Abstract
Objective
Neurologic complications seriously affect the survival rate and quality of life in patients with extracorporeal cardiopulmonary resuscitation (ECPR) undergoing cardiac arrest. This study aimed to repurpose selective hypothermic cerebral perfusion (SHCP) as a novel approach to protect the brains of these patients.
Methods
Rats were randomly allocated to Sham, ECPR, and SHCP combined ECPR (CP-ECPR) groups. In the ECPR group, circulatory resuscitation was performed at 6 minutes after asphyxial cardiac arrest by extracorporeal membrane oxygenation. The vital signs were monitored for 3 hours, and body and brain temperatures were maintained at the normal level. In the CP-ECPR group, the right carotid artery catheterization serving as cerebral perfusion was connected with the extracorporeal membrane oxygenation device to achieve selective brain cooling (26-28 °C). Serum markers of brain injury and pathomorphologic changes in the hippocampus were evaluated. Three biological replicates further received RNA sequencing in ECPR and CP-ECPR groups. Microglia activation and inflammatory cytokines in brain tissues and serum were detected.
Results
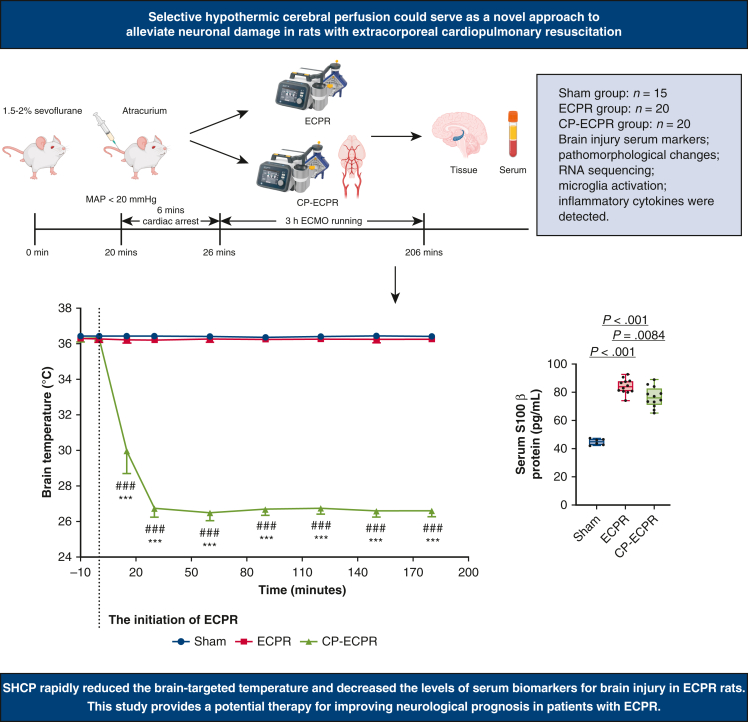
SHCP rapidly reduced the brain-targeted temperature and significantly alleviated nerve injury. This was evident from the reduced brain injury serum biomarker levels, lower pathologic scores, and more surviving neurons in the hippocampus in the CP-ECPR group. Furthermore, more differentially expressed genes for inflammatory responses were clustered functionally according to Kyoto Encyclopedia of Genes and Genomes pathway analysis. And SHCP reduced microglia activation and the release of proinflammatory mediators.
Conclusions
Our preliminary data indicate that SHCP may serve as a potential therapy to attenuate brain injury via downregulation of neuroinflammation in patients with ECPR.
Key Words: cardiac arrest, cerebral protection, extracorporeal cardiopulmonary resuscitation, hypothermic cerebral perfusion, neuroinflammation
Abbreviations and Acronyms: CA, cardiac arrest; DEGs, differentially expressed genes; ECMO, extracorporeal membrane oxygenation; ECPR, extracorporeal cardiopulmonary resuscitation; H&E, hematoxylin–eosin; Iba1, ionized calcium-binding adaptor molecule 1; ICAM-1, Intercellular adhesion molecule-1; IHC, immunohistochemistry; IL-1β/6/8, interleukin-1β/6/8; MAP, mean arterial pressure; NSE, neuron-specific enolase; PCR, polymerase chain reaction; RNA-seq, RNA sequencing; S100β, S-100β protein; SHCP, selective hypothermic cerebral perfusion; TNF-α, tumor necrosis factor-α; UCH-L1, ubiquitin C-terminal hydrolase-L1
Graphical abstract
Schematic diagram of SHCP approach in rats with ECPR.
Central Message.
SHCP had an important neuroprotection effect via downregulation of neuroinflammation in rats with ECPR.
Perspective.
SHCP could rapidly reduce the brain-targeted temperature and significantly alleviate nerve injury in rats receiving ECPR. Its protective mechanism may predominantly relate to the down-regulation of neuroinflammation. SHCP may serve as a potential therapy for improving neurologic prognosis in patients with ECPR.
Cardiac arrest (CA) is one of the main causes of death and disability in patients and remains a major public health concern worldwide.1 The American Heart Association recommended that extracorporeal cardiopulmonary resuscitation (ECPR) be considered for patients undergoing CA with reversible etiologies.2 The extracorporeal membrane oxygenation (ECMO) technique was gradually developed as a feasible resuscitation method for refractory CA.3 Although ECPR has been effective for many patients with CA, the discharge survival rate is only 23% to 41%.4, 5, 6 Notably, nervous system injury is the leading cause of death and one of the most common complications in patients with ECPR,7 with less than one-third of these patients retaining favorable neurologic function.7,8 The neurologic complications include hypoxic–ischemic brain damage, ischemic stroke, cerebral hemorrhage, and brain death,9 which seriously affect the survival rate and quality of life in patients suffering from CA.
Brain protection is the key point of advanced life support, with neurologic recovery used as a sign for evaluating the ultimate success of resuscitation. Targeted temperature management is the only therapeutic strategy that has been clinically proved to benefit the neural functional recovery in patients with CA.10 However, several systemic complications resulting from general hypothermia, such as coagulation disorders, respiratory depression, electrolyte disorders, and cardiac arrhythmias,11,12 have greatly limited its implementation as a clinical treatment in patients with CA undergoing ECPR. Therefore, it is imperative to develop a more targeted and safer treatment modality to improve the neurologic prognosis of these patients. Selective hypothermic cerebral perfusion (SHCP), as a selective brain-cooling method, is commonly used as a cerebral-protection strategy in complex aortic surgeries.13 There is considerable clinical evidence that SHCP provides patients with continuous cerebral blood flow and repays the oxygen debt accumulated during circulatory arrest.14 It can also effectively reduce the incidence of perioperative stroke and operative mortality.15
Therefore, we innovatively combined SHCP with ECPR in CA rats to explore the effect of this method on neurologic outcomes and its potential neuroprotective mechanism. We aimed to propose a novel and effective neuroprotective approach for patients with ECPR undergoing CA.
Methods
Ethical Approval
All experimental procedures were approved by the Ethical Committee of Lanzhou Second Hospital, Lanzhou University, Gansu (no. D2021-139) and performed in compliance with the “Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health (National Academy Press, revised 2011).
ECMO Circuits Installing and Priming
The principal components of the ECMO circuit include a peristaltic pump (PreFluid), membrane oxygenator, and tubing (Xijing Medical). Priming without blood was done using 6% hydroxyethyl starch 130/0.4 (5 mL), 8.4% sodium bicarbonate (1 mL), 0.01 mg/mL epinephrine (0.3 mL), and 300 IU/kg heparin (0.7 mL).
Anesthesia and Surgical Preparation
The animals were anesthetized with 5% sevoflurane in a closed chamber and then placed supine on the operating table, followed by oral endotracheal intubation. The ventilation parameters were set as follows: respiratory frequency 70 to 75 times/min, tidal volume 8 to 10 mL/kg, positive end-expiratory pressure 3 cmH2O, inspiration/expiration ratio 1:2, and oxygen flow 3 L. Anesthesia was maintained with 1.5% to 2% sevoflurane during the experiment. The probes of the electronic thermometer were respectively placed at 2 to 3 cm from the anal verge for the body temperature and the nasopharyngeal area for the brain temperature. Both the body temperature and the brain temperature of the rats were kept at 36 ± 0.5 °C by a heating pad.
CA and CP-ECPR Modeling
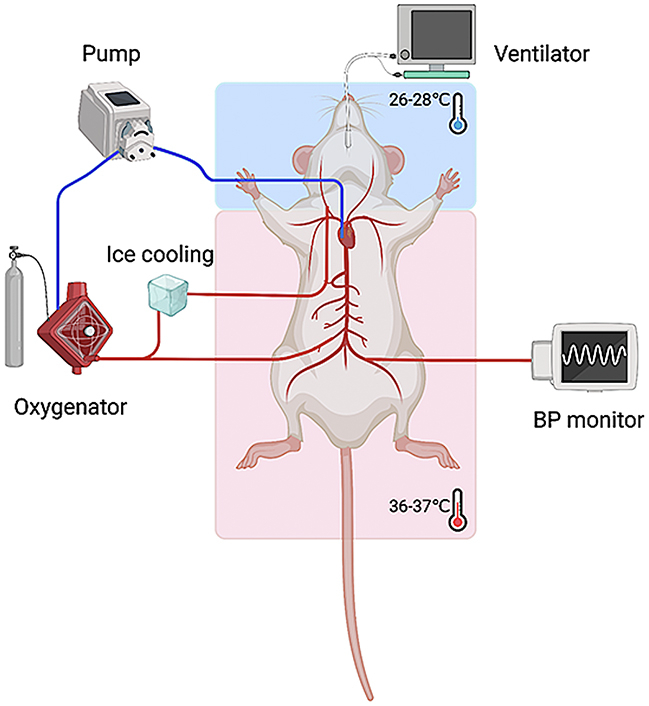
After skin preparation and disinfection in the anterior cervical region and bilateral inguinal area, local infiltration anesthesia was performed with 1% lidocaine. The left femoral artery was separated under a surgical microscope, and a 22-G catheter was inserted for electrocardiogram monitoring, followed by giving heparin (400 U/kg). A 24-G catheter was inserted in the right femoral artery for continuous arterial perfusion during ECMO. The right jugular vein was exposed and cannulated with a specially designed catheter, which served as the venous drainage. In the CP-ECPR group, a 22-G catheter was inserted into the right carotid artery for cerebral perfusion. The cerebral perfusion tube connected to the ECMO circuits was covered with a cooling bag.
Before starting ECMO, the rats were given cisatracurium besylate (2 mg/kg) through the right jugular vein while mechanical ventilation was suspended. When the mean arterial pressure (MAP) dropped below 20 mm Hg, the rat model of asphyxial CA was successfully established. After 6 minutes, cardiopulmonary resuscitation, including restoring mechanical ventilation and starting ECPR, was performed. ECMO was initially established at a lower flow rate, and later increased to the optimal flow rate (80 mL/kg/min) that could maintain the desired arterial pressure, and gas flow (90% O2) was set at 80 to 100 mL/min. Sodium bicarbonate (8.4%; 1 mmol/kg) was used for electrolyte imbalances, and vasoactive drugs, such as epinephrine, norepinephrine, and dopamine, were administered to maintain MAP > 60 mm Hg. If no return of spontaneous circulation exceeded 20 minutes, the resuscitation failed. In particular, the brain temperature was selectively decreased at 26 to 28 °C in the CP-ECPR group during ECPR. After 3 hours, the animals were weaned off ECMO, and all rats were humanely killed under deep anesthesia. The left femoral artery catheterization was performed for hemodynamic monitoring in the Sham group without CA and venoarterial ECMO, and the right femoral artery and right jugular vein were ligated. In the Sham group, the brain temperature and body temperature were maintained at normal levels, and the rats were sacrificed after 3 hours of monitoring.
Enzyme-Linked Immunosorbent Assay
S-100β protein (S100β), neuron-specific enolase (NSE), ubiquitin C-terminal hydrolase-L1 (UCH-L1), interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-8 (IL-8), and tumor necrosis factor-α (TNF-α) serum concentrations and the level of these inflammatory cytokines in the brain tissue homogenates were measured using enzyme-linked immunosorbent assay kits from Mlbio, in accordance with the manufacturer's instructions.
Hematoxylin–Eosin (H&E) and Nissl Staining
The hippocampi were fixed for histopathologic assessment. Paraffin sections (4 μm thick) were prepared. After conventional dewaxing and hydration, the sections were stained with H&E (Beyotime Biotechnology). The neurologic injury of the CA1 regions in the hippocampus was evaluated by a blind method according to a 0 to 4 grading scale reported by Hua and colleagues16 (the significance of pathologic scores was as follows: 0 = no damage; 1 = 0%-12.5% damage; 2 = 12.5%-25% damage; 3 = 25%-50% damage; 4 > 50% damage). These scores were based on the characteristics of cell death, which exhibited shrunken cell bodies, triangulated pyknotic nuclei, and eosinophilic cytoplasm. Nissl staining were performed with Crystal Violet (Beyotime Biotechnology) according to the manufacturer's instruction. The number of surviving hippocampus neurons in the CA1 regions was calculated by a counting procedure.
RNA Sequencing (RNA-seq) Analysis
In the ECPR and CP-ECPR groups, 3 brain tissue samples from each group were randomly selected for RNA-seq, followed by total RNA extraction, clustering, sequencing, quality control, transcriptome assembly, gene expression quantification, and bioinformatics analysis. The “DESeq” R package v1.10.1 (AT&T Bell Laboratories) was used to identify differentially expressed genes (DEGs), which were converted to official genetic symbols based on the Kyoto Encyclopedia of Genes and Genomes pathway analysis and grouped according to common biological characteristics. Based on the transcriptomic analysis, quantitative real-time polymerase chain reaction (PCR) was further used to validate the expression of DEGs in the CP-ECPR group relative to the ECPR group.
RNA Extraction and Quantitative Real-Time PCR
RNA was extracted from the brain tissues using TRIzol reagent (Invitrogen). RNA concentration was determined by nanodrop (Thermo Fisher Scientific). The equal amounts of RNA were reversely transcribed into cDNA by the PrimeScript RT reagent Kit (TaKaRa) according to the manufacturer's instruction. Quantitative PCR was performed in real-time PCR system (Roche LightCycle 96), using primers and SYBR Green Master Mix (TaKaRa). β-actin was selected as housekeeping gene. The gene relative expression levels were calculated using the 2−△△CT method. The experiments were independently repeated 3 times.
The primers used were as follows:
Ccl2: 5′ - ATGAGTCGGCTGGAGAACTA - 3′, 5′ - ACTTCTGGACCCATTCCTTATTG - 3′
Cxcl1: 5′ - GCACCCAAACCGAAGTCATA - 3′, 5′ - GGGACACCCTTTAGCATCTTT - 3′
Icam1: 5′ - CTGCCTCTGAAGCTCAGATATAC - 3′, 5′ - CAGGGTTCTGTCCAACTTCTC - 3′
Il6: 5′ - GAAGTTAGAGTCACAGAAGGAGTG - 3′, 5′ - GTTTGCCGAGTAGACCTCATAG - 3′
β-actin: 5′ - AGATCCTGACCGAGCGTGGC - 3′, 5′ - CCAGGGAGGAAGAGGATGCG - 3′
Immunohistochemistry (IHC)
The hippocampi were collected for IHC of ionized calcium-binding adaptor molecule 1 (Iba1) and postfixed in 4% paraformaldehyde for 24 hours. After a high-pressure antigen retrieval method, the sections were incubated in an endogenous peroxidase blocker (Zhongshan Golden Bridge Biotechnology) for 20 minutes to inactivate endogenous peroxidases. The nonspecific binding was blocked by incubation with blocking reagent (Zhongshan Golden Bridge Biotechnology) for 20 minutes. Then, the slices were incubated with rabbit anti-Iba1 (1:400; Wako) at 4 °C overnight, followed by incubation in goat anti-rabbit IgG (Zhongshan Golden Bridge Biotechnology) for 15 minutes at 25 °C. The sections were visualized with diaminobenzidine for a few seconds before they were counterstained with hematoxylin. Finally, the average number of activated microglia was calculated in the CA1 regions of the hippocampus.
Statistical Analysis
All statistical analyses and graphical representations were performed with GraphPad Prism 8.0. Data were presented as mean ± standard deviation or median with 25th and 75th percentile. When data followed normal distribution, the statistical differences between groups were determined using Student's t-test or one-way analysis of variance. Kruskal–Wallis test was used to compare the differences of nonparametric data among the 3 groups.
Results
SHCP Could Rapidly Achieve Brain Cooling
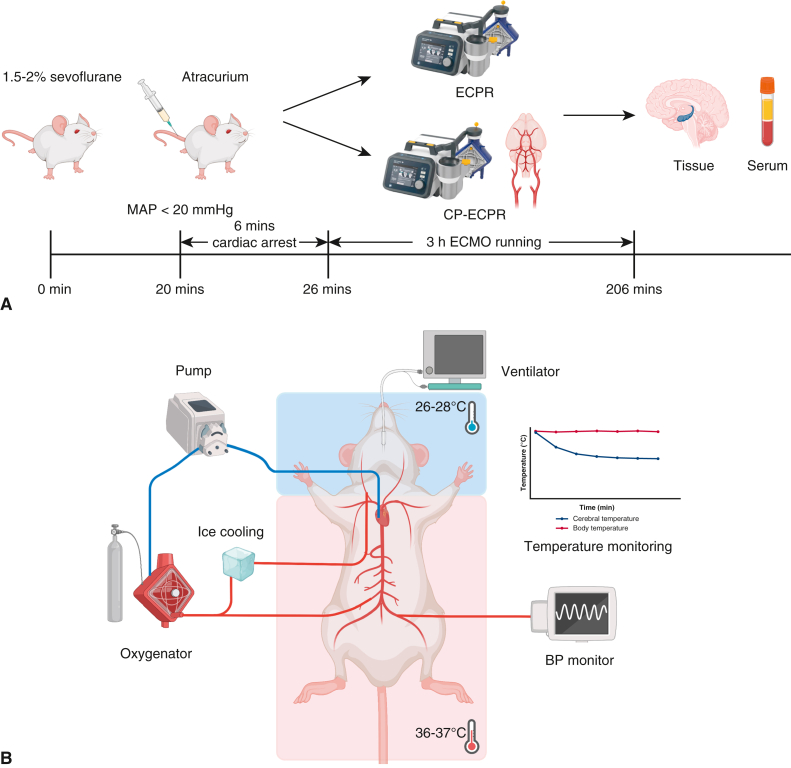
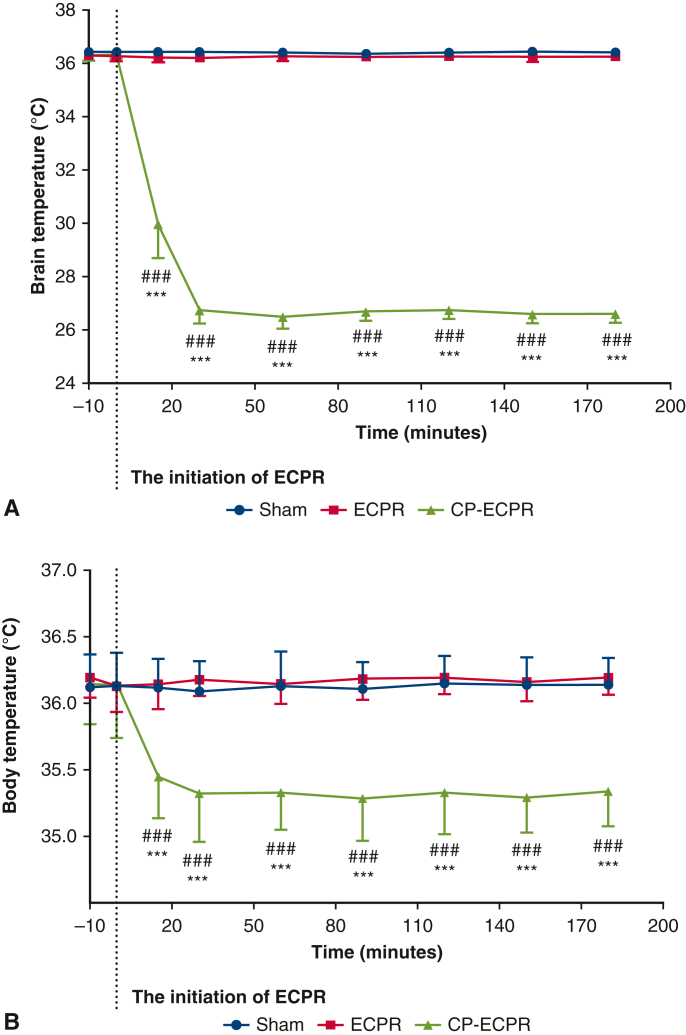
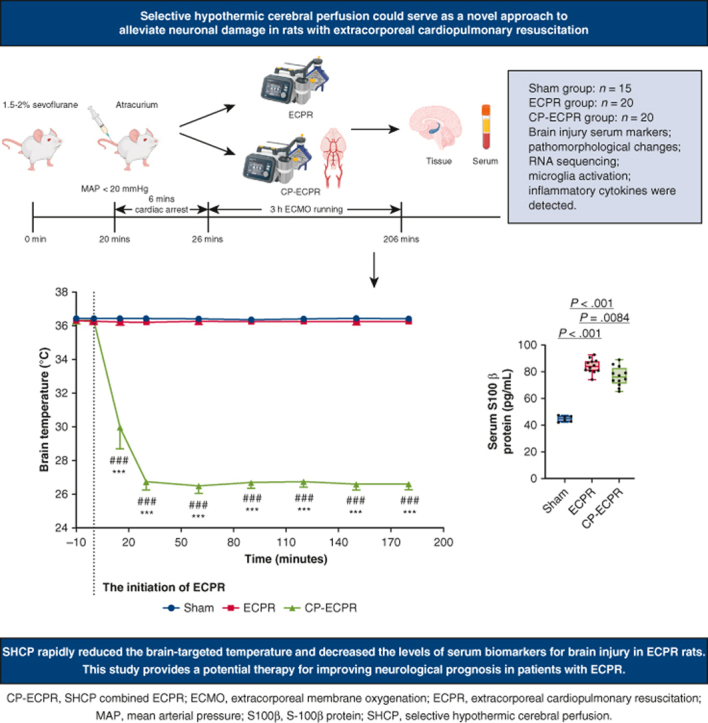
The flow chart of animal modeling is shown in Figure 1, A, and the schematic diagram of the SHCP approach is shown in Figure 1, B. Spontaneous circulation was restored in all animals rescued by ECPR. The physiologic data during ECPR are summarized in Table E1. MAP was stably maintained above 80 mm Hg in the ECPR and CP-ECPR groups. Once hypothermic brain perfusion was initiated, the cerebral temperature decreased sharply in the CP-ECPR group. The brain temperatures, starting at 36.38 ± 0.22 °C at the onset of perfusion, dropped to 26.74 ± 0.47 °C after 30 minutes and did not exceed 28 °C for the remainder of the time. In contrast, the brain temperature of the ECPR rats was maintained within a normal range via the heating pad (Figure 2, A). The use of SHCP had a limited impact on the rectal temperature. Starting at 36.14 ± 0.38 °C, the rectal temperature slightly dropped to 35.37 ± 0.28 °C after 30 minutes in the CP-ECPR rats, and never decreased below 34.5 °C during ECMO (Figure 2, B). Video 1 shows the CP-ECPR modeling in more detail. These data indicated that successful selective brain cooling was implemented.
Figure 1.
The protocols of animal modeling. A, Flow chart of animal modeling. The rats were randomly allocated to 3 groups: Sham (n = 15), ECPR (n = 20), and CP-ECPR (n = 20) groups. B, Schematic diagram of SHCP approach in the ECPR rats. The tube for cerebral perfusion, covered with a cooling bag, was connected to the ECMO circuits. The brain temperature selectively decreased to 26 to 28 °C in the CP-ECPR rats. MAP, Mean arterial pressure; ECPR, extracorporeal cardiopulmonary resuscitation; CP-ECPR, SHCP combined ECPR; SHCP, selective hypothermic cerebral perfusion; ECMO, extracorporeal membrane oxygenation; BP, blood pressure.
Figure 2.
Brain and body temperatures in Sham (n = 15), ECPR (n = 20), and CP-ECPR (n = 20) groups during procedures. Temperatures in (A) the nasopharynx and (B) the rectum measured in a time-dependent manner in 3 groups. The cerebral temperature decreased sharply after the initiation of hypothermic brain perfusion and stabilized after 30 minutes in the CP-ECPR group. The rectal temperature of the CP-ECPR rats slightly dropped but never less than 34.5 °C. Graphs represent means ± standard deviation. ###P < .001 represents Sham versus CP-ECPR, ∗∗∗P < .001 represents ECPR versus CP-ECPR. ECPR, Extracorporeal cardiopulmonary resuscitation; CP-ECPR, SHCP combined ECPR.
SHCP Alleviated Neuronal Damage
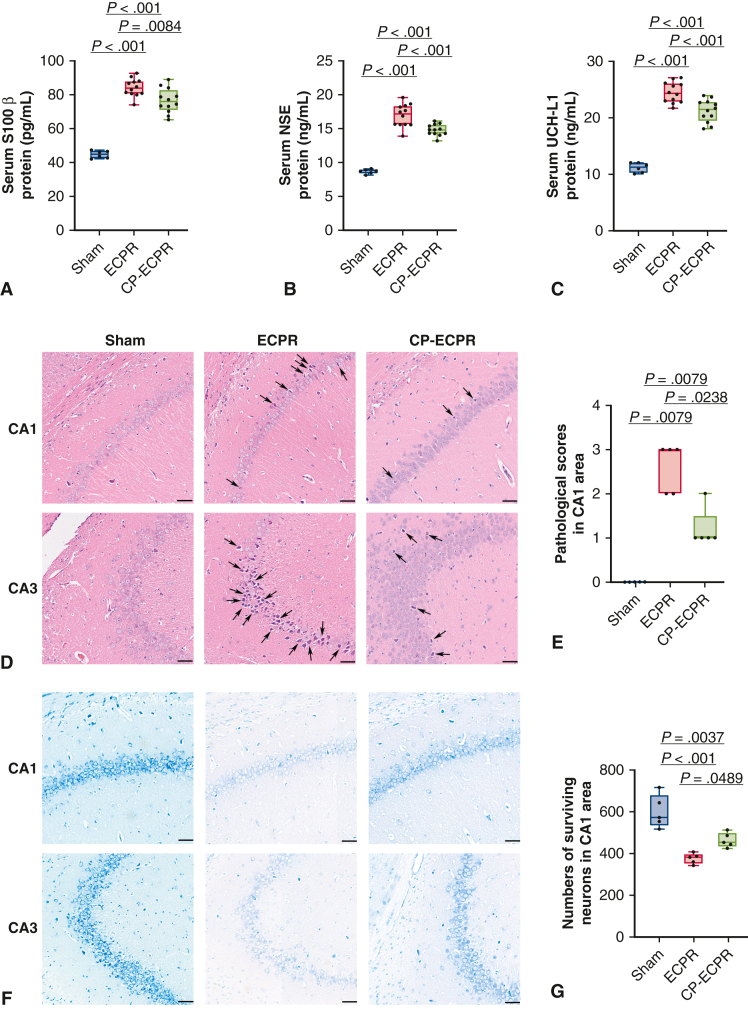
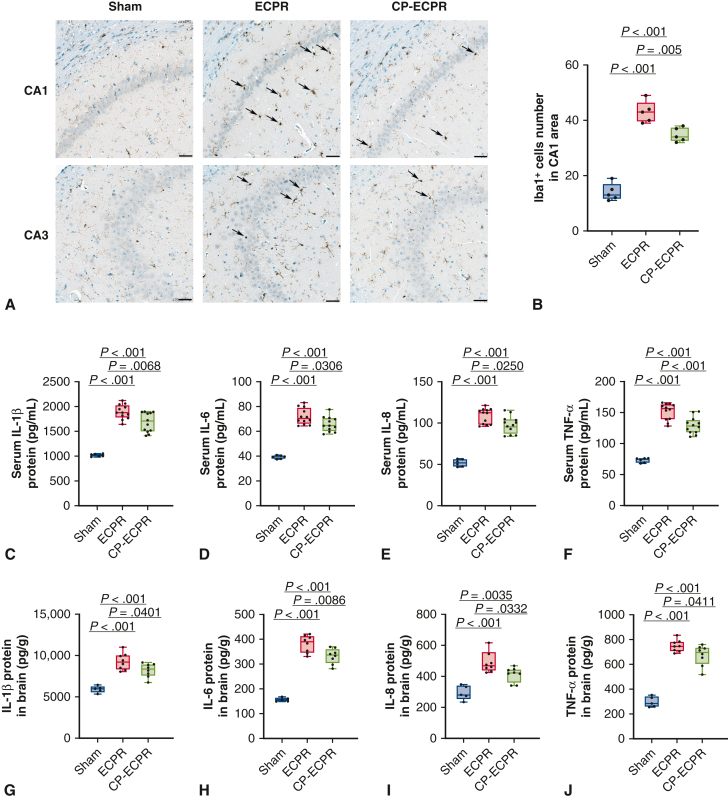
To evaluate the degree of neuronal damage, serum biomarkers of brain injury were measured. These biomarkers in the ECPR rats were significantly greater than those in the Sham group (S100β: 44.85 ± 2.07 pg/mL vs 84.21 ± 5.01 pg/mL, P < .001; NSE: 8.65 ± 0.31 ng/mL vs 16.87 ± 1.60 ng/mL, P < .001; UCH-L1: 11.14 ± 0.78 ng/mL vs 24.48 ± 1.77 ng/mL, P < .001; Figure 3, A-C). In the CP-ECPR group, the biomarkers were significantly decreased compared with those of the ECPR group (S100β: 84.21 ± 5.01 pg/mL vs 76.52 ± 6.96 pg/mL, P = .0084; NSE: 16.87 ± 1.60 ng/mL vs 14.75 ± 0.80 ng/mL, P < .001; UCH-L1: 24.48 ± 1.77 ng/mL vs 21.24 ± 1.92 ng/mL, P < .001; Figure 3, A-C). Furthermore, no neuronal injury was observed in the Sham group, whereas a large number of neuron losses were seen in the ECPR group. H&E staining showed that the pyramidal cells were sparsely arranged and the outlines were fuzzy in the hippocampal CA1 and CA3 areas. The pathologic scores of the CP-ECPR group in the CA1 region were lower than those of the ECPR group (1 [1-1.5] vs 3 [2-3], P = .0238; Figure 3, D and E). For Nissl staining, the number of surviving pyramidal neurons robustly increased in the CP-ECPR rats compared with that in the ECPR group (Figure 3, F and G). These promising results showed that the application of SHCP could ameliorate cerebral injury for rats receiving ECPR (Figure 4).
Figure 3.
The application of SHCP ameliorated cerebral injury for the ECPR rats. A-C, Levels of S100β, NSE, and UCH-L1 serum concentrations in Sham (n = 6), ECPR (n = 12), and CP-ECPR (n = 12) groups. A significant decrease of the biomarkers observed in the CP-ECPR group compared with those in the ECPR group. (D) Damaged neurons identified by H&E staining in representative hippocampal CA1 and CA3 regions (arrows; scale bars represent 50 μm) in each group (n = 5 per group). No neuronal injury observed in the Sham group. Compared with the CP-ECPR rats, a larger number of damaged neurons could be seen in the ECPR group. E, Pathologic scores in the hippocampal CA1 region for each group (n = 5 per group). Pathologic scores of the CP-ECPR group are lower than those of the ECPR group. F and G, Large number of pyramidal neurons in the hippocampal CA1 and CA3 regions of the Sham group using Nissl staining. Clear cell boundaries and abundant Nissl bodies could be observed. The number of surviving pyramidal neurons in the hippocampal CA1 area robustly increased in the CP-ECPR rats compared with that in the ECPR group (n = 5 per group) (scale bars represent 50 μm). Graphs represent means ± standard deviation or median with 25th and 75th percentile. The box-and-whiskers dot plots include the minimum value, the lower quartile (25th percentile), the median, the upper quartile (75th percentile), and the maximum value. SHCP, Selective hypothermic cerebral perfusion; S100β, S-100β Protein; NSE, neuron-specific enolase; ECPR, extracorporeal cardiopulmonary resuscitation; UCH-L1, ubiquitin C-terminal hydrolase-L1; CP-ECPR, SHCP combined ECPR; H&E, hematoxylin–eosin.
Figure 4.
The rats were randomly allocated to Sham (n = 15), ECPR (n = 20), and SHCP combined ECPR (CP-ECPR) (n = 20) groups. Brain injury serum markers, pathomorphologic changes in the hippocampus, RNA sequencing, microglia activation, and inflammatory cytokines in brain tissues and serum were detected. The main results showed that SHCP rapidly reduced the brain-targeted temperature and brain injury serum biomarker levels. SHCP could serve as a novel approach to alleviate neuronal damage in ECPR rats. MAP, Mean arterial pressure; ECPR, extracorporeal cardiopulmonary resuscitation; SHCP, selective hypothermic cerebral perfusion; CP-ECPR, selective hypothermic cerebral perfusion combined ECPR; RNA, ribonucleic acid; S100β, S-100β protein.
SHCP Reduced Microglia Activation and Neuroinflammation
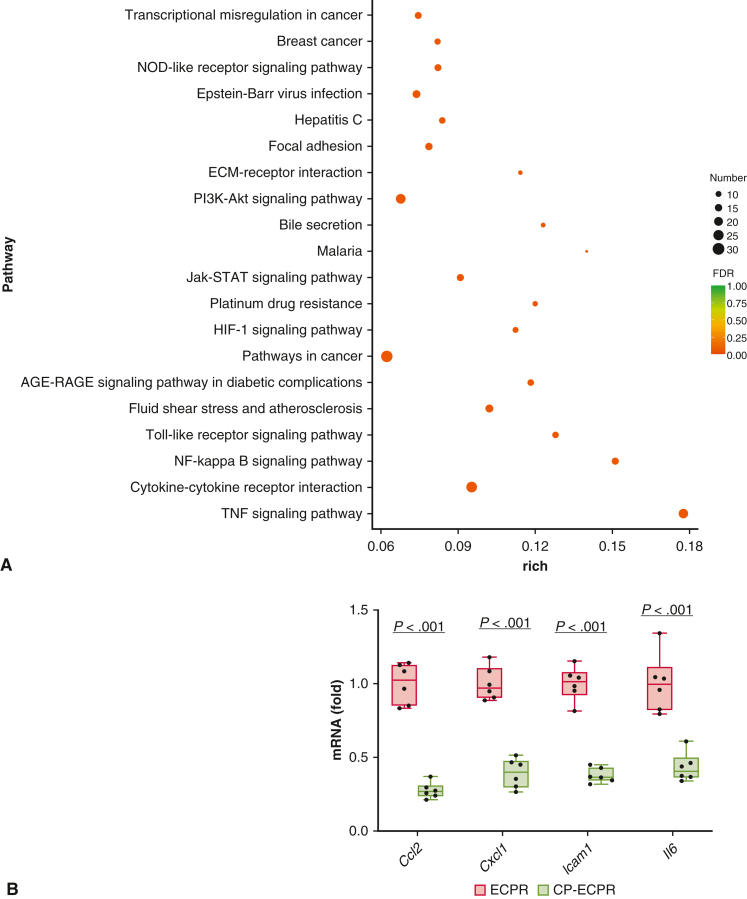
To identify the underlying mechanisms of SHCP, we further performed RNA-seq on rat brains between the ECPR and CP-ECPR groups. A total of 594 DEGs, including 71 up- and 523 down-regulated genes, were identified between the 2 groups. The Kyoto Encyclopedia of Genes and Genomes pathway analysis showed that most down-regulated DEGs were involved in the “TNF signaling pathway,” “cytokine-cytokine receptor interaction,” and “NF-kappa B signaling pathway,” suggesting that the role of SHCP might be correlated to inhibiting the inflammation response during ECPR (Figure 5, A). Next, the transcriptomics data were validated by quantitative real-time PCR (Figure 5, B). Compared with the ECPR group, the mRNA levels of Il6, Ccl2, Cxcl1, and Icam1 in the CP-ECPR group were significantly decreased. Microglia activation was evaluated by calculating the number of Iba1+ microglial cells. Compared with the ECPR group, the Iba1+ microglia numbers in hippocampus CA1 area significantly reduced in the CP-ECPR group (Figure 6, A and B). Moreover, the use of SHCP decreased the levels of IL-1β, IL-6, IL-8, and TNF-α in both serum (Figure 6, C-F) and brain tissues (Figure 6, G-J) when compared with those in the ECPR group. The role of SHCP might be correlated to downregulating neuroinflammation during ECPR.
Figure 5.
RNA-sequencing analysis on brain tissues. A, Primary enriched KEGG pathways between ECPR and CP-ECPR groups (n = 3 per group). The y-axis represents the different KEGG categories, and the x-axis represents the rich factor. The size of the dot indicates the number of DEGs involved in the pathway. The color bar represents the P value of the KEGG pathway. B, Transcriptomics data validated by qRT-PCR. Compared with the ECPR group, the mRNA levels of Il6, Ccl2, Cxcl1, and Icam1 in the CP-ECPR group were significantly decreased (n = 6 per group). Graphs represent means ± standard deviation. The box-and-whiskers dot plots includes the minimum value, the lower quartile (25th percentile), the median, the upper quartile (75th percentile), and the maximum value. NOD, Nucleotide oligomerization domain; ECMO, extracorporeal membrane oxygenation; Jak-STAT, Janus tyrosine kinase - signal transducer and activator of transcription; HIF, hypoxia-inducible factor; AGE-RAGE, advanced glycation end products - receptor for advanced glycation end products; NF, nuclear factor; TNF, tumor necrosis factor-α; ECPR, extracorporeal cardiopulmonary resuscitation; CP-ECPR, selective hypothermic cerebral perfusion combined ECPR; DEGs, differentially expressed genes; KEGG, kyoto Encyclopedia of Genes and Genomes; qRT-PCR, quantitative real-time polymerase chain reaction.
Figure 6.
The role of SHCP might be correlated to downregulating neuroinflammation during ECPR. A and B, Activated microglia identified by Iba1 IHC in representative hippocampal CA1 and CA3 regions (arrows; scale bars represent 50 μm) in each group (n = 5 per group). Compared with the ECPR group, the Iba1+ microglia numbers in the hippocampus CA1 area significantly reduced in the CP-ECPR group. C-F, Levels of IL-1β, IL-6, IL-8, and TNF-α serum concentrations in Sham (n = 6), ECPR (n = 12), and CP-ECPR (n = 12) groups. G-J, Contents of IL-1β, IL-6, IL-8, and TNF-α in brain tissues in Sham (n = 5), ECPR (n = 8), and CP-ECPR (n = 8) groups. The use of SHCP decreased the levels of IL-1β, IL-6, IL-8, and TNF-α in both serum and brain tissues compared with those in the ECPR group. Graphs represent means ± standard deviation. The box-and-whiskers dot plots includes the minimum value, the lower quartile (25th percentile), the median, the upper quartile (75th percentile), and the maximum value. ECPR, Extracorporeal cardiopulmonary resuscitation; CP-ECPR, SHCP combined ECPR; IL-1β/6/8, interleukin-1β/6/8; SHCP, selective hypothermic cerebral perfusion; Iba1, ionized calcium-binding adaptor molecule 1; IHC, immunohistochemistry; TNF-α, tumor necrosis factor-α.
Discussion
In this study, SHCP combined with ECPR was applied for the first time in rats suffering from CA. The aim was to determine the neuroprotective efficacy of this modality on the neurologic prognosis and provide a novel approach for improving neurologic outcomes in patients with ECPR. Our results showed that the application of SHCP rapidly reduced the brain-targeted temperature after the beginning of resuscitation, decreased the levels of serum biomarkers for brain injury in the ECPR rats, and ameliorated the histopathologic damage in the hippocampus. In particular, SHCP significantly reduced the activation of microglia and the release of proinflammatory mediators (TNF-α, IL-1β, IL-6, intercellular adhesion molecule-1 [ICAM-1], etc).
Brain damage is a major cause of death and disability in patients with ECPR after CA.17 In a meta-analysis of 6261 patients with ECPR, Migdady and colleagues18 found that the favorable neurologic recovery rate was only 24% (95% confidence interval, 21%-28%). Two key points of restoring neuronal function are maintaining cerebral blood perfusion and reducing brain temperature. Hypothermia has been shown to potentially reduce mortality and neurologic morbidity in patients with ECPR.19,20 In recent years, targeted temperature management has gained consensus as an important therapeutic measure for neuroprotection after cardiopulmonary resuscitation.10 However, this treatment could bring a variety of systemic complications, which strongly limits its clinical application in patients with ECPR.11 Compared with systemic hypothermia, selective brain cooling has generally gained interest as a compelling and feasible approach to provide a faster, deeper, and more targeted temperature reduction and maximize neurologic benefit over systemic complications.12 Rutherford and colleagues21 respectively carried out whole-body cooling and selective head cooling therapies in newborns with hypoxic–ischemic encephalopathy, their results showed that the infants treated with selective head cooling had a lower proportion of severe cortical lesions than the infants cooled with whole-body cooling. Nevertheless, Rustic-Buckland and colleagues22 found that cerebral blood flow also decreases with decreased brain temperature. This decrease in cerebral blood flow may lead to the persistence of brain injury. Cerebral perfusion with hypothermia is increasingly applied to complex aortic surgeries, especially when brain temperature drops to 20 to 28 °C, the ideal neuroprotective effect for selective cerebral perfusion can be achieved.23 Inspired by the aforementioned results, we aimed to develop a targeted and safe treatment modality for these patients, especially for patients who are not eligible for systemic cooling. Therefore, the purpose of our study was not exactly the same as that of Wang and colleagues24; in addition to better maintaining cerebral perfusion, we took into account the potential harm caused by systemic hypothermia and the neurologic benefits brought by hypothermia to patients undergoing ECPR.
In this study, by monitoring nasopharyngeal temperature, a reliable indicator of brain temperature,25 we found that SHCP could rapidly reduce brain temperature to 26 to 28 °C with the support of venoarterial ECMO and had a minor effect on the core temperature in rats. To date, serum S100β, NSE, and UCH-L1 have been shown to accurately predict adverse neurologic outcomes after CA independent of temperature.26,27 To further clarify the underlying mechanisms, RNA-seq analysis was conducted to determine the brain gene expression profiles in the ECPR and CP-ECPR rats. The preliminary results suggested that this modality may have a protective effect on the brain by inhibiting neuroinflammation. The inflammatory cascade plays a major part in cerebral ischemia–reperfusion injury.28 Cerebral ischemia triggers inflammation as a response to necrotic cells followed by the production of reactive oxygen species and cytokines from inflammatory cells. Nucleic acids released from necrotic cells and other immune molecules then activate microglia, which produces more proinflammatory mediators, including TNF-α, IL-1β, and IL-6.29 These cytokines can up-regulate the expression of adhesion molecules such as ICAM-1 on the surface of cerebrovascular endothelial cells.30 As shown by Vemuganti and colleagues,31 inhibiting ICAM-1 level can protect brain tissue and reduce the volume of cerebral infarction. Meanwhile, chemokines such as CXCL-1 recruit circulating immune cells to the damaged brain parenchyma.32 Leukocytes and central immune cells further release inflammatory mediators, which ultimately aggravate neuronal damage.28 Studies have shown that successful neuroinflammation blocking can ameliorate brain injury in stroke models.33 This suggests a protective role in the brain for SHCP by alleviating neuroinflammation in patients with ECPR.
We can see a promising future for SHCP combine with ECPR in transforming from preclinical trials to the clinical settings. During cerebral perfusion in complex aortic surgeries, axillary, subclavian, and carotid arteries are the common sites for arterial cannulation, which are easily exposed and achieved by direct cannula insertion.34 Therefore, we speculate that while professional teams set up ECMO for patients undergoing CA, the carotid artery may be used as an additional arterial perfusion. It simultaneously ensures appropriate brain blood flow and brain hypothermia to promote cerebral resuscitation. However, it is worth noting that the severe aortic atherosclerosis increases the risk of stroke and cerebral embolism when SHCP is employed. The dislodging of atherosclerotic debris may occur as the result of cannulation and blood perfusion during SHCP.35 Therefore, we suggest that performing epiaortic ultrasonography before SHCP to evaluate for the presence of aortic and peripheral artery vascular pathology and determine an atherosclerotic plaque-free site for the cannulation.
There are some limitations to this study. First, we evaluated the short-term brain protection effect of this novel strategy, and its influence on the long-term neurologic prognosis still needs to be identified. In this study, samples were collected during the acute phase, so we believed that the apoptotic signaling of neurons had not been completely activated due to the limited intervention time (3 hours). In the following research, apoptosis assays are also needed to identify the neurologic prognosis of this strategy. Second, translating rodent experiments to other mammals will also be the next challenge. Third, we explored the effect of SHCP with a restricted temperature range (26-28 °C) and limited cooling time. ECMO support was performed for only 3 hours to alleviate blood damage from the pump. Moreover, hypothermia results in loss of cerebral autoregulation, and in these cases, cerebral perfusion is largely influenced by blood pressure, which is a potential interference factor. Continuous hemodynamic monitoring showed that there were no statistical differences in blood pressure between the ECPR and CP-ECPR groups at different times. Therefore, we believe that this variable would not significantly affect the results of this study. And in the future, we will pay more attention to the effect of blood pressure on brain protection during SHCP. Finally, whether systemic cooling with perfusion would be necessary and whether the neuroprotective effects of this method are superior to that of systemic cooling are problems worthy of inquiry.
Conclusions
In this study, the ECPR models for asphyxial CA rats were successfully established, and selective brain cooling was achieved by SHCP. Our preliminary data indicate that SHCP could reduce brain injury in ECPR rats, and its protective mechanism may predominantly relate to the down-regulation of neuroinflammation. This study provides a potential therapy for improving the neurologic prognosis in patients with ECPR. The safety and mechanism of this treatment should be verified in the future.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Acknowledgments
Figure 1 was created with BioRender.com.
Footnotes
Drs Zhai and Li contributed equally to this article.
This study was supported by the Talent Introduction Plan of the Lanzhou University Second Hospital (No. YJRCKYQDJ-2021-02); the Natural Sciences Foundation of Gansu (No. 20JR10RA760); the Scientific Research Projects of Colleges in Gansu Province (No. 2020B-028, 2020B-037); the Cuiying Scientific and Technological Innovation Program of Lanzhou University Second Hospital (No. CY2019-QN01, CY2019-QN12, CY2021-QN-B04); the Scientific Research Projects in Lanzhou (No. 2021-1-107); and the National Key R&D Program of China (No. 2021YFC2701700).
Appendix 1
Table E1.
The physiological data during ECPR
| Physiological parameters | Sham | ECPR | CP-ECPR |
|---|---|---|---|
| Weight, g | 275.16 ± 9.87 | 272.93 ± 10.24 | 274.54 ± 11.61 |
| Time between asphyxia and CA, min | – | 3.97 ± 0.71 | 3.72 ± 0.66 |
| Time of CA, min | – | 6 | 6 |
| Time of ROSC, s | – | 71.33 ± 54.35 | 52 ± 17.05 |
| Epinephrine usage (0.01 mg/mL), mL | – | 0.40 ± 0.14 | 0.38 ± 0.16 |
| Baseline MAP, mm Hg | 105.86 ± 4.23 | 108.73 ± 7.41 | 102.00 ± 5.71 |
| ECPR 15 min MAP, mm Hg | – | 103.53 ± 17.39 | 94.92 ± 15.23 |
| ECPR 30 min MAP, mm Hg | – | 89.07 ± 7.56 | 87.31 ± 9.82 |
| ECPR 60 min MAP, mm Hg | – | 82.07 ± 11.14 | 84.23 ± 7.74 |
| ECPR 90 min MAP, mm Hg | – | 82.73 ± 11.60 | 82.15 ± 9.36 |
| ECPR 120 min MAP, mm Hg | – | 83.93 ± 13.77 | 81.15 ± 8.38 |
| ECPR 150 min MAP, mm Hg | – | 83.33 ± 11.18 | 82.38 ± 8.17 |
| ECPR 180 min MAP, mm Hg | – | 83.40 ± 12.89 | 82.69 ± 8.94 |
Data are presented as mean ± standard deviation. ECPR, Extracorporeal cardiopulmonary resuscitation; CP-ECPR, SHCP combined ECPR; CA, cardiac arrest; ROSC, return of spontaneous circulation; MAP, mean arterial pressure; SHCP, selective hypothermic cerebral perfusion.
Supplementary Data
Surgical procedure in the rat model of selective hypothermic cerebral perfusion combined with extracorporeal cardiopulmonary resuscitation. Video available at: https://www.jtcvsorg/article/S2666-2736(22)00302-3/fulltext.
References
- 1.Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 2.Panchal A.R., Bartos J.A., Cabañas J.G., Donnino M.W., Drennan I.R., Hirsch K.G., et al. Part 3: adult basic and advanced life support: 2020 American Heart Association Guidelines for cardiopulmonary resuscitation and emergency cardiovascular Care. Circulation. 2020;142:S366–S468. doi: 10.1161/CIR.0000000000000916. [DOI] [PubMed] [Google Scholar]
- 3.Soar J., Böttiger B.W., Carli P., Couper K., Deakin C.D., Djärv T., et al. European Resuscitation Council Guidelines 2021: adult advanced life support. Resuscitation. 2021;161:115–151. doi: 10.1016/j.resuscitation.2021.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Ouweneel D.M., Schotborgh J.V., Limpens J., Sjauw K.D., Engström A.E., Lagrand W.K., et al. Extracorporeal life support during cardiac arrest and cardiogenic shock: a systematic review and meta-analysis. Intensive Care Med. 2016;42:1922–1934. doi: 10.1007/s00134-016-4536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beyea M.M., Tillmann B.W., Iansavichene A.E., Randhawa V.K., Van Aarsen K., Nagpal A.D. Neurologic outcomes after extracorporeal membrane oxygenation assisted CPR for resuscitation of out-of-hospital cardiac arrest patients: a systematic review. Resuscitation. 2018;130:146–158. doi: 10.1016/j.resuscitation.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Wang C.H., Chou N.K., Becker L.B., Lin J.W., Yu H.Y., Chi N.H., et al. Improved outcome of extracorporeal cardiopulmonary resuscitation for out-of-hospital cardiac arrest—a comparison with that for extracorporeal rescue for in-hospital cardiac arrest. Resuscitation. 2014;85:1219–1224. doi: 10.1016/j.resuscitation.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 7.Ryu J.A., Chung C.R., Cho Y.H., Sung K., Jeon K., Suh G.Y., et al. Neurologic outcomes in patients who undergo extracorporeal cardiopulmonary resuscitation. Ann Thorac Surg. 2019;108:749–755. doi: 10.1016/j.athoracsur.2019.03.033. [DOI] [PubMed] [Google Scholar]
- 8.Guru P.K., Seelhammer T.G., Singh T.D., Sanghavi D.K., Chaudhary S., Riley J.B., et al. Outcomes of adult patients supported by extracorporeal membrane oxygenation (ECMO) following cardiopulmonary arrest. The Mayo Clinic experience. J Card Surg. 2021;36:3528–3539. doi: 10.1111/jocs.15804. [DOI] [PubMed] [Google Scholar]
- 9.Podell J.E., Krause E.M., Rector R., Hassan M., Reddi A., Jaffa M.N., et al. Neurologic outcomes after extracorporeal cardiopulmonary resuscitation: recent experience at a single high-volume center. ASAIO J. 2022;68:247–254. doi: 10.1097/MAT.0000000000001448. [DOI] [PubMed] [Google Scholar]
- 10.Donnino M.W., Andersen L.W., Berg K.M., Reynolds J.C., Nolan J.P., Morley P.T., et al. Temperature management after cardiac arrest: an advisory statement by the advanced life support task force of the International Liaison Committee on resuscitation and the American Heart Association Emergency Cardiovascular Care Committee and the council on cardiopulmonary, critical care, perioperative and resuscitation. Circulation. 2015;132:2448–2456. doi: 10.1161/CIR.0000000000000313. [DOI] [PubMed] [Google Scholar]
- 11.Sanfilippo F., La Via L., Lanzafame B., Dezio V., Busalacchi D., Messina A., et al. Targeted temperature management after cardiac arrest: a systematic review and meta-analysis with trial sequential analysis. J Clin Med. 2021;10:3943. doi: 10.3390/jcm10173943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Assis F.R., Narasimhan B., Ziai W., Tandri H. From systemic to selective brain cooling—methods in review. Brain Circ. 2019;5:179–186. doi: 10.4103/bc.bc_23_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qu J.Z., Kao L.W., Smith J.E., Kuo A., Xue A., Iyer M.H., et al. Brain protection in aortic arch surgery: an evolving field. J Cardiothorac Vasc Anesth. 2021;35:1176–1188. doi: 10.1053/j.jvca.2020.11.035. [DOI] [PubMed] [Google Scholar]
- 14.Di Eusanio M., Schepens M.A., Morshuis W.J., Dossche K.M., Di Bartolomeo R., Pacini D., et al. Brain protection using antegrade selective cerebral perfusion: a multicenter study. Ann Thorac Surg. 2003;76:1181–1188. doi: 10.1016/s0003-4975(03)00824-5. [DOI] [PubMed] [Google Scholar]
- 15.Tian D.H., Weller J., Hasmat S., Oo A., Forrest P., Kiat H., et al. Adjunct retrograde cerebral perfusion provides superior outcomes compared with hypothermic circulatory arrest alone: a meta-analysis. J Thorac Cardiovasc Surg. 2018;156:1339–1348. doi: 10.1016/j.jtcvs.2018.04.063. [DOI] [PubMed] [Google Scholar]
- 16.Hua F., Ma J., Li Y., Ha T., Xia Y., Kelley J., et al. The development of a novel mouse model of transient global cerebral ischemia. Neurosci Lett. 2006;400:69–74. doi: 10.1016/j.neulet.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 17.Wollborn J., Steiger C., Doostkam S., Schallner N., Schroeter N., Kari F.A., et al. Carbon monoxide exerts functional neuroprotection after cardiac arrest using extracorporeal resuscitation in pigs. Crit Care Med. 2020;48:e299–e307. doi: 10.1097/CCM.0000000000004242. [DOI] [PubMed] [Google Scholar]
- 18.Migdady I., Rice C., Deshpande A., Hernandez A.V., Price C., Whitman G.J., et al. Brain injury and neurologic outcome in patients undergoing extracorporeal cardiopulmonary resuscitation: a systematic review and meta-analysis. Crit Care Med. 2020;48:e611–e619. doi: 10.1097/CCM.0000000000004377. [DOI] [PubMed] [Google Scholar]
- 19.Tahsili-Fahadan P., Farrokh S., Geocadin R.G. Hypothermia and brain inflammation after cardiac arrest. Brain Circ. 2018;4:1–13. doi: 10.4103/bc.bc_4_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernández-Ruiz I. Hypothermia helps in nonshockable cardiac arrest. Nat Rev Cardiol. 2020;17:6. doi: 10.1038/s41569-019-0301-3. [DOI] [PubMed] [Google Scholar]
- 21.Rutherford M.A., Azzopardi D., Whitelaw A., Cowan F., Renowden S., Edwards A.D., et al. Mild hypothermia and the distribution of cerebral lesions in neonates with hypoxic-ischemic encephalopathy. Pediatrics. 2005;116:1001–1006. doi: 10.1542/peds.2005-0328. [DOI] [PubMed] [Google Scholar]
- 22.Russell-Buckland J., Tachtsidis I. In: Oxygen Transport to Tissue XLI. Copyright 2020. Ryu P.D., LaManna J.C., Harrison D.K., Lee S.S., editors. Springer; 2020. Developing a model to simulate the effect of hypothermia on cerebral blood flow and metabolism; pp. 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spielvogel D., Kai M., Tang G.H., Malekan R., Lansman S.L. Selective cerebral perfusion: a review of the evidence. J Thorac Cardiovasc Surg. 2013;145:S59–S62. doi: 10.1016/j.jtcvs.2012.11.073. [DOI] [PubMed] [Google Scholar]
- 24.Wang C.-H., Shinozaki K., Becker L.B. Abstract 307: selective brain perfusion in addition to emergency cardiopulmonary bypass resuscitation after prolonged asphyxial cardiac arrest: feasibility studies in a novel rodent model. Circulation. 2013;128:A307. [Google Scholar]
- 25.Lim H., Kim B., Kim D.C., Lee S.K., Ko S. A comparison of the temperature difference according to the placement of a nasopharyngeal temperature probe. Korean J Anesthesiol. 2016;69:357–361. doi: 10.4097/kjae.2016.69.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfeifer R., Franz M., Figulla H.R. Hypothermia after cardiac arrest does not affect serum levels of neuron-specific enolase and protein S-100b. Acta Anaesthesiol Scand. 2014;58:1093–1100. doi: 10.1111/aas.12386. [DOI] [PubMed] [Google Scholar]
- 27.Ebner F., Moseby-Knappe M., Mattsson-Carlgren N., Lilja G., Dragancea I., Undén J., et al. Serum GFAP and UCH-L1 for the prediction of neurological outcome in comatose cardiac arrest patients. Resuscitation. 2020;154:61–68. doi: 10.1016/j.resuscitation.2020.05.016. [DOI] [PubMed] [Google Scholar]
- 28.Anrather J., Iadecola C. Inflammation and stroke: an overview. Neurotherapeutics. 2016;13:661–670. doi: 10.1007/s13311-016-0483-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma Y., Wang J., Wang Y., Yang G.Y. The biphasic function of microglia in ischemic stroke. Prog Neurobiol. 2017;157:247–272. doi: 10.1016/j.pneurobio.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Li C., Sun T., Jiang C. Recent advances in nanomedicines for the treatment of ischemic stroke. Acta Pharm Sin B. 2021;11:1767–1788. doi: 10.1016/j.apsb.2020.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vemuganti R., Dempsey R.J., Bowen K.K. Inhibition of intercellular adhesion molecule-1 protein expression by antisense oligonucleotides is neuroprotective after transient middle cerebral artery occlusion in rat. Stroke. 2004;35:179–184. doi: 10.1161/01.STR.0000106479.53235.3E. [DOI] [PubMed] [Google Scholar]
- 32.Kawabori M., Yenari M.A. Inflammatory responses in brain ischemia. Curr Med Chem. 2015;22:1258–1277. doi: 10.2174/0929867322666150209154036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meng H., Zhao H., Cao X., Hao J., Zhang H., Liu Y., et al. Double-negative T cells remarkably promote neuroinflammation after ischemic stroke. Proc Natl Acad Sci U S A. 2019;116:5558–5563. doi: 10.1073/pnas.1814394116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ziya Apaydın A. Antegrade cerebral perfusion: a review of its current application. Turk Gogus Kalp Damar Cerrahisi Derg. 2021;29:1–4. doi: 10.5606/tgkdc.dergisi.2021.21255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki T., Asai T., Nota H., Kuroyanagi S., Kinoshita T., Takashima N., et al. Selective cerebral perfusion with mild hypothermic lower body circulatory arrest is safe for aortic arch surgery. Eur J Cardiothorac Surg. 2013;43:e94–e98. doi: 10.1093/ejcts/ezs690. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Surgical procedure in the rat model of selective hypothermic cerebral perfusion combined with extracorporeal cardiopulmonary resuscitation. Video available at: https://www.jtcvsorg/article/S2666-2736(22)00302-3/fulltext.