Abstract
Interleukin-2 (IL-2)/IL-15 receptor β (IL-15Rβ)−/− mice were susceptible to infection with avirulent Salmonella enterica subsp. enterica serovar Choleraesuis, whereas IL-2−/− mice were resistant. A natural killer cell response was not evident for both types of deficient mice. A Th1 response was detected in IL-2−/− but not in IL-2/IL-15Rβ−/− mice infected with Salmonella, suggesting that IL-2/IL-15Rβ signaling is important for the generation of protective Th1 cells.
In mice lacking the interleukin-2 (IL-2)/IL-15 receptor β (IL-2/IL-15Rβ−/− mice), natural killer (NK) cells and NK T cells are completely absent, and the number of intestinal intraepithelial lymphocytes is severely diminished (9). On the other hand, mainstream T cell and NK cell developments in mice with a disrupted IL-2 gene (IL-2−/− mice) are normal (6). These observations suggest that cytokines other than IL-2, such as IL-15, which uses IL-2/IL-15Rβ, are important for the development of a significant fraction of NK and T cells. It was previously reported that protection against avirulent Salmonella enterica subsp. enterica serovar Choleraesuis 31N-1 in mice mainly depends on NK cells and a type 1 helper T-cell (Th1) response and that IL-15 plays an important role in protection against Salmonella infection via induction of an NK response (2). In the present study, we compared the immunity of IL-2−/− and IL-2/IL-15Rβ−/− mice to systemic infection with avirulent serovar Choleraesuis to investigate the role of the IL-2/IL-15Rβ chain in resistance against primary infection with avirulent Salmonella.
IL-2/IL-15Rβ−/− mice have previously been described (8). Mice genetically deficient in IL-2 gene expression bred to the C57BL/6 background were obtained from Jackson Laboratory (Germantown, N.Y.). S. enterica subsp. enterica serovar Choleraesuis strain 31N-1, which is a derivative cured of a virulence plasmid of 50 kb, was used as the avirulent strain (1). Mice were inoculated intraperitoneally (i.p.) with 2 × 106 viable Salmonella serovar Choleraesuis cells in 0.2 ml of phosphate-buffered saline. On day 3 or 6 after inoculation, peritoneal contents were lavaged with 5 ml of phosphate-buffered saline and harvested after gentle massage. Samples were spread on tryptic soy agar (Nissui Pharmaceutical, Tokyo, Japan) plates. Peritoneal exudate cells (PEC) were prepared by centrifuging peritoneal exudates at 110 × g for 10 min and used for flow cytometric (FCM) analysis and T-cell culture.
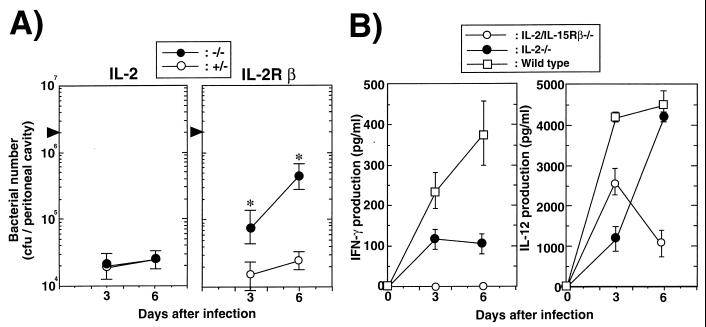
To investigate the susceptibility of IL-2/IL-15Rβ−/− or IL-2−/− mice to systemic Salmonella infection, we compared bacterial growth in the peritoneal cavity after an i.p. inoculation with serovar Choleraesuis 31N-1. As shown in Fig. 1A, the bacterial number in the peritoneal cavities of IL-2/IL-15Rβ−/− mice on days 3 and 6 after infection with serovar Choleraesuis was significantly higher than that from control mice. On the other hand, there was no difference in the susceptibility to Salmonella infection on days 3 and 6 between IL-2−/− and control mice (Fig. 1A). Almost all IL-2/IL-15Rβ−/− mice died within 12 days after infection with Salmonella, whereas all IL-2−/− and control mice survived for more than 30 days (data not shown). In addition, the surviving mice cleared infection within 30 days, as assessed by bacterial counting in the peritoneum, spleen, and liver (data not shown). Thus, IL-2/IL-15Rβ−/− mice are highly susceptible to serovar Choleraesuis 31N-1 infection, but IL-2−/− mice showed the same resistance as control mice.
FIG. 1.
Susceptibility to Salmonella serovar Choleraesuis avirulent strain 31N-1 of IL-2/IL-15Rβ-or IL-2-deficient mice. (A) The number of serovar Choleraesuis cells recovered from peritoneal cavities of IL-2/IL-15Rβ- or IL-2-deficient mice (−/−) or their littermate control mice (+/−) after i.p. infection with serovar Choleraesuis (2 × 106 CFU). The number of bacteria was determined by a colony formation assay in tryptic soy agar plates. The data are representative of three independent experiments and are shown as the means ± standard deviations (SDs) for three to five mice. Statistically significant differences (∗) between deficient mice and control mice are shown (P < 0.001). (B) Early production of cytokines in the sera of IL-2/IL-15Rβ−/− or IL-2−/− mice after i.p. infection with serovar Choleraesuis (2 × 106 CFU). Sera were collected from deficient mice or wild-type control mice on day 0, 3, or 6 after i.p. challenge with serovar Choleraesuis. The amounts of IFN-γ or IL-12 p40 in the serum were determined by an enzyme-linked immunosorbent assay. Data were obtained from three separate experiments and are expressed as the means ± SDs for three to five mice.
IL-12 and gamma interferon (IFN-γ) at the initial phase of the immune response are known to play a pivotal role in the generation and selective activation of Th1 cells (3, 7). We next compared IL-12 and IFN-γ production in the serum of IL-2/IL-15Rβ−/− and IL-2−/− mice infected with serovar Choleraesuis. As shown in Fig. 1B, there was no significant difference in IL-12 production at the early stage of infection between IL-2/IL-15Rβ−/− and IL-2−/− mice. On the other hand, the IFN-γ production was severely impaired in the serum of IL-2/IL-15Rβ−/− mice on day 3 after i.p. inoculation with Salmonella compared to that of wild-type mice (Fig. 1B). A significant amount of IFN-γ was detected in the serum of IL-2−/− mice on day 3 after i.p. inoculation with Salmonella (Fig. 1B). Thus, early production of IFN-γ is impaired in IL-2/IL-15Rβ−/− mice but not in IL-2−/− mice after Salmonella infection.
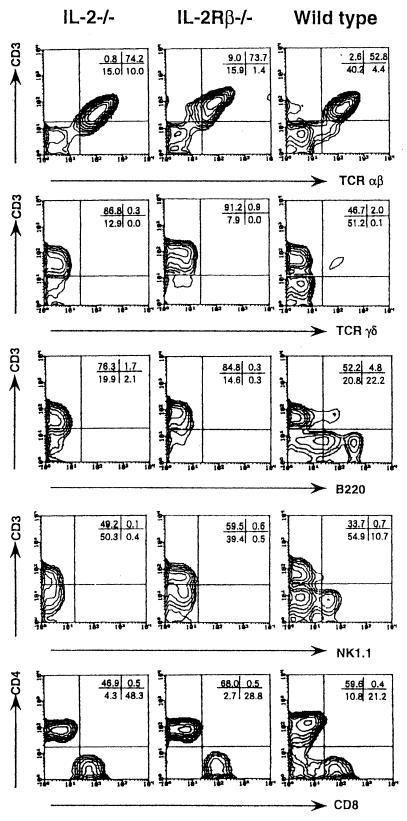
To compare the cellular responses in the peritoneal cavities of IL-2/IL-15Rβ−/− and IL-2−/− mice after infection with Salmonella, the expression of NK1.1, CD3, T-cell receptor (TCR) αβ, TCRγδ, CD4, and CD8 was examined by FCM analysis. The absolute number of peritoneal nonadherent cells was significantly lower in IL-2/IL-15Rβ−/− mice than in IL-2−/− and control mice on day 6 after infection with Salmonella (P < 0.01) (data not shown). As shown in Fig. 2, the proportion of NK1.1+ CD3− cells, which were representative of NK cells, was increased in the peritoneal cavities of control mice after infection with Salmonella, whereas such an increase was not evident in IL-2/IL-15Rβ−/− or IL-2−/− mice. Notably, the relative number of CD8+ cells was markedly increased in the peritoneal cavities of IL-2−/− mice, whereas such an increase of CD8+ cells was not evident for IL-2/IL-15Rβ−/− or control mice. The absolute number of CD4+ cells in the peritoneal cavities of IL-2/IL-15Rβ−/− mice was increased as much as that of control mice.
FIG. 2.
FCM analysis of the nonadherent PEC from IL-2/IL-15Rβ- or IL-2-deficient mice or wild-type mice on day 6 after i.p. infection with serovar Choleraesuis (2 × 106 CFU). The cells were stained with a fluorescein isothiocyanate (FITC)-CD3 monoclonal antibody (MAb) (145-2C11) and a biotin-NK1.1 MAb (PK136) followed by streptavidin-RED 613, with FITC-CD3 (145-2C11) and a phycoerythrin (PE)–anti-TCR αβ MAb (H57-597), a PE–anti-TCR γδ MAb (GL3), or an anti-CD45R/B220 MAb (RA3-6B2) and analyzed by FCM, with the analysis gate set for small lymphocytes. In some experiments, the cells were stained with a FITC-CD3 MAb (145-2C11), a PE–anti-CD8 MAb (53-6.7), and a RED–anti-CD4 MAb (RM4-5), and the analysis gate was set for CD3+ cells. Data are representative of two independent experiments using pooled cells from three to five deficient mice and are shown as typical two-color profiles.
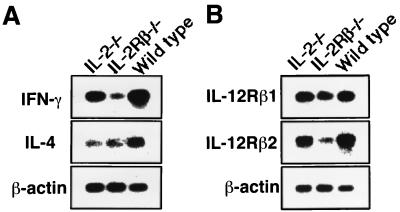
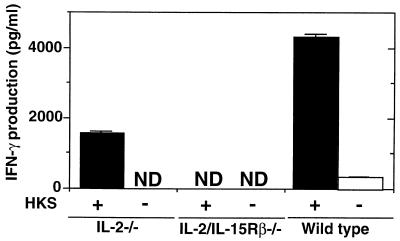
To determine whether Th1 cells are generated in IL-2/IL15Rβ−/− mice following Salmonella infection, we first examined the mRNA expression of cytokines in freshly isolated CD4+ T cells from deficient mice on day 6 after infection with serovar Choleraesuis. The purity of magnetic bead cell sorter column-sorted CD4+ T cells was confirmed to be more than 95% by FCM analysis. As shown in Fig. 3A, the expression level of IFN-γ in the CD4+ T cells from IL-2/IL-15Rβ−/− mice was significantly lower than that of IL-2−/− or C57BL/6 mice on day 6 after infection with Salmonella. We next examined the expression of the IL-12Rβ1 and IL-12Rβ2 subunits in the CD4+ T cells from IL-2/IL-15Rβ−/− mice. As shown in Fig. 3B, the expression of the IL-12Rβ2 subunit was reduced in the IL-2/IL-15Rβ−/− mice compared to that of control mice or IL-2−/− mice. However, there was no difference in the expression level of the IL-12Rβ1 subunit between IL-2/IL-15Rβ−/− and IL-2−/− or control C57BL/6 mice. We next examined the cytokine production of the peritoneal CD4+ T cells in response to heat-killed Salmonella (HKS). As shown in Fig. 4, CD4+ T cells from IL-2−/− mice on day 6 after infection with Salmonella could produce a significant amount of IFN-γ in response to HKS, whereas no IFN-γ was detected in the culture supernatant of CD4+ T cells from IL-2/IL-15Rβ−/− mice on day 6 after infection with Salmonella. No IL-4 was produced by the T cells from Salmonella-infected mice of any group in response to HKS in the presence of mitomycin C-treated spleen cells (data not shown). With the data taken together, serovar CD4+ T cells from IL-2/IL-15Rβ−/− mice fail to mount Th1 responses against infection with serovar Choleraesuis, at least partly due to impaired early production of IFN-γ. Further analysis is required to clarify the possibility.
FIG. 3.
Gene expression of cytokines (A) or IL-12Rβ subunits (B) in the enriched CD4+ T cells from IL-2/IL-15Rβ- or IL-2-deficient mice or wild-type mice on day 6 after i.p. infection with serovar Choleraesuis (2 × 106 CFU). Total RNA was extracted from the magnetic beads cell sorter column-sorted CD4+ T cells by an acid guanidinium thiocyanate-phenol-chloroform method and reverse transcribed, and the cDNA was amplified using specific primers. After amplification, the PCR products were resolved on 1.8% agarose gels and Southern blotted using cytokine-specific internal oligonucleotide probes. After hybridization, the membrane was exposed to X-ray film. The data are representative of three independent experiments using pooled cells from three to five mice of each group.
FIG. 4.
Cytokine production by PEC from IL-2/IL-15Rβ- or IL-2-deficient mice or wild-type mice on day 6 after i.p. infection with serovar Choleraesuis (2 × 106 CFU) in response to HKS. The enriched T cells (5 × 105) were cultured with HKS in the presence of mitomycin C-treated spleen cells (5 × 105) from uninfected C57BL/6 mice for 2 days at 37°C. Thereafter, the supernatants were collected, and cytokine activity was determined by an enzyme-linked immunosorbent assay. The data are representative of three independent experiments using pooled cells from three to five deficient mice and are shown as the means of triplicate determinations ± SDs. ND, not detected.
Early IFN-γ production is thought to be mediated by NK, γδ T, or CD8+ T cells stimulated with IL-15 at the early phase of Salmonella infection (2, 5). NK cell development and activity are impaired in IL-2/IL-15Rβ−/− mice, whereas NK cells in IL-2−/− mice are normal (6, 9). Differences in early IFN-γ production between IL-2/IL-15Rβ−/− and IL-2−/− mice may be due to differences in the NK response following Salmonella infection. However, the NK response was also severely impaired in IL-2−/− mice. Instead, it is notable that CD8+ T cells were significantly increased in IL-2−/− mice. Memory phenotype CD8+ T cells are known to proliferate in response to IL-15 (11). It was previously reported that the memory phenotype of CD8+ T cells produced IFN-γ in response to IL-12 and IL-15 in a bystander manner (4). It can be speculated that early IFN-γ production may be derived from the CD8+ T cells in IL-2−/− mice and that the increased number of CD8+ T cells may consume IL-15, resulting in the impaired NK response in the mice. Thus, impaired early IFN-γ production in IL-2/IL-15Rβ−/− mice after Salmonella infection may be caused by lack of NK and memory phenotype CD8+ T cells that require IL-15 for proliferation and maintenance.
IFN-γ is known to induce IL-12Rβ2 expression via signal transducer and activator of transcription 1 activation and consequently to promote IL-12 responsiveness of CD4+ T cells (10). Therefore, the impairment of early production of IFN-γ in IL-2/IL-15Rβ−/− mice after Salmonella infection may affect the induction of IL-12Rβ2 expression in CD4+ T cells. In fact, our results revealed that IL-12Rβ2 mRNA expression was severely reduced in CD4+ T cells from infected IL2/IL-15Rβ−/− mice. Therefore, the impaired Th1 response in IL-2/IL-15Rβ−/− mice may be due to unresponsiveness to IL-12 in CD4+ T cells that lack IL-12Rβ2 expression. However, we must consider the possibility that intrinsic defects of T cells, such as increased susceptibility to apoptosis, are involved in the impairment of the Th1 response in IL-2/IL-15Rβ−/− mice following Salmonella infection.
Acknowledgments
We thank A. Nishikawa and K. Itano for excellent technical assistance.
This work was supported in part by grants from the Mochida Memorial Foundation, Suzuken Memorial Foundation, the Japanese Ministry of Education, Science and Culture (JSPS-RFTF97L00703), and the Center of Excellence of the Japanese Government.
REFERENCES
- 1.Emoto M, Danbara H, Yoshikai Y. Induction of γ/δ T cells in murine salmonellosis by an avirulent but not by a virulent strain of Salmonella choleraesuis. J Exp Med. 1992;176:363–372. doi: 10.1084/jem.176.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirose K, Nishimura H, Matsuguchi T, Yoshikai Y. Endogenous IL-15 might be responsible for early protection by natural killer cells against infection with an avirulent strain of Salmonella choleraesuis in mice. J Leukoc Biol. 1999;66:382–390. doi: 10.1002/jlb.66.3.382. [DOI] [PubMed] [Google Scholar]
- 3.Mossmann T R, Coffman R L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 4.Nishimura H, Yajima T, Naiki Y, Tsunobuchi H, Umemura M, Itano K, Matsuguchi T, Suzuki M, Ohashi P S, Yoshikai Y. Differential roles of interleukin 15 mRNA isoforms generated by alternative splicing in immune responses in vivo. J Exp Med. 2000;191:157–169. doi: 10.1084/jem.191.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishimura H, Hiromatsu K, Kobayashi N, Grabstein K H, Paxton R, Sugamura K, Bluestone J A, Yoshikai Y. IL-15 is a novel growth factor for murine γδ T cells induced by Salmonella infection. J Immunol. 1996;156:663–669. [PubMed] [Google Scholar]
- 6.Schorle H, Holtschke T, Hunig T, Schimple A, Horak I. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature. 1991;352:621–624. doi: 10.1038/352621a0. [DOI] [PubMed] [Google Scholar]
- 7.Sinigaglia F, D'Ambrosio D, Panina-Bordignon P, Rogge L. Regulation of the IL-12/IL-12R axis: a critical step in T-helper cell differentiation and effector function. Immunol Rev. 1999;170:65–72. doi: 10.1111/j.1600-065x.1999.tb01329.x. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki H, Kundig T M, Furlonger C, Wakeham A, Timms E, Matsuyama T, Scimits R, Simard J J L, Ohashi P S, Griesser H, Taniguchi T, Paige C J, Mak T W. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor β. Science. 1995;268:1472–1476. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki H, Duncan G S, Takimoto H, Mak T W. Abnormal development of intestinal intraepithelial lymphocytes and peripheral natural killer cells in mice lacking the IL-2 receptor β chain. J Exp Med. 1997;185:499–505. doi: 10.1084/jem.185.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szabo S J, Dighe A S, Gubler U, Murphy K M. Regulation of the interleukin (IL)-12Rβ2 subunit expression in developing T helper (Th1) and Th2 cells. J Exp Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zang X, Sun S, Hwang I, Tough D F, Sprent J. Potent and selective stimulation of memory-phenotype CD8+T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]