80%, respectively). This high response rate has a dramatic impact on our daily clinical choices. The consequential movement from “old” tyrosine kinase inhibitors with low response to “new” tyrosine kinase inhibitors with high response opened a new scenario in which physicians are probably more inclined to switch very early from locoregional therapy to systemic therapy. In fact, in a previous study from our research group, performing an inverse probability of treating weighting analysis, the most important survival benefit from lenvatinib compared to sorafenib was shown in the same subgroup of patients [20], which is consistent with the results from the analysis of Shigeo and collaborators [21] and Kudo et al. [22].
Concerning the prognostic factors, our study confirmed our previously paper that highlighted the better OS of patients with NAFLD/NASH-related HCC. It seems worth highlighting that these patients had an OS of 21.2 months, which is an impressive result in this setting. While these data do not derive from a randomized clinical trial with appropriate stratification, they could have a crucial value considering the lack of biomarkers to stratify patients more likely to respond to treatment in advanced HCC. The better response of patients with no viral-related HCC to lenvatinib should also be put in the context of recent evidence underscoring the efficacy of immunotherapy in patients with NASH [17]. As previously mentioned, this meta-analysis was performed on published data and included nonhomogeneous trials, run in first- and second-line settings, with single agents and a combination, the nonviral etiology included both alcohol and NASH, and not only NASH; therefore, the results can be considered only hypothesis generating.
We also found that AST, NLR, and hepatitis B infection have a strong correlation with the disease outcome. Correlation between AST and prognosis could be explained as a higher aggressive tumor caused by high tumor cell turnover and tissue damage [23]. Moreover, the correlation between hepatitis B and worsening prognosis is well known. While in this study, we have not compared lenvatinib with sorafenib, our previous experience highlighted the greater efficacy of lenvatinib in this subcategory of patients. In particular, our network meta-analysis showed that lenvatinib could be preferable for HBV-positive patients in 59% of cases compared to only 1% of patients treated with sorafenib [24]. Is it important to underline that patients with hepatitis B demonstrated an OS of 13.7 months in our analysis, which is higher than the OS obtained by sorafenib in previous real-life studies [25, 26]. Finally, several previous studies proved the NLR in advanced settings has been shown in several previous studies [27, 28, 29].
Concerning the safety profile, our study confirmed the good tolerability of lenvatinib, and our results were in line with other papers of real life. We confirmed that hypertension, fatigue, and proteinuria were the main toxicities when we treated the patients with lenvatinib.
We confirmed our previous study on the negative impact in terms of OS of patients experiencing a decrease in appetite during treatment [30]. Thus, early recognition of nutritional alteration and muscle wasting is crucial, given the possible negative impact of toxicity management on survival in HCC patients [30]. This is also confirmed by the fact that the occurrence of appetite loss is often associated with lenvatinib discontinuation [31, 32]. Conversely, we did not confirm the positive impact of arterial hypertension in multivariate analysis [33].
The current literature hardly provides data about post-progression treatment, and clinical outcomes are available. Our large cohort of patients has allowed us to analyze this aspect, allowing us to highlight three important points. First, the impressive results of the lenvatinib-immunotherapy sequence. Second, the high median OS with ramucirumab and third, the lack of efficacy of regorafenib after lenvatinib.
The demonstration of a median OS of 47 months from the sequence lenvatinib and immunotherapy in advanced stage could open a new prospective for future studies. These results have several limitations, therefore, the results can be considered only hypothesis generating.
Emerging preclinical and clinical evidence supports the concept that lenvatinib can engage the immune system to act beyond tumor cells, possibly providing lasting benefits to cancer patients via enhanced and long-term immunosurveillance [34]. In addition, immune inhibitory molecules were downregulated in tumors after treatment with lenvatinib [35]. In mouse tumor models, lenvatinib decreased tumor-associated macrophages, increased activated cytotoxic T cells, and demonstrated greater antitumor activity in combination with an anti-PD-1 monoclonal antibody compared to either treatment alone [36]. Waiting for the data of the ongoing phase 3 LEAP-002 trial, which compares the association of pembrolizumab plus lenvatinib versus lenvatinib in first line, our data suggest that lenvatinib may contribute in generating a microenvironment more susceptible to immunotherapy. In the future, studies should be focused on the best strategy of the combination agents (concomitant vs. sequentially). The results of the sequence lenvatinib and ramucirumab were unexpected. In the literature, small case series of patients were published without encouraging data [37, 38, 39]. All patients treated with ramucirumab had AFP > 400, nevertheless in second line have had a higher OS over TACE and sorafenib. Data derived from our study suggested a moderate benefit from TACE after lenvatinib; from the beginning of lenvatinib, the OS was high (24.7 months), but from the data of TACE, it was very low (only 8.7 months). Two reasons might contribute to this finding: first, patients harbored a TACE-insensitive tumor and second, the high possibility of liver failure after locoregional therapy is highly pretreated in patients. These two points are linked, since several studies demonstrated no efficacy from continuing TACE after TACE refractoriness. Our data suggest that the sequence of lenvatinib-TACE at the progression is not an effective strategy even if patients remain in stage BCLC-B after lenvatinib treatment [15, 21, 40]. In clinical practice, sorafenib is the drug more frequently prescribed after lenvatinib. This is derived mostly from rules posed by regulatory agency and not from clinical studies. Our study suggests a moderate activity from the sequence lenvatinib and sorafenib, which is consistent with previous research [41, 42, 43]. Finally, regorafenib use after lenvatinib treatment showed no efficacy.
Our work presents some limitations, mainly due to its retrospective nature, which implicates collection of studies with different basal designs and endpoints, as well as the lack of data due to the retrospective collection in a clinical-practice setting. In the attempt to reduce such aspects, cases from our sample were consecutively selected thus reducing potential bias, and three statistical models were constructed to test all the variables in a multivariate model without incurring in the bias of multicollinearity. Furthermore, we must take into account the limitation of the definition of NAFLD and/or NASH, as the allocation of a patient to the NAFLD or NASH group could be complicated by the existence of different definitions in the literature. However, to reduce this bias, we have considered the definition from the European Association for the Study of Liver guidelines. Due to the multicenter and multinational nature of the analysis, a centralized imaging review was not possible, and the criteria for tumor assessments were based on each center's internal protocol. On the other hand, our analysis presents important points of power, mainly recognized in the large sample size and in the multicenter eastern and western enrollment of patients in a field-practice setting.
In conclusion, our study confirms in a large and global population of patients with advanced HCC, not candidates for locoregional treatment the OS reported in the registration study and a high response rate with lenvatinib. Furthermore, the analysis of the prognostic factors highlighted the higher efficacy of lenvatinib in patients with NAFLD/NASH and lower efficacy in patients with high NLR, AST, and hepatitis B. In particular, the NAFLD/NASH etiology which from recent data seems to correlate to scarce response to immunotherapy, also the data are in contrast in the different studies (e.g., STRIDE amr in HIMALAYA trial), should constitute a field of particular interest for future studies. Finally, our results highlighted an impressive result of sequential use of lenvatinib and immunotherapy, and further studies should investigate sequence and combination treatment strategies, while focusing on the HCC cause.
Statement of Ethics
The study protocol was reviewed and approved by the local Ethics Committee (EC). In particular, the protocol was first approved by the San Raffaele Authority Hospital EC for the coordinating center and subsequently approved by the remaining EC (number DSAN854-A-OS/5). A written informed consent was obtained according to the ECs recommendations. The study was conducted according to the ethical guidelines of the 1975 Declaration of Helsinki.
Conflict of Interest Statement
A.C.-G. has received grants and personal fees from MSD, Eisai, and Bayer and is an adviser for MSD, Eisai, Bayer, Bristol-Myers Squibb, AstraZeneca, and GSK. M.K. has received grants from Taiho Pharmaceuticals, Chugai Pharmaceuticals, Otsuka, Takeda, Sumitomo Dainippon-Sumitomo, Daiichi Sankyo, AbbVie, Astellas Pharma, and Bristol-Myers Squibb; has received grants and personal fees from MSD, Eisai, and Bayer; and is an adviser for MSD, Eisai, Bayer, Bristol-Myers Squibb, Eli Lilly, and ONO Pharmaceutical. Prof. Kudo is the Editor-in-chief of Liver Cancer. K.U.: she is an Editorial Board Member. A.J.: personal fees from Eisai.
Funding Sources
The authors did not receive any funding.
Author Contributions
Andrea Casadei-Gardini: design and concept, data curation, critical revision, drafting the manuscript, and statistics. Margherita Rimini, Mesatoshi Kudo, Shigeo Shimose, Toshifumi Tada, Goki Suda, Myung Ji Goh, Andre Jefremow, Mario Scartozzi, Giuseppe Cabibbo, Claudia Campani, Emiliano Tamburini, Francesco Tovoli, Kazuomi Ueshima, Tomoko Aoki, Hideki Iwamoto, Takuji Torimura, Takashi Kumada, Atsushi Hiraoka, Masanori Atsukawa, Ei Itobayashi, Hidenori Toyoda, Naoya Sakamoto, Takuya Sho, Wonseok Kang, Jürgen Siebler, Markus Friedrich Neurath, and Valentina Burgio: data curation, critical revision, and drafting the manuscript. Stefano Cascinu: design and concept, data curation, critical revision, and drafting the manuscript. All authors approved the final version of the manuscript.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author.
Supplementary Material
Supplementary data
Supplementary data
Funding Statement
The authors did not receive any funding.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018 Nov;68((6)):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Cronin KA, Lake AJ, Scott S, Sherman RL, Noone AM, Howlader N, et al. Annual report to the nation on the status of cancer, part I: national cancer statistics. Cancer. 2018 Jul;124((13)):2785–800. doi: 10.1002/cncr.31551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019 Apr;380((15)):1450–62. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 4.Bertot LC, Adams LA. Trends in hepatocellular carcinoma due to non-alcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol. 2019 Feb;13((2)):179–87. doi: 10.1080/17474124.2019.1549989. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008 Jul 24;359((4)):378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 6.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009 Jan;10((1)):25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 7.Cheng AL, Kang YK, Lin DY, Park JW, Kudo M, Qin S, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31((32)):4067–75. doi: 10.1200/JCO.2012.45.8372. [DOI] [PubMed] [Google Scholar]
- 8.Johnson PJ, Qin S, Park JW, Poon RT, Raoul JL, Philip PA, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31((28)):3517–24. doi: 10.1200/JCO.2012.48.4410. [DOI] [PubMed] [Google Scholar]
- 9.Cainap C, Qin S, Huang WT, Chung IJ, Pan H, Cheng Y, et al. Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol. 2015;33((2)):172–9. doi: 10.1200/JCO.2013.54.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu AX, Rosmorduc O, Evans TJ, Ross PJ, Santoro A, Carrilho FJ, et al. SEARCH: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2015;33((6)):559–66. doi: 10.1200/JCO.2013.53.7746. [DOI] [PubMed] [Google Scholar]
- 11.Abou-Alfa GK, Niedzwieski D, Knox JJ, Kaubisch A, Posey J, Tan BR, et al. Phase III randomized study of sorafenib plus doxorubicin versus sorafenib in patients with advanced hepatocellular carcinoma (HCC): CALGB 80802. J Clin Oncol. 2016;34:4003. [Google Scholar]
- 12.Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020 Jan 20;38((3)):193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 13.Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022 Jan;23((1)):77–90. doi: 10.1016/S1470-2045(21)00604-5. [DOI] [PubMed] [Google Scholar]
- 14.Qin S, Chen Z, Fang W, Ren Z, Xu R, Ryoo BY, et al. Pembrolizumab plus best supportive care versus placebo plus best supportive care as second-line therapy in patients in Asia with advanced hepatocellular carcinoma (HCC): phase 3 KEYNOTE-394 study. J Clin Oncol. 2022;40:383. [Google Scholar]
- 15.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020 May 14;382((20)):1894–905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 16.Abou-Alfa GK, Lam Chan S, Kudo M, Lau G, Kelley RK, Furuse J, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. Presented at: 2022 Gastrointestinal Cancers Symposium; January 20–22, 2022; San Francisco, CA. Abstract 379. 2022 [Google Scholar]
- 17.Pfister D, Núñez NG, Pinyol R, Govaere O, Pinter M, Szydlowska M, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021 Apr;592((7854)):450–6. doi: 10.1038/s41586-021-03362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018 Mar 24;391((10126)):1163–73. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 19.Common terminology criteria for adverse events (CTCAE) version 4.0 Available from: www.evs.nci.nih.gov (accessed 12 September 2020)
- 20.Casadei-Gardini A, Scartozzi M, Tada T, Yoo C, Shimose S, Masi G, et al. Lenvatinib versus sorafenib in first-line treatment of unresectable hepatocellular carcinoma: an inverse probability of treatment weighting analysis. Liver Int. 2021 Jun;41((6)):1389–97. doi: 10.1111/liv.14817. [DOI] [PubMed] [Google Scholar]
- 21.Shimose S, Kawaguchi T, Tanaka M, Iwamoto H, Miyazaki K, Moriyama E, et al. Lenvatinib prolongs the progression-free survival time of patients with intermediate-stage hepatocellular carcinoma refractory to transarterial chemoembolization: a multicenter cohort study using data mining analysis. Oncol Lett. 2020 Sep;20((3)):2257–65. doi: 10.3892/ol.2020.11758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kudo M, Ueshima K, Chan S, Minami T, Chishina H, Aoki T, et al. Lenvatinib as an initial treatment in patients with intermediate-stage hepatocellular carcinoma beyond up-to-seven criteria and Child-Pugh a liver function: a proof-of-concept study. Cancers. 2019 Jul 31;11((8)):1084. doi: 10.3390/cancers11081084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bezan A, Mrsic E, Krieger D, Stojakovic T, Pummer K, Zigeuner R, et al. The preoperative AST/ALT (De Ritis) ratio represents a poor prognostic factor in a cohort of patients with nonmetastatic renal cell carcinoma. J Urol. 2015 Jul;194((1)):30–5. doi: 10.1016/j.juro.2015.01.083. [DOI] [PubMed] [Google Scholar]
- 24.Casadei Gardini A, Puzzoni M, Montagnani F, Marisi G, Tamburini E, Cucchetti A, et al. Profile of lenvatinib in the treatment of hepatocellular carcinoma: design, development, potential place in therapy and network meta-analysis of hepatitis B and hepatitis C in all Phase III trials. Onco Targets Ther. 2019 Apr 24;12:2981–8. doi: 10.2147/OTT.S192572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee YC, Wang JH, Chen CH, Hung CH, Lo KC, Yen YH, et al. Sorafenib use in hepatitis B virus- or hepatitis C virus-related hepatocellular carcinoma: a propensity score matching study. Kaohsiung J Med Sci. 2021 Jun 24;37((10)):894–902. doi: 10.1002/kjm2.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng AL, Guan Z, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma according to baseline status: subset analyses of the phase III Sorafenib Asia-Pacific trial. Eur J Cancer. 2012 Jul;48((10)):1452–65. doi: 10.1016/j.ejca.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Bruix J, Cheng AL, Meinhardt G, Nakajima K, De Sanctis Y, Llovet J, et al. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: analysis of two phase III studies. J Hepatol. 2017 Nov;67((5)):999–1008. doi: 10.1016/j.jhep.2017.06.026. Erratum in: J Hepatol. 2018 Oct;69(4):990–1. [DOI] [PubMed] [Google Scholar]
- 28.Casadei-Gardini A, Dadduzio V, Rovesti G, Cabibbo G, Vukotic R, Rizzato MD, et al. Utility of neutrophil-to-lymphocyte ratio to identify long-term survivors among HCC patients treated with sorafenib. Medicine. 2020 May 29;99((22)):e19958. doi: 10.1097/MD.0000000000019958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tada T, Kumada T, Hiraoka A, Michitaka K, Atsukawa M, Hirooka M, et al. Neutrophil-to-lymphocyte ratio is associated with survival in patients with unresectable hepatocellular carcinoma treated with lenvatinib. Liver Int. 2020 Apr;40((4)):968–76. doi: 10.1111/liv.14405. [DOI] [PubMed] [Google Scholar]
- 30.Rinninella E, Cintoni M, Raoul P, Pozzo C, Strippoli A, Ponziani FR, et al. Skeletal muscle loss during multikinase inhibitors therapy: molecular pathways, clinical implications, and nutritional challenges. Nutrients. 2020;12((10)):3101. doi: 10.3390/nu12103101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimose S, Iwamoto H, Niizeki T, Shirono T, Noda Y, Kamachi N, et al. Clinical significance of adverse events for patients with unresectable hepatocellular carcinoma treated with lenvatinib: a multicenter retrospective study. Cancers. 2020 Jul 1;12((7)):1867. doi: 10.3390/cancers12071867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hiraoka A, Kumada T, Kariyama K, Takaguchi K, Atsukawa M, Itobayashi E, et al. Clinical features of lenvatinib for unresectable hepatocellular carcinoma in real-world conditions: multicenter analysis. Cancer Med. 2019 Jan 1;8((1)):137–46. doi: 10.1002/cam4.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sung MW, Finn RS, Qin S, Han K-H, Ikeda K, Cheng A-L, et al. Association between overall survival and adverse events with lenvatinib treatment in patients with hepatocellular carcinoma (REFLECT) J Clin Oncol. 2019 Feb 1;37((4_suppl)):317. [Google Scholar]
- 34.Roma-Rodrigues C, Mendes R, Baptista PV, Fernandes AR. Targeting tumor microenvironment for cancer therapy. Int J Mol Sci. 2019 Feb 15;20((4)):840. doi: 10.3390/ijms20040840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kato Y, Tabata K, Kimura T, Yachie-Kinoshita A, Ozawa Y, Yamada K, et al. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PLoS One. 2019 Feb 27;14((2)):e0212513. doi: 10.1371/journal.pone.0212513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hiraoka A, Kumada T, Tada T, Ogawa C, Tani J, Fukunishi S, et al. Therapeutic efficacy of ramucirumab after lenvatinib for post-progression treatment of unresectable hepatocellular carcinoma. Gastroenterol Rep. 2020 Oct 10;9((2)):133–8. doi: 10.1093/gastro/goaa042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kasuya K, Kawamura Y, Kobayashi M, Shindoh J, Kobayashi Y, Kajiwara A, et al. Efficacy and safety of ramucirumab in patients with unresectable hepatocellular carcinoma with progression after treatment with lenvatinib. Intern Med. 2021 Feb 1;60((3)):345–51. doi: 10.2169/internalmedicine.5185-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuzuya T, Ishigami M, Ito T, Ishizu Y, Honda T, Ishikawa T, et al. Initial experience of ramucirumab treatment after lenvatinib failure for patients with advanced hepatocellular carcinoma. Anticancer Res. 2020 Apr;40((4)):2089–93. doi: 10.21873/anticanres.14167. [DOI] [PubMed] [Google Scholar]
- 39.Arizumi T, Ueshima K, Minami T, Kono M, Chishina H, Takita M, et al. Effectiveness of sorafenib in patients with transcatheter arterial chemoembolization (TACE) refractory and intermediate-stage hepatocellular carcinoma. Liver Cancer. 2015 Dec;4((4)):253–62. doi: 10.1159/000367743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogasawara S, Chiba T, Ooka Y, Kanogawa N, Motoyama T, Suzuki E, et al. Efficacy of sorafenib in intermediate-stage hepatocellular carcinoma patients refractory to transarterial chemoembolization. Oncology. 2014;87((6)):330–41. doi: 10.1159/000365993. [DOI] [PubMed] [Google Scholar]
- 41.Koroki K, Kanogawa N, Maruta S, Ogasawara S, Iino Y, Obu M, et al. Posttreatment after lenvatinib in patients with advanced hepatocellular carcinoma. Liver Cancer. 2021;10((5)):473–84. doi: 10.1159/000515552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomonari T, Sato Y, Tanaka H, Tanaka T, Taniguchi T, Sogabe M, et al. Sorafenib as second-line treatment option after failure of lenvatinib in patients with unresectable hepatocellular carcinoma. JGH Open. 2020 Aug 15;4((6)):1135–9. doi: 10.1002/jgh3.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jefremow A, Wiesmueller M, Rouse RA, Dietrich P, Kremer AE, Waldner MJ, et al. Beyond the border: the use of lenvatinib in advanced hepatocellular carcinoma after different treatment lines: a retrospective analysis. J Physiol Pharmacol. 2020 Oct;71((5)) doi: 10.26402/jpp.2020.5.11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author.