Abstract
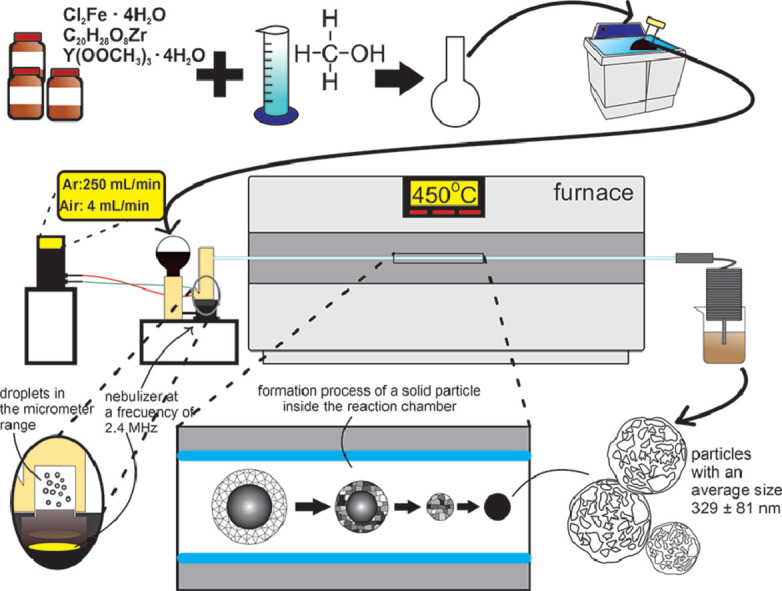
This work details the production of solid composite particles by the aerosol-assisted chemical vapor deposition method. With this method it is feasible to produce at low temperature (450 °C) the tetragonal phase of zirconium oxide stabilizing it with yttrium oxide (YSZ) and cubic iron oxide (Fe3O4) at the same time. The particles have a solid morphology in which both metal oxides coexist without mixing. The average size of the obtained particles is 329 ± 81 nm, moreover, each particle is formed by thousands of crystallites of size 2 ± 0.5 nm. The formation of solid structures is due to the amount of Zr and Y found in each particle. These particles can be applied as reinforcements of metallic structures.
-
•
A simple and low-cost method for producing composite particles to be applied as reinforcing agents for metal structures.
-
•
The particles are formed by two phases of tetragonal yttria-stabilized zirconia (t-YSZ) and cubic Fe3O4, which was synthesized following a one-step process via the aerosol-assisted chemical vapor deposition method (AACVD).
-
•
The tetragonal phase of ZrO2 is obtained at 450 °C stabilizing it with ∼3.8% of yttrium oxide.
Keywords: AACVD, Aerosol assisted-CVD, t-YSZ-Fe3O4 particles, Solid particles
Method name: Aerosol-assisted CVD method for the synthesis of solid particles of t-YSZ-Fe3O4
Graphical abstract
Specifications table
| Subject Area: | Materials Science |
| More specific subject area: | Synthesis and characterization of nanostructured materials, particularly metal oxides. |
| Protocol name: | Aerosol-assisted CVD method for the synthesis of solid particles of t-YSZ-Fe3O4 |
| Reagents/tools: | |
| Experimental design: | The synthesis of nanostructured solid particles of zirconium oxide stabilized with yttrium oxide and magnetite by the AACVD technique is described in detail; the system and all its accessories are shown part by part. The obtaining of these particles by a simple methodology and relatively easy to install in a laboratory is explained step by step. |
| Trial registration: | Not applicable |
| Ethics: | Not applicable |
Value of the protocol
-
•
AACVD method is a low-cost, easily manipulated, simple and scalable, without the need for complex infrastructure.
-
•
The composite material is produced in a single step, continuously without requiring a vacuum system, process control agents, additives, templates, or pH controllers.
-
•
The tetragonal phase of zirconium oxide is obtained at a low temperature, around 450 °C.
Description of protocol
Details and fundamentals of the AACVD method
Aerosol-assisted chemical vapor deposition (AACVD) is one of the physicochemical methods that allows for obtaining nanostructured materials with reproducibility and controlled characteristics [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17] which is a variant of the conventional CVD process. This method is mostly used for the synthesis of thin films and powders and also because the formation of nanostructures takes place at or near atmospheric pressure, and usually at ambient air. With this technique it is possible to obtain nanostructures with different geometries such as thin films [6,11], nanorods [8,9], nanowires [9,16], nano-alloy films [15], nanoparticles [1,2,17] for different and diverse applications in photocatalysis [12,13], production of hydrogen [15,16], adsorption processes and water treatment [1,2,14,17], as reinforcements of metallic structures [4,5], in solar cells as selective absorbing materials [10] and transparent conductive materials [7].The materials synthesized by AACVD are produced in a single step, in a continuous and relatively simple way, unlike other techniques that fabricate similar nanostructures in several stages, require a larger amount of reagents, pH control agents and longer synthesis time [18], [19], [20]. Thus, automatically reducing the cost in terms of infrastructure investment, reduction of energy costs, extra reagents, time, etc.
In this case, precursor solutions composed of organometallic or inorganic salts dissolved in organic or aqueous solvents are used to synthesize particles. The technique consists of producing a cloud of the precursor solution, which is transported by a vector gas or gas mixture to the interior of a tubular reactor, located inside a temperature-controlled chamber (furnace). Due to the temperature of the reactor, the decomposition of the precursor's salts and the formation of the desired material takes place. Directly connected to the tubular reactor at the exit of the chamber a collection device is used to collect the powder, which consists of a coil that helps to diminish the velocity of the formed particles and also cools the carrier gas flow with the material to be collected in a beaker with a recollection liquid. The temperature of the furnace is the fundamental parameter that provides the thermal energy necessary to control the synthesis of the material. This temperature depends on the material to be obtained and the precursors to be used.
The synthesis process begins when the cloud of droplets enters the tubular reactor and is heated in the reaction chamber. The following steps must occur successively: (a) evaporation of the solvent; (b) nucleation of precursor salts; (c) melting, or eventual sublimation of the precursor compound; (d) evaporation or thermal decomposition, (e) chemical reaction of the elements of interest and the formation of the required material. This methodology allows obtaining powders in the form of particles with hollow or solid morphology. The composition of the precursor solution plays an important factor in the morphology of the resulting material. The physical basis for the generation of hollow or solid particles is reported in detail in [5,17,[21], [22], [23].
Description of the experimental AACVD system
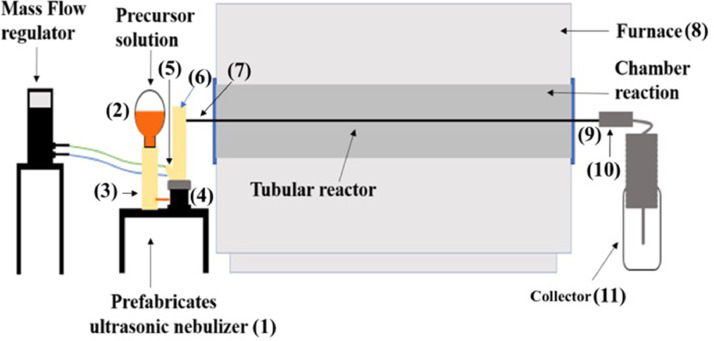
In this section, a detailed description of an AACVD system in the synthesis of the particles with the details of the components as well as how they are interconnected. Fig. 1 shows a schematic diagram of the AACVD system used to produce the t-YSZ-Fe3O4 particles. The principal components of the system are: (a) the nebulizer (1), (b) a tubular furnace (8), and (c) the particle collection device (11).
-
(a)
The nebulizer is of the ultrasonic type and operates at a frequency of 2.4 MHz. The principal advantage of this type of nebulizer is that it provides a narrow size distribution of the precursor solution droplets (diameter around 2 μm) from which the particles will be synthesized. With a narrow size distribution of the droplets, an optimal deposition condition (such as deposition temperature, carrier gas type and flow, and precursor solution composition) can be attained for these droplets of a particular diameter. Otherwise, with a wide size distribution of the droplets, the optimal deposition conditions are different for droplets of very different sizes. In this case, the synthesis will develop under non-optimal conditions for many sizes of the droplets, only droplets of around some particular size will have favorable conditions to produce the desired material with the required microstructure and properties. The nebulizer consists of a precursor solution container (3) and its level regulator (2), a nebulization chamber (4), an inlet of the carrier gas (5) which is controlled, by a mass flow regulator (6), and finally a coupling (7) with the borosilicate tube, which is inserted inside the tubular furnace. The core of this device is inside the nebulization chamber (4), which is filled with the precursor solution, and in contact with a piezoelectric crystal, located at the bottom of the chamber. The piezoelectric vibrate at a high frequency (∼ MHz) generating ultrasonic waves within the volume of the precursor solution. The geometry of the chamber causes a concentration of the ultrasonic waves at a certain distance from the piezoelectric surface, the optimum generation of droplets occurs if the surface of the solution coincides with the distance where the concentration of the waves takes place. Under these circumstances, thousands of micrometric droplets will be generated at the surface of the solution; the size of the droplets depends on the frequency of the vibration, density and surface tension of the solution. Finally, the cloud of droplets is conveyed by the carrier gas (5) and injected into the borosilicate tube (7).
-
(b)
The tubular furnace (8). The borosilicate tube or tubular reactor (7) is introduced inside of the tubular furnace (8). The tubular reactor must exit from the front and rear of the furnace to allow coupling with the nebulizer (7) and the collection system (9), respectively.
-
(c)
The particle collection device (11) consists of a stainless-steel tubular coil (10), connected on one side to the outlet of the tubular reactor (9) and the other end is immersed into some appropriate liquid inside the beaker (11). In the case of the t-YSZ-Fe3O4, the beaker contains tri-distilled water. Another important function of the liquid inside of the collector flask is that it does not allow the oxygen from the environment to pass into the tubular reactor; it only allows the exit of the system's product gasses, constituting what is called a liquid trap.
Fig. 1.
Schematic diagram of the AACVD system, showing the principal components required to synthesize the particles.
Once the particles are obtained, they are washed with tri-distilled water and centrifuged to obtain the material using an ultrasonic sonicator (Cole Parmer), a Clinical 200 centrifuge (VWR) and a microcentrifuge (Thermoscientific) respectively. Subsequently, the powder is dried and stored in a desiccator for future application.
Fig. 2 shows the components of the actual AACVD system, the mass flow controllers (Fig. 2a) which are connected to the nebulizer (Fig. 2b), and the collector system (Fig. 2c). It is worth mentioning that this laboratory-level AACVD system does not require a complex infrastructure to be implemented (such as a vacuum system, process control agents, or plasma systems), making it a low-cost, easily controlled, relatively simple, method for producing high quality oxides and noble metals, and additionally scalable at an industrial level.
Fig. 2.
The fundamental components of the actual AACVD system. (a) The mass flow regulators. (b) The self-fabricated ultrasonic nebulizer and the precursor solution container are already assembled with the tubular reactor inserted inside the furnace. (c) The furnace outlet shows the tubular reactor coupled with the collecting system.
Materials and equipment necessary to synthesize the particles of t-YSZ-Fe3O4
Table 1 shows the reagents needed for the synthesis of t-YSZ-Fe3O4
Table 1.
Reagents used for the synthesis of the t-YSZ-Fe3O4.
| Reagent | Chemical formula | Molecular weight | Supplier | CAS |
|---|---|---|---|---|
| Tridistilled water | H2O | 18 | J.T. Baker | 7732-18-5 |
| Air gas | – | – | – | – |
| Argon gas, grade 4.8 | Ar | 39.95 | Praxair | – |
| Methanol | CH3 · OH | 32 | J.T. Baker | 67-56-1 |
| Iron chloride tetrahydrate | Cl2Fe · 4H2O | 198.81 | Sigma Aldrich | 13478-10-9 |
| Zirconium acetylacetonate | C20H28O8Zr | 487.66 | Sigma Aldrich | 17501-44-9 |
| Yttrium acetate | Y(OOCH3)3 · 4H2O | 338.1 | Alfa Aesar | 23363-14-6 |
Experimental procedure
-
1.
The synthesis begins with the preparation of the precursor solution containing the three salts of the elements of interest: zirconium (Zr), Y (yttrium), and (Fe) iron (see Table 1). The salts are dissolved in methanol. The final molar concentration of the precursor solution is 0.1 mol.dm−3 with a stoichiometric ratio of Fe:Zr:Y of 60:39:1 respectively. It is very important to obtain the total dissolution of the three precursor salts used since it is a determining factor for obtaining homogeneous materials both in composition and morphology.
-
2.
Then the three parts of the AACVD system are coupled together: nebulizer-furnace/tubular reactor-collector system, it is important that the couplings are completely airtight, to avoid the intake of atmospheric air or possible leakage of the droplet cloud that influences the composition of the synthesized material.
-
3.
Following the formulation of the precursor solution and assembling of the system, the precursor solution is placed inside the solution container and nebulizer chamber.
-
4.
Subsequently, the introduction of the carrier gas mixture is started. The carrier gas is composed of a mixture of an inert gas (argon) and an oxidizing gas (filtered clean and dry air). The flows of the inert and oxidizing gas must be controlled with precision by employing mass flow regulators. The flow rates are 250 mL/min for the argon gas and 4 mL/min for the oxidizing gas (air). The composition and flow rate of the aerosol carrier gas is an important parameter since it allows to control of the nature and microstructural characteristics of the material to be obtained, this means generating the metal oxide phases of interest, in this case, t-YSZ-Fe3O4.
-
5.
Subsequently, the tubular furnace temperature is set at 450 °C.
-
6.
Once the synthesis conditions mentioned above are reached, the nebulization of the precursor solution is started in the nebulizer, simultaneously the aerosol cloud is dragged by the carrier gas and introduced into the tubular reactor, being heated inside the tubular furnace. With heating, important stages occur that will determine the morphology and final structure of the materials, one of them is the evaporation of the drop where physical processes are involved, such as the diffusion rate of the solute and the evaporation rate of the solvent, which will allow the physical and chemical transformations that generate the t-YSZ-Fe3O4 particles, phenomena already detailed in [5,17,[21], [22], [23].
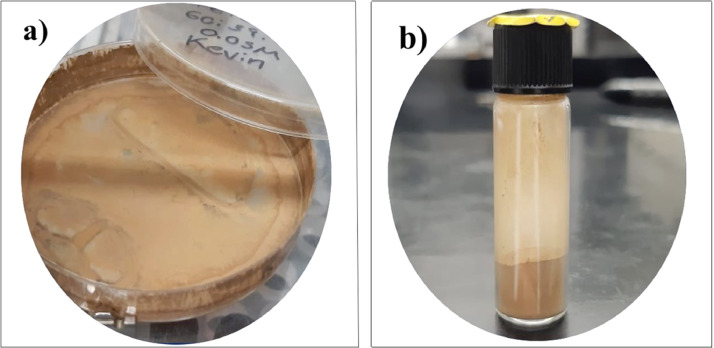
The particles generated continue to be transported by the flow of the carrier gas towards the outside of the furnace and introduced into the collection liquid of the recovery system. Once the nebulization has started, the synthesis time is continued until the desired amount of the t-YSZ-Fe3O4 particles is obtained. As supplementary material, video 1 is presented, which shows the AACVD system in real operation. At the end of the process, the equipment is turned off, the collection container is removed, and the particles obtained by centrifugation are recovered, collected in a Petri dish, placed in a desiccator for drying, and then deposited in a vial with a screw cap for storage. In addition, Fig. 3 shows the powder obtained with a brown coloration deposited in a petri dish after centrifugation and drying and then placed in a glass vial with a screw cap for storage.
Fig. 3.
Powders (t-YSZ-Fe3O4 particles) recollected after synthesis. (a) The particles are collected in a Petri dish for subsequent drying. (b) Then particles are stored in a glass vial with a screw cap for further use.
Finally, Fig. 4a, shows a micrograph obtained by SEM, which presents the morphology of the particles, and inserted in Fig. 4a, there is a table showing the elemental composition in atomic percentage, the presence of the elements of interest Zr, Y, Fe and O is observed. The Fig. 4b, shows the diffraction pattern indicating the crystalline phases obtained, YSZ in tetragonal phase (JCPDF 00–083–0113; 01–081–1317) [24], Fe3O4 (JCPDF 019–0629) [24], traces of Fe2O3 (JCPDF 01–088–2359) [24] and Si, the substrate where the sample was prepared for analysis) [5].
Fig. 4.
(a) SEM micrograph showing the solid morphology of the particles, the elemental composition is inserted in the figure [5]. (b) Shows the diffraction pattern and the crystalline phases present in the material obtained [5].
Method validation
A powdered composite material comprised of mainly t-YSZ and cubic Fe3O4 was obtained using the reproducible AACVD synthesis method. Noteworthy, the material was synthesized in one step, at relatively low temperature (450 °C), and without the utilization of templates or the addition of pH control reagents, additives, and subsequent high-temperature annealing steps. The chemical reaction and the formation of the solid composite particles occur optimally at the established temperature. The performance of the AACVD equipment is very stable, as long as necessary maintenance services are provided. The efficiency of the system in a continuous operation is around 11 ± 1 mg h−1 of particles. The particles exhibited a solid-like structure with an irregular morphology at the surface. The experience in working with this AACVD system and method has been more than a decade. The validation of the method occurs when obtaining the nanostructured powder, with the characteristics and chemical phases proposed at the beginning.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.mex.2022.101973.
Appendix. Supplementary materials
Data Availability
No data was used for the research described in the article.
References
- 1.Monárrez-Cordero B.E., Amézaga-Madrid P., Sáenz-Trevizo A., Pizá-Ruiz P., Antúnez-Flores W., Miki-Yoshida M. Synthesis and characterization of composite Fe-Ti oxides nanoparticles with high surface area obtained via AACVD. Ceram. Int. 2018;44:6990–6996. doi: 10.1016/j.ceramin.2018.01.132. [DOI] [Google Scholar]
- 2.Hernández-Salcedo P.G., Amézaga-Madrid P., Monárrez-Cordero B.E., Pizá-Ruiz P., Antúnez-Flores W., Leyva-Porras C., Ornelas-Gutiérrez C., Miki-Yoshida M. Theoretical and experimental influence of aerosol assisted CVD parameters on the microstructural properties of magnetite nanoparticles and their response on the removal efficiency of arsenic. J. Alloy. Compd. 2014;643(1):287–296. doi: 10.1016/j.jallcom.2014.12.064. [DOI] [Google Scholar]
- 3.Cho S.Y., Lee J.H., Park S.J. Preparation of spherical Zr0.8Sn0.2TiO4 powder by ultrasonic spray pyrolysis. J. Mater. Sci. 1995;30:3274–3278. https://link.springer.com/content/pdf/10.1007/BF01209249.pdf [Google Scholar]
- 4.Contreras-Vargas K.I., Carreño-Gallardo C., Campos-Venegas K., Hurtado-Macías A., Pizá-Ruiz P., Amézaga-Madrid P. Synthesis by AACVD, microstructural characterization and mechanical properties of a Cr2O3/Fe3O4 nanocomposite. Microsc. Microanal. 2022;28(Suppl 1):2876. doi: 10.1017/S1431927622010320. [DOI] [Google Scholar]
- 5.Contreras-Vargas K.I., Sáenz-Trevizo A., Carreño-Gallardo C., Pizá-Ruiz P., Hurtado-Macías A., Silva-Vidaurri L.G., Amézaga-Madrid P. Nanostructured t-YSZ/Fe3O4 powdered composite obtained via AACVD method as a promising reinforcing material for metal matrices. Phys. B Phys. Condens. Matter. 2022;644 doi: 10.1016/j.physb.2022.414194. [DOI] [Google Scholar]
- 6.Sáenz-Trevizo A., Kuchle-Mena D., Pizá-Ruiz P., Amézaga-Madrid P., Solís-Canto O., Silva-Vidaurri L.G., Miki-Yoshida M. Microstructural and optical properties of high quality Mg-Zn oxide thin films. Mater. Sci. Semicond. Process. 2021;127 doi: 10.1016/j.mssp.2021.105690. [DOI] [Google Scholar]
- 7.Heiras-Trevizo A., Amézaga-Madrid P., Corral-Bustamante L., Antúnez-Flores W., Pizá-Ruiz P., Miki-Yoshida M. Structural, morphological, optical and electrical properties of Sb-doped SnO2 thin films obtained by aerosol assisted chemical vapor deposition. Thin Solid Films. 2017;638:22–27. doi: 10.1016/j.tsf2017.07.017. [DOI] [Google Scholar]
- 8.Sáenz-Trevizo A., Amézaga-Madrid P., Pizá-Ruiz P., Antúnez-Flores W., Ornelas-Gutiérrez C., Miki-Yoshida M. Single and multi-layered core-shell structures based on ZnO nanorods obtained by aerosol assisted chemical vapor deposition. Mater. Charact. 2015;105:64–70. doi: 10.1016/j.matchar.2015.04.020. [DOI] [Google Scholar]
- 9.Lugo-Ruelas M., Amézaga-Madrid P., Esquivel-Pereyra O., Antúnez-Flores W., Pizá-Ruiz P., Ornelas-Gutiérrez C., Miki-Yoshida M. Synthesis, microstructural characterization and optical properties of CuO nanorods and nanowires obtainede by aerosol assisted CVD. J. Alloy. Compd. 2015;643:S46–S50. doi: 10.1016/j.jallcom.2014.11.119. [DOI] [Google Scholar]
- 10.Vázquez-Vargas D.A., Amézaga-Madrid P., Jáuregui-Martínez L.E., Esquivel-Pereyra O., Antúnez-Flores W., Pizá-Ruiz P., Miki-Yoshida M. Synthesis and microstructural characterization of cupric oxide and cobalt oxide nanostructures for their application as selective solar coatings. Thin Solid Films. 2020;706 doi: 10.1016/j.tsf.2020.138046. [DOI] [Google Scholar]
- 11.Pizá-Ruiz P., Sáenz-Trevizo A., Verde-Gómez Y., Amézaga-Madrid P., Miki-Yoshida M. Delafossite CuFeO2 thin film via aerosol assisted CVD: synthesis and characterization. Ceram. Int. 2019;45:1156–1162. doi: 10.1016/j.ceramint.2018.09.298. [DOI] [Google Scholar]
- 12.Sáenz-Trevizo A., Amézaga-Madrid P., Pizá-Ruiz P., Solís-Canto O., Ornelas-Gutiérrez C., Pérez-García S., Miki-Yoshida M. Microstructural characterization, optical and photocatalytic properties of bilayered CuO and ZnO based thin films. J. Alloy. Compd. 2014;615:S375–S381. doi: 10.1016/j.jalcom.2014.01.149. [DOI] [Google Scholar]
- 13.Sáenz-Trevizo A., Amézaga-Madrid P., Pizá-Ruiz P., Antunez-Flores W., Ornelas-Gutiérrez C., Miki-Yoshida M. Efficient and durable ZnO core-shell structures for photocatalytic applications in aqueous media. Mater. Sci. Semicond. Process. 2016;45:57–58. doi: 10.1016/j.mssp.2016.01.018. [DOI] [Google Scholar]
- 14.Heiras-Trevizo A., Sáenz-Trevizo A., Pizá-Ruiz P., Amézaga-Madrid P. Al-doped Fe3O4 hollow nanostructured particles: a promising material for application in the coagulation-flocculation process. Mater. Lett. 2023;330 doi: 10.1016/j.matlet.2022.133404. [DOI] [Google Scholar]
- 15.Ul-Ain Babbar N., Khan A., Hakeem A.S., Mohamed H.D., Al-Saeed M.H.A., Ehsan M.A. NiPd nano-alloy film as a promising low overpotential electrocatalyst for high activity water oxidation reaction. J. Environ. Chem. Eng. 2022;10 doi: 10.1016/j.jece.2022.107959. [DOI] [Google Scholar]
- 16.Ehsan M.A., Khan A., Zafar M.N., Akber U.A., Hakeem A.S., Nazar M.F. Aerosol-assisted chemical vapor deposition of nickel sulfide nanowires for electrochemical water oxidation. Int. J. Hydrog. Energy. 2022 doi: 10.1016/j.ijhydene.2021.10.231. xxx (xxxx) xxx. [DOI] [Google Scholar]
- 17.Monárrez-Cordero B.E., Sáenz-Trevizo A., Bautista-Carrillo L.M., Silva-Vidaurri L.G., Miki-Yoshida M., Amézaga-Madrid P. Simultaneous and fast removal of As3+, As5+, Cd2+, Cu2+, Pb2+, and F− from water composite Fe-Ti oxides nanoparticles. J. Alloy. Compd. 2018;757:150–160. doi: 10.1016/j.jallcom.2018.05.013. [DOI] [Google Scholar]
- 18.Khan N.A., Ahmad I., Rashid N., Zafar M.N., Shehzad F.K., Ullah Z., Ul-Hamid A., Nazar M.F., Junaid M., Faheem M., Shafqat S.S., Jabeen U., Dahshan A. Enhanced electrochemical activity of Co3O4/Co9S8 heterostructure catalyst for water splitting. Int. J. Hydrog. Energy. 2022;47:30970–30980. doi: 10.1016/j.ijhydene.2021.11.124. [DOI] [Google Scholar]
- 19.Khan N.A., Rashid N., Ahmad I., Zahidullah R.Zairov, ur Rehman H., Nazar M.F., Jabeen U. An efficient Fe2O3/FeS heterostructures water oxidation catalyst. Int. J. Hydrog. Energy. 2022;47:22340–22347. doi: 10.1016/j.ijhydene.2022.05.045. [DOI] [Google Scholar]
- 20.Ahmad I., Ahmed J., Batool S., Zafar M.N., Hanif A., Zahidullah M.F.Nazar, Ul-Hamid A., Jabeen U., Dahshan A., Idrees M., Shehzadi S.A. Design and fabrication of Fe2O3/FeP heterostructure for oxygen evolution reaction electrocatalysis. J. Alloy. Compd. 2022;894 doi: 10.1016/j.jallcom.2021.162409. [DOI] [Google Scholar]
- 21.Jayanthi G., Zhang S., Messing G. Modeling of solid particle formation during solution aerosol thermolysis: the evaporation stage. Aerosol Sci. Technol. 1993;19:478–490. doi: 10.1080/02786829308959653. [DOI] [Google Scholar]
- 22.Zhang S.C., Messing G.L., Borden M. Synthesis of solid, spherical zirconia particles by spray pyrolysis. J. Am. Ceram. Soc. 1990;73:61–67. doi: 10.1111/j.1151-2916.1990.tb05091.x. [DOI] [Google Scholar]
- 23.Wuled I., Hata T., Iskandar F., Lunden M., Okuyama K. An experimental and modeling investigation of particle production by spray pyrolysis using a laminar flow aerosol. React. J. Mater. Res. 2000;15:733–743. doi: 10.1557/JMR.2000.0106. [DOI] [Google Scholar]
- 24.Join Comitte on Powder Diffraction Standars . International Center for Diffraction Data; Swarthmore, PA: 2006. Powder Diffraction File. JCPDF cards 00-083-0113; 01-081-1317; 01-088-2359; 00-019-0629. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.