Summary
Given the low prevalence of hearing aid use among individuals with hearing loss due to their high costs and social stigma, personal sound amplification products (PSAPs) may serve as alternatives with adequate hearing compensation and greater accessibility. This study examined the electroacoustic features of hearing aids and selected smartphone-bundled earphones, specifically AirPods, as PSAPs, and compared hearing performances among adults with mild-to-moderate hearing loss when aided with each hearing assistive device. Our results indicated that AirPods Pro met four out of five PSAP standards. No significant differences were found regarding speech perception between AirPods Pro and hearing aids in quiet but not with the presence of background noises. AirPods Pro may have the potential to be a hearing assistive device for adults with mild-to-moderate hearing loss. More research is needed to investigate the safety and feasibility of using earphones bundled with other smartphones as PSAPs.
Subject areas: Health sciences, Medicine, Medical device, Audiology, Medical biotechnology
Graphical abstract
Highlights
-
•
AirPods Pro with smartphone meets most of the ANSI/CTA-2051 electroacoustic standards
-
•
Speech recognition of AirPods Pro is similar to conventional hearing aids in noise
-
•
AirPods Pro may serve as a hearing assistive device for mild-to-moderate hearing loss
Health sciences; Medicine; Medical device; Audiology; Medical biotechnology
Introduction
Hearing loss is the third most common chronic health condition in the United States1 and the fourth highest cause of years lived with a disability at a global level.2 It has been linked to emotional distress,3 social isolation,4 and loneliness,4 which may further contribute to the association between hearing loss and negative physical,5 cognitive,6 and psychological health outcomes.7 Unaddressed hearing loss functionally limits effective communication and can result in a reduced quality of life,8 as well as risky patterns of healthcare utilization that lead to increased healthcare expenses.9 Moreover, since hearing loss has been identified as a potentially modifiable risk factor for dementia during midlife with the highest population attributable fraction,10 improving early intervention among people with impaired hearing needs to be underscored.
The prevalence of hearing loss is expected to increase as adults live longer, such that the prevalence of hearing loss in the United States is expected to double by 2060.11 This public health concern is particularly problematic because nearly 75% of people with hearing loss do not obtain hearing aids.12 Hearing aids are regulated medical devices that are typically distributed by licensed professionals in most countries.13 The major impediment to accessing hearing aids is their high cost (ranging from US$3000 to US$6000 a pair) and limited insurance coverage.14 The complicated and time-consuming acquisition process, which requires multiple otolaryngologist and audiologist visits, also creates a barrier to adequate hearing healthcare.14 Furthermore, the long-existing social stigma associated with hearing loss and hearing aids may also contribute to the low rate of obtaining hearing aids.15
Personal sound amplification products (PSAPs) are wearable electronic devices intended for individuals with normal hearing; these devices are much less expensive over-the-counter products that can be fitted by the users themselves. Although not labeled a regulated medical device that can “treat” hearing loss, evidence shows that PSAPs not only meet the objective electroacoustic criteria when compensating for hearing loss16 but also provide comparable benefits in terms of listening effort and speech-in-noise perception among patients with mild-to-moderate hearing loss.17,18 A recent meta-analysis has confirmed that PSAPs may be equally beneficial as the traditional hearing aids.19 The large size of the consumer population and increasing popularity of PSAPs20 may also support the efficacy of these consumer products as a more affordable and accessible option when addressing hearing loss. However, studies have shown considerable heterogeneity among different products in terms of electroacoustic features, and the quality of PSAPs has been shown to vary; thus the results from previous studies may not be generalizable.18,21 Therefore, further investigations on the feasibility of using specific PSAPs that have higher popularity are needed.
The percentage of smartphone ownership has been increasing over time regardless of the age group.22 Approximately 50% of the Taiwanese population and 58% of the U.S. population are users of Apple mobile phone products,23 including wireless Bluetooth earbuds (i.e., AirPods). First introduced in 2016, the Apple Company implemented the “Live Listen” feature that receives the environmental sounds and transmits the amplified sound through the bundled earphone AirPods, and it was claimed that this feature may resemble, by definition, the functionality of PSAPs.24 Despite the high market share of AirPods, its possible role as a PSAP for aiding individuals with hearing loss has yet to be evaluated. The aim of this study was to assess the electroacoustic properties of AirPods and compare their performance among patients with mild-to-moderate hearing impairment when compensating for hearing loss to that of basic and advanced hearing aids.
Results
Electroacoustic analysis
The electroacoustic test results for the hearing aids showed that the OTICON Opn 1 premium hearing aid and the Bernafon MD 1 basic hearing aid both complied with the ANSI S3.22-2003 standards.
Table 1 demonstrates the ANSI/CTA-2051 comparisons of the AirPods 2 and AirPods Pro regarding the PSAP electroacoustic features. The AirPods 2 met two out of five ANSI/CTA-2051 standards for a PSAP, namely, the frequency response bandwidth and total harmonic distortion. The frequency response curve of the AirPods 2 exhibited a sharp peak at 5000–8000 Hz, which failed to satisfy the smoothness criterion of ANSI/CTA-2051. The Max OSPL 90 values of the left and right AirPods 2 were 123 dB SPL and 122 dB SPL, respectively, and both were 2–3 dB SPL higher than the standard. The AirPods 2 did not fit the equivalent internal noise (EIN) norm either.
Table 1.
The electroacoustic characteristics of AirPods 2 and AirPods Pro
| Parameter | ANSI/CTA-2051 standards | AirPods 2- Right | AirPods 2- Left | AirPods Pro- Right | AirPods Pro- Left |
|---|---|---|---|---|---|
| Frequency response smoothness | No sharp peak shown | aSharp peak shown | aSharp peak shown | No sharp peak shown | No sharp peak shown |
| Frequency response bandwidth | 250–5000 Hz | 200–7100 Hz | 200–7100 Hz | 200–5000 Hz | 200–5000 Hz |
| Max OSPL90 | <120 dB SPL | a122 dB SPL | a123 dB SPL | 110 dB SPL | 110 dB SPL |
| THD | <5% | 0% | 0% | 0% | 0% |
| EIN | <32 dB SPL | a50 dB SPL | a51 dB SPL | a37 dB SPL | a37 dB SPL |
Note: Max OSPL90 = maximum output sound pressure level at 90 dB sound pressure level input; THD = total harmonic distortion; EIN = equivalent internal noise.
Indicates the characteristics that does not meet the ANSI/CTA-2051 criteria.
The AirPods Pro passed four out of five standards per the ANSI/CTA-2051 criteria for PSAPs. The exception was the EIN of the AirPods Pro, which exceeded the standard value (<32 dB SPL) by 5 dB SPL.
Sample characteristics
Table 2 summarizes the characteristics of the study sample. Twenty-one adults (9 males and 12 females) with a mean age of 42.9 years were included in this study. The means of air conduction pure-tone average were similar between the two ears (right: mean, 42.7 dB HL, left: mean, 43.5 dB HL), and none of the participants had prior experience with hearing aids.
Table 2.
Demographics and auditory characteristics of the study sample
| Variables | N = 21 |
|---|---|
| Age (years, mean ± SD) | 42.9 ± 17.0 |
| Sex (n, %) | |
| Male | 9 (42.9%) |
| Female | 12 (57.1%) |
| Pure-tone average (dB HL, mean ± SD) | |
| Right ear | 42.7 ± 7.32 |
| Left ear | 43.5 ± 8.71 |
| Previous experience with hearing aid (n, %) | 0 (0%) |
Note: Data are represented as mean ± SD.
MHINT
Table 3 summarizes the means of the unaided and aided Mandarin hearing in noise test (MHINT) performances. Participants’ speech reception thresholds (SRT) and signal-to-noise ratio (SNR) were the best when aided with the premium hearing aid (quiet: SRT = 30.2 ± 8.9 dBA); noise front: SNR = −1.5 ± 2.0 dB; noise left: SNR = −13.8 ± 1.7 dB). When aided with the AirPods Pro, the SRT in quiet (SRT = 32.2 ± 9.2 dBA) and the SNR with the background noise from the left side (SNR = −13.1 ± 3.2 dB) were similar to the results aided with the premium hearing aid, but when the noises came from the front (i.e., the noises were from the same direction as the target speech), no improvement regarding SNR was found when the AirPods Pro were used as the assistive device (SNR = 0.6 ± 3.3 dB). Table 4 compares the MHINT results when aided with the selected hearing devices to the unaided circumstances. Without the presence of background noises, the SRT aided with premium hearing aid (coefficient = −9.1 dBA, 95% CI = −15.1 to −3.1 dBA), basic hearing aid (coefficient = −7.1 dBA, 95% CI = −12.6 to −1.7 dBA), and AirPods Pro (coefficient = −7.3 dBA, 95% CI = −12.8 to −2.0 dBA) was all significantly better than unaided. In noisy environment, however, regardless of the direction of the noises, the MHINT performances were significantly better when aided with the premium hearing aid than unaided (noise front: coefficient = −1.9 dB SNR, 95% CI = −3.8 to −0.04 dB SNR; noise left: coefficient = −2.4 dB SNR, 95% CI = −4.8 to −0.1 dB SNR), while no differences were found when aided with either the basic hearing aid (noise front: coefficient = 0.1 dB SNR, 95% CI = −1.7 to 2.0 dB SNR; noise left: coefficient = −1.0 dB SNR, 95% CI = −3.3 to 1.4 dB SNR) or the AirPods Pro (noise front: coefficient = 1.1 dB SNR, 95% CI = −0.8 to 3.0 dB SNR; noise left: coefficient = −1.8 dB SNR, 95% CI = −4.1 to 0.6 dB SNR).
Table 3.
Summary of MHINT results
| Cost, US $a | Quiet (dB A, mean ± SD) | Noise Frontb (dB SNR, mean ± SD) | Noise Leftc (dB SNR, mean ± SD) | |
|---|---|---|---|---|
| Unaided | 39.5 ± 9.0 | −0.1 ± 1.9 | −11.3 ± 3.4 | |
| Premium HA | 10,000.0 | 30.2 ± 8.9 | −1.5 ± 2.0 | −13.8 ± 1.7 |
| Basic HA | 1,500.0 | 32.4 ± 7.2 | −0.3 ± 2.1 | −12.3 ± 3.2 |
| AirPods Pro | 249.0 | 32.2 ± 9.2 | 0.6 ± 3.3 | −13.1 ± 3.2 |
| AirPods 2 | 129.0 | 39.4 ± 9.3 | 2.1 ± 2.1 | −11.3 ± 4.2 |
| p | <0.05 | <0.05 | <0.05 |
Note: Data are represented as mean ± SD. HA, hearing aid; dBA, A-weighted decibels; SNR, signal-to-noise ratio; SD, standard deviation.
The prices of the hearing aids were based on the Taiwanese market price; the costs of the AirPods were the official prices from the company website (https://www.apple.com/airpods/).
Noise and the targeting speech were both from the front direction of each participant.
Noise and the targeting speech were from different direction (noises were from the left side of each participant).
Table 4.
Comparison of effect size regarding MHINT results: aided versus unaided
| Condition | Coefficient | SE | 95% CI | p |
|---|---|---|---|---|
| Quiet (dB A) | ||||
| Premium HA vs Unaided | −9.1 | 3.0 | −15.1 to −3.1 | <0.05 |
| Basic HA vs Unaided | −7.1 | 2.6 | −12.6 to −1.7 | <0.05 |
| AirPods Pro vs Unaided | −7.3 | 2.7 | −12.8 to −2.0 | <0.05 |
| AirPods 2 vs Unaided | −0.1 | 3.0 | −6.0 to 5.8 | 0.97 |
| Noise fronta (dB SNR) | ||||
| Premium HA vs Unaided | −1.9 | 0.9 | −3.8 to −0.04 | <0.05 |
| Basic HA vs Unaided | 0.1 | 0.9 | −1.7 to 2.0 | 0.87 |
| AirPods Pro vs Unaided | 1.1 | 0.9 | −0.8 to 3.0 | 0.25 |
| AirPods 2 vs Unaided | 2.3 | 1.0 | 0.3 to 4.4 | <0.05 |
| Noise leftb (dB SNR) | ||||
| Premium HA vs Unaided | −2.4 | 1.2 | −4.8 to −0.1 | <0.05 |
| Basic HA vs Unaided | −1.0 | 1.2 | −3.3 to 1.4 | 0.40 |
| AirPods Pro vs Unaided | −1.8 | 1.2 | −4.1 to 0.6 | 0.14 |
| AirPods 2 vs Unaided | 0.02 | 1.2 | −2.3 to 2.4 | 0.98 |
Note: HA, hearing aid; CI, confidence interval; dBA, A-weighted decibels; SNR, signal-to-noise ratio.
Noise and the targeting speech were both from the front direction of each participant.
Noise and the targeting speech were from different direction (noises were from the left side of each participant).
Table 5 demonstrates the comparison of MHINT performances when aided with each amplification device. In quiet, the SRT aided with the premium hearing aid was 9.2 dBA lower than that with the AirPods 2 (95% confidence interval [CI] = −15.1 to −3.2 dBA) but not significantly lower than the outcome aided with the AirPods Pro (coefficient = −1.9 dBA, 95% CI = −7.4 to 3.6 dBA). When the noises were from the front side (i.e., the target speech and noises were from the same direction), the SNR aided with the premium hearing aid was 3.6 dB SNR lower than the performances aided with the AirPods 2 (95% CI = −5.6 to −1.6 dB SNR) and 2.1 dB SNR lower than that with the AirPods Pro (95% CI = −3.8 to −0.5 dB SNR), respectively. However, when the noises were from the left side of the participants, the SNR aided with the premium hearing aid was not significantly lower than that aided with the AirPods Pro (coefficient = −0.7 dB SNR, 95% CI = −3.0 to 1.7 dB SNR). Regardless of the presence of background noises, the SRTs and SNRs aided with the basic hearing aid were not significantly lower than those aided with the AirPods Pro (quiet: coefficient = 0.57 dBA, 95% CI = −5.9 to 7.0 dB; noise front: coefficient = −0.9 dB SNR, 95% CI = −2.9 to 1.0 dB SNR; noise left: coefficient = 0.8 dB SNR, 95% CI = −1.6 to 3.2 dB SNR).
Table 5.
Comparison of effect size regarding MHINT results when aided with different hearing assistive devices
| Condition | Coefficient | SE | 95% CI | p |
|---|---|---|---|---|
| Quiet (dB A) | ||||
| Premium HA vs Basic HA | −2.1 | 2.8 | −7.6 to 3.4 | 0.45 |
| Premium HA vs AirPods Pro | −1.9 | 2.8 | −7.4 to 3.6 | 0.49 |
| Premium HA vs AirPods 2 | −9.2 | 3.0 | −15.1 to −3.2 | <0.05 |
| Basic HA vs AirPods Pro | 0.2 | 2.8 | −5.3 to 5.7 | 0.94 |
| Basic HA vs AirPods 2 | −7.0 | 3.0 | −13.0 to −1.1 | <0.05 |
| Noise fronta (dB SNR) | ||||
| Premium HA vs Basic HA | −1.2 | 0.8 | −2.9 to 0.4 | 0.15 |
| Premium HA vs AirPods Pro | −2.1 | 0.8 | −3.8 to −0.5 | <0.05 |
| Premium HA vs AirPods 2 | −3.6 | 1.0 | −5.6 to −1.6 | <0.05 |
| Basic HA vs AirPods Pro | −0.9 | 0.8 | −2.6 to 0.7 | 0.27 |
| Basic HA vs AirPods 2 | −2.4 | 1.0 | −4.4 to −0.3 | <0.05 |
| Noise leftb (dB SNR) | ||||
| Premium HA vs Basic HA | −1.5 | 1.2 | −3.8 to 0.9 | 0.22 |
| Premium HA vs AirPods Pro | −0.7 | 1.2 | −3.0 to 1.7 | 0.58 |
| Premium HA vs AirPods 2 | −2.5 | 1.2 | −4.8 to −0.1 | <0.05 |
| Basic HA vs AirPods Pro | 0.8 | 1.2 | −1.6 to 3.2 | 0.50 |
| Basic HA vs AirPods 2 | −1.0 | 1.2 | −3.4 to 1.3 | 0.4 |
Note: HA, hearing aid; CI, confidence interval; dBA, A-weighted decibels; SNR, signal-to-noise ratio.
Noise and the targeting speech were both from the front direction of each participant.
Noise and the targeting speech were from different direction (noises were from the left side of each participant).
Discussion
To our knowledge, this study is the first to use smartphone-bundled earphones as the tested PSAPs to compare hearing performance among patients with symmetrical mild-to-moderate hearing loss. We found that AirPods Pro met most of the electroacoustic criteria and may be considered a competent PSAP, whereas AirPods 2 only met two out of five criteria. We also found that the hearing performance aided with the AirPods Pro was not inferior when compared to conventional hearing aids. In contrast, the outcome aided with AirPods 2 was poorer than hearing aids. The results suggest that for the population with mild-to-moderate hearing impairment, selected products (i.e., AirPods Pro in this study) may have the potential to serve as an adequate hearing assistive device.
Our results have demonstrated that even though patients with mild-to-moderate sensorineural hearing loss (SNHL) had the best speech perception performances with the premium hearing aid, there was no significant difference in SRT in a quiet environment when aided with the AirPods Pro. This suggests that AirPods Pro are not inferior and are able to effectively improve an individual’s hearing, thus serving as an appropriate listening assistive device. In the presence of background noise, the statistics reflected the value of the premium hearing aid as an optimal hearing assistive device compared to the basic hearing aid and the PSAPs, as there were significant differences in the SNR levels. However, when the noises were introduced from the lateral side of the examinee, the estimates showed no differences regarding the speech-in-noise understanding abilities between the conventional hearing aids and the AirPods Pro. Overall, our investigation indicated that, regardless of the type of amplification device, aided speech perception performance was better than unaided speech perception performance among the population with mild-to-moderate hearing impairment.
Although not categorized as a “medical device” or designed for treating hearing-impaired populations based on the guidance drafted by the U.S. Food and Drug Administration,25 PSAPs have been established as having a role in compensating for hearing loss ever since their release given their remarkably low cost and high accessibility compared to conventional hearing aids. Previous studies have shown that selected PSAPs have comparable electroacoustic output to that of traditional hearing aids and are able to provide appropriate amplification for people with mild-to-moderate hearing impairment.16 Based on the results of our electroacoustic analysis, although the AirPods Pro was found to have an EIN that exceeded the standard regulated by the ANSI/CTA-2051, which may affect speech perception among users, this device met all the other criteria, suggesting that AirPods Pro should be considered to be an adequate PSAP. AirPods 2, however, did not fulfill three out of five parameters, and thus the listening experience would be more significantly impacted when using this device as a PSAP.
Prior investigations also compared hearing performances with PSAPs to conventional hearing aids, and all these studies led to a similar conclusion that PSAPs improve functional hearing outcomes and the selected items may be equally effective as hearing aids.17,18,19,26 Furthermore, previous research also shed light on the affordability issue—in the US, the average out-of-pocket cost of a single hearing aid is more than $2000.27 In the study conducted by Reed et al., the hearing aid with the US Food and Drug Administration approval costs $1910.0/pair, whereas the prices of the selected PSAPs were all below $500.0.18 Our results not only aligned with these studies but also add to the literature by providing comprehensive electroacoustic evaluations of the specific PSAPs examined. To our knowledge, this study is the first to use AirPods as the tested PSAPs. In addition to the known pros of PSAPs, including an affordable cost, widespread availability resulting from their over-the-counter nature, and self-fitting that exempts consumers from multiple burdensome office visits,27 the design of AirPods does not mimic the traditional hearing aid, as most PSAPs do, but is even considered trendy. One major reason for the low fitting rate of hearing aids is social stigma; 28 the use of AirPods may eliminate such concerns and enhance the willingness of individuals to wear hearing assistive devices. Furthermore, as there is only a single manufacturer that produces AirPods and their bundled iOS system, it may be more manageable to implement adequate quality control and calibration compared to the more heterogeneous Android system.
Another interesting finding in this study was that the aided speech-in-noise perception varied based on the direction of the noise source. Greater speech understanding was found with premium hearing aids when both the target sentences and background noises were from the same position, whereas no significant differences were identified between the hearing aids and the PSAPs when the noises were from the side. Studies have demonstrated that speech perception is more challenging when speech and noise are colocalized,29 which may explain the results of our research and highlight the limitation of PSAPs when assisting communication in noisy environments since limited speech-in-noise understanding is the major hurdle among patients with SNHL, especially presbycusis.30
Despite the seemingly promising results indicated in this study that certain PSAPs may have the potential to provide similar hearing compensation for people with mild-to-moderate hearing loss, several concerns need to be addressed. Since one of the major selling points of PSAPs is the convenience rooted in their self-fitting nature, the fitting process is not overseen by professionals, and an adequate volume is not well established. Because PSAPs are not devices listed in the Federal Food, Drug, and Cosmetic Act, stringent quality management or product standards have not been needed. This may have led to considerable heterogeneity across the current PSAP market. All these are potential issues suggesting that the products may not only fail to appropriately assist a consumer’s verbal communication but also impact their health and already impaired hearing. Furthermore, although there is no long-existing social stigma like hearing aids, given the trendy looks and universality of PSAP or smartphone-bundled earphones, the communicating partner may consider wearing such products during a conversation being inappropriate if they do not understand the actual purpose of using these devices is compensating hearing.
In conclusion, our study demonstrated that smartphone-bundled AirPods Pro met most of the criteria of the ANSI/CTA-2051 for PSAPs and may provide similar improvements in terms of speech understanding among patients with mild-to-moderate hearing loss as conventional hearing aids. With sufficient quality management under certified standards, PSAPs may serve as a highly feasible and more affordable option when compensating for hearing impairment and thus could potentially bridge the gap between persons with hearing difficulties and their first step to seeking hearing assistance.
Limitations of this study
This study is limited by a relatively small sample size, yet the statistical power was satisfactory. People with severe SNHL and other types of hearing impairment were excluded from the study population, which may have resulted in decreased generalizability. Residual confounding effects regarding speech perception and the different devices may be present because information related to MHINT performance in relation to the subjects, such as education and cognitive capacity, was not collected. Since the cognitive process is a crucial factor affecting speech understanding, the MHINT scores in this study may not fully represent the participants’ function given the lack of the cognitive measures. Because this was a single-blind study, there may be potential observer bias. The whole experiment was conducted in a laboratory setting with a single sound source, and the outcomes thus may not completely reflect real-world conditions. In addition, as the first fit setting was used instead of real-ear measurement, the output sound volumes of the two setting programs may result in different reception thresholds. Furthermore, since the only PSAPs studied in this investigation were created by the same manufacturer, the results may not be applicable to other products. Future studies focusing on the efficacy and safety issues of regular PSAP usage on long-term health outcomes and quality of life measures among patients with mild-to-moderate hearing loss are clearly needed. In addition, a cost-effectiveness analysis compared to over-the-counter hearing aids should be considered as this may clearly establish the role of PSAPs within the hearing loss population.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Software and algorithms | ||
| HINT Pro | Bio-Logic | N/A |
| Stata 16 | StataCorp | RRID:SCR_012763 |
| Audioscan Verifit 2.0 test box | Audioscan | https://www.audioscan.com/en/verifit2/?cn-reloaded=1 |
| Interacoustics Affinity Compact 2.0 | Interacoustics | https://www.interacoustics.com/hearing-aid-fitting/affinity |
Resource availability
Lead contact
Further information and requests for resources and materials may be directed to and will be fulfilled by the lead contact, Dr. Yen-Fu Cheng (yfcheng2@vghtpe.gov.tw).
Materials availability
This study did not generate new unique reagents or materials.
Experimental models and subject details
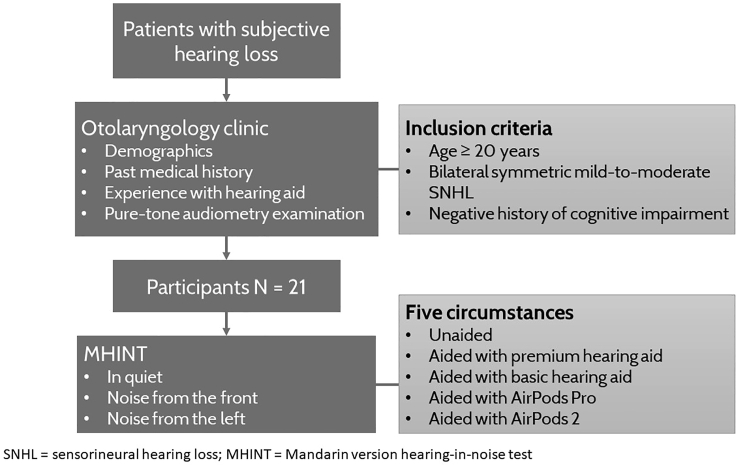
This was a cross-sectional self-controlled study conducted at Taipei Veterans General Hospital, a tertiary medical center in Taiwan. The persons who provided written informed consents to participate in the study were recruited from the otolaryngology clinic where basic demographics, including age and sex, and information regarding prior experiences with hearing aids, were initially collected. The inclusion criteria were as follows: age ≥20 years, bilateral symmetric mild-to-moderate sensorineural hearing loss (SNHL) based on pure tone audiometry results, and adequate psychosocial capacity to understand and perform the tasks required during this investigation. The definitions of mild and moderate hearing loss were based on the World Health Organization classification with a pure tone average of 26–40 decibel hearing level (dB HL) as mild and 41–55 dB HL as moderate hearing loss. Persons with cognitive impairment or asymmetric, conductive, or mixed-type hearing loss were excluded. Twenty-one participants with no hearing aids experience were recruited (see below figure). The participants were blinded in this study while the investigators were not. This study was approved by the institutional review board of Taipei Veterans General Hospital (IRB number: 2020-11-003BC).
Figure 1.
Flowchart of the experimental procedures conducted in this study
Method details
Amplification devices examined
The smartphone-bundled earphones examined in this study were AirPods 2 and AirPods Pro (both from Apple Inc., Cupertino, California, USA). iPhone XS Max smartphones with iOS version 13.0 (Apple Inc., Cupertino, California, USA) were paired-up with the AirPods. Two behind-the-ear (BTE) conventional hearing aids were also investigated and served as the control intervention; these were a premium hearing aid (OTICON Opn 1; Oticon Ltd., Smørum, Denmark) and a basic hearing aid (Bernafon MD1; Bernafon Ltd., Bern, Switzerland). The premium hearing aid had 16 channels for fine-tuning, while the basic hearing aid had six channels. Fitting of these two devices was performed by a certified audiologist using the NAL-NL 2 formula.
Electroacoustic analysis
Electroacoustic assessments on all four devices (premium hearing aid, basic hearing aid, AirPods 2, and AirPods Pro) were performed using an HA-1 2-cc coupler. The hearing aids were first adjusted to the full-on gain, then to reference test gain settings respectively, by related hearing aid adjustment software. Connect the hearing aid with HA-1 2 c.c coupler and place them into the test box (Interacoustics Affinity Compact 2.0). The coupler is a cavity of specified shape and volume used for the calibration of earphones in conjunction with a calibrated microphone adapted to measure the sound pressure developed in the cavity.
The electroacoustic parameters were measured automatically by the test box. Items in full-on gain setting measured include:
-
(a)
full-on gain test gain curve;
-
(b)
maximum output sound pressure level at 90 dB (Max OSPL 90; the maximum output of the device with 90 dB SPL input) and maximum output sound pressure level at 90 dB SPL frequency (Max OSPL frequency; the frequency of the peak output produced by the device).
Items in reference gain setting measured include:
-
(a)
reference test gain curve;
-
(b)
Total harmonic distortion (THD; the introduction of undesired sounds into the signal) at 500, 800, and 1600 Hz;
-
(c)
equivalent internal noise (EIN; the noise produced by the device during amplification).
The AirPods first paired-up with the iPhone, then adjust to the maximum volume for electroacoustic analysis. Press the start button after place the AirPods and iPhone with HA-1 2 c.c. coupler into the Audioscan Verifit 2.0 test box. The ANSI/CTA-2051 parameters include: frequency response smoothness, frequency response bandwidth, Max OSPL 90, THD at 500 Hz, and EIN.
The electroacoustic results of hearing aid will be compared by the technical sheet of that hearing aid manufacturer, while that of AirPods will be compared by the American National Standards Institute/Consumer Technology Association-2051 Standard for Personal Sound Amplification Product (ANSI/CTA-2051).
Hearing in noise test, Mandarin version (MHINT)
The Hearing in Noise Test (HINT) was first introduced by Nilsson et al. to measure speech perception in quiet or noisy environments.31 To implement the speech-in-noise test among the Mandarin-speaking population, the Mandarin version of the HINT (MHINT) was developed by Wong et al.32 with a reliability that is comparable to the English version. The tests were conducted by licensed personnel. Under quiet surroundings, twenty randomly selected MHINT sentences were presented from the front (0 degrees) of the participants. Each person was asked to verbally repeat what they heard; it was determined to be a correct response if the participant flawlessly repeated all the target words within the sentence. The initial speech volume was 20 dBA (A-weighted decibels), with a 4-dB decrease for a correct response and a 4-dB increase for an incorrect response for the first four sentences. For sentence numbers 5 to 20, a 2-dB step was used. For the speech-in-noise examination, the speech was presented from the front as well, but with 65 dBA noises introduced from the front and then from the subject’s left side. The subsequent test procedures were the same as under the quiet conditions. The speech reception threshold (SRT) and the signal-to-noise ratio (SNR) were calculated as speech perception measures. A lower SRT and SNR indicated better speech perception abilities.
Quantification and statistical analysis
The SRT and SNR derived from the MHINT were modelled as continuous variables. Descriptive analyses were performed to investigate the speech perception with or without the four examined amplification devices. Simple linear regression was conducted to compare the participants’ speech understanding ability with the four devices; given the self-controlled design, each participant served as their own control and thus accounted for various potential confounders. Significance testing for all analyses was 2-sided with a type I error of 0.05. The statistical software used was Stata 16 (StataCorp, College Station, TX).
Acknowledgments
This work was supported by grants from National Science and Technology Council (MOST-110-2622-8-075-001, MOST 110-2320-B-075-004-MY3, and MOST 111-2221-E-A49-041-MY2) and Veterans General Hospitals and University System of Taiwan Joint Research Program (VGHUST111-G6-11-1 and VGHUST111-G6-11-2).
Author contributions
Y.-H.L. and Y.-F. Cheng were responsible to the conceptualization and design for the manuscript. All authors contributed to the acquisition, analysis, and interpretation of the data. H.-Y.H.L. and H.-S.L. drafted the manuscript. Y.-H.L. and Y.-F. Cheng performed critical revision of the manuscript for important intellectual content. H.-Y.H.L., Y.-H.L., H.-S.L., and S.-L.W. performed the statistical analysis. Y.-H.L. and Y.-F. Cheng obtained the funding. H.-Y.H.L., Y.-H.L., Y.-F. Chen, and Y.-F. Cheng were supervisors of the manuscript. All authors reviewed, edited, and approved the final version of the article.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: November 15, 2022
Data and code availability
-
•
All data produced in this study are included in the published article and its supplementary information, or are available from the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Blackwell D.L., Lucas J.W., Clarke T.C. Vital Health Statistics. National Center for Health Statistics; 2014. Summary health statistics for US adults: national Health Interview Survey, 2012; p. 260. [PubMed] [Google Scholar]
- 2.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwagami M., Kobayashi Y., Tsukazaki E., Watanabe T., Sugiyama T., Wada T., Hara A., Tamiya N. Associations between self-reported hearing loss and outdoor activity limitations, psychological distress and self-reported memory loss among older people: analysis of the 2016 Comprehensive Survey of Living Conditions in Japan. Geriatr. Gerontol. Int. 2019;19:747. doi: 10.1111/ggi.13708. [DOI] [PubMed] [Google Scholar]
- 4.Shukla A., Harper M., Pedersen E., Goman A., Suen J., Price C., Applebaum J., Hoyer M., Lin F.R., Reed N.S. Hearing loss, loneliness, and social isolation: a systematic review. Otolaryngol. Head Neck Surg. 2020;162:622–633. doi: 10.1177/0194599820910377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deal J.A., Sharrett A.R., Bandeen-Roche K., Kritchevsky S.B., Pompeii L.A., Windham B.G., Lin F.R. Hearing impairment and physical function and falls in the atherosclerosis risk in communities (ARIC) hearing pilot study. J. Am. Geriatr. Soc. 2016;64:906. doi: 10.1111/jgs.14075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin F.R., Metter E.J., O’Brien R.J., Resnick S.M., Zonderman A.B., Ferrucci L. Hearing loss and incident dementia. Arch. Neurol. 2011;68:214–220. doi: 10.1001/archneurol.2010.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shukla A., Reed N.S., Armstrong N.M., Lin F.R., Deal J.A., Goman A.M. Hearing loss, hearing aid use, and depressive symptoms in older adults—findings from the atherosclerosis risk in communities neurocognitive study (ARIC-NCS) J. Gerontol. 2021;76:518–523. doi: 10.1093/geronb/gbz128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalton D.S., Cruickshanks K.J., Klein B.E., Klein R., Wiley T.L., Nondahl D.M. The impact of hearing loss on quality of life in older adults. Gerontologist. 2003;43:661–668. doi: 10.1093/geront/43.5.661. [DOI] [PubMed] [Google Scholar]
- 9.Reed N.S., Altan A., Deal J.A., Yeh C., Kravetz A.D., Wallhagen M., Lin F.R. Trends in health care costs and utilization associated with untreated hearing loss over 10 years. JAMA Otolaryngol. Head Neck Surg. 2019;145:27–34. doi: 10.1001/jamaoto.2018.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Livingston G., Huntley J., Sommerlad A., Ames D., Ballard C., Banerjee S., Mukadam N. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goman A.M., Reed N.S., Lin F.R. Addressing estimated hearing loss in adults in 2060. JAMA Otolaryngol. Head Neck Surg. 2017;143:733–734. doi: 10.1001/jamaoto.2016.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chien W., Lin F.R. Prevalence of hearing aid use among older adults in the United States. Arch. Intern. Med. 2012;172:292–293. doi: 10.1001/archinternmed.2011.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yong M., Willink A., McMahon C., McPherson B., Nieman C.L., Reed N.S., Lin F.R. Access to adults’ hearing aids: policies and technologies used in eight countries. Bull. World Health Organ. 2019;97:699. doi: 10.2471/BLT.18.228676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mamo S.K., Reed N.S., Nieman C.L., Oh E.S., Lin F.R. Personal sound amplifiers for adults with hearing loss. Am. J. Med. 2016;129:245–250. doi: 10.1016/j.amjmed.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruusuvuori J.E., Aaltonen T., Koskela I., Ranta J., Lonka E., Salmenlinna I., Laakso M. Studies on stigma regarding hearing impairment and hearing aid use among adults of working age: a scoping review. Disabil. Rehabil. 2021;43:436–446. doi: 10.1080/09638288.2019.1622798. [DOI] [PubMed] [Google Scholar]
- 16.Reed N.S., Betz J., Lin F.R., Mamo S.K. Pilot electroacoustic analyses of a sample of direct-to-consumer amplification products. Otol. Neurotol. 2017;386:804. doi: 10.1097/MAO.0000000000001414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho Y.S., Park S.Y., Seol H.Y., Lim J.H., Cho Y.S., Hong S.H., Moon I.J. Clinical performance evaluation of a personal sound amplification product vs a basic hearing aid and a premium hearing aid. JAMA Otolaryngol. Head Neck Surg. 2019;145:516–522. doi: 10.1001/jamaoto.2019.0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reed N.S., Betz J., Kendig N., Korczak M., Lin F.R. Personal sound amplification products vs a conventional hearing aid for speech understanding in noise. JAMA. 2017;318:89–90. doi: 10.1001/jama.2017.6905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen C.H., Huang C.Y., Cheng H.L., Lin H.Y.H., Chu Y.C., Chang C.Y., Lai Y.H., Wang M.C., Cheng Y.F. Comparison of personal sound amplification products and conventional hearing aids for patients with hearing loss: a systematic review with meta-analysis. EClinicalMedicine. 2022;46:101378. doi: 10.1016/j.eclinm.2022.101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kochkin S.E. MarkeTrak VIII: utilization of PSAPs and direct-mail hearing aids by people with hearing impairment. Hear. Rev. 2010;17:12–16. [Google Scholar]
- 21.Manchaiah V., Taylor B., Dockens A.L., Tran N.R., Lane K., Castle M., Grover V. Applications of direct-to-consumer hearing devices for adults with hearing loss: a review. Clin. Interv. Aging. 2017;12:859. doi: 10.2147/CIA.S135390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Statista Research Department Smartphone ownership in the U.S. 2015-2021, by age group. https://www.statista.com/statistics/489255/percentage-of-us-smartphone-owners-by-age-group/
- 23.S. Mobile Vendor Market Share Taiwan from February 2020 to February 2021. https://gs.statcounter.com/vendor-market-share/mobile/taiwan
- 24.Gilmore J.N. Design for everyone: apple AirPods and the mediation of accessibility. Crit. Stud. Mass Commun. 2019;36:482–494. doi: 10.1080/15295036.2019.1658885. [DOI] [Google Scholar]
- 25.The U.S. Industry and Food and Drug Administration Staff Regulatory requirements for hearing aid devices and personal sound amplification products; draft guidance for industry and Food and Drug administration staff; availability. Fed. Regist. 2021;86:58192. [Google Scholar]
- 26.Brody L., Wu Y.H., Stangl E. A comparison of personal sound amplification products and hearing aids in ecologically relevant test environments. Am. J. Audiol. 2018;27:581–593. doi: 10.1044/2018_AJA-18-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warren E., Grassley C. Over-the-counter hearing aids: the path forward. JAMA Intern. Med. 2017;177:609–610. doi: 10.1001/jamainternmed.2017.0464. [DOI] [PubMed] [Google Scholar]
- 28.David D., Zoizner G., Werner P. Self-stigma and age-related hearing loss: a qualitative study of stigma formation and dimensions. Am. J. Audiol. 2018;27:126–136. doi: 10.1044/2017_AJA-17-0050. [DOI] [PubMed] [Google Scholar]
- 29.Hawley M.L., Litovsky R.Y., Culling J.F. The benefit of binaural hearing in a cocktail party: effect of location and type of interferer. J. Acoust. Soc. Am. 2004;115:833–843. doi: 10.1121/1.1639908. [DOI] [PubMed] [Google Scholar]
- 30.Lin F.R. Hearing loss in older adults: who's listening? JAMA. 2012;307:1147–1148. doi: 10.1001/jama.2012.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nilsson M., Soli S.D., Sullivan J.A. Development of the Hearing in Noise Test for the measurement of speech reception thresholds in quiet and in noise. J. Acoust. Soc. Am. 1994;95:1085–1099. doi: 10.1121/1.408469. [DOI] [PubMed] [Google Scholar]
- 32.Wong L.L., Soli S.D., Liu S., Han N., Huang M.W. Development of the Mandarin hearing in noise test (MHINT) Ear Hear. 2007;28:70S–74S. doi: 10.1097/AUD.0b013e31803154d0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
-
•
All data produced in this study are included in the published article and its supplementary information, or are available from the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.