Abstract
Introduction
The efficacy of systemic first-line treatments in older adults with unresectable hepatocellular carcinoma (HCC) has not been well-studied. We compared the safety and efficacy of atezolizumab plus bevacizumab versus sorafenib as a first-line treatment in younger versus older patients with unresectable HCC.
Methods
This global, phase 3, open-label, randomized clinical trial (IMbrave150) recruited patients aged ≥18 years with locally advanced metastatic or unresectable HCC, an Eastern Cooperative Oncology Group performance status score of 0 or 1, and Child-Pugh class A liver function who had not previously received systemic therapy for liver cancer. Patients received either 1,200 mg atezolizumab plus 15 mg/kg bevacizumab intravenously every 3 weeks or 400 mg sorafenib orally twice daily until loss of clinical benefit or unacceptable toxicity. Primary endpoints were overall survival (OS) and progression-free survival (PFS). Secondary outcomes were the incidence of adverse events and time to deterioration of patient-reported outcomes (PROs). This subgroup analysis evaluated safety and efficacy endpoints in patients <65 years, ≥65 to <75 years, and ≥75 years.
Results
Of 501 patients, 165 patients were randomized to sorafenib and 336 were randomized to atezolizumab plus bevacizumab (175 patients <65 years; 106 patients ≥65 to <75 years; 55 patients ≥75 years). Across all age groups, patients receiving atezolizumab plus bevacizumab had longer median OS (<65: 18.0 vs. 12.2 months [HR, 0.57; 95% CI: 0.40–0.82]; ≥65 to <75: 19.4 vs. 14.9 months [HR, 0.80; 95% CI: 0.52–1.23]; ≥75: 24.0 vs. 18.0 months [HR, 0.72, 95% CI: 0.37–1.41]) and PFS than those receiving sorafenib. Time to deterioration for multiple PROs was delayed for patients receiving atezolizumab plus bevacizumab, including older adults. There were no clinically meaningful differences in toxicity between age groups.
Conclusion
Atezolizumab plus bevacizumab is safe and effective in adults <65, ≥65 to <75, and ≥75. Treatment was well-tolerated even in elderly patients.
Keywords: Hepatocellular carcinoma, Atezolizumab, Bevacizumab, Older adults
Introduction
Hepatocellular carcinoma (HCC) presents a global challenge, with a rising incidence [1]. Despite recent progress in treatments, HCC still remains one of the leading causes of cancer-related mortality worldwide [1]. The incidence of HCC increases with age, with a majority of patients in the United States diagnosed at age ≥65 years [2]. There has been a rapid expansion of US Food and Drug Administration (FDA)–approved systemic treatment options for patients with unresectable HCC, ranging from anti-angiogenesis agents [3, 4] to multitargeted tyrosine kinase inhibitors [5, 6, 7, 8], immune checkpoint inhibitors [9, 10], and combination approaches [11]. With the development of multiple combination treatments for HCC [12, 13, 14, 15], it is of particular importance to focus not only on their efficacy but on their tolerability and impact on quality of life (QOL) in the older adult HCC patient population. However, data regarding the efficacy and safety of systemic therapies for older adults with unresectable HCC are limited [4, 16, 17, 18, 19].
Older adults with HCC are particularly vulnerable to treatment toxicity because of their higher number of comorbid medical conditions and age-related pharmacokinetic changes associated with decline in hepatic blood flow that lead to a reduction in hepatic clearance of drugs [20]. In addition, treatment with anti-angiogenesis agents such as bevacizumab carries the risk of arterial and venous thromboembolic events and bleeding [3, 21, 22, 23], which potentially increases with age [24, 25, 26]. Furthermore, older adults experience a general decline in immune function and an increase in autoimmune antibodies [27, 28, 29], which could result in decreased immunotherapy efficacy and increased risk for immunotherapy-related toxicities. However, prior retrospective studies suggest there are no differences in efficacy or toxicity between older and younger patients who received immunotherapy [30, 31, 32].
IMbrave150 was the first randomized phase 3 study in more than a decade to demonstrate a statistically significant and clinically meaningful improvement in overall survival (OS) with the combination of anti-programmed cell death 1 ligand 1 (anti-PD-L1) antibody atezolizumab and bevacizumab versus sorafenib in patients with unresectable HCC who had not received prior systemic therapy [11]. In addition, there was a significant delay in deterioration of patient-reported outcomes (PROs), including QOL, physical and role functioning, and HCC symptoms, with atezolizumab plus bevacizumab versus sorafenib [33]. Here, we performed post hoc analyses evaluating the efficacy, PROs, and safety results in older adults (≥65 years) enrolled in IMbrave150.
Materials and Methods
Patients, Treatment, and Endpoints
The study design (online supplement: CONSORT checklist; see www.karger.com/doi/10.1159/000525671 for all online suppl. material) and eligibility criteria of this global phase 3, randomized, open-label study have been previously described (NCT03434379) [11]. Briefly, patients aged 18 years or older with locally advanced metastatic or unresectable HCC, an Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1, Child-Pugh class A liver function, and no previous systemic therapy for liver cancer were recruited from 111 cancer centers in 17 countries and regions [11]. Key exclusion criteria included a history of autoimmune disease; coinfection with hepatitis B virus (HBV), hepatitis C virus (HCV), or both; and untreated or incompletely treated esophageal or gastric varices with bleeding or high risk of bleeding. Patients were required to have an esophagogastroduodenoscopy within 6 months of enrollment, and all size varices were to be treated per standard of care prior to enrollment.
An interactive voice-web response system was used to assign treatment with atezolizumab plus bevacizumab or sorafenib (in a 2:1 ratio) to patients using permuted-block randomization with a block size of 6. The randomization sequence was generated by an independent statistician and stored in the interactive voice or web response system, and the treatment assignment was obtained directly from the system by the investigators. Randomization was stratified by geographic region (Asia excluding Japan vs. rest of the world); macrovascular invasion, extrahepatic spread, or both (presence vs. absence); baseline α-fetoprotein concentration (<400 vs. ≥400 ng/mL); and ECOG performance status (0 vs. 1).
IMbrave150 was carried out in accordance with the International Conference on Harmonization guidelines for Good Clinical Practice and the principles of the Declaration of Helsinki. All patients gave written informed consent to participate in the study. No compensation was provided to patients. Protocol approval was obtained from the Institutional Review Board or Ethics Committee at each site. An independent data monitoring committee reviewed unmasked safety and trial conduct data approximately every 6 months until study unblinding.
Patients received either 1,200 mg atezolizumab plus 15 mg/kg bevacizumab intravenously every 3 weeks or 400 mg of sorafenib orally twice daily until loss of clinical benefit or unacceptable toxicity [11]. Treatment could continue beyond disease progression per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 if, in the opinion of the investigator, the patient was experiencing clinical benefit and signs indicating unequivocal disease progression were absent. If patients transiently or permanently discontinued either atezolizumab or bevacizumab because of an adverse event (AE), single-agent therapy was allowed if the patient was experiencing clinical benefit. Dose modifications were permitted in the sorafenib arm but not in the atezolizumab-plus-bevacizumab arm.
The co-primary endpoints were OS (time from randomization to death from any cause) and progression-free survival (PFS; time from randomization to disease progression per independent review facility-assessed RECIST 1.1 or death from any cause, whichever occurred first) [11]. Key secondary efficacy endpoints included objective response rate (the percentage of patients with a confirmed complete or partial response) and duration of response (time from first documented complete or partial response to disease progression or death) per independent review facility-assessed RECIST 1.1. Results from the primary analysis of these efficacy endpoints, an updated descriptive analysis, and an updated safety and efficacy analysis have been reported previously [11, 33, 34, 35].
Statistical Considerations
For this analysis, efficacy was assessed in the intent-to-treat population (i.e., all patients randomly assigned to treatment), specifically in the subgroups of patients <65 years, ≥65 to <75 years, and ≥75 years of age. All analyses are based on the data cutoff for the updated analysis of OS (August 31, 2020). The Kaplan-Meier method was used to estimate median OS and PFS in confirmed responders for each treatment arm with 95% confidence intervals (CIs). A stratified Cox proportional-hazards model was used to estimate the treatment effect, expressed as a hazard ratio (HR) (atezolizumab plus bevacizumab vs. sorafenib) with a 95% CI. The randomization stratification factors were applied to all stratified efficacy analyses except ECOG, which was not used to avoid overstratification. The safety population included patients who had received at least 1 dose of study treatment. Analyses were conducted using SAS® software, version 9.4.
Results
Demographic and Baseline Clinical Characteristics
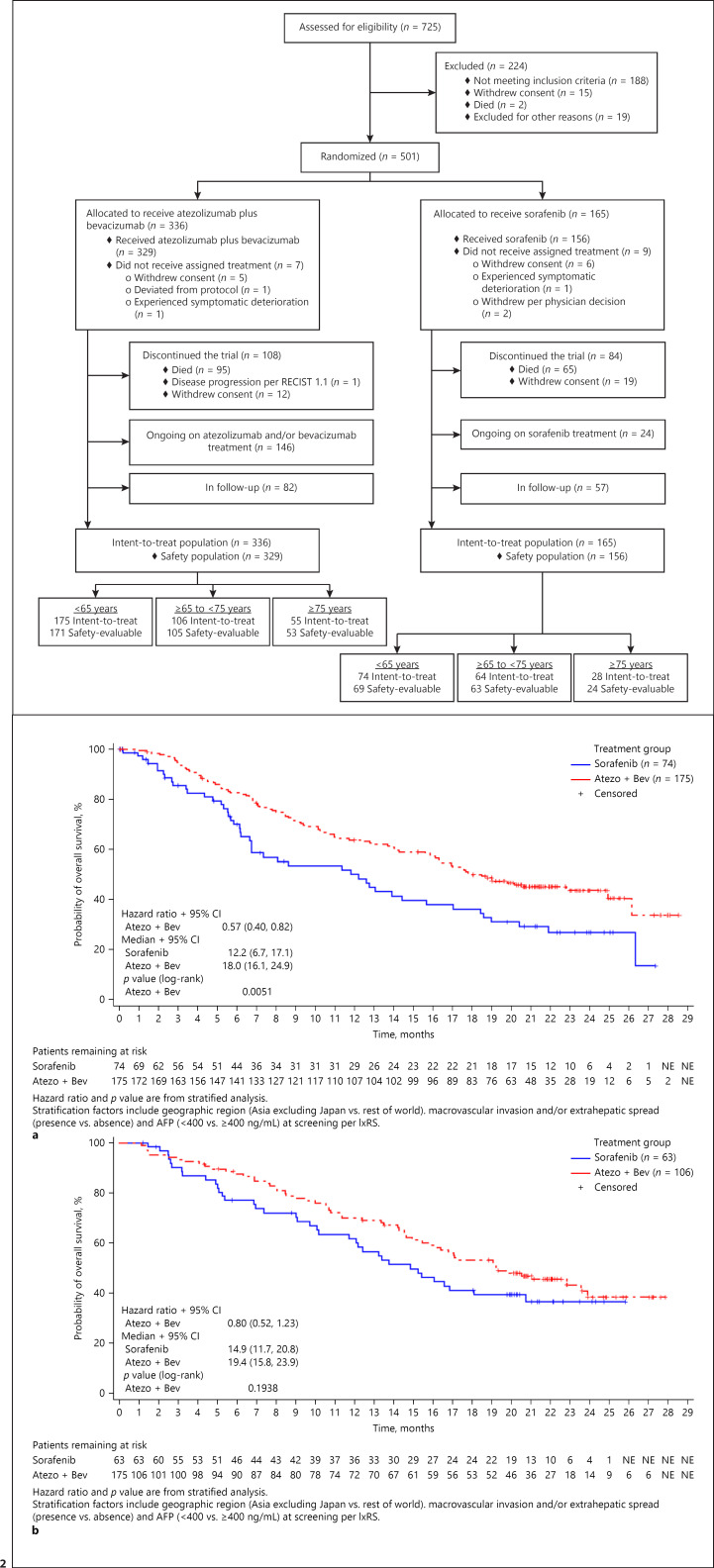
This post hoc analysis included 336 patients in the intent-to-treat atezolizumab-plus-bevacizumab arm (175 patients <65 years; 106 patients ≥65 to <75 years; 55 patients ≥75 years) and 165 patients in the intent-to-treat sorafenib arm (74 patients <65 years; 63 patients ≥65 to <75 years; 28 patients ≥75 years) (Fig. 1). Baseline characteristics were generally similar across both treatment groups (Table 1). The percentages of patients with varices (present, treated, or both) at baseline were also comparable. Younger patients (<65 years) had higher percentages of extrahepatic spread and macrovascular invasion and AFP values ≥400 ng/mL.
Fig. 1.
CONSORT diagram of IMbrave150 patients, subdivided by age.
Table 1.
Patient demographics and baseline characteristics, intent-to-treat population
| Atezolizumab + bevacizumab |
Sorafenib |
|||||
|---|---|---|---|---|---|---|
| <65 (n = 175) | ≥65 to <75 (n = 106) | ≥75 (n = 55) | <65 (n = 74) | ≥65 to <75 (n = 63) | ≥75 (n = 28) | |
| Median age (range), years | 56.0 (26–64) | 69.0 (65–74) | 79.0 (75–88) | 57.5 (33–64) | 69.0 (65–74) | 78.0 (75–87) |
| Female, n (%) | 24 (13.7) | 23 (21.7) | 12 (1.8) | 10 (13.5) | 14 (22.2) | 4 (14.3) |
| Region, n (%) | ||||||
| Asia (excluding Japan) | 91 (52.0) | 32 (30.2) | 10 (18.2) | 46 (62.2) | 14 (22.2) | 8 (28.6) |
| Rest of the world | 84 (48.0) | 74 (69.8) | 45 (81.8) | 28 (37.8) | 49 (77.8) | 20 (71.4) |
| ECOG PS = 1, n (%) | 111 (63.4) | 39 (36.8) | 24 (43.6) | 28 (37.8) | 22 (34.9) | 12 (42.9) |
| Child-Pugh class, n (%) | ||||||
| A5 | 127 (73.0) | 73 (69.5) | 39 (70.9) | 53 (71.6) | 48 (76.2) | 20 (71.4) |
| A6 | 47 (27.0) | 31 (29.5) | 16 (29.1) | 21 (28.4) | 15 (23.8) | 8 (28.6) |
| B7 | 0 | 1 (1.0) | 0 | 0 | 0 | 0 |
| BCLC stage at study entry, n (%) | ||||||
| A | 3 (1.7) | 3 (2.8) | 2 (3.6) | 2 (2.8) | 3 (4.8) | 1 (3.6) |
| B | 25 (14.3) | 16 (15.1) | 10 (18.2) | 6 (8.1) | 14 (22.2) | 5 (17.9) |
| C | 147 (84.0) | 87 (82.1) | 43 (78.2) | 66 (89.2) | 46 (73.0) | 22 (78.6) |
| Etiology of HCC, n (%) | ||||||
| HBV | 105 (60.0) | 40 (37.7) | 19 (34.5) | 51 (68.9) | 17 (27.0) | 8 (28.6) |
| HCV | 41 (23.4) | 20 (18.9) | 11 (20.9) | 11 (14.9) | 17 (27.0) | 8 (28.6) |
| Nonviral | 29 (16.6) | 46 (43.4) | 25 (45.5) | 12 (16.2) | 29 (46.0) | 12 (42.9) |
| AFP ≥400 ng/mL, n (%) | 81 (46.3) | 29 (27.4) | 16 (29.1) | 29 (39.2) | 20 (31.7) | 12 (42.9) |
| Presence of EHS, MVI, or both, n (%) | ||||||
| EHS | 121 (69.1) | 62 (58.5) | 29 (52.7) | 49 (66.2) | 30 (47.6) | 14 (50.0) |
| MVI | 79 (45.1) | 32 (30.2) | 18 (32.7) | 36 (48.6) | 26 (41.3) | 9 (32.1) |
| EHS, MVI, or both | 146 (83.4) | 78 (73.6) | 34 (61.8) | 62 (83.8) | 41 (65.1) | 17 (60.7) |
| Varices, n (%) | ||||||
| Present at baseline | 45 (25.7) | 30 (28.3) | 14 (25.5) | 21 (28.4) | 18 (29.6) | 4 (14.3) |
| Treated at baseline | 23 (13.1) | 8 (7.5) | 5 (9.1) | 13 (17.6) | 9 (14.2) | 1 (3.6) |
| Prior TACE, n (%) | 65 (37.1) | 49 (46.2) | 17 (30.9) | 35 (47.3) | 23 (36.5) | 12 (42.9) |
| Prior radiotherapy, n (%) | 18 (10.3) | 12 (11.3) | 4 (7.3) | 8 (10.8) | 6 (9.5) | 3 (10.7) |
AFP, α-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; ECOG PS, Eastern Cooperative Oncology Group performance status; EHS, extrahepatic spread; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; MVI, macrovascular invasion; TACE, transarterial chemoembolization.
While HBV infection was more common in younger patients, the percentage of patients with HCV infection was similar across age groups. The prevalence of other comorbidities, including diabetes mellitus, cardiac disorders, and vascular disorders, increased with age (Table 2). Notably, 48 of 53 patients ≥75 years (90.6%) in the atezolizumab-plus-bevacizumab arm reported vascular disorders, and 46 of these patients (95.8%) reported hypertension. In the sorafenib arm, 18 of 24 patients ≥75 years (75.0%) reported vascular disorders, and 17 of these patients (94.4%) reported hypertension. The percentage of patients reporting esophageal varices and gastrointestinal disorders, including gastritis, was also similar between age groups.
Table 2.
Comorbid baseline medical conditions with an incidence rate ≥10% (any age group), safety-evaluable population
| Comorbid medical condition, n (%) | Atezolizumab + bevacizumab |
Sorafenib |
||||
|---|---|---|---|---|---|---|
| <65 (n = 171) | ≥65 to <75 (n = 105) | ≥75 (n = 53) | <65 (n = 69) | ≥65 to <75 (n = 63) | ≥75 (n = 24) | |
| Benign prostatic hyperplasia | 8 (4.7) | 13 (12.4) | 7 (13.2) | 1 (1.4) | 7 (11.1) | 2 (8.3) |
| Cardiac disorders | 14 (8.2) | 14 (13.3) | 12 (22.6) | 1 (1.4) | 14 (22.2) | 1 (4.2) |
| Chronic gastritis | 27 (15.8) | 14 (13.3) | 8 (15.1) | 11 (15.9) | 6 (9.5) | 2 (8.3) |
| Constipation | 15 (8.8) | 11 (10.5) | 10 (18.9) | 6 (8.7) | 8 (12.7) | 4 (16.7) |
| Diabetes mellitusa | 33 (19.3) | 42 (39.6) | 22 (41.5) | 16 (23.2) | 26 (41.3) | 12 (50.0) |
| Esophageal varices | 16 (9.4) | 12 (11.4) | 5 (9.4) | 8 (11.6) | 6 (9.5) | 2 (8.3) |
| Gastroesophageal reflux disease | 14 (8.2) | 15 (14.3) | 5 (9.4) | 4 (5.8) | 14 (22.2) | 4 (16.7) |
| Hepatic cirrhosis | 59 (34.5) | 33 (31.4) | 13 (24.5) | 27 (39.1) | 15 (23.8) | 2 (8.3) |
| Hepatitis Bb | 91 (53.2) | 30 (28.3) | 12 (22.6) | 40 (58.0) | 13 (20.6) | 5 (20.8) |
| Hepatitis Cb | 26 (15.2) | 8 (7.5) | 7 (13.2) | 11 (15.9) | 14 (22.2) | 3 (12.5) |
| Hyperlipidemia | 8 (4.7) | 14 (13.3) | 5 (9.4) | 2 (2.9) | 5 (7.9) | 2 (8.3) |
| Hypertension | 73 (42.7) | 58 (55.2) | 46 (86.8) | 19 (27.5) | 40 (63.5) | 17 (70.8) |
| Vascular disorders | 73 (42.7) | 61 (58.1) | 48 (90.6) | 19 (27.5) | 43 (68.3) | 18 (75.0) |
Includes all diabetes-related preferred terms: diabetes mellitus, type 2 diabetes mellitus, and insulin-requiring type 2 diabetes mellitus.
Includes both acute and chronic infections.
Common medications included treatments for vascular, cardiovascular, and gastrointestinal disorders (Table 3). Patients ≥65 years were generally more likely to report use of a given class of medication. Median numbers of concomitant medications were greater in the atezolizumab-plus-bevacizumab arm than the sorafenib arm and increased with age.
Table 3.
Number of concomitant medications and medication classes among patients with at least one concomitant medication
| Atezolizumab + bevacizumab |
Sorafenib |
|||||
|---|---|---|---|---|---|---|
| <65 (n = 175) | ≥65 to <75 (n = 106) | ≥75 (n = 55) | <65 (n = 74) | ≥65 to <75 (n = 63) | ≥75 (n = 28) | |
| Concomitant medications,a median (range) | 14 (1–78) | 16 (2–56) | 17 (2–51) | 11.5 (2–60) | 14 (4–43) | 12 (5–21) |
| Medication class,b, c n (%) | (n = 171) | (n = 105) | (n = 53) | (n = 69) | (n = 63) | (n = 24) |
| Calcium channel blockers | 65 (38.0) | 43 (40.6) | 34 (64.2) | 13 (18.8) | 28 (44.4) | 9 (37.5) |
| Proton pump inhibitors | 84 (49.1) | 59 (55.7) | 26 (49.1) | 28 (40.6) | 36 (57.1) | 13 (54.2) |
| Ophthalmologicals | 79 (46.2) | 55 (51.9) | 24 (45.3) | 21 (30.4) | 28 (44.4) | 11 (45.8) |
| Local oral analgesics/rinses | 63 (36.8) | 42 (39.6) | 28 (52.8) | 15 (21.7) | 22 (33.9) | 6 (25.0) |
| Analgesics | 60 (35.1) | 39 (36.8) | 25 (47.2) | 10 (14.5) | 21 (33.3) | 5 (20.8) |
| Steroids | 73 (42.7) | 44 (41.5) | 24 (45.3) | 11 (15.9) | 18 (28.6) | 6 (25.0) |
| Osmotically acting laxatives | 45 (26.3) | 39 (36.8) | 22 (41.5) | 18 (26.1) | 15 (23.8) | 6 (25.0) |
Intent-to-treat population.
Safety-evaluable population.
A cutoff of ≥40% among patients ≥75 years receiving atezolizumab plus bevacizumab was used to select the medication classes shown in this table.
Efficacy
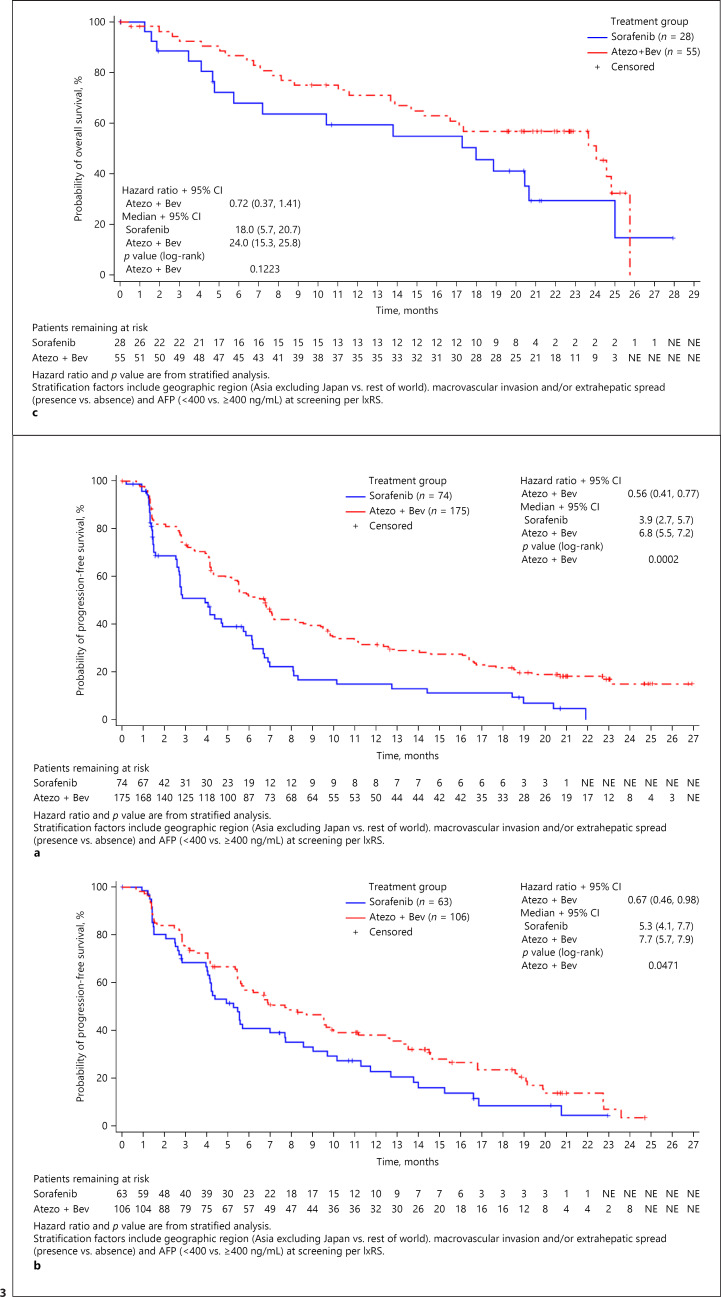
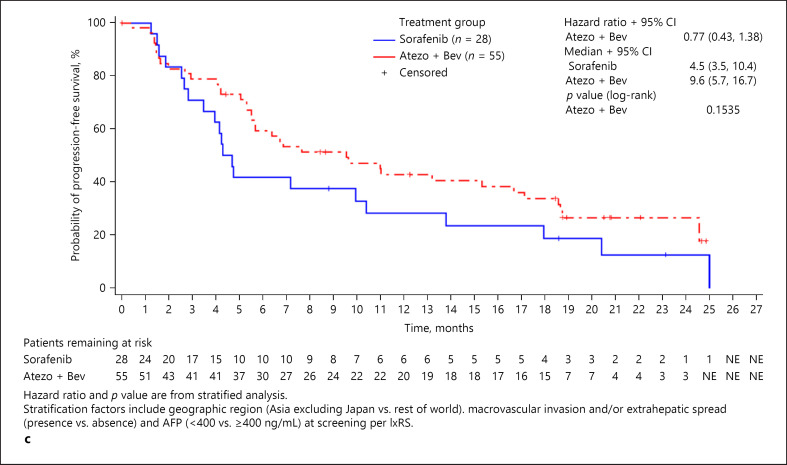
The median OS for patients treated with atezolizumab plus bevacizumab was longer across all age groups compared with the median OS of patients treated with sorafenib (<65 years: 18.0 vs. 12.2 months [HR, 0.57; 95% CI: 0.40–0.82]; ≥65 to <75 years: 19.4 vs. 14.9 months [HR, 0.80; 95% CI: 0.52–1.23]; ≥75 years: 24.0 vs. 18.0 months [HR, 0.72, 95% CI: 0.37–1.41]) (Fig. 2). Additionally, median PFS was longer in all age groups receiving atezolizumab plus bevacizumab than in those receiving sorafenib (<65 years: 6.8 vs. 3.9 months [HR, 0.56; 95% CI: 0.41–0.77]; ≥65 to <75 years: 7.7 vs. 5.3 months [HR, 0.67; 95% CI: 0.46–0.98]; ≥75 years: 9.6 vs. 4.5 months [HR, 0.77; 95% CI: 0.43–1.38]) (Fig. 3). Objective response rate was also greater in patients treated with atezolizumab plus bevacizumab than in patients treated with sorafenib across all age groups (<65 years: 30.0 vs. 10.0%; ≥65 to <75 years: 27.5 vs. 13.1%; ≥75 years: 33.3 vs. 10.7%) (Table 4). Furthermore, all age groups receiving atezolizumab plus bevacizumab had a greater percentage of patients who achieved complete response compared with groups receiving sorafenib (<65 years: 9.4 vs. 0.0%; ≥65 to <75 years: 4.9 vs. 1.6%; ≥75 years: 7.4 vs. 0.0%).
Fig. 2.
Overall survival, intent-to-treat population. a Patients aged <65 years. b Patients aged ≥65 to <75 years. c Patients aged ≥75 years.
Fig. 3.
Progression-free survival, intent-to-treat population. a Patients aged <65 years. b Patients aged ≥65 to <75 years. c Patients aged ≥75 years.
Table 4.
Objective response rate per independent review facility-assessed RECIST 1.1, intent-to-treat population
| Response | <65 |
≥65 to <75 |
≥75 |
|||
|---|---|---|---|---|---|---|
| atezolizumab + bevacizumab | sorafenib | atezolizumab + bevacizumab | sorafenib | atezolizumab + bevacizumab | sorafenib | |
| Response-evaluable population | 170 | 70 | 102 | 61 | 54 | 28 |
| Objective response rate, n (%) | 51 (30.0) | 7 (10.0) | 28 (27.5) | 8 (13.1) | 18 (33.3) | 3 (10.7) |
| Odds ratio for objective response rate, (95% CI)a | 5.09 (2.08, 12.41) | 2.78 (1.15, 6.73) | 4.04 (1.08, 15.07) | |||
| Complete response, n (%) | 16 (9.4) | 0 | 5 (4.9) | 1 (1.6) | 4 (7.4) | 0 |
| Partial response, n (%) | 35 (20.6) | 7 (10.0) | 23 (22.5) | 7 (11.5) | 14 (25.9) | 3 (10.7) |
| Stable disease, n (%) | 71 (41.8) | 22 (31.4) | 52 (51.0) | 35 (57.4) | 21 (38.9) | 12 (42.9) |
| Progressive disease, n (%) | 37 (21.8) | 25 (35.7) | 17 (16.7) | 12 (29.7) | 9 (16.7) | 3 (10.7) |
| Not evaluable,b n (%) | 5 (2.9) | 8 (11.4) | 2 (2.0) | 3 (4.9) | 1 (1.9) | 3 (10.7) |
| Missing,b n (%) | 6 (3.5) | 8 (11.4) | 3 (2.9) | 3 (4.9) | 5 (9.3) | 7 (25.0) |
Confidence interval.
Patients were classified as not evaluable or missing if no post-baseline response assessments were available or all post-baseline assessments were not evaluable.
Safety
Of the 336 patients in the intent-to-treat atezolizumab-plus-bevacizumab arm, 329 patients (171 patients <65 years; 105 patients ≥65 to <75 years; 53 patients ≥75 years) were evaluated for safety. Patients treated with atezolizumab plus bevacizumab received median dose intensities greater than 94.0% and had longer median treatment durations than patients receiving sorafenib (≥6.7 months vs. 2.8–4.1 months) (Table 5). Rates of dose interruption or treatment withdrawal (atezolizumab, bevacizumab, or both) were similar between treatment arms.
Table 5.
Safety summary of atezolizumab plus bevacizumab, safety-evaluable population
| Atezolizumab + bevacizumab |
|||
|---|---|---|---|
| <65 (n = 171) | ≥65 to <75 (n = 105) | ≥75 (n = 53) | |
| Median treatment duration, months (range) | Atezo = 7.7 (0–28) | Atezo = 9.2 (0–26) | Atezo = 7.8 (0–24) |
| Bev = 6.9 (0–28) | Bev = 8.4 (0–26) | Bev = 6.7 (0–24) | |
| Median dose intensity (%) (range) | Atezo = 97.0 (66–104) | Atezo = 95.0 (64–102) | Atezo = 97.0 (75–100) |
| Bev = 96.0 (27–104) | Bev = 95.0 (39–101) | Bev = 94.0 (58–100) | |
| All-grade AEs, any cause, n (%) | 165 (96.5) | 104 (99.0) | 53 (100) |
| AEs related to any study treatment | 150 (87.7) | 88 (83.8) | 46 (86.8) |
| AEs related to atezolizumab | 137 (80.1) | 82 (78.1) | 45 (84.9) |
| AEs related to bevacizumab | 136 (79.5) | 79 (75.2) | 39 (73.6) |
| Grade 3–4 AEs, n (%) | 100 (58.5) | 69 (65.7) | 38 (71.7) |
| Treatment-related grade 3–4 AEs | 68 (39.8) | 46 (43.8) | 29 (54.7) |
| SAEs, n (%) | 76 (44.4) | 54 (51.4) | 30 (56.6) |
| Treatment-related SAEs | 36 (21.1) | 24 (22.9) | 16 (30.2) |
| Grade 5 AEs, n (%) | 11 (6.4) | 7 (6.7) | 5 (9.4) |
| Treatment-related grade 5 AEs | 2 (1.2) | 2 (1.9) | 2 (3.8) |
| AEs leading to withdrawal from any component, n (%) | 31 (18.1) | 29 (27.6) | 12 (22.6) |
| AEs leading to withdrawal from atezolizumab, n (%) | 16 (9.4) | 14 (13.3) | 9 (17.0) |
| AEs leading to withdrawal from bevacizumab, n (%) | 29 (17.0) | 29 (27.6) | 11 (20.8) |
| AEs leading to withdrawal from both components, n (%) | 13 (7.6) | 13 (12.4) | 8 (15.1) |
| AEs leading to dose interruption of any study treatment, n (%) | 93 (54.4) | 67 (63.8) | 35 (66.0) |
AE, adverse event; atezo, atezolizumab; bev, bevacizumab; SAE, serious adverse event.
The incidence of any-grade treatment-related AEs for atezolizumab, bevacizumab, or both was similar in all age groups (Table 5). While the incidence of treatment-related grade 3–4 AEs and serious AEs was higher in patients ≥75 years, the percentage of treatment-related grade 5 AEs was less than 4% and comparable across all patient groups receiving atezolizumab plus bevacizumab (Table 5). Notably, most treatment-related serious AEs and all-grade 5 AEs occurring in patients ≥75 years were single occurrences spread across several different system organ classes (online suppl. Tables S1, S2). Of all AEs, hypertension was the most common across age groups. Other common AEs included fatigue, diarrhea, and decreased appetite (online suppl. Table S3). While patients ≥65 years were more likely to experience arthralgia and peripheral edema, patients ≥75 years were more likely to report general malaise.
Of the 165 patients in the intent-to-treat sorafenib arm, 156 patients (69 patients <65 years; 63 patients ≥65 to <75 years; 24 patients ≥75 years) were evaluated for safety. The median treatment duration ranged from 2.8 months, in patients <65 years and those ≥65 to <75 years, to 4.1 months, in those ≥75 years. The incidence of AEs was similar to that in previously reported safety profiles of sorafenib [5, 8], and there were no clinically meaningful differences in AE incidence or median dose intensity between age groups.
Patient-Reported Outcomes
PROs included physical function, role functioning, QOL or global health status, appetite loss, diarrhea, nutrition, fatigue, and pain (measured by the EORTC QLQ-C30 and EORTC QLQ-HCC18). Time to deterioration (TTD) for PROs was longer for patients treated with atezolizumab plus bevacizumab compared with sorafenib across all age groups (Table 6). Notably, median TTD of physical function was more than 4 times greater in patients ≥75 years who received atezolizumab plus bevacizumab compared with sorafenib (12.09 vs. 3.02 months [HR, 0.51; 95% CI: 0.21–1.25]).
Table 6.
Patient-reported outcomes: Time to deterioration, intent-to-treat population
| Median time to confirmed deterioration, months (95% Cl)a | Atezolizumab + bevacizumab |
Sorafenib |
||||
|---|---|---|---|---|---|---|
| <65 (n = 175) | ≥65 to <75 (n = 106) | ≥75 (n = 55) | <65 (n = 74) | ≥65 to <75 (n = 63) | ≥75 (n = 28) | |
| Physical functioning (QLQ-C30)b | NE (10.45, NE)d | 7.69 (5.59,10.78) | 12.09 (5.62, NE) | 9.82 (2.10, NE) | 4.21 (3.48, 5.78) | 3.02 (1.87, NE) |
| Role functioning (QLQ-C30) | 8.31 (6.34,16.36) | 10.51 (5.29,13.37) | 5.68 (3.48,23.49) | 3.52 (1.64,13.73) | 4.27 (3.48, 7.43) | 3.02 (0.82, NE) |
| Quality of life/Global Health Status (QLQ-C30) | 11.24 (6.51, NE) | 6.21 (3.48,13.37) | 5.68 (3.52, NE) | 6.97 (1.48,11.07) | 3.58 (2.73,4.96) | 3.19 (1.48, NE) |
| Abdominal swelling (QLQ-HCC18)c | NE (20.3, NE) | NE (15.11, NE) | NE (11.04, NE) | NE (5.65, NE) | 13.40 (5.52, NE) | 5.32 (2.86, NE) |
| Appetite loss (QLQ-C30) | 22.37 (15.11, NE) | 10.64 (7.85, NE) | 21.42 (11.70, NE) | 9.82 (2.20, NE) | 7.62 (3.61, NE) | 3.02 (0.85,5.32) |
| Diarrhea (QLQ-C30) | NE (23.00, NE) | NE (15.34, NE) | NE (23.49, NE) | 4.21 (2.10,5.59) | 5.09 (3.61,9.26) | 3.48 (1.48, NE) |
| Fatigue (QLQ-C30) | 8.28 (5.75, NE) | 4.30 (2.76, 5.98) | 2.92 (0.85, 5.68) | 4.17 (1.45,21.68) | 2.10 (0.99,4.83) | 1.45 (0.82, NE) |
| Fatigue (QLQ-HCC18) | 6.41 (4.14,11.04) | 5.59 (2.99, 9.92) | 4.76 (1.48, 23.49) | 2.07 (1.12, 2.27) | 2.83 (2.10,6.28) | 2.14 (0.82, NE) |
| Jaundice (QLQ-HCC18) | 11.96 (7.16, NE) | 8.38 (4.76, NE) | 7.06 (6.34,18.76) | 9.82 (5.55, NE) | 6.28 (3.02, NE) | NE (1.41, NE) |
| Nutrition (QLQ-HCC18) | NE (11.17, NE) | 13.37 (8.54, NE) | 13.31 (6.24, NE) | NE (2.92, NE) | 6.93 (3.61, NE) | 3.02 (2.10, NE) |
| Pain (QLQ-C30) | 8.28 (5.55,12.32) | 7.66 (5.52,13.01) | 18.07 (5.62, NE) | 2.46 (1.58,6.97) | 3.81 (2.14,6.28) | 2.14 (0.79,4.37) |
| Pain(QLQ-HCC18) | 20.04 (14.55, NE) | 9.66 (7.56, NE) | NE (7.13, NE) | 9.82 (3.48, NE) | NE (5.19, NE) | 3.58 (1.48,20.40) |
Confidence interval.
EORTC (European Organisation for Research and Treatment of Cancer) Quality of Life Questionnaire − Core Questionnaire.
EORTC (European Organisation for Research and Treatment of Cancer) Quality of Life Questionnaire − Hepatocellular Carcinoma/Primary Liver Cancer Module.
Not estimable.
Discussion/Conclusion
This is the first study to report outcomes from a large randomized clinical trial investigating combination immunotherapy plus vascular endothelial growth factor (VEGF) inhibition in older adults with cancer. We found consistent benefit in using atezolizumab plus bevacizumab versus sorafenib even among older adults, with improvements in median OS and PFS for patients treated with atezolizumab plus bevacizumab across all age groups. Additionally, median TTD for multiple PROs was longer in patients receiving atezolizumab plus bevacizumab across all age groups. Atezolizumab plus bevacizumab has a safety profile in older patients (≥65 years) similar to that in younger patients (<65 years), despite older patients having the majority of baseline comorbidities, particularly vascular comorbidities. There were no clinically meaningful differences in ability to tolerate treatment. Importantly, even for patients ≥75 years treated with atezolizumab plus bevacizumab, there were no clinically meaningful changes in the overall safety profile, including the incidence of treatment-related AEs leading to dose interruption or withdrawal, suggesting there is overall tolerance of atezolizumab plus bevacizumab, even among the elderly population.
In this analysis, atezolizumab-plus-bevacizumab combination treatment showed the longest OS reported in older adults with HCC, compared with what was previously reported for single-agent treatment with either programmed cell death 1 protein (PD-1)/PD-L1 immunotherapy or VEGF inhibition in older adults. We found that patients with HCC treated with atezolizumab plus bevacizumab who were ≥65 to <75 years had a median OS of 19.4 months and patients ≥75 years had a median OS of 24.0 months. In contrast, Melero et al. [36] reported that among patients with HCC treated with nivolumab in the CheckMate 040 study the median OS was 17.0 months in patients ≥65 to <75 years and 19.9 months in patients ≥75 years [37]. In the field of single-agent VEGF inhibition, Kudo et al. [4] reported that among patients with HCC treated with ramucirumab in the second-line setting median OS was 7.6 months in patients ≥65 to <75 years and 8.9 months in patients ≥75 years.
Interestingly, compared with younger patients, older adults treated in IMbrave150 had strikingly longer OS. One possible reason for the superior OS seen in older patients is that patients <65 years have poorer prognostic features of more extrahepatic disease, macrovascular invasion, and AFP ≥400 ng/mL. Additionally, although the HR for OS increased with age, this was possibly due to the reduced prevalence of older HBV-positive patients receiving sorafenib (<65 years: 58.0%; ≥65 to 75 years: 20.6%; ≥75 years: 20.8%). Sorafenib is potentially less efficacious in patients with HBV [38], which could have contributed to the increased OS seen in the older adult population treated with sorafenib in IMbrave150 (<65 years: 12.2 months; ≥65 to 75 years: 14.9 months; ≥75 years: 18.0 months).
PROs and other QOL measures are increasingly being regarded as important prognostic factors for response to treatment and disease progression [39, 40], particularly in older patients. A noninferiority trial of lenvatinib versus sorafenib noted that while both treatments resulted in deterioration of QOL measures, this deterioration was delayed in patients receiving lenvatinib [8]. In a post hoc analysis of the REACH and REACH-2 trials, Kudo et al. [4] reported that patients receiving ramucirumab as second-line treatment displayed a trend toward a delay in deterioration; this benefit was consistent for all age groups, including patients ≥75 years. We showed that patients treated with atezolizumab plus bevacizumab experienced a delay in TTD of various QOL measures [33]. In this study, TTD for QOL measures was consistently delayed across all age groups receiving atezolizumab plus bevacizumab versus sorafenib, indicating that atezolizumab plus bevacizumab can prolong TTD even in elderly patients.
In addition to increased efficacy and delayed TTD of key PROs, including physical functioning, role functioning, and global health status, treatment safety is of particular concern for elderly patients because age-related changes, such as increased comorbidities and concomitant medications [41, 42], may reduce treatment tolerance. In the landscape of PD-1/PD-L1 monotherapy, Melero et al. [36] reported that 22.5% of patients ≥65 years with HCC treated with nivolumab experienced grade 3–4 treatment-related AEs. For VEGF inhibition, Kudo et al. [4] reported that 60.2% of patients with HCC ≥65 to <75 years and 61.5% of patients ≥75 years who received ramucirumab experienced at least one grade ≥3 AE. In IMbrave150, the incidence of grade 3–4 AEs in patients treated with atezolizumab plus bevacizumab was 58.5% in patients <65 years, 65.7% in patients ≥65 to <75 years, and 71.1% in patients ≥75 years; the incidence of treatment-related grade 3–4 AEs was 39.8%, 43.8%, and 54.7%, respectively. The median number of comorbidities and concomitant medications increased with age (<65 years: 6 comorbidities, 14 concomitant medications; ≥65 to <75 years: 6 comorbidities, 16 concomitant medications; ≥75 years: 7 comorbidities, 17 concomitant medications), which could explain the higher incidence of grade 3–4 AEs observed in patients ≥75 years who received atezolizumab plus bevacizumab versus that in patients <75 years. Despite this, all age groups were able to tolerate a dose intensity of at least 94.0% for each component, and only 21 of 158 older patients (13 patients ≥65 to <75 years, 8 patients ≥75 years) discontinued both atezolizumab and bevacizumab as a result of AEs. Importantly, there were no clinically meaningful differences between age groups in the incidence of AEs related to atezolizumab (<65 years: 80.1%; ≥65 to <75 years: 78.1%; ≥75 years, 84.9%) or bevacizumab (<65 years: 79.5%; ≥65 to <75 years: 75.2%; ≥75 years, 73.6%), suggesting that neither atezolizumab's nor bevacizumab's safety profile is negatively affected by advanced age.
Limitations to our study include the post hoc retrospective nature of the analysis and small sample size of individual subgroups. This limited our potential to perform additional subgroup analyses with significant power. Although our use of 3 subgroups reduced the size of each population, it allowed us to generate data that are more clinically relevant to this vulnerable patient population (i.e., the impact of treatment on patients ≥75 years). Only patients with preserved liver function (Child-Pugh class A) and minimized risk of variceal bleeding (having untreated esophageal, gastric varices, or both was an exclusion criterion) were eligible for IMbrave150, and therefore these patients may not be completely representative of the clinical older adult patient population with HCC (e.g., patients with Child-Pugh B/C, untreated active varices, or additional autoimmune comorbidities). Additionally, restricting enrollment to patients with an ECOG performance status of 0 or 1 and adequate hematologic/organ function may have further limited the diversity of our older adult patient population. However, the inclusion of patients with main portal vein (or contralateral main branch) invasion (Vp4) or bile duct invasion, who are usually excluded from phase 3 trials of HCC treatments [8, 43], increased the diversity of our patient population. Moreover, additional baseline characteristics of patients in our study, such as comorbidities and concomitant medications, are similar to those expected in the general patient population, increasing the validity of our results.
In this post hoc subgroup analysis, atezolizumab plus bevacizumab increased median OS and PFS and delayed TTD for multiple PROs over those with sorafenib for patients <65 years, ≥65 to <75 years, and ≥75 years. Even among the most elderly patients in this trial population, who have more comorbidities and concomitant medications, treatment with atezolizumab plus bevacizumab was well-tolerated with no clinically meaningful differences between age groups in treatment-related AE incidence or discontinuation. Atezolizumab plus bevacizumab should be considered a first-line treatment option even for older adults with unresectable HCC.
Statement of Ethics
IMbrave150 was carried out in accordance with the International Conference on Harmonisation guidelines for Good Clinical Practice and the principles of the Declaration of Helsinki. All patients gave written informed consent to participate in the study. Protocol approval was obtained from the Institutional Review Board (IRB) or Ethics Committee (EC) at each site. The first IRB approval for IMbrave150 was granted on December 19, 2017 from the City of Hope National Medical Center, Duarte, CA, USA (IRB No. 20172734; Western Institutional Review Board, Inc. Puyallup, WA, USA), in addition to multiple other EC/IRB approvals obtained across all participating sites in the different countries of enrollment. An independent data monitoring committee reviewed unmasked safety and trial conduct data approximately every 6 months until study unblinding. The study sponsor supplied the study drugs and collaborated with academic authors on the study design, data collection, data analysis, and data interpretation.
Conflict of Interest Statement
Dr. Daneng Li reports research funding to his institution from AstraZeneca and Brooklyn ImmunoTherapeutics. He serves as a consultant and has received honoraria from Adagene, Advanced Accelerator Applications, Bayer Healthcare, Coherus BioSciences, Eisai, Exelixis, Genentech, Ipsen Biopharmaceuticals, Lexicon Pharmaceuticals, Merck, MiNA Therapeutics, QED Therapeutics, Servier, Sun Pharma, Taiho Pharmaceutical, and TerSera Therapeutics. Dr. Han Chong Toh has received research funding from Bristol Myers Squibb, Merck, and Bayer Healthcare; honoraria from Roche, Merck, and Eisai; and consultancy fee from Tessa Therapeutics Ltd. Dr. Philippe Merle has received advisory board fees from Bayer Healthcare, Bristol Myers Squibb, Eisai, Eli Lilly and Company, Ipsen Biopharmaceuticals, Merck, and Roche. Dr. Kaoru Tsuchiya has received lecture fees from Eisai, Chugai Pharmaceutical, and Eli Lilly. Dr. Sairy Hernandez is employed by Genentech USA. Dr. Wendy Verret was employed by Genentech USA at the time the analysis was completed. Dr. Alan Nicholas is employed by Genentech USA. Dr. Masatoshi Kudo has received grant support from AbbVie, Dainippon Sumitomo Pharma, Gilead Sciences, Otsuka Pharmaceutical, Taiho Pharmaceutical, and Takeda Medical Research Foundation; grant support and consulting fees from Bayer and Merck; grant support, lecture fees, and consulting fees from Bristol Myers Squibb and Eisai; consulting fees from Chugai Pharmaceutical and Ono Pharmaceutical; and is the Editor-in-Chief of Liver Cancer. No other potential conflict of interest relevant to this article was reported.
Funding Sources
This study was funded by F. Hoffmann-La Roche/Genentech. The study was designed and data analyzed by the sponsor in collaboration with the investigators. Editorial support was provided by Thea Gray of Health Interactions and funded by the sponsor.
Author Contributions
Daneng Li had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Daneng Li, Han Chong Toh, and Wendy Verret. Acquisition, analysis, or interpretation of data: Daneng Li, Han Chong Toh, Philippe Merle, Kaoru Tsuchiya, Sairy Hernandez, Wendy Verret, Alan Nicholas, and Masatoshi Kudo. Drafting of the manuscript: Daneng Li, Han Chong Toh, and Wendy Verret. Critical revision of the manuscript for important intellectual content: Daneng Li, Han Chong Toh, Philippe Merle, Kaoru Tsuchiya, Sairy Hernandez, Alan Nicholas, and Masatoshi Kudo. Statistical analysis: Alan Nicholas. Administrative, technical, or material support: Daneng Li and Masatoshi Kudo. Supervision: Daneng Li, Han Chong Toh, and Masatoshi Kudo.
Data Availability Statement
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Acknowledgments
The authors would like to thank Christiana J. Crook, MS, and Ya-Han Zhang, BA, for their editorial assistance and Yong Wang, PhD, for his assistance with statistical analyses.
Funding Statement
This study was funded by F. Hoffmann-La Roche/Genentech. The study was designed and data analyzed by the sponsor in collaboration with the investigators. Editorial support was provided by Thea Gray of Health Interactions and funded by the sponsor.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68((6)):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Percent of new cases by age group: liver and intrahepatic bile duct cancer . Cancer stat facts: liver and intrahepatic bile duct cancer. Bethesda, MD: National Institutes of Health; National Cancer Institute; 2021. In: Surveillance, Epidemiology, and End Results (SEER) Program. seer.cancer.gov . [Google Scholar]
- 3.Boige V, Malka D, Bourredjem A, Dromain C, Baey C, Jacques N, et al. Efficacy, safety, and biomarkers of single-agent bevacizumab therapy in patients with advanced hepatocellular carcinoma. Oncologist. 2012;17((8)):1063–72. doi: 10.1634/theoncologist.2011-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kudo M, Galle PR, Llovet JM, Finn RS, Vogel A, Motomura K, et al. Ramucirumab in elderly patients with hepatocellular carcinoma and elevated alpha-fetoprotein after sorafenib in REACH and REACH-2. Liver Int. 2020;40((8)):2008–20. doi: 10.1111/liv.14462. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359((4)):378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 6.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389((10064)):56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 7.Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379((1)):54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391((10126)):1163–73. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 9.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389((10088)):2492–502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19((7)):940–52. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 11.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T-Y, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382((20)):1894–905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 12.Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38((26)):2960–70. doi: 10.1200/JCO.20.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yau T, Kang Y-K, Kim T-Y, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol. 2020;6((11)):e204564. doi: 10.1001/jamaoncol.2020.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelley RK, Sangro B, Harris W, Ikeda M, Okusaka T, Kang YK, et al. Safety, efficacy, and pharmacodynamics of tremelimumab plus durvalumab for patients with unresectable hepatocellular carcinoma: randomized expansion of a phase I/II study. J Clin Oncol. 2021;39((27)):2991–3001. doi: 10.1200/JCO.20.03555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelley RK, Yau T, Cheng AL, Kaseb A, Qin S, Zhu AX, et al. VP10-2021: cabozantinib (C) plus atezolizumab (A) versus sorafenib (S) as first-line systemic treatment for advanced hepatocellular carcinoma (aHCC): results from the randomized phase III COSMIC-312 trial. Ann Oncol. 2022;33((1)):114–6. [Google Scholar]
- 16.Wong H, Tang YF, Yao T-J, Chiu J, Leung R, Chan P, et al. The outcomes and safety of single-agent sorafenib in the treatment of elderly patients with advanced Hepatocellular Carcinoma (HCC) Oncologist. 2011;16((12)):1721–8. doi: 10.1634/theoncologist.2011-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jo M, Yasui K, Kirishima T, Shima T, Niimi T, Katayama T, et al. Efficacy and safety of sorafenib in very elderly patients aged 80 years and older with advanced hepatocellular carcinoma. Hepatol Res. 2014;44((13)):1329–38. doi: 10.1111/hepr.12308. [DOI] [PubMed] [Google Scholar]
- 18.Ziogas DC, Papadatos-Pastos D, Thillai K, Korantzis I, Chowdhury R, Suddle A, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: age is not a problem. Eur J Gastroenterol Hepatol. 2017;29((1)):48–55. doi: 10.1097/MEG.0000000000000739. [DOI] [PubMed] [Google Scholar]
- 19.Hajiev S, Allara E, Motedayеn Aval L, Arizumi T, Bettinger D, Pirisi M, et al. Impact of age on sorafenib outcomes in hepatocellular carcinoma: an international cohort study. Br J Cancer. 2021;124((2)):407–13. doi: 10.1038/s41416-020-01116-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soto-Perez-de-Celis E, Li D, Yuan Y, Lau YM, Hurria A. Functional versus chronological age: geriatric assessments to guide decision making in older patients with cancer. Lancet Oncol. 2018;19((6)):e305–16. doi: 10.1016/S1470-2045(18)30348-6. [DOI] [PubMed] [Google Scholar]
- 21.Siegel AB, Cohen EI, Ocean A, Lehrer D, Goldenberg A, Knox JJ, et al. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol. 2008;26((18)):2992–8. doi: 10.1200/JCO.2007.15.9947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tewari KS, Sill MW, Long HJ, 3rd, Penson RT, Huang H, Ramondetta LM, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370((8)):734–43. doi: 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li LJ, Chen DF, Wu GF, Guan WJ, Zhu Z, Liu YQ, et al. Incidence and risk of thromboembolism associated with bevacizumab in patients with non-small cell lung carcinoma. J Thorac Dis. 2018;10((8)):5010–22. doi: 10.21037/jtd.2018.07.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alikhan R, Cohen AT, Combe S, Samama MM, Desjardins L, Eldor A, et al. Risk factors for venous thromboembolism in hospitalized patients with acute medical illness: analysis of the MEDENOX study. Arch Intern Med. 2004;164((9)):963–8. doi: 10.1001/archinte.164.9.963. [DOI] [PubMed] [Google Scholar]
- 25.Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol. 2008;28((3)):370–2. doi: 10.1161/ATVBAHA.108.162545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grilz E, Königsbrügge O, Posch F, Schmidinger M, Pirker R, Lang IM, et al. Frequency, risk factors, and impact on mortality of arterial thromboembolism in patients with cancer. Haematologica. 2018;103((9)):1549–56. doi: 10.3324/haematol.2018.192419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vadasz Z, Haj T, Kessel A, Toubi E. Age-related autoimmunity. BMC Med. 2013;11:94. doi: 10.1186/1741-7015-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fülöp T, Dupuis G, Witkowski JM, Larbi A. The role of immunosenescence in the development of age-related diseases. Rev Invest Clin. 2016;68((2)):84–91. [PubMed] [Google Scholar]
- 29.Pawelec G. Immunosenescence and cancer. Biogerontology. 2017;18((4)):717–21. doi: 10.1007/s10522-017-9682-z. [DOI] [PubMed] [Google Scholar]
- 30.Elias R, Hartshorn K, Rahma O, Lin N, Snyder-Cappione JE. Aging, immune senescence, and immunotherapy: a comprehensive review. Semin Oncol. 2018;45((4)):187–200. doi: 10.1053/j.seminoncol.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 31.van Holstein Y, Kapiteijn E, Bastiaannet E, van den Bos F, Portielje J, de Glas NA. Efficacy and adverse events of immunotherapy with checkpoint inhibitors in older patients with cancer. Drugs Aging. 2019;36((10)):927–38. doi: 10.1007/s40266-019-00697-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samani A, Zhang S, Spiers L, Mohamed AA, Merrick S, Tippu Z, et al. Impact of age on the toxicity of immune checkpoint inhibition. J Immunother Cancer. 2020;8((2)):e000871. doi: 10.1136/jitc-2020-000871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galle PR, Finn RS, Qin S, Ikeda M, Zhu AX, Kim T-Y, et al. Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): an open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22((7)):991–1001. doi: 10.1016/S1470-2045(21)00151-0. [DOI] [PubMed] [Google Scholar]
- 34.Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76((4)):862–73. doi: 10.1016/j.jhep.2021.11.030. [DOI] [PubMed] [Google Scholar]
- 35.Salem R, Li D, Sommer N, Hernandez S, Verret W, Ding B, et al. Characterization of response to atezolizumab + bevacizumab versus sorafenib for hepatocellular carcinoma: results from the IMbrave150 trial. Cancer Med. 2021;10((16)):5437–47. doi: 10.1002/cam4.4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melero I, El-Khoueiry A, Yau T, Hsu C, Kudo M, Crocenzi T, et al. Efficacy and safety of nivolumab in patients with advanced hepatocellular carcinoma analyzed by patient age: a sub-analysis of the CheckMate 040 study. Ann Oncol. 2017;28((Suppl 3)):iii139. [Google Scholar]
- 37.Powers V. Hilton, TN: OncLive; 2017. Advanced age presents no barrier to successful nivolumab treatment in HCC. [Google Scholar]
- 38.Bruix J, Raoul J-L, Sherman M, Mazzaferro V, Bolondi L, Craxi A, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol. 2012;57((4)):821–9. doi: 10.1016/j.jhep.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bottomley A, Flechtner H, Efficace F, Vanvoorden V, Coens C, Therasse P, et al. Health related quality of life outcomes in cancer clinical trials. Eur J Cancer. 2005;41((12)):1697–709. doi: 10.1016/j.ejca.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 40.Mierzynska J, Piccinin C, Pe M, Martinelli F, Gotay C, Coens C, et al. Prognostic value of patient-reported outcomes from international randomised clinical trials on cancer: a systematic review. Lancet Oncol. 2019;20((12)):e685–98. doi: 10.1016/S1470-2045(19)30656-4. [DOI] [PubMed] [Google Scholar]
- 41.Salive ME. Multimorbidity in older adults. Epidemiol Rev. 2013;35:75–83. doi: 10.1093/epirev/mxs009. [DOI] [PubMed] [Google Scholar]
- 42.Morin L, Johnell K, Laroche ML, Fastbom J, Wastesson JW. The epidemiology of polypharmacy in older adults: register-based prospective cohort study. Clin Epidemiol. 2018;10:289–98. doi: 10.2147/CLEP.S153458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38((3)):193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Data Availability Statement
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.