Abstract
This study aimed to explore the effects of trans-anethole (TA) on lipopolysaccharide (LPS)-induced acute jejunal inflammation model of broilers. A total of 160 one-day-old broilers (male; Arbor Acres) were randomly allocated into four treatment groups with 8 replicates of 5 birds each. On d 20, the dose of 5 mg/kg body weight LPS solution and the equal amount of sterile saline were intraperitoneally injected into LPS-challenged and unchallenged broilers, respectively. Compared with the control group, LPS decreased (P < 0.05) the villus height (VH) and the ratio of villus height to crypt depth (VCR) but increased (P < 0.05) the crypt depth (CD), meanwhile, enhanced (P < 0.01) the levels of interleukin-6 (IL-6), interleukin-1beta (IL-1β) and tumor necrosis factor-alpha (TNF-α) but decreased (P < 0.01) the level of interleukin-10 (IL-10). The group supplemented with 600 mg/kg of TA had lower (P < 0.01) CD and higher (P < 0.01) VCR than the LPS group. TA increased (P < 0.01) the level of IL-10 and decreased (P < 0.01) the level of IL-1β. The mRNA expression levels of IL-6, nuclear factor kappa B (NF-κB), TNF-α were up-regulated (P < 0.05) and the levels of IL-10 and inhibitor of NF-κB alpha (IκBα) were down-regulated (P < 0.05) by LPS as compared with the control group. TA down-regulated (P < 0.05) the increased mRNA expression levels of genes caused by LPS, as well as up-regulated (P < 0.05) the levels of IL-10 and IκBα. Furthermore, LPS down-regulated (P < 0.05) and up-regulated (P < 0.05) the protein expression levels of IκBα and NF-κB p65, respectively. TA up-regulated (P < 0.05) the level of IκBα and down-regulated (P < 0.05) the level of NF-κB p65. The conclusion of this study is that TA could exert protective effect on the LPS-induced acute jejunal inflammation of broilers via repressing the activation of NF-κB and the 600 mg/kg is the optimal dose against LPS-induced acute jejunal inflammation of broilers.
Key words: trans-anethole, acute inflammation, intestine, lipopolysaccharide, broiler, NF-κB signaling pathway
INTRODUCTION
A former publication has elucidated a summary of the intestinal inflammation phenotypes of poultry that are categorized as physiological inflammation, pathological inflammation, metabolic inflammation and sterile inflammation (Kogut et al., 2018). The physiological inflammation is a primal and acute phase reaction to the disturbed balance between intestinal microbes and intestinal tolerance characterized by the increased intestine permeability, which occurs specifically following the release of virulence factors from dying bacteria (Abraham and Medzhitov, 2011). However, once the virulence factors such as lipopolysaccharide (LPS) are out of control, the physiological inflammation could be transformed to pathological inflammation and chronic inflammation, which ultimately leads to tissue damage and even organ failure (Barton, 2008). The LPS, an endotoxin existing in the gut, emerges from the gram-negative bacteria and consists of O-antigen, core oligosaccharide domain and lipid A. The lipid A is a conserved region responsible for the LPS recognition by the pattern recognition receptors (PRRs) including toll-like receptors (TLRs) (Bidne et al., 2018). Numerous studies have confirmed that the LPS is mainly recognized by toll-like receptor 4 (TLR4), which can induce the production of proinflammatory cytokines such as interleukin-6 (IL-6) through activating the NF-κB signaling pathway (Sanjabi et al., 2000; Lai et al., 2017; Tang et al., 2021).
Trans-anethole (TA), as a major active component of essential oil that extracted from various herbal plants such as Illicium verum, has been confirmed to possess multiple functions including antioxidant, antifungal, anti-inflammatory and gastroprotective (Domiciano et al., 2013; Luís et al., 2019; Sharafan et al., 2022). In addition, the preparations of TA have been used in cosmetic, food and medical industries for TA is identified as a safe compound by the Expert Panel of the Flavour and Extract Manufacturer Association (FEMA) (Newberne et al., 1999). TA is reported to have an aromatic odor and a sweet taste thus contributing to being the constitute of perfume and spice. However, the visible light and high temperature can transform TA to its isomer cis-anethole which is toxic, but the cis-form has a negligible amount in essential oil (Aprotosoaie et al., 2016). Although the majority of previous studies have shown the potent anti-inflammatory effect of TA and its mechanisms on mammals (Ponte et al., 2012; Kim et al., 2017; Samadi-Noshahr et al., 2021a), few studies have elucidated the anti-inflammatory effect of TA and its mechanism on poultry. Further, the NF-κB signaling pathway has attracted the interest of most researchers for decades, which is also confirmed to be repressed by the administration of TA in the inflammatory models of animals (Feng et al., 2019). Hence, we hypothesized that TA could show protective effects on the LPS-induced acute jejunal inflammation of broilers via repressing the NF-κB signaling pathway.
MATERIALS AND METHODS
Preparation of Materials
TA was purchased from Nanjing Dilger Medical Technology Co., Ltd (Nanjing, China), whose purity was 98.35%. The storage method of TA was keeping in glass bottles avoiding light at 4°C. The doses of TA in the experiment were referred to the earlier study (Yu et al., 2021). LPS (O55:B5) was purchased from Sigma-Aldrich Chemical Inc. (#L2880, St. Louis, MO), dissolved with equal proportion in 0.86% (w/v) sterile saline when used for administration, the dosage of which was referred to previous studies (Zhang et al., 2020; Chen and Yu, 2021).
Animals and Experimental Design
The experiment was performed by raising 160 one-day-old broilers (male; Arbor Acres) following the protocols approved by the Institution of Animal Care Committee of Nanjing Agriculture University. The healthy birds with similar weight were obtained from Yantai Land Animal Husbandry Co., Ltd (Yantai, China) and randomly allocated into 4 treatment groups with 8 replicates of 5 birds each. Four treatment groups were classified as control group (CON), lipopolysaccharide group (LPS), lipopolysaccharide group plus 400 mg/ kg trans-anethole (LPS + TA400) and lipopolysaccharide group plus 600 mg/kg trans-anethole (LPS + TA600). The CON and LPS groups were fed with basal diet which was formulated to meet the nutrient requirements of broilers following the Feeding Standard of chicken of the People's Republic of China (NY/T 33-2004), the other groups were fed basal diet supplemented with 400 or 600 mg/kg TA, respectively. The ingredients and nutrient composition of the basal diet are shown in Table 1. On d 20, the dose of 5 mg/kg body weight (BW) LPS solution and the equal amount of sterile saline were intraperitoneally injected into LPS-challenged broilers and unchallenged broilers, respectively. Birds were kept in a house with controllable temperature and humidity, and had free access to water and feed. The duration of the experiment was 21 d. The growth performance before LPS administration has shown in our previous study (Tong et al., 2022), which exhibited that TA at the dose of 600 mg/kg had higher average daily feed intake (ADFI) of broilers compared with other groups.
Table 1.
Ingredients and nutrient composition of the basal diet1 (%, as fed-basis).
| Ingredient | % | Nutrient levels1 | % |
|---|---|---|---|
| Corn | 55.60 | Metabolizable energy, Mcal/kg | 2.87 |
| Expanded soybean meal | 29.00 | Crude protein | 20.95 |
| Cottonseed meal | 2.50 | Total calcium | 0.96 |
| Wheat flour | 4.00 | Total phosphorus | 0.66 |
| Hydrolyzed feather meal | 1.50 | Total lysine | 1.11 |
| Soybean oil | 2.00 | Total methionine | 0.35 |
| Dicalcium phosphate | 0.90 | Total threonine | 0.82 |
| Limestone | 1.50 | ||
| Bentonite | 1.00 | ||
| Premix2 | 2.00 | ||
| Total | 100.00 |
All nutrient levels were analyzed values, except metabolizable energy.
Supplied per kilogram of diet: vitamin A, 11,500 IU; cholecalciferol, 3,500 IU; vitamin E, 30 mg; vitamin K3, 5 mg; thiamin, 3.38 mg; riboflavin, 9.0 mg; pyridoxine, 8.96 mg; vitamin B12, 0.025 mg; choline chloride, 800 mg; calcium pantothenate, 13 mg; niacin, 45 mg; biotin, 0.15 mg; folic acid, 1.20 mg; Mn, 60 mg; Fe, 66.5 mg; Zn, 88 mg; Cu, 8.8 mg; I, 0.70 mg; Se, 0.288 mg.
Sample Collection
On d 21, eight broilers per group were chosen randomly to sacrifice with cervical dislocation. The segments about 1.5 cm cut from the middle of jejunum were fixed in 4% paraformaldehyde for morphological measurement. After that, the segments about 3 cm of jejunum were dissected and the remaining digesta of the segments were rinsed by precooling sterile saline. The segments were collected into sterile tubes and then stored in liquid nitrogen for further analyses.
Morphological Measurement of Jejunum
Paraformaldehyde-fixed segments of jejunum were dehydrated in xylene, embedded in paraffin and sectioned (5 μm) with a microtome for hematoxylin-eosin (H&E) staining. The villus height (VH) and crypt depth (CD) were observed using light microscope (Olympus CX31, Tokyo, Japan), and then the length of ten villi and crypts in each section were measured by Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD). The results of measurement were used to calculate the ratio of villus height to crypt depth (VCR).
Determination of Inflammatory Cytokines
The levels of IL-6, interleukin-10 (IL-10), interleukin-1beta (IL-1β), and tumor necrosis factor-alpha (TNF-α) in the jejunum were determined by ELISA kits (Jiangsu Meimian Industry Co., Ltd, Yancheng, China) according to the manufacturer's instructions.
Extraction of RNA and Fluorescence Quantitative Real-Time PCR Analysis
The total RNA was extracted from jejunum samples using Trizol reagent (Nanjing Vazyme Biotech Co., Ltd, China) following the manufacturer's instructions. The extractive RNA was used to determine the purity and concentration using a spectrophotometer (NanoDrop Products, Wilmington, DE) and the concentration of RNA was unified using DNase/RNase-free ddH2O (Beyotime Biotechnology, Shanghai, China) to 500 ng/mL for reverse transcription. The reverse transcription reaction of RNA was performed with the EasyScript All-in-one First-Strand cDNA Synthesis SuperMix for quantitative real-time qPCR products (TransGen Biotech, Beijing, China). The fluorescence quantitative real-time PCR of cDNA was performed with PerfectStart Green qPCR SuperMix (TransGen Biotech, Beijing, China) based on Applied Biosystems QuantStudio 7 Flex apparatus. The mRNA expression levels of target genes relative to β-actin were calculated using 2−△△CT method. The primer sequences of target genes and housekeeping gene are shown in Table 2.
Table 2.
Gene-specific primers sequences for quantitative real-time PCR.
| Gene name1 | GenBank2 | Primer sequence3 (5′→3′) | Length |
|---|---|---|---|
| IL-2 | AY510091.1 | TGCAGTGTTACCTGGGAGAAG | 148 |
| TTGCATTCACTTCCGGTGTG | |||
| IL-4 | AJ621249.1 | AGCCTCCACAATTGTTTGGG | 139 |
| TGAAGTAGTGTTGCCTGCTG | |||
| IL-6 | AB302327.1 | AACAACCTCAACCTGCCCAA | 112 |
| AGGTCTGAAAGGCGAACAGG | |||
| IL-8 | DQ393272.2 | CCTCCTCCTGGTTTCAGCTG | 136 |
| TGGCGTCAGCTTCACATCTT | |||
| IL-10 | NM_012854.2 | CAGACCAGCACCAGTCATCA | 96 |
| TCCCGTTCTCATCCATCTTCTC | |||
| IL-1β | NM_204524.2 | TTTTTGAGCCCGTCACCTTC | 111 |
| AGCACTTCTGGTTGATGTCG | |||
| NF-κB | NM_001012887.2 | AAGATCTGGTGGTGTGCCTG | 137 |
| AGTGGAACCTTTCGCGGATT | |||
| IκBα | NM_001001472.2 | CAGCACTACACTTGGCCGTA | 101 |
| GGAGTAGCCCTGGTAGGTCA | |||
| TNF-α | HQ739087.1 | GAACCCTCCGCAGTACTCAG | 116 |
| AACTCATCTGAACTGGGCGG | |||
| TLR4 | KP410249.1 | CGGCTCCGCATCTTGGATAT | 148 |
| GGGCTTGGAGTGGCTTGTAT | |||
| IFN-γ | NM_205149.1 | TGTAGCTGACGGTGGACCTA | 134 |
| GCGGCTTTGACTTGTCAGTG | |||
| β-Actin | NM_205518.1 | ACCGGACTGTTACCAACACC | 116 |
| CCTGAGTCAAGCGCCAAAAG |
IL, interleukin; NF-κB, nuclear factor kappa B; IκBα, inhibitor of NF-κB alpha; TNF-α, tumor necrosis factor-α; TLR4, toll-like receptor 4; IFN-γ, interferon-gamma.
GenBank Accession Number.
Shown as the forward primer then the reverse primer.
Western Blot Analysis
Four replicates per group were randomly selected to perform western blot analysis in order to determine the protein expression level related to NF-κB signal pathway in the jejunum. Nuclear proteins were extracted from the whole proteins using the Nuclear Protein Extraction Kit (Beijing Solarbio Science & Technology Co., Ltd, Beijing, China) following the manufacturer's instructions. The concentrations of nuclear and cytoplasmic proteins were detected using the Bicinchoninic Acid (BCA) Protein Assay Kit (Beyotime Biotechnology, Shanghai, China) following the manufacturer's instructions. After unifying the concentration of protein to 5 ng/mL, adding the SDS-PAGE Sample Loading Buffer (5X; Beyotime Biotechnology, Shanghai, China) into the protein and then denaturalizing the protein at 95°C for 5 min. The protein gel was prepared using the 12.5% PAGE Gel FAST Preparation Kit (Epizyme Biomedical Technology Co., Ltd, Shanghai, China) following the manufacturer's instructions, on which the protein samples and protein marker were loaded for protein electrophoresis. After that, the target gels were separated from the whole protein gel and transferred to the polyvinylidene fluoride (PVDF) membranes at 4°C. The membranes of target proteins were closed in blocking buffer for 1.5 h, then incubated overnight at 4°C with the primary antibodies against nuclear factor kappa B p65 (NF-κB p65; Proteintech Group, Inc., Wuhan, China), inhibitor of NF-κB alpha (IκBα; Proteintech Group, Inc., Wuhan, China), β-Actin (Affinity Bioscicences Co., Ltd, Jiangsu, China), Lamin B1 (Proteintech Group, Inc., Wuhan, China). Subsequently, the membranes were washed using Tris-buffered saline containing 0.1% Tween 20 (TBST) buffer for 3 times and incubated with the secondary antibody for 1.5 h. At last, putting the bands in the enhanced chemiluminescence (ECL) reagent (Nanjing Vazyme Biotech Co., Ltd, Nanjing, China) for the detection of protein expression level using the ChemiDoc MP Imaging System apparatus (Bio-Rad Laboratories, Inc., Hercules, CA). β-Actin and Lamin B1 were used as an internal reference for cytoplasmic protein and nuclear protein, respectively.
Statistical Analysis
The data were analyzed using one-way ANOVA of SPSS software (ver. 21.0; IBM-SPSS, Inc., Chicago, IL) and represented as the mean ± SEM. The significant differences between groups were analyzed by the Tukey's HSD test of SPSS software. P < 0.05 and P < 0.01 means significant and highly significant differences, respectively. P > 0.05 means no significant differences.
RESULTS
Jejunal Morphology
As shown in Table 3, compared with the control group, LPS challenge significantly decreased (P < 0.05) the VH and VCR of jejunum but significantly increased (P < 0.05) the CD of jejunum. The group supplemented with 600 mg/kg of TA had lower (P < 0.01) CD and higher (P < 0.01) VCR than the LPS group. As shown in Figure 1, the LPS group exhibited structural deteriorations including the VH and CD, which were attenuated by dietary 600 mg/kg of TA.
Table 3.
Effects of trans-anethole on jejunal morphology of LPS-challenged broiler.1
| Items2 | CON | LPS | LPS + TA400 | LPS + TA600 | P-value |
|---|---|---|---|---|---|
| VH (μm) | 1031.34 ± 35.03a | 936.35 ± 30.11b | 938.81 ± 29.85b | 883.25 ± 25.80b | 0.016 |
| CD (μm) | 214.98 ± 10.60b | 252.36 ± 8.42a | 240.28 ± 11.56ab | 184.01 ± 9.92c | <0.001 |
| VCR (μm/μm) | 4.87 ± 0.27a | 3.73 ± 0.15b | 3.99 ± 0.27b | 4.88 ± 0.24a | 0.002 |
The values in the same row with different superscripts means significantly different (P < 0.05).
The values are represented as mean ± SEM, n = 8.
VH, villus height; CD, crypt depth; VCR, villus height to crypt depth ratio. CON, control group fed with basal diet and treated with saline; LPS, group fed with basal diet and treated with lipopolysaccharide; LPS+TA400, group fed with 400 mg/kg trans-anethole and treated with lipopolysaccharide; LPS + TA600, group fed with 600 mg/kg trans-anethole and treated with lipopolysaccharide.
Figure 1.
Effects of trans-anethole on jejunal morphology of LPS-challenged broilers. (A) control group fed with basal diet and treated with saline, (B) group fed with basal diet and treated with lipopolysaccharide, (C) group fed with 400 mg/kg trans-anethole and treated with lipopolysaccharide, (D) group fed with 600 mg/kg trans-anethole and treated with lipopolysaccharide.
Inflammatory Cytokines Levels in the Jejunum
As shown in Table 4, LPS challenge enhanced (P < 0.01) the levels of IL-6, IL-1β and TNF-α, meanwhile, decreased (P < 0.01) the level of IL-10 in the jejunum compared with the control group. However, compared with the LPS group, dietary supplementation of TA increased (P < 0.01) the level of IL-10 and decreased (P < 0.01) the level of IL-1β, but made no effect (P > 0.05) on the levels of IL-6 and TNF-α.
Table 4.
Effects of trans-anethole on the jejunal inflammatory cytokine levels of LPS-challenged broilers.1
| Items2 | CON | LPS | LPS+TA400 | LPS+TA600 | P-value |
|---|---|---|---|---|---|
| IL-6, ng/g protein | 29.60 ± 0.85b | 37.46 ± 2.65a | 25.00 ± 1.33b | 27.18 ± 1.72b | <0.001 |
| IL-10, ng/g protein | 56.79 ± 1.61b | 50.17 ± 1.14c | 57.97 ± 1.25b | 67.97 ± 2.01a | <0.001 |
| IL-1β, ng/g protein | 85.80 ± 2.65b | 115.70 ± 4.68a | 67.97 ± 4.05b | 84.43 ± 6.81b | <0.001 |
| TNF-α, ng/g protein | 54.80 ± 1.56b | 86.82 ± 3.93a | 74.63 ± 3.71a | 76.52 ± 5.04a | <0.001 |
The values in the same row with different superscripts means significantly different (P < 0.05).
The values are represented as mean ± SEM, n = 8.
IL-6, interleukin-6; IL-10, interleukin-10; IL-1β, interleukin-1beta; TNF-α, tumor necrosis factor-alpha; CON, control group fed with basal diet and treated with saline; LPS, group fed with basal diet and treated with lipopolysaccharide; LPS+TA400, group fed with 400 mg/kg trans-anethole and treated with lipopolysaccharide; LPS + TA600, group fed with 600 mg/kg trans-anethole and treated with lipopolysaccharide.
Expression Levels of Inflammation-related Genes in the Jejunum
The relative expression levels of inflammation-related genes in the jejunum of broilers are shown in Figure 2. LPS challenge up-regulated (P < 0.05) the jejunum mRNA expression levels of interleukin-2 (IL-2), IL-6, NF-κB, TNF-α, TLR4, and interferon-gamma (IFN-γ) but down-regulated (P < 0.05) the levels of interleukin-4 (IL-4), IL-10 and IκBα as compared with unchallenged birds. However, there were no significant differences (P > 0.05) in the levels of interleukin-8 (IL-8) and IL-1β between the control group and LPS group. Compared with the LPS group, dietary supplementation of TA down-regulated (P < 0.05) the mRNA expression levels of IL-2, IL-6, NF-κB, TNF-α, TLR4, and IFN-γ, also up-regulated (P < 0.05) the mRNA expression levels of IL-10 and IκBα. Furthermore, birds supplemented with 600 mg/kg TA had lower (P < 0.05) levels of NF-κB, TNF-α and IFN-γ than the birds supplemented with 400 mg/kg TA.
Figure 2.
Effects of trans-anethole on the relative mRNA expression of genes related to inflammation in the jejunum of LPS-challenged broilers. The values are represented as mean (n = 8) with their standard errors. Bars with unlike letters means significantly different. Abbreviation: IL-2, interleukin-2; IL-4, interleukin-4; IL-6, interleukin-6; IL-8, interleukin-8; IL-10, interleukin-10; IL-1β, interleukin-1beta; NF-κB, nuclear factor kappa B; IκBα, inhibitor of NF-κB alpha; TNF-α, tumor necrosis factor-alpha; TLR4, toll-like receptor 4; IFN-γ, interferon-gamma; CON, control group fed with basal diet and treated with saline; LPS, group fed with basal diet and treated with lipopolysaccharide; LPS + TA400, group fed with 400 mg/kg trans-anethole and treated with lipopolysaccharide; LPS + TA600, group fed with 600 mg/kg trans-anethole and treated with lipopolysaccharide.
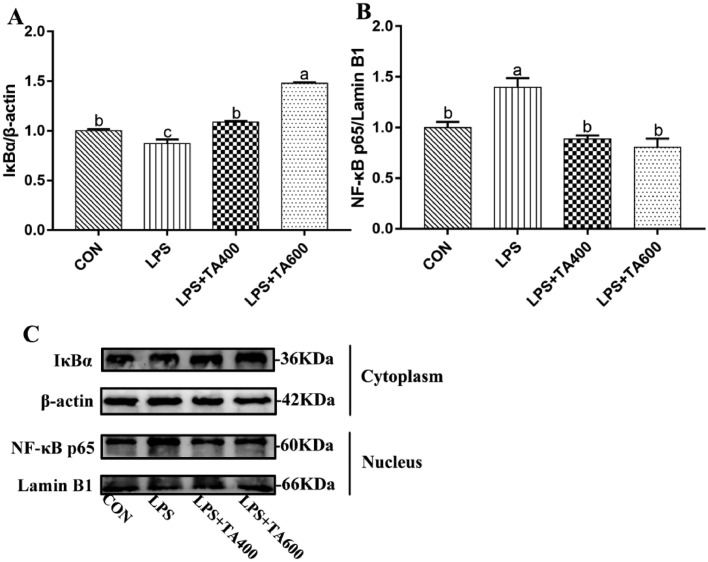
Relative Protein Expression Levels in the Jejunum
As shown in Figure 3, compared with the control group, LPS challenge down-regulated (P < 0.05) and up-regulated (P < 0.05) the relative protein expression levels of IκBα and NF-κB p65, respectively. However, compared with the LPS group, dietary supplementation of TA up-regulated (P < 0.05) the level of IκBα and down-regulated (P < 0.05) the level of NF-κB p65.
Figure 3.
Effects of trans-anethole on the protein expression of NF-κB pathway in the jejunum of LPS-challenged broilers. The values are represented as mean (n = 4) with their standard errors. Bars with unlike letters means significantly different. Abbreviations: NF-κB p65, nuclear factor kappa B p65; IκBα, inhibitor of NF-κB alpha; CON, control group fed with basal diet and treated with saline; LPS, group fed with basal diet and treated with lipopolysaccharide; LPS + TA400, group fed with 400 mg/kg trans-anethole and treated with lipopolysaccharide; LPS + TA600, group fed with 600 mg/kg trans-anethole and treated with lipopolysaccharide.
DISCUSSION
The intestinal tract has a complex immune system, despite that, a variety of commensal bacteria and fungi attach to the mucosal cells in the gut. Thus, the risks of bacterial infection increase concomitant with the death and dissolution of detrimental bacteria, such as Escherichia coli (Kelly et al., 2012). The LPS is located in the outermost membrane of gram-negative bacteria, which can be released into circulation when the permeability of intestine gets increased (Xing et al., 2021). The circulating LPS is commonly identified as an endotoxin that can trigger acute inflammatory response, at this time, the host is more vulnerable of the environmental stimuli and stressors, which can in turn result in chronic inflammatory diseases, endotoxemia and death (Cavaillon, 2018). In addition, Han (2002) demonstrated that the intestinal endotoxemia can further aggravate acute liver injury and even induce acute liver failure, which suggested that there is an interplay between liver and intestine on the pathogenesis of liver injury. Therefore, more and more research on quelling the LPS-induced detriments were carried out upon the animal models of mimicking endogenous LPS stimulation. Numerous studies have concluded that it is feasible to embark on suppressing the acute inflammation responded to LPS (Donovan and Grundy, 2012; Hsiang et al., 2015; Matos et al., 2021). To date, studies concerning the anti-inflammatory effect of TA have focused on the experiments of rats and cells, but few on the acute inflammation model of poultry (Kang et al., 2013; Estevao-Silva et al., 2014; Samadi-Noshahr et al., 2021b). The small intestine of chicken is short and simple consisting of duodenum, jejunum and ileum. Moreover, the jejunal epithelium is an effective barrier that can prevent endotoxin from constantly enhancing the inflammatory response (Rodrigues and Choct, 2018). Our recent study has reported that TA had protective effect on acute liver inflammation of broilers (Tong et al., 2022). Similarly, the aim of this study was to investigate the influence of TA on LPS-induced acute jejunal inflammation of broilers through observing the jejunal morphology, determining the inflammatory cytokines levels and detecting the expression levels of genes and proteins related to NF-κB signaling pathway in the jejunum.
The jejunal morphology is dynamic due to its specific villus-crypt tissue structure, which plays an important role in intestinal health. The continuous turnover of villus-crypt tissue renders the gut epithelium to be a barrier (Gehart and Clevers, 2019). Thereby, decreased villus height and increased crypt depth are hallmarks of intestinal injury. Many researchers have proven that the LPS administration has negative effects on the VH and CD (Yang et al., 2014; Zheng et al., 2020; Zhang et al., 2021). In line with that, in this study, LPS-challenged broilers had not only lower VH and VCR but also higher CD than unchallenged broilers. However, dietary supplemented with 600 mg/kg of TA attenuated the variation of CD and VCR, which is in accordance with the results of Yu et al. (2022a) that TA improved the VH and VH/CD in the model of necrotic enteritis-induced broilers. It may attribute to the improved effect of TA on feed intake because of the association between the villus-crypt structure and nutrient absorption (Yu et al., 2021). It is worthwhile to mention that the CD of group supplemented with 600 mg/kg TA was much lower than that of control group, which may be due to the rate of crypt cells proliferation (Tappenden, 2014). Furthermore, we also observed that the dose of 600 mg/kg of TA had more apparent effects on CD and VCR than the dose of 400 mg/kg of TA, but there is no significant difference between broilers supplemented with 600 mg/kg and 400 mg/kg of TA on VH. It may be influenced by the sustained replenishment from crypt to villus (Gehart and Clevers, 2019).
When LPS exists in the blood circulation, the signal recognition mechanism of LPS initiates rapidly. The LPS is primarily recognized by and binds with the lipopolysaccharide-binding protein (LBP) secreted from liver, then, the LPS-LBP dimer binds with the cluster of differentiation 14 (CD14) in order to transferring the LPS to the myeloid differentiation factor 2 (MD2). The LPS is finally recognized by TLR4 with the help of MD2 (Fenton and Golenbock, 1998). The recognition procedure above occurs outside the cells. The TLR4 can transduce the signal into the intracellular effectors, which ultimately leads to the activation of NF-κB signaling pathway. NF-κB signaling pathway have long been researched for its complexity and multifunctional effects on inflammation and diseases such as cancer (Yu et al., 2020; Liu et al., 2022). The activation of NF-κB pathway can elicit the transcription of proinflammatory genes, which is critical for inflammatory response (Jing et al., 2022). It is well-established that IL-1β, IL-6 and TNF-α are the prototypical proinflammatory cytokines rapidly released from immune cells contributing to the acute phase reaction, whereas, IL-10 and IL-4 are the cytokines against the inflammatory response by suppressing the production of proinflammatory cytokines (Wojdasiewicz et al., 2014). Furthermore, an earlier study has illustrated that the excessive IFN-γ can cause tissue damage and inflammation (Kak et al., 2018). Accordingly, the results in the current study exhibited that LPS challenge increased the levels of IL-6, IL-1β, and TNF-α, decreased the level of IL-10 in the jejunum. This is in accordance with the results of Han et al. (2020), who showed that LPS provoked the increased levels of IL-6, IL-1β, and TNF-α in the liver of broiler chickens. A former study has reported that anethole reduced the increased expression levels of IL-1β, IL-6, and TNF-α in the model of enterotoxigenic Escherichia coli-challenged piglets (Yi et al., 2021). In line with that, in this study, dietary with TA improved the decreased level of IL-10 and attenuated the increased level of IL-1β in the jejunum, but made no effect on the levels of IL-6 and TNF-α. Additionally, TA also down-regulated the mRNA expression levels of IL-2, TLR4 and IFN-γ and up-regulated the level of IL-10. However, we noted that TA down-regulated the mRNA expression levels of IL-6 and TNF-α from the results of qPCR analysis. The discrepancy between the expression level of mRNA and protein may be explained by the post-transcriptional modification of mRNA and the post-translational modification of protein (Courtney, 2021; Leutert et al., 2021).
The central components of NF-κB signaling pathway are the transcription factor NF-κB and its inhibitor protein IκB (Hayden and Ghosh, 2008). The NF-κB family is consisted of five subunits, p50, p52, p65, c-Rel and RelB. Two of them bind to form a NF-κB dimer, which is generally composed by p50 or p52 and p65, c-Rel or RelB as p65, c-Rel and RelB have the transcription activation domain responsible for the positive regulation of proinflammatory genes expression (Magnani et al., 2000). The most canonical one in the NF-κB dimers is the combination of p50 and p65, which generally binds with IκBα protein keeping inactive state in the cytoplasm. A diversity of stimuli such as LPS are capable of induing the ubiquitination of IκBα, which contributes to the release and translocation of NF-κB into the nucleus and ultimately initiating the inflammatory reaction (Lawrence, 2009; Liu et al., 2021a,b). The results of this study showed that LPS challenge down-regulated and up-regulated the mRNA expression level of IκBα and NF-κB, respectively. Similarly, the protein expression levels of IκBα in the cytoplasm as well as NF-κB p65 in the nucleus were down- and up-regulated by LPS, respectively. These results are in agreement with the results of Ju et al. (2019), who revealed that LPS enhanced the NF-κB expression and decreased the IκBα expression in the model of acute lung injury rats. A previous study has reported that anethole decreased the level of NF-κB in myocardial infarction rats (Younis and Mohamed, 2022). In addition, another earlier study has demonstrated that anethole suppressed nuclear localization of NF-κB protein in human prostate cancer cells (Elkady, 2018). In line with them, in this study, birds dietary of TA had lower mRNA expression level of NF-κB and higher level of IκBα, simultaneously lower protein expression level of NF-κB p65 in the nucleus and higher level of IκBα in the cytoplasm of jejunum, which implied that TA could suppress the activation of NF-κB signaling pathway in the jejunum of broilers.
Taken together, TA could improve the broken jejunal morphology, reduce the production of proinflammatory cytokines via suppressing the activation of NF-κB signaling pathway in the broilers. Furthermore, the dose of 600 mg/kg is more efficient than the dose of 400 mg/kg from the observation of jejunal morphology and qPCR analysis, which may exhibit that the 600 mg/kg of TA is the optimal dose in inhibiting the inflammation of broilers. This is in agreement with the conclusion of Yu et al. (2022b), who revealed that TA administration at 600 mg/kg ameliorated subclinical NE infection of broilers more efficiently. However, much scientific work remains to be done to investigate the anti-inflammatory effect of TA on LPS-induced acute inflammation in broilers more specifically, for example, to narrow down the concentration gradient of TA.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (No.2018YFD0501101).
Disclosures
The authors declare that there is no conflict of interest.
REFERENCES
- Abraham C., Medzhitov R. Interactions between the host innate immune system and microbes in inflammatory bowel disease. Gastroenterology. 2011;140:1729–1737. doi: 10.1053/j.gastro.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aprotosoaie A.C., Costache I.I., Miron A. Anethole and its role in chronic diseases. Adv. Exp. Med. Biol. 2016;929:247–267. doi: 10.1007/978-3-319-41342-6_11. [DOI] [PubMed] [Google Scholar]
- Barton G.M. A calculated response: control of inflammation by the innate immune system. J. Clin. Invest. 2008;118:413–420. doi: 10.1172/JCI34431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidne K.L., Dickson M.J., Ross J.W., Baumgard L.H., Keating A.F. Disruption of female reproductive function by endotoxins. Reproduction. 2018;155:R169–R181. doi: 10.1530/REP-17-0406. [DOI] [PubMed] [Google Scholar]
- Cavaillon J.M. Exotoxins and endotoxins: inducers of inflammatory cytokines. Toxicon. 2018;149:45–53. doi: 10.1016/j.toxicon.2017.10.016. [DOI] [PubMed] [Google Scholar]
- Chen J.Y., Yu Y.H. Bacillus subtilis-fermented products ameliorate the growth performance and alter cecal microbiota community in broilers under lipopolysaccharide challenge. Poult. Sci. 2021;100:875–886. doi: 10.1016/j.psj.2020.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney D.G. Post-transcriptional regulation of viral RNA through epitranscriptional modification. Cells. 2021;10:1129. doi: 10.3390/cells10051129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domiciano T.P., Dalalio M.M., Silva E.L., Ritter A.M., Estevao-Silva C.F., Ramos F.S., Caparroz-Assef S.M., Cuman R.K., Bersani-Amado C.A. Inhibitory effect of anethole in nonimmune acute inflammation. Naunyn Schmiedebergs Arch. Pharmacol. 2013;386:331–338. doi: 10.1007/s00210-012-0820-5. [DOI] [PubMed] [Google Scholar]
- Donovan J., Grundy D. Endocannabinoid modulation of jejunal afferent responses to LPS. Neurogastroenterol. Motil. 2012;24:956–e465. doi: 10.1111/j.1365-2982.2012.01961.x. [DOI] [PubMed] [Google Scholar]
- Elkady A.I. Anethole inhibits the proliferation of human prostate cancer cells via induction of cell cycle arrest and apoptosis. Anticancer Agents Med. Chem. 2018;18:216–236. doi: 10.2174/1871520617666170725165717. [DOI] [PubMed] [Google Scholar]
- Estevao-Silva C.F., Kummer R., Fachini-Queiroz F.C., Grespan R., Nogueira de Melo G.A., Baroni S., Cuman R.K., Bersani-Amado C.A. Anethole and eugenol reduce in vitro and in vivo leukocyte migration induced by fMLP, LTB4, and carrageenan. J. Nat. Med. 2014;68:567–575. doi: 10.1007/s11418-014-0839-7. [DOI] [PubMed] [Google Scholar]
- Feng T., Zhou L., Gai S., Zhai Y., Gou N., Wang X., Zhang X., Cui M., Wang L., Wang S. Acacia catechu (L.f.) Willd and Scutellaria baicalensis Georgi extracts suppress LPS-induced pro-inflammatory responses through NF-κB, MAPK, and PI3K-Akt signaling pathways in alveolar epithelial type II cells. Phytother. Res. 2019;33:3251–3260. doi: 10.1002/ptr.6499. [DOI] [PubMed] [Google Scholar]
- Fenton M.J., Golenbock D.T. LPS-binding proteins and receptors. J. Leukoc. Biol. 1998;64:25–32. doi: 10.1002/jlb.64.1.25. [DOI] [PubMed] [Google Scholar]
- Gehart H., Clevers H. Tales from the crypt: new insights into intestinal stem cells. Nat. Rev. Gastroenterol. Hepatol. 2019;16:19–34. doi: 10.1038/s41575-018-0081-y. [DOI] [PubMed] [Google Scholar]
- Han D.-W. Intestinal endotoxemia as a pathogenetic mechanism in liver failure. World J. Gastroenterol. 2002;8:961–965. doi: 10.3748/wjg.v8.i6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H., Zhang J., Chen Y., Shen M., Yan E., Wei C., Yu C., Zhang L., Wang T. Dietary taurine supplementation attenuates lipopolysaccharide-induced inflammatory responses and oxidative stress of broiler chickens at an early age. J. Anim. Sci. 2020;98:skaa311. doi: 10.1093/jas/skaa311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden M.S., Ghosh S. Shared principles in NF-κB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Hsiang C.Y., Cheng H.M., Lo H.Y., Li C.C., Chou P.C., Lee Y.C., Ho T.Y. Ginger and zingerone ameliorate lipopolysaccharide-induced acute systemic inflammation in mice, assessed by nuclear factor-kappab bioluminescent imaging. J. Agric. Food Chem. 2015;63:6051–6058. doi: 10.1021/acs.jafc.5b01801. [DOI] [PubMed] [Google Scholar]
- Jing C., Niu J., Liu Y., Jiao N., Huang L., Jiang S., Yan L., Yang W., Li Y. Tannic acid extracted from galla chinensis supplementation in the diet improves intestinal development through suppressing inflammatory responses via blockage of NF-κB in broiler chickens. Animals (Basel) 2022;12:2397. doi: 10.3390/ani12182397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju M., He H., Chen S., Liu Y., Liu Y., Pan S., Zheng Y., Xuan L., Zhu D., Luo Z. Ulinastatin ameliorates LPS-induced pulmonary inflammation and injury by blocking the MAPK/NFκB signaling pathways in rats. Mol. Med. Rep. 2019;20:3347–3354. doi: 10.3892/mmr.2019.10561. [DOI] [PubMed] [Google Scholar]
- Kak G., Raza M., Tiwari B.K. Interferon-gamma (IFN-γ): Exploring its implications in infectious diseases. Biomol. Concepts. 2018;9:64–79. doi: 10.1515/bmc-2018-0007. [DOI] [PubMed] [Google Scholar]
- Kang P., Kim K.Y., Lee H.S., Min S.S., Seol G.H. Anti-inflammatory effects of anethole in lipopolysaccharide-induced acute lung injury in mice. Life Sci. 2013;93:955–961. [PubMed] [Google Scholar]
- Kelly C.J., Colgan S.P., Frank D.N. Of microbes and meals: the health consequences of dietary endotoxemia. Nutr. Clin. Pract. 2012;27:215–225. doi: 10.1177/0884533611434934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.Y., Lee H.S., Seol G.H. Anti-inflammatory effects of trans-anethole in a mouse model of chronic obstructive pulmonary disease. Biomed. Pharmacother. 2017;91:925–930. doi: 10.1016/j.biopha.2017.05.032. [DOI] [PubMed] [Google Scholar]
- Kogut M.H., Genovese K.J., Swaggerty C.L., He H., Broom L. Inflammatory phenotypes in the intestine of poultry: not all inflammation is created equal. Poult. Sci. 2018;97:2339–2346. doi: 10.3382/ps/pey087. [DOI] [PubMed] [Google Scholar]
- Lai J.L., Liu Y.H., Liu C., Qi M.P., Liu R.N., Zhu X.F., Zhou Q.G., Chen Y.Y., Guo A.Z., Hu C.M. Indirubin inhibits LPS-induced inflammation via TLR4 abrogation mediated by the NF-κB and MAPK signaling pathways. Inflammation. 2017;40:1–12. doi: 10.1007/s10753-016-0447-7. [DOI] [PubMed] [Google Scholar]
- Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009;1 doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutert M., Entwisle S.W., Villen J. Decoding post-translational modification crosstalk with proteomics. Mol. Cell. Proteomics. 2021;20 doi: 10.1016/j.mcpro.2021.100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.C., Ou B.H., Liang Z.L., Zhang R., Zhao Z.H. Algae-derived polysaccharides supplementation ameliorates heat stress-induced impairment of bursa of Fabricius via modulating NF-κB signaling pathway in broilers. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.C., Zhu Y.R., Zhao Z.H., Jiang P., Yin F.Q. Effects of dietary supplementation of algae-derived polysaccharides on morphology, tight junctions, antioxidant capacity and immune response of duodenum in broilers under heat stress. Animals (Basel) 2021;11:2279. doi: 10.3390/ani11082279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.C., Zhuang D.P., Zhao Y., Balasubramanian B., Zhao Z.H. Seaweed-derived polysaccharides attenuate heat stress-induced splenic oxidative stress and inflammatory response via regulating Nrf2 and NF-κB signaling pathways. Mar. Drugs. 2022;20:358. doi: 10.3390/md20060358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luís Â., Sousa S., Wackerlig J., Dobusch D., Duarte A.P., Pereira L., Domingues F. Star anise (Illicium verum Hook. f.) essential oil: Antioxidant properties and antibacterial activity against Acinetobacter baumannii. Flavour Fragr. J. 2019;34:260–270. [Google Scholar]
- Magnani M., Crinelli R., Bianchi M., Antonelli A. The ubiquitin-dependent proteolytic system and other potential targets for the modulation of nuclear factor-kB (NF-kB) Curr. Drug Targets. 2000;1:387–399. doi: 10.2174/1389450003349056. [DOI] [PubMed] [Google Scholar]
- Matos R.S., Oria R.B., Bruin P.F.C., Pinto D.V., Viana A., Santos F.A., Duarte A.S.G., Bruin V.M.S. Acute blockade of endogenous melatonin by Luzindole, with or without peripheral LPS injection, induces jejunal inflammation and morphological alterations in Swiss mice. Braz. J. Med. Biol. Res. 2021;54:e11215. doi: 10.1590/1414-431X2021e11215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberne P., Smith R.L., Doull J., Goodman J.I., Munro I.C., Portoghese P.S., Woods L.A., Adams T.B., Wagner B.M., Weil C.S., Lucas C.D., Ford R.A. The FEMA GRAS assessment of trans-anethole used as a flavouring substance. Food Chem. Toxicol. 1999;37:789–811. doi: 10.1016/s0278-6915(99)00037-x. [DOI] [PubMed] [Google Scholar]
- Ponte E.L., Sousa P.L., Rocha M.V.A.P., Soares P.M.G., Coelho-de-Souza A.N., Leal-Cardoso J.H., Assreuy A.M.S. Comparative study of the anti-edematogenic effects of anethole and estragole. Pharmacol. Rep. 2012;64:984–990. doi: 10.1016/s1734-1140(12)70895-2. [DOI] [PubMed] [Google Scholar]
- Rodrigues I., Choct M. The foregut and its manipulation via feeding practices in the chicken. Poult. Sci. 2018;97:3188–3206. doi: 10.3382/ps/pey191. [DOI] [PubMed] [Google Scholar]
- Samadi-Noshahr Z., Ebrahimzadeh-Bideskan A., Hadjzadeh M.A., Shafei M.N., Salmani H., Hosseinian S., Khajavi-Rad A. Trans-Anethole attenuated renal injury and reduced expressions of angiotensin II receptor (AT1R) and TGF-beta in streptozotocin-induced diabetic rats. Biochimie. 2021;185:117–127. doi: 10.1016/j.biochi.2021.03.011. [DOI] [PubMed] [Google Scholar]
- Samadi-Noshahr Z., Hadjzadeh M.A., Moradi-Marjaneh R., Khajavi-Rad A. The hepatoprotective effects of fennel seeds extract and trans-Anethole in streptozotocin-induced liver injury in rats. Food Sci. Nutr. 2021;9:1121–1131. doi: 10.1002/fsn3.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjabi S., Hoffmann A., Liou H.-C., Baltimore D., Smale S.T. Selective requirement for c-Rel during IL-12 P40 gene induction in macrophages. PNAS. 2000;97:12705–12710. doi: 10.1073/pnas.230436397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharafan M., Jafernik K., Ekiert H., Kubica P., Kocjan R., Blicharska E., Szopa A. Illicium verum (Star Anise) and trans-anethole as valuable raw materials for medicinal and cosmetic applications. Molecules. 2022;27:650. doi: 10.3390/molecules27030650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J., Xu L., Zeng Y., Gong F. Effect of gut microbiota on LPS-induced acute lung injury by regulating the TLR4/NF-κB signaling pathway. Int. Immunopharmacol. 2021;91 doi: 10.1016/j.intimp.2020.107272. [DOI] [PubMed] [Google Scholar]
- Tappenden K.A. Intestinal adaptation following resection. JPEN. J. Parenter. Enteral Nutr. 2014;38:23S–31S. doi: 10.1177/0148607114525210. [DOI] [PubMed] [Google Scholar]
- Tong Y., Yu C., Xie Z., Ziang X., Yang Z., Wang T. Trans-anethole ameliorates lipopolysaccharide-induced acute liver inflammation in broilers via inhibiting NF-κB signaling pathway. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojdasiewicz P., Poniatowski L.A., Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014 doi: 10.1155/2014/561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y.Y., Zheng Y.K., Yang S., Zhang L.H., Guo S.W., Shi L.L., Xu Y.Q., Jin X., Yan S.M., Shi B.L. Artemisia ordosica polysaccharide alleviated lipopolysaccharide-induced oxidative stress of broilers via Nrf2/Keap1 and TLR4/NF-κB pathway. Ecotoxicol. Environ. Saf. 2021;223 doi: 10.1016/j.ecoenv.2021.112566. [DOI] [PubMed] [Google Scholar]
- Yang H., Song Z., Jin H., Cui Y., Hou M., Gao Y. Protective effect of rhBNP on intestinal injury in the canine models of sepsis. Int. Immunopharmacol. 2014;19:262–266. doi: 10.1016/j.intimp.2014.01.023. [DOI] [PubMed] [Google Scholar]
- Yi Q., Liu J., Zhang Y., Qiao H., Chen F., Zhang S., Guan W. Anethole attenuates enterotoxigenic Escherichia coli-induced intestinal barrier disruption and intestinal inflammation via modification of TLR signaling and intestinal microbiota. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.647242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younis N.S., Mohamed M.E. Anethole's effects against myocardial infarction: The role of TLR4/NFκB and Nrf2/HO1 pathways. Chem. Biol. Interact. 2022;360 doi: 10.1016/j.cbi.2022.109947. [DOI] [PubMed] [Google Scholar]
- Yu C., Tong Y., Li Q., Wang T., Yang Z. Trans-anethole ameliorates intestinal injury through activation of Nrf2 signaling pathway in subclinical necrotic enteritis-induced broilers. Front. Vet. Sci. 2022;9 doi: 10.3389/fvets.2022.877066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C., Wang D., Tong Y., Li Q., Yang W., Wang T., Yang Z. Trans-anethole alleviates subclinical necro-haemorrhagic enteritis-induced intestinal barrier dysfunction and intestinal inflammation in broilers. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.831882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C., Zhang J., Zhang H., Chen Y., Wang C., Zhang L., Ding L., Wang T., Yang Z. Influence of trans-anethole on the nutrient digestibility and intestinal barrier function in broilers. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Lin L., Zhang Z., Zhang H., Hu H. Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct. Target. Ther. 2020;5:209. doi: 10.1038/s41392-020-00312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Chen Y., Chen Y., Li Y., Jia P., Ji S., Zhou Y., Wang T. Dietary pterostilbene supplementation attenuates intestinal damage and immunological stress of broiler chickens challenged with lipopolysaccharide. J. Anim. Sci. 2020;98:skz373. doi: 10.1093/jas/skz373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Yu X., Li Q., Cao G., Feng J., Shen Y., Yang C. Effects of rhamnolipids on growth performance, immune function, and cecal microflora in linnan yellow broilers challenged with lipopolysaccharides. Antibiotics (Basel) 2021;10:905. doi: 10.3390/antibiotics10080905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C., Song B., Duan Y., Zhong Y., Yan Z., Zhang S., Li F. Dietary beta-hydroxy-beta-methylbutyrate improves intestinal function in weaned piglets after lipopolysaccharide challenge. Nutrition. 2020;78 doi: 10.1016/j.nut.2020.110839. [DOI] [PubMed] [Google Scholar]