Abstract
The crop of pigeon has specific characteristics as producing crop milk in the lactating period. However, the exact mechanisms underlying the regulation of crop lactation remain unclear. miRNAs, the essential regulators of gene expression, are implicated in various physiological and biological activities. In this study, we discovered a new miRNA that regulated phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit delta (PIK3CD) and crop fibrocyte proliferation. Results of the luciferase reporter assay suggested that miR-193-5p suppressed PIK3CD expression by targeting a conserved binding site in the 3′-untranslated region (UTR) of PIK3CD mRNA. MiR-193-5p promoted crop fibrocyte proliferation and migration, whereas PIK3CD inhibited these effects. These findings suggested an important regulatory role of miR-193-5p in crop fibrocyte proliferation, suggesting that miR-193-5p and PIK3CD might be important regulators of crop milk production.
Key words: PIK3CD, miR-193-5p, crop fibrocyte, cell proliferation, pigeon
INTRODUCTION
Both male and female pigeons could produce a curd-like substance in their crops called crop milk (Gillespie et al., 2011). Similarly, male and female flamingos and male emperor penguins could produce crop milk, whereas in other birds, crops only had the function of food storage (Gillespie et al., 2011). Crop produced crop milk 2 d before the first egg hatches following the regulation of hormone as prolactin (Dumont, 1965). Studies of the synthesis of crop milk by crop have been conducted since 1786, and prolactin has been shown to play an important role in lipid accumulation, fatty acid transportation and de novo synthesis at the hormone level (Dumont, 1965; Wan et al., 2019). In the lactation period, the germinal cell layer of the crop proliferated quickly and formed the nutritive cell layer in response to prolactin (Dumont, 1965; Wan et al., 2019). With the consist proliferation of crop fibrocyte, it began to be cornified and sloughed off to produce milk (Gillespie et al., 2013). But the physiological mechanism of lactation in pigeon is not similar to that in mammals. Mammalian mammary glands are glandular tissues, but the crop of pigeon is not glandular (Gillespie et al., 2011). Thus, it is necessary to reveal the molecular mechanism regulating crop milk protein synthesis, fatty acid synthesis, fatty acid transporters, and the differentially expressed gene enriched pathways (Xie et al., 2017; Chen et al., 2019; Xie et al., 2019; Chen et al., 2020).
Phosphoinositide 3-kinase (PI3K) plays a key role in physiological activities as metabolic regulations by activating serine-threonine protein kinase AKT and its downstream effector (Du et al., 2018). PI3K can be divided into 3 classes according to their substrate specificity and structures. Class Ⅰ PI3K can be divided into subclasses ⅠA and ⅠB. The catalytic isoforms of Class ⅠA including p110α, p110β, and p110δ are encoded by PIK3CA, PIK3CB, and PIK3CD, respectively (Thorpe et al., 2015; Singh et al., 2016; Bilanges et al., 2019). When the growth factors or ligand bind to tyrosine kinases receptor, phosphatidylinositol 4,5-bisphosphate (PIP2) is phosphorylated by PI3Ks, then phosphatidylinositol 3,4,5-trisphosphate (PIP3) is generated (Raimondi and Falasca, 2012). Then the pleckstrin homology (PH) domain-containing proteins such as AKT could be recruited by PIP3 to the plasma membrane. These events activate the signaling cascade to promote metabolic activities (Kriplani et al., 2015).
MicroRNAs (miRNAs) are noncoding RNAs made up of approximately 21 to 25 nucleotides, which can regulate gene expression post-transcriptionally and control diverse biological processes including cell proliferation, division, migration, and apoptosis (Bartel, 2004; He and Hannon, 2004; Bartel, 2009; Pennisi, 2014). miRNAs inhibit gene expression by targeting the 3′-untranslated region (UTR) of mRNA to induce mRNA degradation and suppress of translation (Bartel, 2004; Bartel, 2009). Many studies have showed that miRNAs played important roles in regulating milk production and milk composition synthesis. As report, miR-103, miR-17-5p, and miR-148a expressed in goat mammary epithelial cells enhanced milk fat synthesis by facilitating lipid droplet formation, whereas miR-181a, miR-34b, and miR-130a expressed in bovine mammary epithelial cells inhibited the synthesis of milk fat by downregulating the genes participated in lipid synthesis (Lin et al., 2013; Chen et al., 2017; Wang et al., 2019). However, few studies have examined the crop milk synthesis regulated by miRNAs. We identified the miRNAs associated ceRNA networks regulating crop milk production by transcriptome sequencing, and we found miR-193-5p was an important miRNAs in lactation which targeted PIK3CD (Ge et al., 2020; Ma et al., 2020).
MATERIALS AND METHODS
Fibrocytes Isolation, Culture, and Identification
Fibrocytes were obtained from the crop of pigeon. Crops were isolated from the incubated pigeon eggs at 17 d under sterile conditions. The tissues were rinsed with PBS and minced to 1 mm3 of pieces. The minced tissues were added to centrifuge tubes containing 0.25% pancreatic enzymes, then the tubes were put in a 37℃ waterbath and digested for 30 min. The tissue digests were mixed with DMEM (Gibco, Grand island, NY) and centrifuged 2 times (5 min, 1,000 r/min), then a 200-μm sterile nylon mesh filter was used to prepare cellular suspensions (Zhang et al., 2021). Cells were cultured in DMEM (Gibco) containing 100 IU/mL penicillin, 100 μg/mL streptomycin, and 10% fetal bovine serum (Gibco), incubated in a humidified incubator at 37℃ in 5% CO2, and identified by immunofluorescence (Galligan and Fish, 2017; Zhou et al., 2020).
Plasmid Construction
To construct dual-luciferase miRNA target reporter vector, the segment sequences of PIK3CD 3′-UTR that contained the putative miR-193-5p binding sequence were amplified by PCR, and then subcloned into XhoⅠand NotⅠrestriction sites in the psi-check-2 dual luciferase reporter vector. The wild-type and mutant PIK3CD 3′- UTRs were named as psi-check-wt and psi-check-mut, respectively. MiR-193-5p mimic and miR-193-5p inhibitor were designed and synthesized by Shanghai Generay (Shanghai, China). Interfering RNA (siRNA) against PIK3CD was synthesized by Shanghai Gima (Shanghai, China).
Cell Transfection and Dual-Luciferase Activity Assay
Lipofectamine LTX Reagent (Invitrogen Ligies, Carlsbad, CA) was used for cell transfection according to manufacturer's directions. Dual-luciferase reporter assays were performed as previously described (Cai et al., 2017; Ma et al., 2018). HEK293T cells were cotransfected with 100 ng of luciferase-PIK3CD mRNA 3′-UTRs constructs and with 50 nM of either miR-193-5p mimic or mimic NC together with the Renilla luciferase construct using Lipofectamine 3,000 (Invitrogen). After 48 h, HEK293T cells were collected, and the luciferase and Renilla luciferase activities were determined by Dual-Luciferase Reporter Assay System (Promega, Madison, WI) according to the manufacturer's instructions (Hou et al., 2020).
Cell Proliferation Assay
The cell proliferation assay was performed using the Cell Counting Kit-8 according to the manufacturer's instructions. A total of 2 × 104 fibrocytes cells per well were seeded into 96-well plates, and transfected with miR-193-5p mimic, mimic NC, miR-193-5p inhibitor, inhibitor NC, siRNA NC, siRNA PIK3CD, and siRNA FITC, respectively. The CCK-8 was added to each well (10 μL/well) at 24 h, 36 h, 48 h, 60 h, and 72 h post-transfection, and incubated at 37℃ for 2 h. The absorbance at 450 nm was measured using the microplate reader. All experiments were performed in triplicate (Han et al., 2016; Yang et al., 2020).
Migration Assay
A total of 1.5 × 105 cells/well were seeded in the 24-well plates. The migration ability of fibrocytes was detected at 24 h after transfection using wound healing assay as previous description (Jonkman et al., 2014). The gap was created by scratching a confluent monolayer with a pipette tip. Then the plates were put into the incubator at 37℃ for 24 h. Microscopy was used to monitor the status of migration into the cell-free area.
Statistical Analysis
Data were expressed as the means ± SD. All experiments were repeated at least 3 times. Statistical differences between 2 groups were determined by Student's t test and 1-way ANOVA was used to compare 3 or more groups. The value of P < 0.05 was considered to be statistically significant.
RESULTS
MiR-193-5p Targets PIK3CD and Inhibits Its Expression
By using TargetScan6.2 software prediction, we found the presence of miR-193-5p targeted at 3814-3833 nt at PIK3CD 3′–UTR (Figure 1). To clarify the relationship between miR-193-5p and PIK3CD gene, the luciferase reporter system was used. We constructed the wild-type and mutant plasmids. The wild-type PIK3CD reporter vector (PIK3CD-3′-UTR-wt), the mutant plasmids (PIK3CD-3′-UTR-mut), miR-193-5p mimic and mimic NC were co-transfected into HEK-293T cells. The luciferase assay showed that miR-193-5p significantly inhibited the relative luciferase activity of the PIK3CD-3′-UTR-wt, whereas the relative luciferase activity of the PIK3CD-3′-UTR-mut was not altered. Therefore, miR-193-5p could negatively regulated PIK3CD expression by targeted binding to the site in 3′-UTR region of PIK3CD (Figure 2).
Figure 1.
miR-193-5p binding sites. The specific binding sites of miR-193-5p in PIK3CD 3′-UTR, mutation sites were designed according to this sequence.
Figure 2.
The PIK3CD 3′-UTR was a target of miR-193-5p. miR-193-5p targeted PIK3CD directly as measured by luciferase reporter assays. Luciferase activities of PIK3CD-luc were markedly decreased in cells transfected with miR-193-5p compared to control. The values were means ± SD. *P < 0.05, **P < 0.01.
MiR-193-5p Promotes Proliferation of Pigeon Fibrocyte
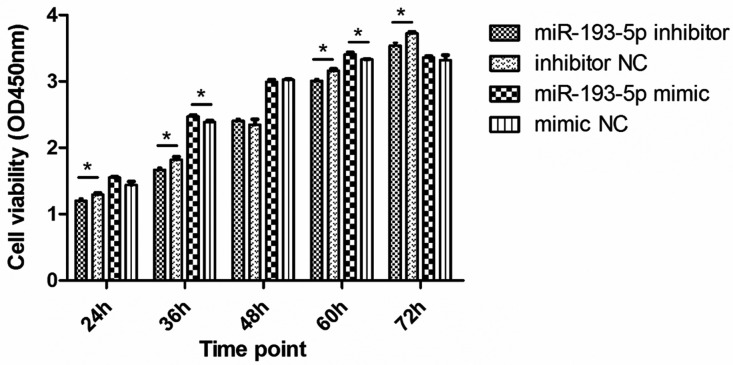
To evaluate the biological function of miR-193-5p, we used the CCK-8 assay to assess the effects of miR-193-5p on proliferation of pigeon fibrocyte. We transfected miR-193-5p mimic, a negative control (mimic NC), miR-193-5p inhibitor and a negative control (inhibitor NC) to the pigeon fibrocyte, then cell proliferation was measured. As shown in Figure 3, cells proliferation increased significantly in miR-193-5p mimic group compared with mimic NC group at 36 h and 60 h after transfection (P < 0.05), whereas cells proliferation decreased significantly in miR-193-5p inhibitor group compared with inhibitor NC group at 24 h, 36 h, 60 h, and 72 h after transfection (P < 0.05). The results suggested that miR-193-5p overexpression promoted cell proliferation, and miR-193-5p inhibition decreased cell proliferation.
Figure 3.
Effects of miR-193-5p on proliferation of pigeon crop fibroblasts. The cell proliferation assay was performed using the Cell Counting Kit-8. The fibrocytes cells were transfected with miR-193-5p mimic, mimic NC, miR-193-5p inhibitor, and inhibitor NC. The values were means ± SD. *P < 0.05, **P < 0.01.
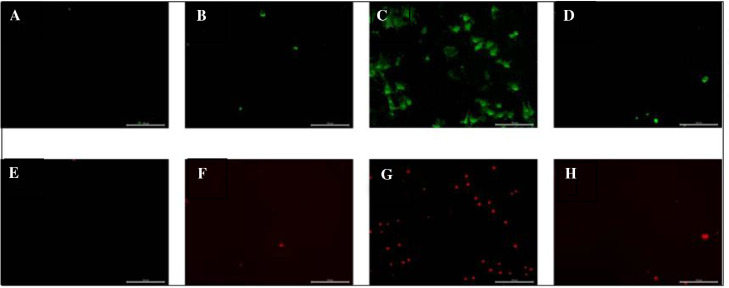
We used Annexin V-FITC/PI apoptosis kit to test apoptosis of pigeon fibrocyte transfected with miR-193-5p mimic, mimic NC, miR-193-5p inhibitor, and inhibitor NC. Cells transfected with miR-193-5p mimic and mimic NC didn't show apoptosis signal both in early and late detection, whereas cells transfected with miR-193-5p inhibitor showed significant apoptosis both in early and late detection (Figure 4).
Figure 4.
Effects of miR-193-5p on apoptosis of pigeon crop fibroblasts. (A) Transfected with mimic in 0 h; (B) transfected with mimic NC in 0 h; (C) transfected with inhibitor in 0 h; (D) transfected with inhibitor NC in 0 h; (E) transfected with mimic in 24 h; (F) transfected with mimic NC in 24 h; (G) transfected with inhibitor in 24 h; (H) transfected with inhibitor NC in 24 h.
Migration of pigeon fibrocyte transfected with these 4 vectors was tested by scratch assays in vitro. Compared with cells transfected with mimic NC, cells transfected with miR-193-5p mimic completely repaired after 24 h of scratch (Figure 5), whereas cells transfected with miR-193-5p inhibitor repaired slowly than that transfected with inhibitor NC, which was completely repaired after 24 h of scratch (Figure 5).
Figure 5.
Effects of miR-193-5p on wound healing repair of pigeon crop fibroblasts. (A) Transfected with mimic in 0 h; (B) transfected with mimic NC in 0 h; (C) transfected with inhibitor in 0 h; (D) transfected with inhibitor NC in 0 h; (E) transfected with mimic in 24 h; (F) transfected with mimic NC in 24 h; (G) transfected with inhibitor in 24 h; (H) transfected with inhibitor NC in 24 h.
PIK3CD Inhibits Proliferation of Pigeon Fibroblast Cell
To investigate the role of PIK3CD in proliferation of pigeon fibroblast cell, PIK3CD siRNA and a negative control (siRNA NC) were transfected in the pigeon fibroblast cell. The CCK-8 assay showed cells transfected with PIK3CD siRNA increased significantly than that transfected with siRNA NC at 24 h, 36 h, 48 h, 60 h, and 72 h after transfection (Figure 6). The scratch assays showed cells transfected with PIK3CD siRNA proliferated much quicker than that transfected with siRNA NC (Figure 7). All these results showed that PIK3CD could inhibit the proliferation of pigeon fibroblast cell.
Figure 6.
Effects of PIK3CD on proliferation of crop fibroblasts in pigeons. The cell proliferation assay was performed using the Cell Counting Kit-8. The fibrocytes cells were transfected with PIK3CD-siRNA and siRNA NC. The values were means ± SD. *P < 0.05, **P < 0.01.
Figure 7.
Effects of PIK3CD on wound healing repair of pigeon crop fibroblasts. (A) Transfected with PIK3CD-siRNA in 0 h; (B) transfected with siRNA NC in 0 h; (C) transfected with PIK3CD-siRNA in 24 h; (D) transfected with siRNA NC in 24 h.
DISCUSSION
Although much progress has been made in investigating the regulatory mechanism of crop milk production (Bartel, 2004; Lin et al., 2013; Chen et al., 2017; Zhang et al., 2020), the role of gene regulation is still poorly understood. miRNA plays an important role in regulating tissue growth and metabolism acting as an endogenous post-transcriptional gene regulator, and it always negatively regulates the translation of specific target genes by binding to their 3′-UTRs (Bartel, 2009). However, compared to other species, little is known about the function of miRNAs in crop milk production. In previous study, we identified miRNA associated ceRNA networks regulating crop milk production, and 71 miRNAs were identified differentially expressed. MiR-193-5p was predicted as an important ceRNA located in the central position of many regulatory axes affecting lactation, and PIK3CD gene was predicted as a potential target of miR-193-5p (Ge et al., 2020). Lactating crops showed a significant high-level expression of miR-193-5p compared with non-lactating crops by small RNA sequencing (P < 0.01), whereas in lactating crops, the expression of PIK3CD was significantly lower than in non-lactating crops (P < 0.01, Ge et al., 2020).
In the present study, we researched the targeting relationship of miR-193-5p and PIK3CD, and assessed the function of miR-193-5p in fibrocyte proliferation and crop milk production. miRNAs regulate post-transcriptional gene expression by binding to the 3′-UTR of target genes (Rashidi et al., 2022). miR-193a generate 2 arms including 3P and 5P, also known as dominant arm and passenger arm, respectively (Wang et al., 2019; Azar et al., 2021). Dysregulation of miR-193-3P and miR-193-5P could cause many diseases such as cancer (Lv et al., 2014; Wang et al., 2019), Parkinson and chronic myeloid leukaemia (Dong et al., 2016; Prinsloo et al., 2017). However, few reports described their functions in nutrition synthesis, and especially the function of miR-193-5p in regulation of crop milk production has remained unknown. Here, we first analyzed the expression of miR-193-5p and PIK3CD in lactating and non-lactating crops, and their regulation of pigeon crop fibrocyte in vitro.
We found that expression of miR-193-5p was upregulated in the lactating crop compared with the non-lactating crop, and miR-193-5p promoted crop fibrocyte proliferation in vitro. Similarly, Fan et al. (2021) reported that miR-193a-5P could promote the synthesis of polyunsaturated fatty acids (PUFAs) by targeting fatty acid desaturase 1 (FADS1) in bovine mammary epithelial cells, and miR-193a-5P expression was positively correlated with the expression of genes associated with milk fatty acid metabolism, including RLOVL fatty acid elongase (ELOVL6) and diacylglycerol O-acyltransferase 2 (DGAT2) (Fan et al., 2021). Lactation is a complex activity regulated by endocrine regulation and changes in gene activity, and the activity is accompanied with various cellular processes as proliferation, differentiation, survival, and apoptosis (Dysin et al., 2021). As previous report, many studies have shown that mammary gland development was controlled by miRNAs by regulating proliferation and apoptosis of mammary epithelial cells, as well as the formation of mammary ducts and acinus (Avril-Sassen et al., 2009; Shimono et al., 2009). The number of epithelial cells influenced the dairy yield of milk (Boutinaud et al., 2004), and in pigeon the lactating crop was also accompanied by extensive proliferation and folding of the germinal epithelium (Gillespie et al., 2011). So our results verified that miR-193-5p could accelerate the proliferation of crop fibrocyte and process crop milk production.
To further validate the relationship of miR-193-5p and its predicted target gene PIK3CD, the luciferase activity assay was used. The results suggested that miR-193-5p negatively impacted PIK3CD by targeting the 3′-UTR sequence of PIK3CD. PI3Ks are a family of lipid kinases regulating variety of biological responses in different cells (Li et al., 2015). PIK3CD, a subunit of PI3K, has mostly been found to regulate progression of cancer and various metabolic disease (Fang et al., 2012; Tzenaki and Papakonstanti, 2013; Shi et al., 2014). The reports of PIK3CD functions were not consistent. Most of the results showed it could promote cell proliferation, whereas only few reported it suppressed cell proliferation. Xie et al. (2020) showed miR-224 could suppress diffuse large B-cell lymphoma (DLBCL) cells by targeted inhibition of PIK3CD, which could promote proliferation of HBL1, a DLBCL cell line (Xie et al., 2020). Chen et al. (2019) showed that PIK3CD induced cell growth and invasion in colorectal cancer by activating AKT/GSK-3β/β-catenin signaling, but the studies of Song et al. (2019) suggested that overexpression of PIK3CD-AS1 inhibited proliferation, colony formation, and cell cycle progression of hepatocellular carcinoma (HCC) cells (Chen et al., 2019). However, little is known about the function of PIK3CD in mammary glands development and metabolism of milk. We first reported PIK3CD suppressed crop fibrocyte proliferation as the targeted gene of miR-193-5p.
In conclusion, we found miR-193-5p was upregulated in lactating pigeon crop and it promoted crop fibrocytes proliferation and migration, which indicated its promotion of crop milk production. PIK3CD was proved to be a functional target of miR-193-5p, which was negatively regulated by miR-193-5p. PIK3CD was downregulated in lactating pigeon crop and it inhibited cell crop fibrocytes proliferation and migration. The results provided novel insights into the regulation of crop milk production by miRNAs.
ACKNOWLEGMENTS
This study was funded by Beijing Innovation Consortium of Agriculture Research System (BAIC06-2022); National Natural Science Foundation of China (31802058); Agricultural Science and Technology Innovation Program (ASTIPIAS04). The authors would like to thank all members of this work for their advice and technical.
DISCLOSURES
The authors declare that there is no conflict of interest.
REFERENCES
- Avril-Sassen S., Goldstein L.D., Stingl J., Blenkiron C., Le Quesne J., Spiteri I., Karagavriilidou K., Watson C.J., Tavaré S., Miska E.A., Caldas C. Characterisation of microRNA expression in post-natal mouse mammary gland development. BMC Genomics. 2009;10:548–562. doi: 10.1186/1471-2164-10-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azar M., Aghazadeh H., Mohammed H.N., Sara M., Hosseini A., Shomali N., Tamjidifar R., Tarzi S., Mansouri M., Sarand S.P., Marofi F., Akbari M., Xu H., Shotorbani S.S. miR-193a-5p as a promising therapeutic candidate in colorectal cancer by reducing 5-FU and Oxaliplatin chemoresistance by targeting CXCR4. Int. Immunopharmacol. 2021;92 doi: 10.1016/j.intimp.2020.107355. [DOI] [PubMed] [Google Scholar]
- Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Bartel D.P. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilanges B., Posor Y., Vanhaesebroeck B. PI3K isoforms in cell signalling and vesicle trafficking. Nat. Rev. Mol. Cell Biol. 2019;20:515–534. doi: 10.1038/s41580-019-0129-z. [DOI] [PubMed] [Google Scholar]
- Boutinaud M., Guinard-Flamenta J., Jammes H. The number and activity of mammary epithelial cells, determining factors for milk production. Reprod. Nutr. Dev. 2004;44:499–508. [PubMed] [Google Scholar]
- Cai B., Li Z., Ma M., Wang Z., Han P., Abdalla B.A., Nie Q., Zhang X. LncRNA-Six1 encodes a micropeptide to activate Six1 in Cis and is involved in cell proliferation and muscle growth. Front. Physiol. 2017;8:230. doi: 10.3389/fphys.2017.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.J., Fu Z., Jiang S.G., Wang X.Q., Yan H.C., Gao C.Q. Targeted disruption of TORC1 retards young squab growth by inhibiting the synthesis of crop milk protein in breeding pigeon (Columba livia) Poult. Sci. 2020;99:416–422. doi: 10.3382/ps/pez513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.S., Huang J.Q., Luo B., Dong S.H., Wang R.C., Jiang Z.K., Xie Y.K., Yi W., Wen G.M., Zhong J.F. PIK3CD induces cell growth and invasion by activating AKT/GSK-3β/β-catenin signaling in colorectal cancer. Cancer Sci. 2019;110:997–1011. doi: 10.1111/cas.13931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Luo J., Sun S., Cao D., Shi H., Loor J.J. miR-148a and miR-17-5p synergistically regulate milk TAG synthesis via PPARGC1A and PPARA in goat mammary epithelial cells. RNA Biol. 2017;14:326–338. doi: 10.1080/15476286.2016.1276149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H., Wang C., Lu S., Yu C., Huang L., Feng W., Xu H., Chen X., Zen K., Yan Q., Liu W., Zhang C., Zhang C.Y. A panel of four decreased serum microRNAs as a novel biomarker for early Parkinson's disease. Biomarkers. 2016;21:129–137. doi: 10.3109/1354750X.2015.1118544. [DOI] [PubMed] [Google Scholar]
- Du X., Li X., Chen L., Zhang M., Lei L., Gao W., Shi Z., Dong Y., Wang Z., Li X., Liu G. Hepatic miR-125b inhibits insulin signaling pathway by targeting PIK3CD. J. Cell Physiol. 2018;233:6052–6066. doi: 10.1002/jcp.26442. [DOI] [PubMed] [Google Scholar]
- Dumont J.N. Prolactin-induced cytologic changes in the mucosa of the pigeon crop during crop- “milk” formation. Z. Zellforsch. Mikrosk. Anat. 1965;68:755–782. doi: 10.1007/BF00343930. [DOI] [PubMed] [Google Scholar]
- Dysin A.P., Barkova O.Y., Pozovnikova M.V. The role of microRNAs in the mammary gland development, health, and function of cattle, goats, and sheep. Noncoding RNA. 2021;7:78–115. doi: 10.3390/ncrna7040078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y., Arbab A., Zhang H., Yang Y., Lu X., Han Z., Yang Z. MicroRNA-193a-5p regulates the synthesis of polyunsaturated fatty acids by targeting fatty acid desaturase 1 (FADS1) in bovine mammary epithelial cells. Biomolecules. 2021;11(2):157–174. doi: 10.3390/biom11020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y., Xue J.L., Shen Q., Chen J., Tian L. MicroRNA-7 inhibits tumor growth and metastasis by targeting the phosphoinositide 3-kinase/Akt pathway in hepatocellular carcinoma. Hepatology. 2012;55:1852–1862. doi: 10.1002/hep.25576. [DOI] [PubMed] [Google Scholar]
- Galligan C.L., Fish E.N. Interleukin-34 promotes fibrocyte proliferation. J. Interferon Cytokine Res. 2017;37:440–448. doi: 10.1089/jir.2017.0063. [DOI] [PubMed] [Google Scholar]
- Ge P., Ma H., Li Y., Ni A., Isa A.M., Wang P., Bian S., Shi L., Zong Y., Wang Y., Jiang L., Hagos H., Yuan J., Sun Y., Chen J. Identification of microRNA-associated-ceRNA networks regulating crop milk production in pigeon (Columba livia) Genes (Basel). 2020;12:39–57. doi: 10.3390/genes12010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie M.J., Crowley T.M., Haring V.R., Wilson S.L., Harper J.A., Payne J.S., Green D., Monaghan P., Stanley D., Donald J.A., Nicholas K.R., Moore R.J. Transcriptome analysis of pigeon milk production—role of cornification and triglyceride synthesis genes. BMC Genomics. 2013;14:169–182. doi: 10.1186/1471-2164-14-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie M.J., Haring V.R., McColl K.A., Monaghan P., Donald J.A., Nicholas K.R., Moore R.J., Crowley T.M. Histological and global gene expression analysis of the 'lactating' pigeon crop. BMC Genomics. 2011;12:452–460. doi: 10.1186/1471-2164-12-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B., Lian L., Li X., Zhao C., Qu L., Liu C., Song J., Yang N. Chicken gga-miR-103-3p targets CCNE1 and TFDP2 and inhibits MDCC-MSB1 cell migration. G3 (Bethesda). 2016;6:1277–1285. doi: 10.1534/g3.116.028498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L., Hannon G.J. MicroRNAs: small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- Hou J., Zhuo H., Chen X., Cheng J., Zheng W., Zhong M., Cai J. MiR-139-5p negatively regulates PMP22 to repress cell proliferation by targeting the NF-κB signaling pathway in gastric cancer. Int. J. Biol. Sci. 2020;16:1218–1229. doi: 10.7150/ijbs.40338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkman J.E., Cathcart J.A., Xu F., Bartolini M.E., Amon J.E., Stevens K.M., Colarusso P. An introduction to the wound healing assay using live-cell microscopy. Cell Adh. Migr. 2014;8:440–451. doi: 10.4161/cam.36224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriplani N., Hermida M.A., Brown E.R., Leslie N.R. Class I PI 3-kinases: function and evolution. Adv. Biol. Regul. 2015;59:53–64. doi: 10.1016/j.jbior.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Li J., Zhou J., Zhang D., Song Y., She J., Bai C. Bone marrow-derived mesenchymal stem cells enhance autophagy via PI3K/AKT signalling to reduce the severity of ischaemia/reperfusion-induced lung injury. J Cell Mol. Med. 2015;19:2341–2351. doi: 10.1111/jcmm.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Luo J., Zhang L., Wang W., Gou D. MiR-103 controls milk fat accumulation in goat (Capra hircus) mammary gland during lactation. PLoS One. 2013;8:e79258. doi: 10.1371/journal.pone.0079258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv L., Deng H., Li Y., Zhang C., Liu X., Liu Q., Zhang D., Wang L., Pu Y., Zhang H., He Y., Wang Y., Yu Y., Yu T., Zhu J. The DNA methylation-regulated miR-193a-3p dictates the multi-chemoresistance of bladder cancer via repression of SRSF2/PLAU/HIC2 expression. Cell Death Dis. 2014;5:e1402. doi: 10.1038/cddis.2014.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M., Cai B., Jiang L., Abdalla B.A., Li Z., Nie Q., Zhang X. lncRNA-Six1 is a target of miR-1611 that functions as a ceRNA to regulate Six1 protein expression and fiber type switching in chicken myogenesis. Cells (Basel). 2018;7:243–258. doi: 10.3390/cells7120243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H., Ni A., Ge P., Li Y., Shi L., Wang P., Fan J., Isa A.M., Sun Y., Chen J. Analysis of long non-coding RNAs and mRNAs associated with lactation in the crop of pigeons (Columba livia) Genes (Basel). 2020;11:201–214. doi: 10.3390/genes11020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennisi E. Cell biology. Lengthy RNAs earn respect as cellular players. Science. 2014;344:1072. doi: 10.1126/science.344.6188.1072. [DOI] [PubMed] [Google Scholar]
- Prinsloo A., Pool R., Van Niekerk C. Preliminary data on microRNA expression profiles in a group of South African patients diagnosed with chronic myeloid leukaemia. Mol. Clin. Oncol. 2017;7:386–390. doi: 10.3892/mco.2017.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondi C., Falasca M. Phosphoinositides signalling in cancer: focus on PI3K and PLC. Adv. Biol. Regul. 2012;52:166–182. doi: 10.1016/j.advenzreg.2011.09.016. [DOI] [PubMed] [Google Scholar]
- Rashidi S., Mansouri R., Ali-Hassanzadeh M., Ghani E., Karimazar M., Muro A., Nguewa P., Manzano-Román R. miRNAs in the regulation of mTOR signaling and host immune responses: the case of Leishmania infections. Acta. Trop. 2022;231 doi: 10.1016/j.actatropica.2022.106431. [DOI] [PubMed] [Google Scholar]
- Shi Y., Chen C., Zhang X., Liu Q., Xu J.L., Zhang H.R., Yao X.H., Jiang T., He Z.C., Ren Y., Cui W., Xu C., Liu L., Cui Y.H., Yu S.Z., Ping Y.F., Bian X.W. Primate-specific miR-663 functions as a tumor suppressor by targeting PIK3CD and predicts the prognosis of human glioblastoma. Clin. Cancer Res. 2014;20:1803–1813. doi: 10.1158/1078-0432.CCR-13-2284. [DOI] [PubMed] [Google Scholar]
- Shimono Y., Zabala M., Cho R.W., Lobo N., Dalerba P., Qian D., Diehn M., Liu H., Panula S.P., Chiao E., Dirbas F.M., Somlo G., Pera R.A., Lao K., Clarke M.F. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138:592–603. doi: 10.1016/j.cell.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P., Dar M.S., Dar M.J. p110α and p110β isoforms of PI3K signaling: are they two sides of the same coin? Febs. Lett. 2016;590:3071–3082. doi: 10.1002/1873-3468.12377. [DOI] [PubMed] [Google Scholar]
- Song W., Zhang J., Zhang J., Sun M., Xia Q. Overexpression of lncRNA PIK3CD-AS1 promotes expression of LATS1 by competitive binding with microRNA-566 to inhibit the growth, invasion and metastasis of hepatocellular carcinoma cells. Cancer Cell Int. 2019;19:150–164. doi: 10.1186/s12935-019-0857-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Thorpe L.M., Yuzugullu H., Zhao J.J. PI3K in cancer: divergent roles of isoforms, modes of activation and therapeutic targeting. Nat. Rev. Cancer. 2015;15:7–24. doi: 10.1038/nrc3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzenaki N., Papakonstanti E.A. p110δ PI3 kinase pathway: emerging roles in cancer. Front Oncol. 2013;3:40–54. doi: 10.3389/fonc.2013.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan X.P., Xie P., Bu Z., Zou X.T., Gong D.Q. Prolactin induces lipid synthesis of organ-cultured pigeon crops. Poult. Sci. 2019;98:1842–1853. doi: 10.3382/ps/pey540. [DOI] [PubMed] [Google Scholar]
- Wang Y., Guo W., Tang K., Wang Y., Zan L., Yang W. Bta-miR-34b regulates milk fat biosynthesis by targeting mRNA decapping enzyme 1A (DCP1A) in cultured bovine mammary epithelial cells. J. Anim. Sci. 2019;97:3823–3831. doi: 10.1093/jas/skz230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Shen X.B., Jia W., Huang D.B., Wang Y., Pan Y.Y. The p53/miR-193a/EGFR feedback loop function as a driving force for non-small cell lung carcinoma tumorigenesis. Ther. Adv. Med. Oncol. 2019;11 doi: 10.1177/1758835919850665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W.Y., Fu Z., Pan N.X., Yan H.C., Wang X.Q., Gao C.Q. Leucine promotes the growth of squabs by increasing crop milk protein synthesis through the TOR signaling pathway in the domestic pigeon (Columba livia) Poult. Sci. 2019;98:5514–5524. doi: 10.3382/ps/pez296. [DOI] [PubMed] [Google Scholar]
- Xie Y., Tan L., Li Y.T., Zeng Y. Mechanism of MiR-224 affecting DLBCL cell proliferation and invasion by targeted inhibition of PIK3CD. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2020;28:1578–1584. doi: 10.19746/j.cnki.issn.1009-2137.2020.05.025. [DOI] [PubMed] [Google Scholar]
- Xie P., Wang X.P., Bu Z., Zou X.T. Differential expression of fatty acid transporters and fatty acid synthesis-related genes in crop tissues of male and female pigeons (Columba livia domestica) during incubation and chick rearing. Br. Poult. Sci. 2017;58:594–602. doi: 10.1080/00071668.2017.1357798. [DOI] [PubMed] [Google Scholar]
- Yang L., Lv Q., Liu J., Qi S., Fu D. miR-431 regulates granulosa cell function through the IRS2/PI3K/AKT signaling pathway. J. Reprod. Dev. 2020;66:231–239. doi: 10.1262/jrd.2019-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Wu Z.Q., Wang Y.J., Wang M., Yang W.C. MiR-143 regulates milk fat synthesis by targeting Smad3 in bovine mammary epithelial cells. Animals (Basel). 2020;10:1453–1463. doi: 10.3390/ani10091453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Dong X., Hou L., Cao Z., Zhu G., Vongsangnak W., Xu Q., Chen G. Identification of differentially expressed non-coding RNA networks with potential immunoregulatory roles during Salmonella enteritidis infection in Ducks. Front. Vet. Sci. 2021;8 doi: 10.3389/fvets.2021.692501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Zhao Y., Li Z., Zhu M., Wang Z., Li Y., Xu T., Feng D., Zhang S., Tang F., Yao J. miR-103a-3p regulates mitophagy in Parkinson's disease through Parkin/Ambra1 signaling. Pharmacol. Res. 2020;160 doi: 10.1016/j.phrs.2020.105197. [DOI] [PubMed] [Google Scholar]