Abstract
Speckles are common on the brown eggshells of chicken eggs, especially for aged hens. They are important as they affect the consumer preference and economic value of eggs. The cause of speckles in eggshells is still unclear. In this study, we verified the difference of eggshell quality between speckled eggs and normal eggs. Structural characteristics of speckled eggshells were investigated using a scanning electron microscope. Results showed no significant difference in the eggshell quality between normal eggs and speckled eggs, except for a lower eggshell color–L value in the latter. More materials deposited between vertical crystal layer and cuticle layer in the speckled shell region, leading to the thicker eggshell than adjacent normal area. The relative content of protoporphyrin IX was significantly higher in the speckled area than in the adjacent normal area of the eggshells. In addition, there was no significant differences in productive and reproductive performance between hens that laid normal eggs or speckled eggs, except for a lower hatchability of the speckled eggs. In conclusion, we infer that the uneven distribution of eggshell pigment protoporphyrin IX leads to the formation of speckled eggs. This is the first study to establish the characteristics and causes of speckled eggshells. Moreover, this study provides novel insights into external egg quality and a foundation for the in-depth study of speckled eggshells.
Key words: chicken egg, eggshell quality, speckle, protoporphyrin IX pigment
INTRODUCTION
Eggshell quality, including strength and thickness (Benavides-Reyes et al., 2021), gloss (Li et al., 2020), and cuticle quality (Chen et al., 2021) have always been traits of concern for breeders. Reduced eggshell strength and thickness can easily lead to an increase in broken eggs, and eggs with thin cuticle are more susceptible to bacterial invasion, resulting in huge economic losses during production (Sirri et al., 2018; Zhang et al., 2019). Speckles are also a crucial quality trait as they impact the eggshell appearance. Speckles typically appear on the blunt ends of brown eggshell surfaces, particularly those laid by aged laying hens, greatly affecting consumer choices. Researchers have found that speckles are a moderate heritable trait (0.15–0.22), and their presence is related to extreme selection for eggshell color during breeding (Arango et al., 2006).
Notably, for many species, speckles on eggshells are a common phenomenon. Turkey eggs are normally covered with speckles and they are more resistant to external microbial invasion than white-shell turkey eggs (Williams and Dillard, 1969). Japanese quails choose substrates that are more closely related to their speckle patterns for camouflaging (Lovell et al., 2013). In great tit eggs, speckles reduce eggshell permeability during incubation (Higham and Gosler, 2006). Newton (1893) and Wallace (1889) believed that the color and pattern of eggs were adapted to the specific microenvironment of the nest, functioning to conceal eggs from predators. Other researchers have suggested that eggshell speckles are conducive to brood parasitism, particularly in cuckoos (Morelli et al., 2020) and magpies (Martinez et al., 2020), thereby reducing the costs of reproduction (Erritzøe et al., 2012). In contrast, few studies have focused on speckles in chicken eggs.
The eggshell function hypothesis states that calcium availability accounts for changes in eggshell thickness and pigmentation. Birds with lower calcium availability have thinner eggshells and more protoporphyrin pigments in the thinner eggshell areas than those of birds with higher calcium availability (Gosler et al., 2005). However, speckle formation and its functions remain controversial.
Scoring is a common method for evaluating speckle traits. Eggs are artificially divided into four grades according to the speckle size on the eggshell surface. Grade 1 eggs (normal eggs) are defined as eggs with almost no speckles on the eggshell surface, and grade 2 eggs are defined as those with small speckles on the eggshell surface with a diameter of 2 to 3 mm. The speckles of grade 3 eggs are larger than those of grade 2 eggs, with a diameter of 3 to 4 mm; grade 4 egg speckles are over 4 mm in diameter (Cheng et al., 2019). However, the method lacks strict common criteria and varies between different evaluators, thereby resulting in observational errors. Another method of measuring the degree of speckles is by calculating the percentage of speckles in the total eggshell area in a photograph taken using a digital camera and by analyzing it using an image-editing software (Martínez-de la Puente et al., 2007). This method is more objective but requires more operational time.
Only a few studies have examined the function of speckles in chicken eggshells, although none of the hypotheses have been tested. Thus, this study aimed to analyze the eggshell quality of speckled eggs and normal eggs, the basic characteristics of speckled eggshells, and the relative content of protoporphyrin IX pigments in the speckled and adjacent normal region. In addition, we compared the production performance and reproductive performance between hens laying normal eggs and those laying speckled eggs.
MATERIALS AND METHODS
Ethics Statement
This study was conducted in accordance with the guidelines of the experimental animals established by the Animal Care and Use Committee of China Agricultural University.
Animals and Management
A total of 3,000 Rhode Island Red hens (30 wk of age) were raised in Hebei Rongde Poultry Breeding Company Limited, Hebei, China. All hens were raised in individual cages under a standard photoperiod of 16L:8D. Hens at 30 wk of age were divided into two groups according to the eggs they laid, namely a speckle group (speckled eggs) and a normal group (normal eggs) with 120 hens per group, according to the visual scoring method described by Cheng et al. (2019). The basal diets (Table 1) conformed to the nutrient requirements of laying hens in China (NY/T33-2004). Feed and water were provided to hens ad libitum. This study was conducted for 10 wk; it started with 30-wk-old hens until they were 40 wk old.
Table 1.
Ingredients and nutrient composition of basal diet.
| Ingredients | Percent | Nutrient levelc | Percent |
|---|---|---|---|
| Corn (CP 8.3%) | 62.8 | Metabolizable energy (ME, MJ/Kg) | 16.03 |
| Soybean meal (CP 44.0%) | 23.8 | Crude protein (CP, %) | 16.11 |
| Soybean oil | 1.6 | Crude fibre (CF, %) | 3.23 |
| Wheat bran | 0.62 | Methionine (%) | 0.25 |
| Limestone | 9.0 | Lysine (%) | 0.70 |
| Calcium hydrogen phosphate | 1.19 | Calcium (%) | 3.49 |
| Sodium chloride | 0.21 | Total P (%) | 0.32 |
| DL-Methionine (98%) | 0.12 | ||
| L-Lysine HCL (78%) | 0.14 | ||
| Vitamin premixa | 0.05 | ||
| Mineral premixb | 0.20 | ||
| Choline chloride (50%) | 0.2 | ||
| Phytase | 0.02 | ||
| NSP enzyme | 0.05 | ||
| Total | 100.0 |
Note:
Supplied per kilogram of diet: vitamin A, 13,500 IU; vitamin D3, 4,500 IU; vitamin E, 75 IU; vitamin K3, 3.6 mg; vitamin B1, 3.0 mg; vitamin B2, 9.24 mg; vitamin B6, 6.0 mg; nicotinic acid, 66 mg; pantothenic acid, 16.8 mg; biotin, 0.54 mg; folic acid, 2.10 mg; vitamin B12, 0.03 mg; vitamin C, 135 mg; choline, 675 mg; ethoxyquinoline, 15 mg.
Mineral premix provided per kilogram of complete diet: iron, 80 mg; copper, 10 mg; manganese, 100 mg; zinc, 100 mg; iodine, 0.35 mg; selenium, 0.30 mg.
ME, CP, and CF were measured values, and the other nutrients were calculated values.
Eggshell Quality of Normal Eggs and Speckled eggs
Twenty-one eggs of normal (grade 1) and speckled eggshells (seven eggs of each of the grades 2, 3, and 4) were collected from the 40-wk-old hens for eggshell quality determination. Egg weight (EW), eggshell strength (ESS), eggshell thickness (EST), and eggshell color (ESC) were assessed. EW was measured using an electronic balance accurate to 0.01 g. ESS was detected by the Eggshell Force Gauge (ModelII, Robotmation, Tokyo, Japan). EST was measured using a digital display micrometer (model 395-741-10, Mitutoyo, Tokyo, Japan). EST was measured at the blunt pole, equatorial regions, and sharp pole of the egg, and the average thickness of the three positions was taken as the EST of the egg. ESC was measured using a portable spectrophotometer (CM-2600d, Konica Minolta, Tokyo, Japan).
Scanning Electron Microscopy (SEM) Analysis of the Eggshells
Thirteen speckled eggs were collected from the 40-wk-old hens to study their microstructure using SEM. The egg contents were removed, the eggshell was washed with deionized water and dried at room temperature for 24 h. Eggshell fragments (1–2 cm2) in the equatorial regions were cut from the speckled eggs using scissors, tweezers, and scalpels. The eggshell fragments contained the speckle and its adjacent normal regions (Orlowski et al., 2020). The longitudinal section of the eggshells was mounted on a metal platform and spray coated with gold for 15 min. The prepared shells were finally observed under a scanning electron microscope (TESCAN Co., Ltd., Brno, Czech Republic) (Ma et al., 2020).
The eggshell thickness (mammillary layer, palisade layer, vertical crystal layer, and cuticle layer), effective eggshell thickness (mammillary layer, palisade layer, and vertical crystal layer), and cuticle thickness were measured using Image J software (https://imagej.nih.gov/ij/download.html) after scanning the longitudinal eggshell section of the speckled region and the background color region. One eggshell fragment was examined from each of the 13 eggs, and three images were taken from each sample.
Determination of Protoporphyrin IX Content in the Eggshells
To analyze the main ingredients of the loose materials, we measured the relative content of protoporphyrin IX pigment between the speckled region and the adjacent normal region of the same speckled egg. For that, seventeen speckled eggs from the 40-wk-old speckled hens were collected. Eggshells were washed with deionized water and air-dried for 12 h to a constant mass. All speckled regions were accurately cut off and separated from the normal regions of eggshells. A total of 17 pairs of samples were obtained and weighed. For determination of protoporphyrin IX content, speckled and normal region samples (3.6 mg each) were solubilized in 6 mL eggshell solution water (concentrated hydrochloric acid: methanol = 1:2) until the samples completely dissolved. The solubilized samples were then centrifuged at 3,500 × g for 45 min, and the supernatant (200 µL) was transferred to a 96-well plate for the measurement of absorbance at a 412-nm wavelength using a microplate reader (Spectra Max i3 × 03030923, Thermo Fisher Scientific, Waltham, MA). Standard curves of protoporphyrin IX were constructed to calculate the concentration in the samples.
Production Performance
Production performance data were collected of the two groups (hens that laid speckled eggs or normal colored eggs) during 10 wk (30 wk old to 40 wk old). The collected data included hen's body weight at 40 wk, total number of eggs laid per hen from 30 to 40 wk, total EW laid at 40 wk, total number of eggs laid at 40 wk, and the degree of translucent eggs laid at 40 wk. Single egg average weight at 40 wk was also verified (total EW laid at 40 wk/total number of eggs laid at 40 wk). Hen's body weight at 40 wk, and total EW laid at 40 wk were measured using 2 electronic balances of different ranges accurate to 0.01 g. The degree of translucent eggs at 40 wk were measured via the grading method of Wang et al. (2019).
Reproductive Performance
The breeding eggs used in this section were also laid by the Rhode Island Red hens (40 wk of age) from Zhuozhou Mufeng Co., LTD., Hebei, China. There were 6000 Rhode Island Red hens. Reproductive performance data of speckled breeding eggs and normal breeding eggs, including fertilization rate, hatchability rate, and water loss rate, were compared. The hens were artificially inseminated with 50 μL pooled semen of Rhode Island Red roosters of same age at 2 PM consecutively for 2 d. The artificial insemination was done to ensure that there were no discrepancies owing to the fertilization and incubation effects caused by differences in the individual roosters. Breeding egg collection was initiated on the third day after insemination and was continued for 3 more days. Dirty, broken, double yolk, and other unqualified eggs were removed from the collection. From the collected eggs, 840 normal and 840 speckled eggs each with similar weights were selected. From those, 180 eggs from each group were randomly selected to be weighed and marked before hatching. The 180 eggs from each group were weighed again on the 19th day to calculate the water loss rate. All selected eggs were uniformly distributed in the same incubator (EIFXDT-90720, Bengbu Sanyuan Incubation Equipment Co., Ltd., Anhui, China) to ensure that the incubation process and environment remained the same (temperature 38°C and relative humidity 55%). On the 19th day of hatching, the numbers of unfertilized eggs and dead embryos were recorded by illuminating the eggs. On the 21st day of hatching, the numbers of hatched, male and female chicks were counted. Indices such as fertilization rate, embryonic mortality rate, hatchability of fertilized eggs, hatchability of eggs, healthy chicken rate, healthy female chicken rate, and water loss rate at the 19th day were calculated according to the formulas below:
Statistical Analysis
All the data were recorded and analyzed using MS Excel 2020 and SPSS 24.0 for Windows (SPSS Inc., Chicago, IL). Shapiro–Wilk test was used to assess the normality of all data. The differences of eggshell quality, productive performance, and reproductive performance indexes were analyzed using the independent sample t-test. Protoporphyrin IX content and the thickness of the speckled region and the background color region on the same egg were compared using the paired samples t-test at a significance level of 5% (P ≤ 0.05) in SPSS. The nonparametric test Mann-Whitney U test was used for the data that was not normally distributed. A significant difference was considered when P < 0.05, and a trend was considered when 0.05 < P < 0.10.
RESULTS AND DISCUSSION
Eggshell Quality of Normal Eggs and Speckled Eggs
The eggshell quality data of normal eggs and speckled eggs are shown in Table 2. Results showed that eggshell color L value (ESC–L) of the speckled eggs was significantly lower than that of normal eggs. ESC–L is the brightness value, which ranges from 0 to 100, representing all black to pure white. The larger the value, the whiter the eggshell color. According to the results, the speckled eggs had a darker eggshell than that of the normal eggs. Other eggshell qualities, such as eggshell color a value (ESC–a), eggshell color b value (ESC–b), EW, ESS, and EST did not differ significantly between the two groups, suggesting that the speckled eggs did not differ significantly from normal eggs except for the darker eggshell color. The eggshell pigment of brown eggs is mainly protoporphyrin IX, which is evenly deposited on the eggshell surface during the formation of the eggshell (Samiullah et al., 2015). We hypothesize that the significant difference in L value of eggshell color between normal eggs and speckled eggs might be related to the deposition of pigmentation. Our results differed from those of Underwood and Sealy (2002), who reported that speckles on blue tit eggs increased eggshell strength. This may be due to the different location of speckles, since speckles of blue tit eggs are on the interior surface of the eggshell, while the chicken egg speckles are located on the exterior eggshell surface (Jagannath et al., 2007).
Table 2.
Eggshell quality of normal and speckled eggs.
| Items | Normal eggs (n = 21) | Speckled eggs (n = 21) | SEM | P-value |
|---|---|---|---|---|
| EW (g) | 57.98 | 55.86 | 0.57 | 0.06 |
| ESC-L | 63.56a | 61.25b | 0.59 | 0.04 |
| ESC-a | 18.82 | 19.41 | 0.29 | 0.32 |
| ESC-b | 26.91 | 27.40 | 0.25 | 0.33 |
| ESS (kgf) | 3.83 | 3.91 | 0.14 | 0.78 |
| EST (mm) | 0.32 | 0.34 | 0.00 | 0.17 |
The values in the same row with different superscript letters differ statistically (P < 0.05).
Ultrastructural Observation of Speckled and Normal Eggshells
To investigate the structure of speckled eggshells, we detected their ultrastructure using a SEM. The results are shown in Figure 1 and Table 3. The eggshell and cuticle layer of the speckled region were significantly thicker than those of the background color region (P < 0.01), but the thickness of the effective eggshell layer was not significantly different between the two regions (Figure 1 A and B, Table 3). This result suggests that the thicker eggshell in the speckled region is due to the thicker cuticle layer in the speckled region. Moreover, more loose material was observed between the vertical crystal layer and cuticle layer in the speckled region than in the background color region of the same egg (Figure 1 C and D). Studies have shown that most of the eggshell pigment is deposited between the vertical crystal layer and cuticle layer (Kulshreshtha et al., 2022). Therefore, we speculate that the loose material may be the eggshell pigment protoporphyrin IX. In a previous study, Gosler et al. (2005) reported that the speckled eggshell is significantly thinner than the adjacent normal eggshell in great tits. This contrasts with our results, which may be due to the differences present in both the species. The great tit is a small passerine whose eggs lack the vertical crystal layer. In our study, the eggs had an integrated shell structure. Jagannath et al. (2007) noted that only speckles internalized within the shell can compensate for calcium deficiency, and superficial speckles showed no correlation with localized shell thinning but may have contributed to crypsis. From the SEM images, we found speckles located in the superficial eggshell, although the function of speckles still needs further study.
Figure 1.
Scanning electron microscopy (SEM) images. (A) Longitudinal section of the speckled eggshell region. (B) Longitudinal section of the background color region. (C) Cuticle layer of speckled region. (D) Cuticle layer of background color region. Red line: eggshell thickness; blue line: effective eggshell thickness; yellow line: cuticle layer thickness. Scale bars are: (A) and (B) 50 µm, (C), and (D) 5 µm.
Table 3.
Eggshell thickness of the speckled region and background color region of speckled eggs.
| Items | Background color region (n = 13) | Speckled region (n = 13) | SEM | P-value |
|---|---|---|---|---|
| Eggshell thickness (μm) | 329.76B | 360.53A | 4.26 | < 0.01 |
| Cuticle thickness (μm) | 3.32B | 31.93A | 4.25 | < 0.01 |
| Effective eggshell thickness (μm) | 326.44 | 328.59 | 1.59 | 0.20 |
The values in the same row with different superscript letter differ statistically (P < 0.01).
Protoporphyrin IX Content in Speckled and Adjacent Normal Area
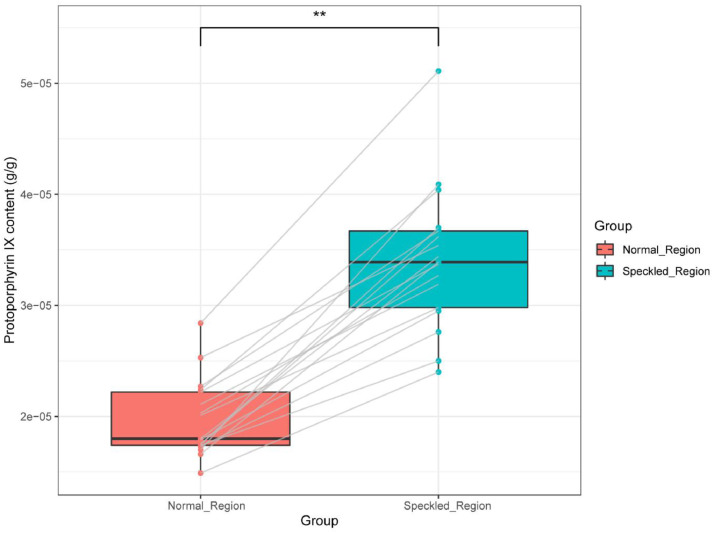
To analyze the main ingredients of the loose materials, we measured the relative content of protoporphyrin IX pigment between the speckled region and the adjacent normal region of the speckled egg. The results are presented in Table 4 and Figure 2. Significant differences in protoporphyrin IX content between the speckled and the adjacent normal regions was observed (P < 0.01). The protoporphyrin IX content of the speckled region was significantly higher than that of the adjacent normal region. Underwood and Sealy (2002) reported that the primary constituent of speckles in the eggshell is protoporphyrin IX. Our results are consistent with these findings. Other studies have also focused on the levels of trace elements, particularly calcium and magnesium, between the speckled and adjacent normal region, indicating that speckles may play a functional role in the chemistry of avian eggs (Orłowski et al., 2017; Orlowski et al., 2019).
Table 4.
Relative content of protoporphyrin IX in the speckled region and normal region of the speckled eggs.
| Items | Normal region (n = 17) | Speckled region (n = 17) | SEM | P-value |
|---|---|---|---|---|
| Protoporphyrin IX content (g/g) | 2.13B | 3.52 A | 0.12 | <0.01 |
The values in the same row with different superscripts represent significantly different (P < 0.01).
Figure 2.
Protoporphyrin IX content between the speckled and adjacent normal region. ⁎⁎P-value < 0.01.
Production Performance
The egg production capacity of hens laying speckled eggs and hens laying normal eggs was compared to further understand the physiological differences between them. The performance data are presented in Table 5. No difference in any parameter was observed (P > 0.05). This result indicates that, although speckles affect eggshell appearance, they have no significant effect on the production performance of laying hens.
Table 5.
Production performance of hens laying normal eggs and hens laying speckled eggs.
| Items | Hens laying normal eggs (n = 120) | Hens laying speckled eggs (n = 120) | SEM | P-value |
|---|---|---|---|---|
| Hen's body weight at 40 wk (g) | 1903.51 | 1955.72 | 14.78 | 0.08 |
| Single egg average weight at 40 wk (g) | 57.91 | 57.21 | 0.3 | 0.24 |
| Total number of eggs laid per hen from 30 to 40 wk | 64.20 | 64.83 | 0.44 | 0.834 |
| Degree of translucent eggs | 1.67 | 1.83 | 0.06 | 0.21 |
Reproduction Performance
Reproduction performance is a crucial factor that determines profitability in the poultry industry. Reproductive performance data of speckled breeding eggs and normal breeding eggs, including fertilization rate, hatchability rate, and water loss rate, were compared. The results are presented in Table 6. No difference was observed in embryonic mortality rate, hatchability rate of fertilized eggs, healthy chicken rate, healthy female chicken rate, and water loss rate at the 19th day. The fertilization rate of speckled eggs showed a reduction trend (P = 0.06). Meanwhile, the hatchability rate of speckled eggs was significantly lower than that of normal eggs (P < 0.05), and the hatchability rate of fertilized eggs showed no significant differences (P > 0.1). Qualities such as shell thickness and strength are reportedly associated with hatchability (Roque and Soares, 1994; Gonzalez et al., 1999). However, in our eggshell quality results, there was no significant difference in eggshell strength or thickness between normal and speckled eggs. Therefore, we hypothesize that the significantly reduced hatchability rate of speckled eggs may be associated with fertilization rate.
Table 6.
Reproduction parameters of normal and speckled breeding eggs.
| Items | Normal breeding eggs (n = 840) | Speckled breeding eggs (n = 840) | SEM | P-value |
|---|---|---|---|---|
| Fertilization rate (%) | 93.33 | 89.61 | 1.00 | 0.06 |
| Embryonic mortality rate (%) | 1.14 | 1.06 | 0.29 | 0.90 |
| Hatchability rate of fertilized eggs (%) | 92.24 | 90.55 | 0.72 | 0.26 |
| Hatchability rate of eggs (%) | 86.05a | 81.14b | 1.14 | 0.03 |
| Healthy chicken rate (%) | 98.75 | 98.80 | 0.33 | 0.95 |
| Healthy female chicken rate (%) | 51.50 | 49.32 | 1.12 | 0.36 |
| Water loss rate at the 19th day (%) (n = 180) | 11.84 | 12.21 | 0.13 | 0.405 |
The value in the same row with different superscripts represent significantly different (P < 0.05).
In this study, from the eggshell ultrastructural results, there are lots of loose materials covering the vertical crystal layer in the speckled shell region, which can get clogged in pores on the eggshell surface. Eggshell pores are the only way for the embryo to exchange gas and water vapor with the outside environment (Ar et al., 1974; Rahn and Ar, 1980). If numerous pores are blocked during incubation, gas and water exchange through the eggshell is suppressed, resulting in the death of the embryo. Therefore, the hatchability rate of fertilized speckled eggs may be lower than that of normal eggs. However, in this study, we found no significant difference in water loss rate between the speckled eggs and the normal eggs, and there was no significant difference in the hatchability rate of the fertilized eggs. This may be because the speckles on the eggshell surface are not so numerous that they completely block all the pores.
Higham and Gosler (2006) reported that eggshell speckles in great tit eggs can reduce eggshell permeability and affect the rate of water loss during incubation, which differs from our results. This may be due to the different eggshell structure and speckle distribution range between chicken and great tit eggs. Compared with chicken eggs, the eggshell structure of great tit eggs lacks vertical crystal layers. And the eggshell speckles of the great tit are interior within the eggshell, while eggshell speckles of chicken eggs are located between the vertical crystal layer and the cuticle.
CONCLUSIONS
The structural characteristics of speckled eggs and their effect on reproduction performance were investigated. The eggshell color L value of speckled eggs was lower than that of normal eggs. There was no difference in eggshell thickness between speckled eggs and normal eggs, but the speckled region was significantly thicker than the nearby background color region in the speckled eggs, caused by the increased thickness between the vertical crystal layer and cuticle layer. And the speckle contains more pigment protoporphyrin IX, which may be the main reason for the formation of speckles. Speckles do not affect the production performance of laying hens and water loss rate of the breeding eggs. These results can promote further studies on the basic characteristics, causes, and formation mechanism of speckled eggs, and provide a foundation in reducing speckled eggs in laying hens.
ACKNOWLEDGMENTS
This study was supported by the China Agriculture Research System (CARS-40) and Beijing Agriculture Innovation Consortium for Poultry (BAIC06-2022-G01).
DISCLOSURES
The authors have no conflicts of interest to declare.
REFERENCES
- Ar A., Paganelli C.V., Reeves R.B., Greene D.G., Rahn H. The avian egg: water vapor conductance, shell thickness, and functional pore area. Condor. 1974;76 [Google Scholar]
- Arango J., Settar P., Arthur J., O'sullivan N. Year. Relationship between shell color and incidence of speckles in brown egg lines. Proc. Proc. XIIth European Poultry Conference. 2006:10–14. [Google Scholar]
- Benavides-Reyes C., Folegatti E., Dominguez-Gasca N., Litta G., Sanchez-Rodriguez E., Rodriguez-Navarro A.B., Umar Faruk M. Research note: changes in eggshell quality and microstructure related to hen age during a production cycle. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., He Z., Li X., Song J., Huang M., Shi X., Li X., Li J., Xu G., Zheng J. Cuticle deposition duration in the uterus is correlated with eggshell cuticle quality in White Leghorn laying hens. Sci. Rep. 2021;11:22100. doi: 10.1038/s41598-021-01718-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X., Fan C., Ning Z. Quality of freckle eggs and its influencing factors. Chinese Poult. Sci. 2019;41:6–9. (In Chinese) [Google Scholar]
- Erritzøe J., Mann C.F., Brammer F.P., Fuller R.A. 1st ed. Christopher Helm Publishers Ltd; London, UK: 2012. Cuckoos of the World. [Google Scholar]
- Gonzalez A., Satterlee D.G., Moharer F., Cadd G.G. Factors affecting ostrich egg hatchability. Poult. Sci. 1999;78:1257–1262. doi: 10.1093/ps/78.9.1257. [DOI] [PubMed] [Google Scholar]
- Gosler A.G., Higham J.P., James Reynolds S. Why are birds’ eggs speckled? Ecol. Lett. 2005;8:1105–1113. [Google Scholar]
- Higham J.P., Gosler A.G. Speckled eggs: water-loss and incubation behaviour in the great tit Parus major. Oecologia. 2006;149:561–570. doi: 10.1007/s00442-006-0484-2. [DOI] [PubMed] [Google Scholar]
- Jagannath A., Shore R.F., Walker L.A., Ferns P.N., Gosler A.G. Eggshell pigmentation indicates pesticide contamination. J Appl. Ecol. 2007;45:133–140. [Google Scholar]
- Kulshreshtha G., D'Alba L., Dunn I.C., Rehault-Godbert S., Rodriguez-Navarro A.B., Hincke M.T. Properties, genetics and innate immune function of the cuticle in egg-laying species. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.838525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Ren J., Wang K., Zheng J., Xu G., Ge C., Yang N., Sun C. The effect of breed and age on the gloss of chicken eggshells. Poult. Sci. 2020;99:2494–2499. doi: 10.1016/j.psj.2020.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell P.G., Ruxton G.D., Langridge K.V., Spencer K.A. Egg-laying substrate selection for optimal camouflage by quail. Curr. Biol. 2013;23:260–264. doi: 10.1016/j.cub.2012.12.031. [DOI] [PubMed] [Google Scholar]
- Ma Y., Yao J., Zhou S., Mi Y., Li J., Zhang C. Improvement of eggshell quality by dietary N-carbamylglutamate supplementation in laying chickens. Poult. Sci. 2020;99:4085–4095. doi: 10.1016/j.psj.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-de la Puente J., Merino S., Moreno J., Tomás G., Morales J., Lobato E., García-Fraile S., Martínez J. Are eggshell spottiness and colour indicators of health and condition in blue tits Cyanistes caeruleus? J. Avian Biol. 2007;38:377–384. [Google Scholar]
- Martinez J.G., Molina-Morales M., Precioso M., Aviles J.M. Age-related brood parasitism and egg rejection in magpie hosts. Am. Nat. 2020;195:876–885. doi: 10.1086/708155. [DOI] [PubMed] [Google Scholar]
- Morelli F., Benedetti Y., Pape Moller A. Diet specialization and brood parasitism in cuckoo species. Ecol. Evol. 2020;10:5097–5105. doi: 10.1002/ece3.6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton A. Adam and Charles Black; London: 1893. A Dictionary of Birds. [Google Scholar]
- Orlowski G., Merta D., Pokorny P., Lukaszewicz E., Dobicki W., Kobielski J., Kowalczyk A., Rzonca Z., Krzywinski A. Eggshell resorption, and embryonic mobilization and accumulation of calcium and metals in eggs of wild and captive Capercaillies Tetrao urogallus. Environ. Pollut. 2019;249:152–162. doi: 10.1016/j.envpol.2019.03.010. [DOI] [PubMed] [Google Scholar]
- Orlowski G., Niedzielski P., Merta D., Pokorny P., Proch J. Quantifying the functional disparity in pigment spot-background egg colour ICP-OES-based eggshell ionome at two extremes of avian embryonic development. Sci. Rep. 2020;10:22107. doi: 10.1038/s41598-020-79040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orłowski G., Pokorny P., Dobicki W., Łukaszewicz E., Kowalczyk A. Speckled and plain regions of avian eggshells differ in maternal deposition of calcium and metals: a hitherto overlooked chemical aspect of egg maculation. Auk. 2017;134:721–731. [Google Scholar]
- Rahn H., Ar A. Gas exchange of the avian egg time, structure, and function. Am. Zool. 1980;20:477–484. [Google Scholar]
- Roque L., Soares M.C. Effects of eggshell quality and broiler breeder age on hatchability. Poult. Sci. 1994;73:1838–1845. doi: 10.3382/ps.0731838. [DOI] [PubMed] [Google Scholar]
- Samiullah S., Roberts J.R., Chousalkar K. Eggshell color in brown-egg laying hens - a review. Poult. Sci. 2015;94:2566–2575. doi: 10.3382/ps/pev202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirri F., Zampiga M., Berardinelli A., Meluzzi A. Variability and interaction of some egg physical and eggshell quality attributes during the entire laying hen cycle. Poult. Sci. 2018;97:1818–1823. doi: 10.3382/ps/pex456. [DOI] [PubMed] [Google Scholar]
- Underwood T.J., Sealy S.G. In: Avian Incubation. Behaviour, Environment and Evolution. Deeming D.C., editor. Oxford University Press; Oxford: 2002. Adaptative significance of egg coloration. [Google Scholar]
- Wallace A.R. Macmillan; London: 1889. Darwinism: An Exposition of the Theory of Natural Selection With Some its Applications. [Google Scholar]
- Wang D.H., Chen H., Zhou R.Y., Huang C.X., Gao H.X., Fan B.L., Liu G.J., Ning Z.H. Study of measurement methods on phenotype of translucent eggs. Poult. Sci. 2019;98:6677–6683. doi: 10.3382/ps/pez539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J.E., Dillard L.H. Salmonella penetration of the outer structures of white- and speckled - shell Turkey eggs. Avian Dis. 1969;13:203–210. [PubMed] [Google Scholar]
- Zhang J., Wang Y., Zhang C., Xiong M., Rajput S.A., Liu Y., Qi D. The differences of gonadal hormones and uterine transcriptome during shell calcification of hens laying hard or weak-shelled eggs. BMC Genomics. 2019;20:707. doi: 10.1186/s12864-019-6017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]