Abstract
Tumor-associated macrophages (TAMs) have emerged as key players in tumor immunology but demonstrate a continuum of functional states being either tumor suppressive or promoting. Moreover, chemotherapeutic agents have been shown to alter the tumor microenvironment. Perioperative chemotherapy is a standard treatment option for resectable esophageal and gastric (EG) adenocarcinoma. The aim of this study was to investigate the influence of neoadjuvant chemotherapy (NAC) on TAMs to improve the prognostication and treatment course for these patients. The study cohort comprised 148 patients, all of whom were diagnosed with resectable EG adenocarcinoma and treated with NAC. Immunohistochemistry was applied to assess the total infiltration and infiltration into tumor nests (TN) of CD68+/CD163−, CD68+/CD163+, and MARCO+ TAMs, on paired biopsies from primary tumors (PT) pre-NAC, and resected PT and lymph node metastases post-NAC. In pre-NAC specimens, high CD68+/CD163+ infiltration into TN was an unfavorable prognostic factor. No association was found between TAM density in PT pre-NAC and histopathological regression. The density of CD68+/CD163+ TAMs was increased in PT post-NAC, while the density of MARCO+ TAMs was decreased. CD68+/CD163− TAM density was not altered. In post-NAC specimens, higher total as well as TN infiltration of CD68+/CD163− TAMs were adverse prognostic factors. In conclusion, these results suggest that NAC may alter certain TAM subsets in EG adenocarcinoma, along with their functional properties and thus their prognostic value.
Keywords: Esophageal cancer, Gastric cancer, Neoadjuvant chemotherapy, Tumor-associated macrophages
Introduction
Esophageal and gastric (EG) cancers are both associated with poor survival and are leading causes of cancer-related mortality worldwide. A standard treatment option for fit patients with locally advanced disease is today perioperative (i.e., neoadjuvant and adjuvant treatment) chemotherapy with FLOT (fluorouracil, leucovorin, oxaliplatin, and docetaxel) [1]. This triplet combination has enhanced the 5-year overall survival (OS) rate to 45% compared to 23–24% for surgery alone, however, only 15–20% of the patients benefit from this multimodality treatment [1, 2, 3]. According to results derived from the MAGIC trial [2], wherein patients were randomized to perioperative ECF (epirubicin, cisplatin, and fluorouracil) or surgery alone, negative nodal status was the only independent predictor of improved OS, in the pre-treated patient group. Good histopathological response (i.e., complete pathologic response or a low number of residual tumor cells [TCs]) also showed an association with improved outcome, however not independently of lymph node (LN) status [4]. To enable personalized treatment for patients with EG adenocarcinoma, in addition to clinicopathological factors, it is of great importance to define complementary biomarkers for guidance in prognostication and response prediction. In doing so, we may be able to identify high- versus low-risk patients and reduce overtreatment before surgery as well as tailor the treatment after surgery.
Tumor-infiltrating immune cells have over the recent years proven to have great influence on tumorigenesis and are important players in the tumor microenvironment (TME) [5, 6]. In EG adenocarcinoma, the adaptive immunity has been investigated in numerous studies, foremost T cells, but lately also B cells. In general, high density of T cells has been associated with an improved outcome [7, 8, 9, 10], whereas the prognostic impact of B cells has been slightly more ambiguous, although the majority of studies have shown associations with an improved outcome [10, 11, 12].
The innate immunity is also of paramount importance for immunosurveillance against tumors and its cellular components consist of mast cells, neutrophils, basophils, eosinophils, dendritic cells, macrophages, natural killer cells, innate lymphoid cells, and semi-innate lymphoid cells such as natural killer T cells. Tumor-associated macrophages (TAMs) are the most abundant immune cells in the TME and are considered frontier soldiers of the innate immunity. However, these cells demonstrate a continuum of functional states with behavioral variation depending on activation. Traditionally, two extreme subtypes have been described, denoted as M1 (pro-inflammatory, classically activated) and M2 (anti-inflammatory, alternatively activated) [13, 14], but recent research has revealed that this terminology is an oversimplification and that many TAM subpopulations exist in between the two extreme activation states, with varying degrees of pro- and anti-inflammatory features [15]. Furthermore, it has been proposed that the tumor milieu itself initiates the differentiation of monocytes toward either tumor-suppressing (M1 like) or tumor-promoting (M2 like) macrophages [16]. Given the different phenotypes of macrophages and their dual roles in tumor progression, their prognostic impact in cancer is still ambiguous, and to clarify their role, a variety of markers have been utilized with the aim to differentiate the two extreme TAM subtypes (M1 like and M2 like). The most commonly used marker to estimate total TAM infiltration is CD68 [17], and, furthermore, CD163 is known to be an efficient M2 like marker [18]. In previous studies on the prognostic impact of TAMs, various markers have been utilized, which must be taken into consideration when interpreting the results. However, in a meta-analysis by Wang et al. [17] on gastric cancer (n = 1,388), including 12 studies, high M2 like density and high total TAM density was associated with poor survival, while high M1 like density was associated with an improved OS. Regarding esophageal adenocarcinoma, the literature is sparse. This is also the case for studies investigating the infiltration of TAMs into tumor nests (TN). In other tumor entities such as breast cancer, melanoma, and endometrial carcinomas, total TAM infiltration has been correlated with poor survival [19, 20, 21, 22] while contrasting findings have been reported in colon cancer [23].
The macrophage receptor with collagenous structure (MARCO) is a scavenger receptor expressed by some TAMs that mediates phagocytosis upon activation [24]. The prognostic impact of MARCO+ TAMs is to date highly unexplored and only one former study has investigated their prognostic impact in chemoradiotherapy-naïve EG adenocarcinoma, whereby no association with survival was found [25]. In hepatocellular carcinoma, a significant association between decreased intratumoral expression of MARCO+ TAMs and shorter survival has been demonstrated [26], while in intestinal-type periampullary carcinoma, high density of MARCO+ TAMs was significantly associated with shorter survival [27].
The foremost goal in multimodal cancer treatment, including neoadjuvant chemotherapy (NAC), is to achieve a maximal cytoreductive effect on the primary tumor (PT) and regional metastases as well as eliminating radiologically occult distant metastases, by inducing apoptosis and necrosis, or by inhibiting TC proliferation [28]. It is however well known that chemotherapeutic agents also have the capability to alter the immune composition in the TME in several solid tumor types including EG cancer [29, 30, 31, 32, 33].
There is a great need to investigate the effect of NAC on TAMs, as well as the prognostic impact of the different TAM subsets including their compartmental localization. Hence, the aim of this study was to delineate the potential effect of NAC on different TAM subsets by examining their prevalence in biopsies from PT before NAC and resected PT and LN metastases after NAC in EG adenocarcinoma.
Materials and Methods
Study Design and Participants
The cohort has been described previously [29, 34, 35] and consists of 148 patients, all of whom were diagnosed and treated for resectable esophageal (n = 39) or gastric (n = 109) adenocarcinoma at Skåne University Hospital, Sweden, between January 1, 2008, and December 31, 2014. Last update on recurrence and vital status was performed on December 31, 2017. All study participants received NAC ± adjuvant chemotherapy, and the majority (>95%) were treated with fluoropyromidine and oxaliplatin-based chemotherapy (EOX, FLOX, or FOLFOX). After NAC, 118 (79.7%) patients underwent surgical resection and all patients were discussed at a multidisciplinary tumor board after diagnosis. Clinical stage was determined based on endoscopy findings and computerized tomography. Patients who received neoadjuvant chemoradiotherapy or palliative chemotherapy followed by salvage surgery were excluded. Residual tumor status was classified as R0 = no residual tumor (free resection margins according to the pathology report), R1 = possible microscopic residual tumor (narrow or compromised resection margins according to the pathology report), or R2 = macroscopic residual tumor (according to the operative report). Clinical and histopathological classification of tumor stage was based on the 7th edition of the UICC/AJCC TNM classification, wherein EG junction tumors Siewert type I–III are classified as esophageal tumors [36]. However, since EG junction Siewert type III tumors are managed as gastric cancer in clinical practice and classified as gastric cancer in the new 8th edition of the UICC/AJCC TNM classification [37], this definition is used in the present study.
Histopathological Response
The degree of residual carcinoma in the post-NAC resected PT was determined according to the tumor regression grading system described by Chirieac [38]. This grading system divides the histopathological response into the following groups; 0%, 1–10%, 11–50%, or >50% residual carcinoma.
TMA Construction and Immunohistochemistry
All cases were histopathologically re-evaluated on hematoxylin and eosin stained sections by a board-certified pathologist (KJ). Tissue microarrays (TMAs) were constructed using cores from separate donor blocks from the resected PT, whenever feasible from both central and peripheral non-necrotic tumor areas, in 118 cases and from paired LN metastases in 56 cases, using a semiautomated arraying device (TMArrayer, Pathology Devices, Westminister, MD, USA), as described previously [39]. For immunohistochemical (IHC) analysis of the different TAM markers, 3-μm sections from the biopsies and 4-μm sections from the TMAs were automatically pre-treated using the PT Link system and then stained in an Autostainer Plus (Dako, Glostrup, Denmark) with the following antibodies: anti-CD68 (clone KP1, mouse, dilution 1:1,200, Dako), anti-CD163 (clone MRQ-26, mouse, dilution 1:100, Cell Marque Corporation, Rocklin, CA, USA), anti-MARCO (clone, HPA063793, dilution 1:250, Atlas Antibodies, Bromma, Sweden). Double staining was performed with anti-CD68 and anti-CD163, and single staining was performed with anti-MARCO.
Evaluation of IHC Staining
A semiquantitative assessment of the estimated total (intratumoral, tumor-adjacent, and stromal) percentage (0–100%) of CD68+/CD163−, CD68+/CD163+ TAMs and a quantitative assessment of the absolute number of MARCO+ TAMs were manually annotated in the entire area of each biopsy and TMA core by two independent observers (M.C.S., K.J.), both blinded to clinical and outcome data. The infiltration of the different TAM subsets into TN (defined as stained immune cells being in juxtaposition into a TC) was further classified as 0 (none), 1 (sparse), or 2 (high) in each biopsy and TMA core. The total infiltration as well as the infiltration into TN was recategorized according to the mean value of infiltration across all assessable TMA cores and biopsies for each case and used in the statistical analyses. The CD68+CD163−/CD68+CD163+ ratio was calculated by dividing the mean values for the two TAM subsets, respectively. For all TAM markers, discrepant cases were re-evaluated and discussed to reach consensus. Staining intensity was not taken into account, and only biopsies, TMA cores, and LNs with presence of clearly visible TCs were accounted for.
Statistical Analyses
The Wilcoxon signed-rank test was applied to determine differences in the density of the different TAM subsets in paired pre-NAC and post-NAC samples. The Mann-Whitney U test was used to investigate if CD68+/CD163− TAM density in post-NAC specimens depended on increased or decreased CD68+/CD63+ density post-NAC. Spearman's rank correlation test was used to investigate the intercorrelation between the different immune markers in paired pre-NAC and post-NAC samples. LN metastases were not included in the subgroup analyses due to the small number of cases. The Mann-Whitney U test was used to evaluate the association of different TAM subsets in PT pre-NAC with histopathological regression. To evaluate the associations of the different TAM subsets with clinicopathological factors in PT pre-NAC and post-NAC, Kruskal-Wallis or Mann-Whitney U test was used. Cox proportional hazards models were used to calculate univariable and multivariable hazard ratios for time to recurrence (TTR) and OS for continuous as well as categorical variables. Age, tumor grade (low vs. high), resection margin (R0 vs. R1/R2), ypT (T0-1 vs. T2-4), ypN (N0 vs. N+), ypM (M0 vs. M1) were the factors included in the multivariable analyses, all being significantly associated with OS in univariable analysis, as shown in online supplementary Table 1 (see www.karger.com/doi/10.1159/000524434 for all online suppl. material). As age was not significantly associated with TTR in univariable analysis, this factor was excluded in the multivariable analysis for TTR.
TTR was defined as time from resection to the date of biopsy or radiology proven recurrent disease. OS was defined as time from resection to the date of death. Patients who died within 90 days after surgery were excluded as this was considered a postoperative complication. LN metastases were not included in the survival analyses for any of the TAM markers due to the small number of cases. All tests were 2-sided and a p value of <0.05 was regarded as statistically significant. All calculations were performed using IBM SPSS Statistics for Mac version 26.0 (IBM Armonk, NY, USA).
Results
TAM Subset Density in Pre-NAC and Post-NAC Specimens
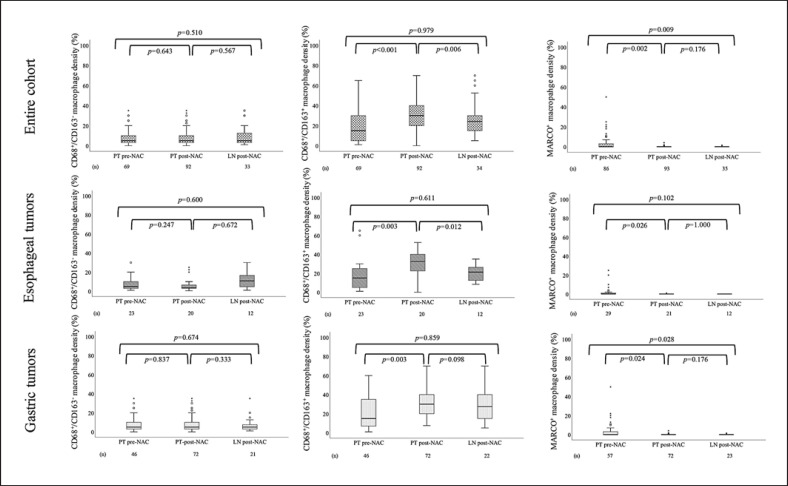
IHC sample images are shown in Figure 1. Box plots visualizing the density of the different TAM subsets in pre-NAC and post-NAC specimens in the entire cohort and by tumor location are shown in Figure 2. The density of CD68+/CD163− TAMs did not differ significantly between PT pre-NAC, PT post-NAC, and LN metastases post-NAC, neither in the entire cohort nor when stratifying for tumor location. The density of CD68+/CD163+ TAMs was significantly higher in PT post-NAC compared to PT pre-NAC and LN metastases post-NAC, with similar findings when stratifying for tumor location. The density of MARCO+ TAMs was significantly higher in PT pre-NAC compared to PT post-NAC and LN metastases post-NAC, with similar findings for gastric and esophageal tumors. In addition, CD68+/CD163− TAM density in post-NAC specimens was not affected by increased or decreased CD68+/CD163+ density post-NAC (p = 0.573). Taken together, these results demonstrate that the density of CD68+/CD163+ and MARCO+ TAMs is altered following NAC, while the density of CD68+/CD163− macrophages is unaffected.
Fig. 1.
Sample IHC images (×20 magnification) of double staining for CD68 (red) and CD163 (brown) and single staining for MARCO (brown). Double positive (CD68+/CD163+) TAMs are indicated by black arrowheads, single positive (CD68+/CD163−) TAMs are indicated by white arrowheads, and MARCO+ TAMs are indicated by red arrowheads. a Predominance of CD68+/CD163+ TAMs, mainly infiltrating in the stroma and periphery of the TN. b Predominance of CD68+/CD163− TAMs, mainly infiltrating in the stroma and periphery of the TN. c Predominance of CD68+/CD163+ TAMs with infiltration into TN. d Predominance of CD68+/CD163− TAMs with infiltration into TN. e, f MARCO+ TAMs, mainly located in the tumor stroma.
Fig. 2.
Boxplots visualizing the density of CD68+/CD163−, CD68+/CD163+, and MARCO+ TAMs in PT pre-NAC, PT post-NAC, and LN post-NAC, in the entire cohort, tumors, respectively.
Intercorrelation between the Investigated Immune Marker Subsets in Pre-NAC and Post-NAC Specimens
Intercorrelations between the different TAM subsets in pre-NAC and post-NAC specimens in the entire cohort are shown in Table 1. All significant associations in PT pre-NAC, PT post-NAC, and LN post-NAC were very weak or weak.
Table 1.
Intercorrelation between the investigated TAM subsets in the entire cohort
| PT pre-NAC | PT post-NAC | LN metastasis post-NAC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CD68+/CD163– | CD68+/CD163+ | MARCO+ | CD68+/CD163– | CD68+/CD163+ | MARCO+ | CD68+/CD163– | CD68+/CD163+ | MARCO+ | |
| CD68+/CD163– | |||||||||
| R | – | 0.262 | 0.018 | – | 0.082 | 0.037 | – | 0.149 | –0.206 |
| p value | – | <0.001 | <0.001 | – | <0.001 | <0.001 | – | <0.001 | <0.001 |
| n | – | 69 | 65 | – | 92 | 89 | – | 33 | 31 |
| CD68+/CD163+ | |||||||||
| R | 0.262 | – | 0.051 | 0.082 | – | 0.217 | 0.149 | – | –0.225 |
| p value | <0.001 | – | <0.001 | <0.001 | – | <0.001 | <0.001 | – | <0.001 |
| n | 69 | – | 65 | 92 | – | 89 | 33 | – | 31 |
| MARCO+ | |||||||||
| R | 0.018 | 0.051 | – | 0.037 | 0.217 | – | –0.206 | –0.225 | – |
| p value | <0.001 | <0.001 | – | <0.001 | <0.001 | – | <0.001 | <0.001 | – |
| n | 65 | 65 | – | 89 | 89 | – | 31 | 31 | – |
R, Spearman's correlation coefficient; p, p value; n, number of cases available for analysis. * Significance at the 5% level.
Intercorrelations between the different TAM subsets in EG tumors in PT pre-NAC and PT post-NAC are shown in online supplementary Tables 2 and 3, respectively. All significant associations were very weak or weak.
Intercorrelations between the different TAM subsets with T cells, B cells, and programmed death-ligand 1 (PD-L1) expression, further described in our recent study [29], were also examined in the entire cohort (shown in online suppl. Table 4). Very weak or weak associations were identified in samples from all time points. In summary, these findings show allover weak intercorrelations between the investigated immune markers of the innate as well as the adaptive immunity.
Associations of Different TAM Subsets with Histopathological Regression
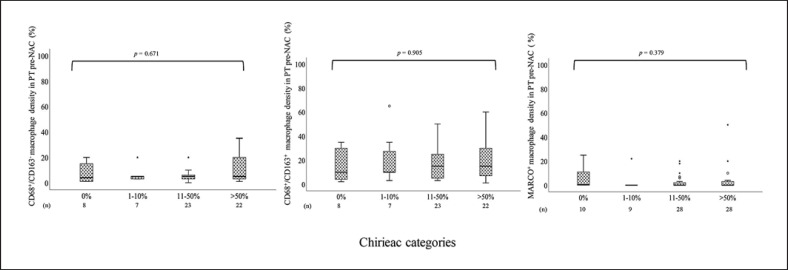
Box plots visualizing TAM subset density in diagnostic biopsies pre-NAC in relation to histopathological response in the resected PT in the entire cohort are shown in Figure 3 and for tumors in online supplementary Figure 1. Information regarding histopathological response was available for 117 (99.2%) of the 118 patients who underwent surgical resection after NAC. Complete response was noted in 13 (11.1%) patients, and the distribution of the other categories were as follows: 13 (11.1%) with 1–10%, 46 (39.3%) with 11–50%, and 45 (38.5%) with >50% residual TCs. The density of CD68+/CD163−, CD68+/CD163+, and MARCO+ TAMs in PT pre-NAC was not significantly altered in relation to histopathological response, neither for the entire cohort nor when stratifying for tumor location. Taken together, this means that the noted changes of the different TAM subsets pre-NAC to post-NAC were not associated with the degree of histopathological regression.
Fig. 3.
Boxplots visualizing the association between the density of CD68+/CD163−, CD68+/CD163+, and MARCO+ TAMs, in PT pre-NAC and histopathological regression according to the different Chirieac categories (0%, 1–10%, 11–50%, or >50% residual carcinoma) in the entire cohort.
Associations of Different TAM Subsets with Clinicopathological Factors in PT Pre-NAC and Post-NAC
Associations of the different TAM subsets with clinicopathological factors in PT pre-NAC and PT post-NAC are shown in online supplementary Tables 5 and 6, respectively. In PT pre-NAC, high density of CD68+/CD163− macrophages was significantly associated with high tumor grade, whereas high density of CD68+/CD163+ macrophages was significantly associated with Laurén mixed type. In PT post-NAC, high density of MARCO+ macrophages was significantly associated with higher age. In conclusion, CD68+/CD163− TAMs was the only TAM marker with a significant association to an established unfavorable prognostic factor (high tumor grade).
Prognostic Significance of TAM Subsets in PT Pre-NAC and Post-NAC
Classification and regression tree derived cutoffs were not meaningful due to skewness of the data, hence, continuous variables were used in univariable and multivariable analyses for the different TAM subsets. The infiltration of CD68+/CD163− and MARCO+ TAMs into TN was dichotomized as none versus sparse in pre-NAC specimens since no cases with high infiltration were identified. This was also the case for infiltration of CD68+/CD163− TAMs into TN in post-NAC specimens; however, no infiltration into TN was identified for MARCO+ TAMs post-NAC. The infiltration of CD68+/CD163+ TAMs into TN was dichotomized as non/sparse versus high in pre- and post-NAC specimens as described previously by Jeremiasen et al. [25]. Hazard ratio and confidence interval of total infiltration, TN infiltration, and CD68+CD163−/CD68+CD163+ ratios are visualized by Forest plots.
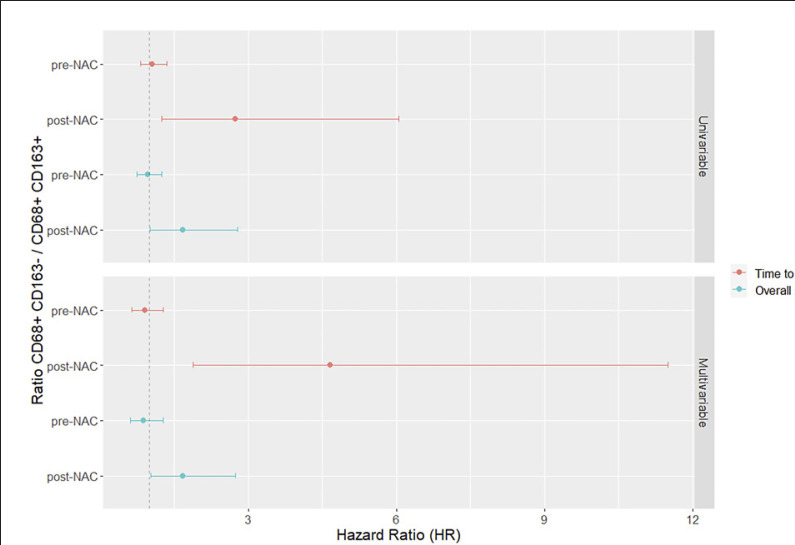
In pre-NAC specimens, no significant associations were identified between total infiltration and TTR or OS for neither of the TAM subsets (shown in Fig. 4, 5 for TTR and OS, respectively). Furthermore, the CD68+CD163−/CD68+CD163+ ratio had no prognostic value, as shown in Figure 6.
Fig. 4.
Forest plots visualizing Cox proportional hazards analysis of the impact of total infiltration of CD68+/CD163−, CD68+/CD163+, and MARCO+ TAMs in PT pre-NAC and PT post-NAC, on TTR, in unadjusted and adjusted analyses. HR, hazard ratio.
Fig. 5.
Forest plots visualizing Cox proportional hazards analysis of the impact of total infiltration of CD68+/CD163−, CD68+/CD163+, and MARCO+ TAMs in PT pre-NAC and PT post-NAC, on OS, in unadjusted and adjusted analyses. HR, hazard ratio.
Fig. 6.
Forest plots visualizing Cox proportional hazards analysis of the impact of CD68+CD163−/CD68+CD163+ TAM ratio in PT pre-NAC and PT post-NAC, on TTR and OS, in unadjusted and adjusted analyses. HR, hazard ratio.
In post-NAC specimens, a significant association was seen between a higher total infiltration of CD68+/CD163− TAMs and a shorter TTR and OS. Furthermore, higher CD68+/CD163− infiltration remained an independent unbeneficial prognostic factor for both TTR and OS, as shown in Figures 4 and 5, for TTR and OS, respectively. Moreover, a higher CD68+CD163−/CD68+CD163+ ratio was significantly associated with a shorter TTR and OS, in univariable as well as multivariable analysis, as shown in Figure 6. In pre-NAC specimens, high infiltration of CD68+/CD163+ TAMs into TN was significantly associated with a shorter TTR and OS in univariable analyses and remained an independent unbeneficial prognostic factor for TTR in multivariable analysis, as shown in Figure 7 and Figure 8, respectively. In post-NAC specimens, the presence of tumor-infiltrating CD68+/CD163− TAMs was an independent prognostic factor for a shorter TTR (shown in Fig. 7).
Fig. 7.
Forest plots visualizing Cox proportional hazards analysis of the impact of TN infiltration of CD68+/CD163−, CD68+/CD163+, and MARCO+ TAMs in PT pre-NAC and PT post-NAC, on TTR, in unadjusted and adjusted analyses. HR, hazard ratio.
Fig. 8.
Forest plots visualizing Cox proportional hazards analysis of the impact of TN infiltration of CD68+/CD163−, CD68+/CD163+, and MARCO+ TAMs in PT pre-NAC and PT post-NAC, on OS, in unadjusted and adjusted analyses. HR, hazard ratio.
Taken together, a higher total infiltration as well as infiltration into TN of CD68+/CD163− TAMs was a strong unbeneficial factor for survival in post-NAC specimens. Contrastingly, in pre-NAC specimens, high infiltration of CD68+/CD163+ macrophages into TN was a prognostic factor for shorter survival.
Discussion
Our results revealed that the density of CD68+/CD163+ TAMs increased in PT after NAC while the density of MARCO+ TAMs decreased. In paired LN metastases, the density of CD68+/CD163− TAMs and MARCO+ TAMs was similar to the density in PT post-NAC, whereas the density of CD68+/CD163+ TAMs was significantly lower. These results are partly in line with a previous study by Wei et al. [40] who examined 50 paired pre- and post-NAC samples of gastric cancer and demonstrated a significant increase in CD68+ and CD163+ TAM expression in PT post-NAC. In that study, single immunohistochemistry was utilized, with CD68 as a pan-macrophage marker, and CD163 as an M2 like marker [40]. Of note, an increase in CD8+ T cells post-NAC was also demonstrated, which is in line with previous results from the present cohort [29].
Our study could not identify any significant associations between the density of the different TAM subsets in pre-treatment biopsies and histopathological regression, which is also in line with the study by Wei et al. [40]. Studies on other immune markers such as T cells and PD-L1 expression have also revealed a lack of association with histopathological response in EG adenocarcinoma [29, 41]. However, Yamamoto et al. [42] demonstrated high CD68+ or CD206+ TAM infiltration in pre-treatment specimens to be an independent predictor of poor histopathological response following NAC in 86 cases of esophageal squamous cell carcinoma.
While the present study could not demonstrate any prognostic value for either of the TAM subsets regarding the total infiltration pre-NAC, higher infiltration of CD68+/CD163− TAMs post-NAC was an independent factor of shorter survival. This may suggest a functional effect by NAC, polarizing the CD68+/CD163− TAMs toward a more immunosuppressive or tumor-promoting state, however without displaying CD163 positivity. This is further strengthened by the fact that CD68+/CD163− TAM density in post-NAC specimens was not affected by altered levels of CD68+/CD163+ density. These results are partly in line with the study by Wei et al. [40] wherein high infiltration of CD163+ TAMs was an independent predictor of shorter OS post-NAC but not pre-NAC. Furthermore, Wei et al. [40] found a correlation between the ratio of CD163+/CD68+ TAMs and CD8+/CD3+ T cells post-NAC, suggesting that an increased CD8/CD3 ratio neutralizes the immunosuppressive effects of M2 polarization. A study by Ishigami et al. [43] (n = 97) on chemoradiotherapy-naïve gastric cancer found that high expression of CD68+ TAMs was associated with advanced clinical stage and poor outcome. Moreover, a negative association between CD68+ TAMs and CD3+ T-cell infiltration was found, proposing an impaired T-cell function in tumors with high TAM infiltration [43].
Of several studies investigating the prognostic impact of TAMs in the chemoradiotherapy-naïve setting, many have only used CD68 as a marker, which may cloud the results. The majority of studies utilizing M2 like markers such as CD204 or CD163 demonstrate an association with adverse clinical outcome [44, 45]. Anti-inflammatory M2 like macrophages suppress immune responses, stimulate angiogenesis and enhance tumor metastasis [46, 47], and have been shown to be a significant risk factor for developing adenocarcinoma from gastric adenoma [48]. However, one study on microsatellite instability-high (MSI-H) gastric tumors demonstrated high CD163+ density to be independently associated with a prolonged disease free survival [49]. There are also studies that, similar to the present study, failed to show any significant association between total infiltration of M2 like TAMs and prognosis, although they examined treatment-naïve PT and not pre- and post-treatment specimens [25, 50, 51]. One of these previous studies (n = 52) employed double IHC with CD68/NOS2 (inducible nitric oxide synthase) and CD68/CD163 to better distinguish M1 like from M2 like macrophages, and demonstrated high M1 like density to be a positive prognostic factor in univariable but not in multivariable analysis, and high M1/M2-like ratio to be a positive independent predictor of OS [51]. Contrarily, the present study demonstrated a higher CD68+CD163-/CD68+CD163+ ratio to be an independent predictor of poor survival. In another study on esophageal adenocarcinoma (n = 53), encompassing both treatment-naïve and chemotherapy-treated patients, double immunofluorescence of CD40/CD68, CD163/CD68, and CD40/CD163 was applied in an effort to distinguish M1 like from M2 like subgroups. The results showed that higher M2 like density was associated with poor survival in treatment-naïve patients, independently if located in the tumor center or tumor edge (whole tissue blocks were examined). Furthermore, a higher M2/M1 like ratio at either the tumor edge or tumor center was significantly associated with LN metastasis and poor survival. Contrarily, for patients treated with chemotherapy, no significant association was seen with nodal spread or OS. The study also demonstrated that M2-like macrophages stimulated EAC cell migration and increased cancer cell invasion in vitro [52].
To the best of our knowledge, no studies have yet investigated the prognostic associations of MARCO+ TAMs post-NAC. As previously described, Jeremiasen et al. [25] could not find any association with survival in a chemoradiotherapy-naïve cohort of EG adenocarcinoma. However, MARCO might be of interest as a potential novel target for immune checkpoint inhibitors, as highlighted by a study on non-small cell lung cancer, revealing co-localization of MARCO and PD-L1 in the membrane and cytoplasm of macrophages [53]. Moreover, in a study on melanoma, Eisinger et al.[54] reported that targeting MARCO+ TAMs led to activation of natural killer cell-mediated tumor elimination.
The present study also investigated the prognostic impact of TAM infiltration into TN and found that high infiltration of CD68+/CD163+ TAMs pre-NAC is a prognostic factor for shorter survival. This result is in line with two previous studies on chemoradiotherapy-naïve gastric cancer, one demonstrating high CD163+ TAM TN infiltration to be correlated with decreased disease free survival [55] and one demonstrating higher infiltration of CD68+ and CD163+ TAMs to be stepwise associated with reduced survival [25]. Contrastingly, a study on gastric cancer by Huang et al.[56] investigating full face gastric cancer tissue specimens (n = 56) with multiplex IHC demonstrated that patients with higher effective density (defined as direct contact between macrophage and TC) of CD68+/163+ TAMs had a significantly longer recurrence-free survival as well as OS compared to those with lower effective TAM density. This study did however not compare the TAM density in tumor specimens before and after treatment.
Of note, in the present study, the prognostic impact of TN-infiltrating CD68+/CD163+ TAMs was lost post-NAC. TN infiltration by MARCO+ TAMs, only denoted in pre-NAC specimens, was not found to be prognostic, which is in line with the study by Jeremiasen et al. [25]. Speculatively, some MARCO+ TAMs in TN might be skewed toward another TAM phenotype after NAC. To the best of our knowledge, this is the first study to investigate the prognostic impact of compartmental localization of TAMs pre- and post-NAC in esophageal and post-NAC in gastric adenocarcinoma.
In chemoradiotherapy-naïve gastric cancer, M1 like TAMs are in general considered to be a beneficial prognostic factor, however in the present study, in addition to the unbeneficial prognostic impact, CD68+/CD163− TAMs was the only immune marker with a significant association to an established unfavorable prognostic factor, i.e., high tumor grade.
As stated before, chemotherapeutic agents have the capability to alter the immune composition in the TME and, conversely, TAMs have been shown to modify chemotherapeutic agents resulting in either an enhanced or a diminished effect, depending on both the tumor type and type of chemotherapy administered [57]. In breast cancer, TAMs have been demonstrated to accumulate in tumors after NAC and to suppress cytotoxic T-cell immunity, thus promoting tumor progression [58]. Furthermore, a study on esophageal squamous cell carcinoma concluded that high infiltration of CD68+ and CD163+ TAMs in post-treatment specimens was associated with poor response to NAC, and the pre-treated subgroup with high TAM infiltration had poor prognosis, while no association was found in the untreated subgroup [59]. Together, these findings mirror the complex interactions between TAMs and chemotherapeutic agents as well as the plasticity of macrophages.
Given the poor prognosis of EG adenocarcinoma, it is of great clinical importance to improve the prognostication as well as the treatment strategies for these patients. To enable this, it is crucial to delineate the effect of NAC on the TME, including different TAM subsets, not least considering the implementation of immune checkpoint inhibition in combination with chemotherapy in different settings.
There are some limitations to this study that need to be acknowledged. There is a lack of uniform standard for macrophage markers which may lead to diverging results. However, the present study utilized double staining in an effort to better distinguish the different TAM subsets. The use of different antibody clones may also cloud the results when comparing studies. For example, in the study by Wei et al. [40], the PG-M1 clone (Dako) was used for detection of CD68, whereas the present study utilized the KP1 clone (Dako), which besides from detecting CD68 presented by macrophages also detects CD68 presented by myeloid cells. Ischigami et al. [43] used the same anti-CD68 antibody as in the present study.
Another possible limitation is the use of the TMA technique which may contribute to a sampling bias considering the heterogeneity of the TME. Moreover, TMAs will likely better reflect the tumor than the stromal compartment. To reduce the risk of sampling bias, the herein used TMAs had been constructed with multiple tissue cores from at least two different donor blocks with PT and from separate LN metastases whenever possible. In addition, both central and peripheral tumor areas were sampled. Lastly, the investigated pre-NAC specimens were derived from diagnostic biopsies, while the post-NAC specimens were derived from resected PT, hence potentially also contributing to a sampling bias.
Conclusion
This study demonstrates that high infiltration of CD68+/CD163− TAMs as well as infiltration into TN is an adverse prognostic factor in post-NAC but not in pre-NAC specimens, whereas high infiltration of CD68+/CD163+ is an adverse prognostic factor only pre-NAC. Moreover, the results propose that NAC may alter certain TAM subsets and potentially polarize their functional state and, thus, their prognostic value.
Statement of Ethics
Approval was acquired from the Ethics Committee of Lund University (reference nr 445/07) whereby the committee waived no need for consent other than the option to opt out. All EU and national regulations and requirements for handling human samples have been fully complied with during the conduct of this project, i.e., decision no. 1110/94/EC of the European Parliament and of the Council (OJL126 18,5,94), the Helsinki Declaration on ethical principles for medical research involving human rights and Biomedicine. All authors have given their consent for publication.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This work was supported by peer-reviewed grants from the Swedish Cancer Society under Grant 2016/483; the Mrs Berta Kamprad Foundation under Grant FBKS-2016-21; regional grants, Region Halland, under Grant 962865; Skåne University Hospital Funds and Donations, Lund University Faculty of Medicine, and Governmental Funding of Clinical Research within the National Health Service (ALF) under Grant 2018-0060. The funders of this study had no role in the study design, data collection, analysis, interpretation, or writing of the report.
Author Contributions
M.C.S.: carried out the immunohistochemical and statistical analyses and wrote the paper. M.S.: carried out statistical analyses and helped writing the paper. B.N.: constructed the tissue microarrays and carried out the immunohistochemical staining. C.H. and D.B.: collected histopathological and clinical data. C.Hj.: supervised the study and helped writing the paper. K.L.: provided immunological expertise and critical reading of the manuscript. K.J.: conceived and supervised the study and helped writing the paper. All authors read and approved the final manuscript.
Data Availability Statement
Part of the data generated in this study is included in the article. The raw data of immune cell expression can be made available upon request. Patient and clinicopathological data cannot be made publicly available due to their content of identifiable human data. Requests to access the datasets should be directed to the corresponding author.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Funding Statement
This work was supported by peer-reviewed grants from the Swedish Cancer Society under Grant 2016/483; the Mrs Berta Kamprad Foundation under Grant FBKS-2016-21; regional grants, Region Halland, under Grant 962865; Skåne University Hospital Funds and Donations, Lund University Faculty of Medicine, and Governmental Funding of Clinical Research within the National Health Service (ALF) under Grant 2018-0060. The funders of this study had no role in the study design, data collection, analysis, interpretation, or writing of the report.
References
- 1.Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393((10184)):1948–57. doi: 10.1016/S0140-6736(18)32557-1. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355((1)):11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 3.Ychou M, Boige V, Pignon JP, Conroy T, Bouche O, Lebreton G, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29((13)):1715–21. doi: 10.1200/JCO.2010.33.0597. [DOI] [PubMed] [Google Scholar]
- 4.Smyth EC, Fassan M, Cunningham D, Allum WH, Okines AF, Lampis A, et al. Effect of pathologic tumor response and nodal status on survival in the medical research council adjuvant gastric infusional chemotherapy trial. J Clin Oncol. 2016;34((23)):2721–7. doi: 10.1200/JCO.2015.65.7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144((5)):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Taube JM, Galon J, Sholl LM, Rodig SJ, Cottrell TR, Giraldo NA, et al. Implications of the tumor immune microenvironment for staging and therapeutics. Mod Pathol. 2018;31((2)):214–34. doi: 10.1038/modpathol.2017.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee HE, Chae SW, Lee YJ, Kim MA, Lee HS, Lee BL, et al. Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer. Br J Cancer. 2008;99((10)):1704–11. doi: 10.1038/sj.bjc.6604738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stein AV, Dislich B, Blank A, Guldener L, Kroll D, Seiler CA, et al. High intratumoural but not peritumoural inflammatory host response is associated with better prognosis in primary resected oesophageal adenocarcinomas. Pathology. 2017;49((1)):30–7. doi: 10.1016/j.pathol.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Lee JS, Won HS, Sun S, Hong JH, Ko YH. Prognostic role of tumor-infiltrating lymphocytes in gastric cancer: a systematic review and meta-analysis. Medicine. 2018;97((32)):e11769. doi: 10.1097/MD.0000000000011769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Svensson MC, Warfvinge CF, Fristedt R, Hedner C, Borg D, Eberhard J, et al. The integrative clinical impact of tumor-infiltrating T lymphocytes and NK cells in relation to B lymphocyte and plasma cell density in esophageal and gastric adenocarcinoma. Oncotarget. 2017;8((42)):72108–26. doi: 10.18632/oncotarget.19437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hennequin A, Derangere V, Boidot R, Apetoh L, Vincent J, Orry D, et al. Tumor infiltration by Tbet+ effector T cells and CD20+ B cells is associated with survival in gastric cancer patients. Oncoimmunology. 2016;5((2)):e1054598. doi: 10.1080/2162402X.2015.1054598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ni Z, Xing D, Zhang T, Ding N, Xiang D, Zhao Z, et al. Tumor-infiltrating B cell is associated with the control of progression of gastric cancer. Immunol Res. 2020;69((1)):43–52. doi: 10.1007/s12026-020-09167-z. [DOI] [PubMed] [Google Scholar]
- 13.Biswas SK, Allavena P, Mantovani A. Tumor-associated macrophages: functional diversity, clinical significance, and open questions. Semin Immunopathol. 2013;35((5)):585–600. doi: 10.1007/s00281-013-0367-7. [DOI] [PubMed] [Google Scholar]
- 14.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41((1)):14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiss M, Van Gassen S, Movahedi K, Saeys Y, Laoui D. Myeloid cell heterogeneity in cancer: not a single cell alike. Cell Immunol. 2018;330:188–201. doi: 10.1016/j.cellimm.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Bogels M, Braster R, Nijland PG, Gul N, van de Luijtgaarden W, Fijneman RJ, et al. Carcinoma origin dictates differential skewing of monocyte function. Oncoimmunology. 2012;1((6)):798–809. doi: 10.4161/onci.20427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang XL, Jiang JT, Wu CP. Prognostic significance of tumor-associated macrophage infiltration in gastric cancer: a meta-analysis. Genet Mol Res. 2016;15((4)) doi: 10.4238/gmr15049040. [DOI] [PubMed] [Google Scholar]
- 18.Nam SJ, Go H, Paik JH, Kim TM, Heo DS, Kim CW, et al. An increase of M2 macrophages predicts poor prognosis in patients with diffuse large B-cell lymphoma treated with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone. Leuk Lymphoma. 2014;55((11)):2466–76. doi: 10.3109/10428194.2013.879713. [DOI] [PubMed] [Google Scholar]
- 19.Zhao X, Qu J, Sun Y, Wang J, Liu X, Wang F, et al. Prognostic significance of tumor-associated macrophages in breast cancer: a meta-analysis of the literature. Oncotarget. 2017;8((18)):30576–86. doi: 10.18632/oncotarget.15736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen TO, Schmidt H, Moller HJ, Hoyer M, Maniecki MB, Sjoegren P, et al. Macrophage markers in serum and tumor have prognostic impact in American Joint Committee on Cancer stage I/II melanoma. J Clin Oncol. 2009;27((20)):3330–7. doi: 10.1200/JCO.2008.19.9919. [DOI] [PubMed] [Google Scholar]
- 21.Medrek C, Pontén F, Jirström K, Leandersson K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer. 2012;12:306. doi: 10.1186/1471-2407-12-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salvesen HB, Akslen LA. Significance of tumour-associated macrophages, vascular endothelial growth factor and thrombospondin-1 expression for tumour angiogenesis and prognosis in endometrial carcinomas. Int J Cancer. 1999;84((5)):538–43. doi: 10.1002/(sici)1097-0215(19991022)84:5<538::aid-ijc17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 23.Forssell J, Oberg A, Henriksson ML, Stenling R, Jung A, Palmqvist R. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res. 2007;13((5)):1472–9. doi: 10.1158/1078-0432.CCR-06-2073. [DOI] [PubMed] [Google Scholar]
- 24.Jing J, Yang IV, Hui L, Patel JA, Evans CM, Prikeris R, et al. Role of macrophage receptor with collagenous structure in innate immune tolerance. J Immunol. 2013;190((12)):6360–7. doi: 10.4049/jimmunol.1202942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeremiasen M, Borg D, Hedner C, Svensson M, Nodin B, Leandersson K, et al. Tumor-associated CD68(+), CD163(+), and MARCO(+) macrophages as prognostic biomarkers in patients with treatment-naive gastroesophageal adenocarcinoma. Front Oncol. 2020;10:534761. doi: 10.3389/fonc.2020.534761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun H, Song J, Weng C, Xu J, Huang M, Huang Q, et al. Association of decreased expression of the macrophage scavenger receptor MARCO with tumor progression and poor prognosis in human hepatocellular carcinoma. J Gastroenterol Hepatol. 2017;32((5)):1107–14. doi: 10.1111/jgh.13633. [DOI] [PubMed] [Google Scholar]
- 27.Lundgren S, Karnevi E, Elebro J, Nodin B, Karlsson MCI, Eberhard J, et al. The clinical importance of tumour-infiltrating macrophages and dendritic cells in periampullary adenocarcinoma differs by morphological subtype. J Transl Med. 2017;15((1)):152. doi: 10.1186/s12967-017-1256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8((1)):59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 29.Christina Svensson M, Linden A, Nygaard J, Borg D, Hedner C, Nodin B, et al. T cells, B cells, and PD-L1 expression in esophageal and gastric adenocarcinoma before and after neoadjuvant chemotherapy: relationship with histopathological response and survival. Oncoimmunology. 2021;10((1)):1921443. doi: 10.1080/2162402X.2021.1921443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leduc C, Adam J, Louvet E, Sourisseau T, Dorvault N, Bernard M, et al. TPF induction chemotherapy increases PD-L1 expression in tumour cells and immune cells in head and neck squamous cell carcinoma. ESMO Open. 2018;3((1)):e000257. doi: 10.1136/esmoopen-2017-000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fukuoka E, Yamashita K, Tanaka T, Sawada R, Sugita Y, Arimoto A, et al. Neoadjuvant chemotherapy increases PD-L1 expression and CD8(+) tumor-infiltrating lymphocytes in esophageal squamous cell carcinoma. Anticancer Res. 2019;39((8)):4539–48. doi: 10.21873/anticanres.13631. [DOI] [PubMed] [Google Scholar]
- 32.Yu Y, Ma X, Zhang Y, Zhang Y, Ying J, Zhang W, et al. Changes in expression of multiple checkpoint molecules and infiltration of tumor immune cells after neoadjuvant chemotherapy in gastric cancer. J Cancer. 2019;10((12)):2754–63. doi: 10.7150/jca.31755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Martinez E, Gil GL, Benito AC, Gonzalez-Billalabeitia E, Conesa MA, Garcia Garcia T, et al. Tumor-infiltrating immune cell profiles and their change after neoadjuvant chemotherapy predict response and prognosis of breast cancer. Breast Cancer Res. 2014;16((6)):488. doi: 10.1186/s13058-014-0488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borg D, Larsson AH, Hedner C, Nodin B, Johnsson A, Jirström K. Podocalyxin-like protein as a predictive biomarker for benefit of neoadjuvant chemotherapy in resectable gastric and esophageal adenocarcinoma. J Transl Med. 2018;16((1)):290. doi: 10.1186/s12967-018-1668-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hedner C, Borg D, Nodin B, Karnevi E, Jirström K, Eberhard J. Expression and prognostic significance of human epidermal growth factor receptors 1, 2 and 3 in oesophageal and gastric adenocarcinomas preneoadjuvant and postneoadjuvant treatment. J Clin Pathol. 2018;71((5)):451–62. doi: 10.1136/jclinpath-2017-204774. [DOI] [PubMed] [Google Scholar]
- 36.Sobin LGM, Wittekind C. TNM classification of malignant tumours. 7th ed. Wiley-Blackwell; 2009. Available from: http://eu.wiley.com/WileyCDA/WileyTitle/productCd-1444332414.html. [Google Scholar]
- 37.Brierley J, Gospodarowicz M, Wittekind C. TNM classification of malignant tumours. 8th ed. Wiley-Blackwell; 2017. p. p. 272. [Google Scholar]
- 38.Chirieac LR, Swisher SG, Ajani JA, Komaki RR, Correa AM, Morris JS, et al. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer. 2005;103((7)):1347–55. doi: 10.1002/cncr.20916. [DOI] [PubMed] [Google Scholar]
- 39.Hedner C, Borg D, Nodin B, Karnevi E, Jirström K, Eberhard J. Expression and prognostic significance of human epidermal growth factor receptors 1 and 3 in gastric and esophageal adenocarcinoma. PLoS One. 2016;11((2)):e0148101. doi: 10.1371/journal.pone.0148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei Q, Xu Q, Yuan X, Li JJ, Chen L, Luo C, et al. Immunological impact of chemotherapy on the tumor microenvironment in gastric cancer. J Surg Oncol. 2021;123((8)):1708–15. doi: 10.1002/jso.26449. [DOI] [PubMed] [Google Scholar]
- 41.Yu PC, Long D, Liao CC, Zhang S. Association between density of tumor-infiltrating lymphocytes and prognoses of patients with gastric cancer. Medicine. 2018;97((27)):e11387. doi: 10.1097/MD.0000000000011387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamamoto K, Makino T, Sato E, Noma T, Urakawa S, Takeoka T, et al. Tumor-infiltrating M2 macrophage in pretreatment biopsy sample predicts response to chemotherapy and survival in esophageal cancer. Cancer Sci. 2020;111((4)):1103–12. doi: 10.1111/cas.14328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Okumura H, Matsumoto M, et al. Tumor-associated macrophage (TAM) infiltration in gastric cancer. Anticancer Res. 2003;23((5A)):4079–83. [PubMed] [Google Scholar]
- 44.Lin CN, Wang CJ, Chao YJ, Lai MD, Shan YS. The significance of the co-existence of osteopontin and tumor-associated macrophages in gastric cancer progression. BMC Cancer. 2015;15:128. doi: 10.1186/s12885-015-1114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan Y, Zhang J, Li JH, Liu X, Wang JZ, Qu HY, et al. High tumor-associated macrophages infiltration is associated with poor prognosis and may contribute to the phenomenon of epithelial-mesenchymal transition in gastric cancer. Onco Targets Ther. 2016;9:3975–83. doi: 10.2147/OTT.S103112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23((11)):549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 47.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8((12)):958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taniyama D, Taniyama K, Kuraoka K, Zaitsu J, Saito A, Nakatsuka H, et al. Long-term follow-up study of gastric adenoma; tumor-associated macrophages are associated to carcinoma development in gastric adenoma. Gastric Cancer. 2017;20((6)):929–39. doi: 10.1007/s10120-017-0713-x. [DOI] [PubMed] [Google Scholar]
- 49.Kim KJ, Wen XY, Yang HK, Kim WH, Kang GH. Prognostic implication of M2 macrophages are determined by the proportional balance of tumor associated macrophages and tumor infiltrating lymphocytes in microsatellite-unstable gastric carcinoma. PLoS One. 2015;10((12)):e0144192. doi: 10.1371/journal.pone.0144192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kawahara A, Hattori S, Akiba J, Nakashima K, Taira T, Watari K, et al. Infiltration of thymidine phosphorylase-positive macrophages is closely associated with tumor angiogenesis and survival in intestinal type gastric cancer. Oncol Rep. 2010;24((2)):405–15. doi: 10.3892/or_00000873. [DOI] [PubMed] [Google Scholar]
- 51.Pantano F, Berti P, Guida FM, Perrone G, Vincenzi B, Amato MM, et al. The role of macrophages polarization in predicting prognosis of radically resected gastric cancer patients. J Cell Mol Med. 2013;17((11)):1415–21. doi: 10.1111/jcmm.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cao W, Peters JH, Nieman D, Sharma M, Watson T, Yu J. Macrophage subtype predicts lymph node metastasis in oesophageal adenocarcinoma and promotes cancer cell invasion in vitro. Br J Cancer. 2015;113((5)):738–46. doi: 10.1038/bjc.2015.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.La Fleur L, Boura VF, Alexeyenko A, Berglund A, Ponten V, Mattsson JSM, et al. Expression of scavenger receptor MARCO defines a targetable tumor-associated macrophage subset in non-small cell lung cancer. Int J Cancer. 2018;143((7)):1741–52. doi: 10.1002/ijc.31545. [DOI] [PubMed] [Google Scholar]
- 54.Eisinger S, Sarhan D, Boura VF, Ibarlucea-Benitez I, Tyystjarvi S, Oliynyk G, et al. Targeting a scavenger receptor on tumor-associated macrophages activates tumor cell killing by natural killer cells. Proc Natl Acad Sci U S A. 2020;117((50)):32005–16. doi: 10.1073/pnas.2015343117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park JY, Sung JY, Lee J, Park YK, Kim YW, Kim GY, et al. Polarized CD163+ tumor-associated macrophages are associated with increased angiogenesis and CXCL12 expression in gastric cancer. Clin Res Hepatol Gastroenterol. 2016;40((3)):357–65. doi: 10.1016/j.clinre.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 56.Huang YK, Wang M, Sun Y, Di Costanzo N, Mitchell C, Achuthan A, et al. Macrophage spatial heterogeneity in gastric cancer defined by multiplex immunohistochemistry. Nat Commun. 2019;10((1)):3928. doi: 10.1038/s41467-019-11788-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Larionova I, Cherdyntseva N, Liu T, Patysheva M, Rakina M, Kzhyshkowska J. Interaction of tumor-associated macrophages and cancer chemotherapy. Oncoimmunology. 2019;8((7)):1596004. doi: 10.1080/2162402X.2019.1596004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1((1)):54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sugimura K, Miyata H, Tanaka K, Takahashi T, Kurokawa Y, Yamasaki M, et al. High infiltration of tumor-associated macrophages is associated with a poor response to chemotherapy and poor prognosis of patients undergoing neoadjuvant chemotherapy for esophageal cancer. J Surg Oncol. 2015;111((6)):752–9. doi: 10.1002/jso.23881. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Data Availability Statement
Part of the data generated in this study is included in the article. The raw data of immune cell expression can be made available upon request. Patient and clinicopathological data cannot be made publicly available due to their content of identifiable human data. Requests to access the datasets should be directed to the corresponding author.