Abstract
Objective
Enhanced Recovery After Surgery protocols are relatively new in cardiac surgery. Enhanced Recovery After Surgery addresses perioperative analgesia by implementing multimodal pain control regimens that include both opioid and nonopioid components. We investigated the effects of an Enhanced Recovery After Surgery protocol at our institution on postoperative outcomes with particular focus on analgesia.
Methods
Single-center retrospective study comparing perioperative opioid use before and after implementation of an Enhanced Recovery After Surgery protocol at our institution. Subjects were divided into 2 cohorts: Enhanced Recovery After Surgery (study group from year 2020) and pre–Enhanced Recovery After Surgery (control group from year 2018). Baseline and perioperative variables including total opioid use from the day of surgery to postoperative day 5 were collected. Opioid use was calculated as morphine milligram equivalents and compared between the 2 cohorts.
Results
A total of 466 patients were included: 250 in the Enhanced Recovery After Surgery group and 216 in the pre–Enhanced Recovery After Surgery group. Both groups had similar baseline characteristics, but the Enhanced Recovery After Surgery group had significantly more subjects with intravenous drug use history (P < .0001), endocarditis (P < .0001), and liver disease (P = .007) compared with the pre–Enhanced Recovery After Surgery group. Every day from the day of surgery to postoperative day 5, the Enhanced Recovery After Surgery group had significant reduction (57%) in opioid use compared with the pre–Enhanced Recovery After Surgery group. Total opioid use for the entire length of stay was 259 morphine milligram equivalents in the Enhanced Recovery After Surgery group versus 452 morphine milligram equivalents in the pre–Enhanced Recovery After Surgery group (P < .0001). Subgroup analysis of subjects with intravenous drug use history did not demonstrate a significant reduction in opioid use.
Conclusions
Enhanced Recovery After Surgery protocols with an emphasis on multimodal pain management throughout perioperative care are associated with a significant reduction in the postoperative use of opioid analgesics.
Key Words: perioperative care, cardiac surgery
Abbreviations and Acronyms: ERAS, Enhanced Recovery After Surgery; IVDU, intravenous drug use; MME, morphine milligram equivalent; NIVDU, nonintravenous drug use; NSAID, nonsteroidal anti-inflammatory drug
Graphical abstract
Reduction in opioid use by postoperative day in cardiac surgery patients.
Central Message.
ERAS protocols with an emphasis on multimodal analgesia are associated with significantly reduced perioperative opioid use in cardiac surgery patients.
Perspective.
Standardized ERAS protocols can help optimize postoperative outcomes, particularly with respect to perioperative analgesia. A multimodal analgesic regimen consisting of both opioid and nonopioid approaches used throughout all phases of care is associated with significantly reduced perioperative opioid analgesic use in cardiac surgery patients.
See Commentary on page 297.
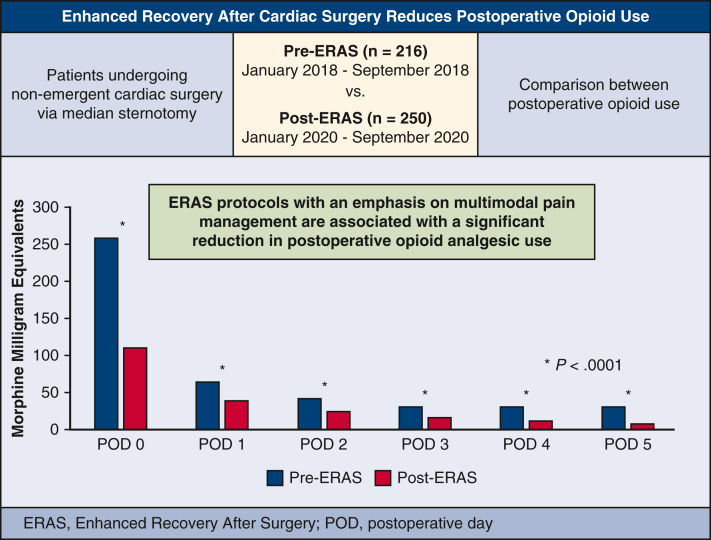
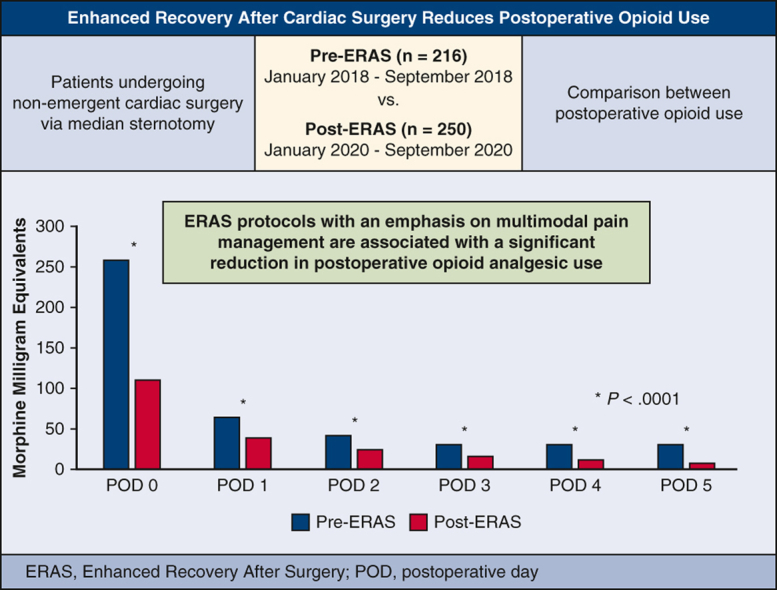
Enhanced Recovery after Surgery (ERAS) is a multimodal, multidisciplinary perioperative management strategy with the goal of optimizing patient recovery and outcomes.1 The first ERAS protocol was developed in 2001 to standardize outcomes in colorectal surgery and was largely built on the principles established by Henrik Kehlet in the 1990s surrounding “fast-track surgery.”2 One major aspect of ERAS addresses postoperative analgesia by implementing multimodal pain control regimens. The use of multimodal pain management protocols has been associated with reduction in opioid analgesic use, patient discomfort, and hospital length of stay across multiple surgical subspecialties.3,4 ERAS protocols remain a relatively new paradigm in cardiac surgery where patients face unique challenges, including multiple incision sites, chest tube drainage, and invasive lines and catheters, which all contribute to patient discomfort.4 The goal of this study was to investigate the effects of a novel ERAS protocol at our institution on postoperative outcomes. Although we intend to report other outcomes of our protocol, this review focuses on perioperative opioid analgesic use (Figure 1).
Figure 1.
ERAS reduces postoperative opioid use. ERAS, Enhanced Recovery After Surgery; POD, postoperative day.
Methods and Materials
Enhanced Recovery After Surgery Protocol Development
Our institution convened a multidisciplinary group of individuals to develop an ERAS protocol. Every discipline that interacted with cardiac surgical patients was included, with each discipline having an “ERAS Champion” to serve as a liaison to other providers within their respective fields. Our protocol was based on the Guidelines for Perioperative Care in Cardiac Surgery Enhanced Recovery After Surgery Society Recommendations and was tailored with consideration of institutional resources, feasibility of successful implementation, and the needs of our patient population.3 After multiple iterations of the protocol, final approval was granted by unanimous agreement by the multidisciplinary champions. ERAS was implemented on all patients who underwent nonemergency cardiac surgery via median sternotomy starting in January 2020. Data variables were collected prospectively into an ERAS database, and an ERAS Leadership Team met regularly to monitor for adverse effects and logistic hurdles. This also allowed the team to identify and address challenges as they arose.
Enhanced Recovery After Surgery Protocol Components
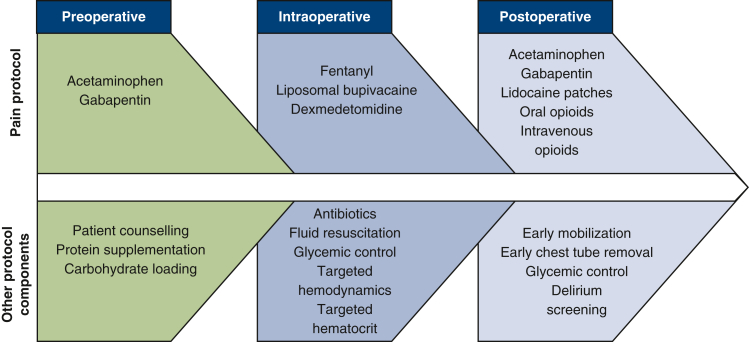
The ERAS protocol addressed all phases of care: preoperative, intraoperative, and postoperative. Key components of the protocol included multimodal pain management, patient education, high protein nutritional supplementation, goal-directed intraoperative fluid and hemodynamic management, early postoperative mobilization and chest tube removal, and delirium screening (Figure 2). Although the entire ERAS protocol encompassed all of these areas, for the purposes of this manuscript we focus on the analgesic aspect of the protocol. The multimodal analgesic components included preoperative acetaminophen and gabapentin, intraoperative topical anesthetic with liposomal bupivacaine and encouragement of reduced intravenous (IV) opioid use, and postoperative opioids in combination with nonopioids such as gabapentin, nonsteroidal anti-inflammatory drugs (NSAIDs), and lidocaine patches (Table 1).
Figure 2.
ERAS protocol.
Table 1.
Perioperative multimodal pain regimen
| Preoperative |
| Acetaminophen 1 g 2 h before surgery |
| Gabapentin 300 mg 2 h before surgery |
| Intraoperative |
| Recommend reduced opioid use to < 500 μg fentanyl |
| Local anesthetic with liposomal bupivacaine 10 mL chest tube sites 15 mL incision |
| Postoperative |
| Dexmedetomidine (initiated in OR, continued until extubation) |
| Acetaminophen 650 mg scheduled every 6 h |
| Lidocaine 5% transdermal patch, applied to bilateral back or chest |
| Gabapentin 100 mg TID 100 mg BID if renal impairment |
| Tramadol 50-100 mg PO every 6 h-PRN for mild pain (every 12 h for CrCl <30, max 200 mg/d) |
| Oxycodone 5-10 mg PO every 4 h-PRN moderate pain |
| IV opioids (eg, hydromorphone, fentanyl), PRN severe/breakthrough pain Dose and drug at provider discretion Discontinued when chest tubes removed |
OR, Operating room; TID, ter in die (3 times daily); BID, bis in die (2 times daily); PO, per os (by mouth); PRN, pro re nata (as needed); CrCl, creatinine clearance; IV, intravenous.
Of note, opioid analgesics were not intentionally withheld or limited in the postoperative period; various narcotics were ordered for all patients and administered when requested by patients or deemed necessary to achieve adequate analgesia by nursing or physician staff. Furthermore, clinical staff were instructed that the goal was not to specifically reduce opioid use but rather that the intent with ERAS analgesia was to achieve adequate pain control to allow for enhanced overall patient recovery. Patients were also administered a 3-question survey (all using Likert scale grading) on the day of discharge, with one of the questions focused on the patient's perception of their perioperative analgesia: “How satisfied are you with how your pain was controlled in the hospital after surgery?” The 5-point Likert scale response options were “very dissatisfied,” “dissatisfied,” “neutral,” “satisfied,” or “very satisfied.”
Study Design
The Institutional Review Board of Indiana University approved this retrospective study on 9/25/2020 (IRB #2009811102). Informed written consent was obtained by all patients who were included in this study. Our ERAS prospective database was used to identify the study group, which included all patients who received the ERAS protocol from January to September 2020. An institutional Society of Thoracic Surgeons database was queried to identify the control group: All patients who underwent nonemergency cardiac surgery via median sternotomy from January to September 2018. This time period was chosen as the control group because the surgical attending staff were mostly unchanged (one surgeon from 2018 had departed the group and thus not included in the 2020 study group), and no ERAS components were applied in 2018. Demographic and relevant clinical data were extracted from these registries and from individual medical records. Medication administration records were reviewed, and all sources of opioid analgesic were converted into morphine milligram equivalents (MMEs) using a standardized conversion chart (Table E1).5
Statistical Analysis
Patient characteristics were evaluated using frequency and proportion for all categorical variables. All continuous variables were tested for normality using Shapiro–Wilk tests and due to evidence of skewness we used median and inter quartile range for all continuous variables. We also examined the statistical difference in the patient characteristics between the control and ERAS cohorts using chi-square or Fisher exact tests and Wilcoxon rank-sum tests, as appropriate. Bivariate analyses using quantile (median) regressions were done to analyze the relationship between opioid analgesic use by postoperative days in all patients, and this analysis was repeated for 2 subgroup analyses (illicit drug use vs nonillicit drug use). Multivariable quantile regression using 50th percentile (median) of MME was performed at each cross-section of the follow-up time (postoperative day 0 to 5). To account for the panel nature of the study where multiple observations are nested within a study participant, we used multivariable quantile regression with bootstrapped clustered standard error to account for the within subject correlation. Marginal plot of linear prediction of MME over days was also created as a postestimation for the nested study design. The result was consistent with the multivariable quantile regression of MME at each cross-section of the follow-up time. All analyses were done using Stata/MP 16.1.6
Results
In total, 466 patients were included and divided into 2 groups: n = 250 in ERAS (study group) and n = 216 in pre-ERAS (control group). Baseline characteristics of the 2 cohorts are listed in Table 2: The 2 groups were similar with the exception that the ERAS group had significantly more patients with endocarditis (n = 60 [24%] vs n = 12 [5.6%], P < .0001), a history of intravenous drug use (IVDU) (n = 56 [22%] vs n = 17 [7.9%], P < .0001), and liver disease (n = 27 [10.8%] vs n = 9 [4.2%], P < .007). These differences between the pre-ERAS and ERAS groups were partially due to the opioid epidemic and were controlled by multivariate analysis (Table E2).7 Patient compliance was greatest in the postoperative period (Table E3).
Table 2.
Patient characteristics
| Characteristic, n (%) | Control (n = 216) | ERAS (n = 250) | Total (n = 466) | P value |
|---|---|---|---|---|
| Procedure type | <.0001 | |||
| Ascending aortic | 47 (21.7) | 36 (14.4) | 83 (17.8) | |
| CABG | 75 (34.7) | 95 (38) | 170 (36.5) | |
| Valve | 65 (30.1) | 54 (21.6) | 119 (25.5) | |
| Valve + CABG | 12 (5.6) | 12 (4.8) | 24 (5.2) | |
| Other | 17 (7.9) | 53 (21.2) | 70 (15.0) | |
| Age, median (Q1, Q3) | 64 (57, 70) | 62 (51, 70) | 63 (55, 70) | .132 |
| Gender | .542 | |||
| Male | 150 (69.4) | 167 (66.8) | 317 (68.0) | |
| Female | 66 (30.6) | 83 (33.2) | 149 (32.0) | |
| Race | .568 | |||
| White | 188 (87.0) | 213 (85.2) | 401 (86.1) | |
| Non-White | 28 (13.0) | 37 (14.8) | 65 (14.0) | |
| Risk factors | ||||
| BMI, median (Q1, Q3) | 30 (25.9, 34.0) | 29 (25.0, 33.8) | 29 (25.4, 34.0) | .240 |
| Diabetes | 81 (37.5) | 93 (37.2) | 174 (37.3) | .947 |
| Endocarditis | 12 (5.6) | 60 (24) | 72 (15.5) | <.0001 |
| Cerebrovascular disease | 31 (14.4) | 52 (20.8) | 83 (17.8) | .070 |
| Chronic lung disease | 55 (25.5) | 62 (24.8) | 117 (25.1) | .869 |
| Family history of CAD | 9 (4.2) | 3 (1.2) | 12 (2.6) | .074 |
| Hypertension | 180 (83.3) | 177 (70.8) | 357 (76.6) | .001 |
| Intravenous drug use | 17 (7.9) | 56 (22.4) | 73 (15.7) | <.0001 |
| Last HbA1c, median (Q1, Q3) | 6 (5.4, 6.6) | 6 (5.6, 7.1) | 6 (5.5, 6.9) | .386 |
| Liver disease | 9 (4.2) | 27 (10.8) | 36 (7.7) | .007 |
| Peripheral artery disease | 25 (11.6) | 38 (15.2) | 63 (13.5) | .254 |
| Previous cardiac interventions | ||||
| Any | 78 (36.1) | 95 (38.0) | 173 (37.1) | .674 |
| CABG | 4 (1.9) | 3 (1.2) | 7 (1.5) | .709 |
| Valve | 20 (9.3) | 30 (12.0) | 50 (10.7) | .340 |
| Other cardiac surgery | 29 (13.4) | 35 (14.0) | 64 (13.7) | .858 |
| PCI | 42 (19.4) | 52 (20.8) | 94 (20.2) | .716 |
| Preoperative cardiac status | ||||
| Prior MI | 34 (15.7) | 56 (22.4) | 90 (19.3) | .120 |
| Heart failure | 66 (30.6) | 68 (27.2) | 134 (28.8) | .425 |
| Cardiogenic shock | 1 (0.5) | 2 (0.8) | 3 (0.6) | .348 |
| Cardiac arrhythmia | 48 (22.2) | 53 (21.2) | 101 (21.7) | .789 |
| Operative | ||||
| CPB use | 205 (94.9) | 244 (97.6) | 449 (96.4) | .122 |
| CPB time (min), median (Q1, Q3) | 150 (118.5, 190.5) | 116.5 (89, 167) | 135 (103, 180) | <.0001 |
| Crossclamp time (min), median (Q1, Q3) | 108.5 (82, 147) | 86.5 (63, 123.5) | 94.5 (71, 133) | <.0001 |
| Postprocedure EF | 204 (94.4) | 237 (94.8) | 441 (94.6) | .865 |
ERAS, Enhanced Recovery After Surgery; CABG, coronary artery bypass grafting; BMI, Body mass index; CAD, coronary artery disease; HbA1c, hemoglobin A1c; PCI, percutaneous coronary intervention; MI, myocardial infarction; CPB, cardiopulmonary bypass; EF, ejection fraction.
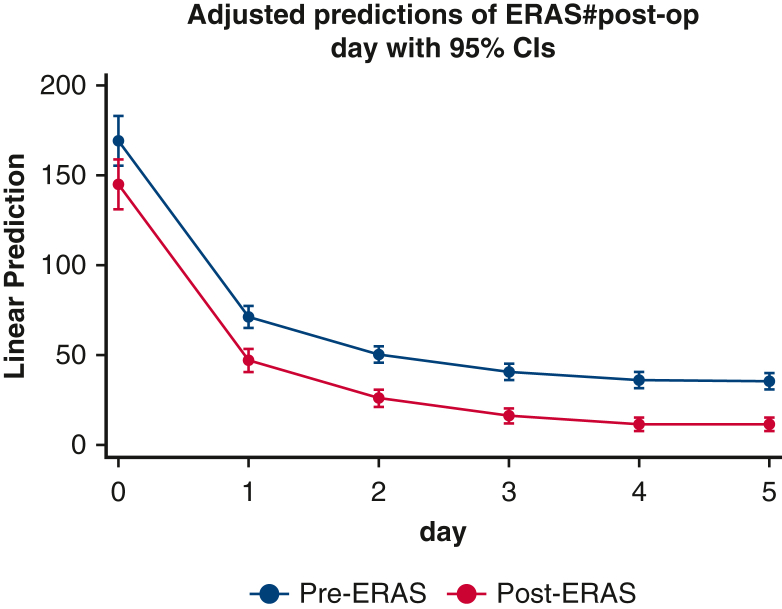
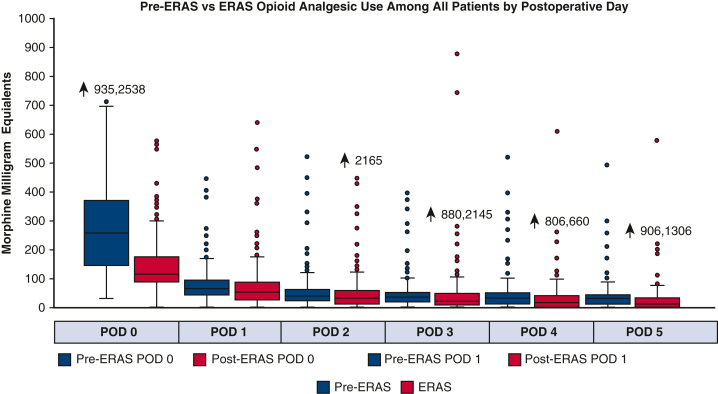
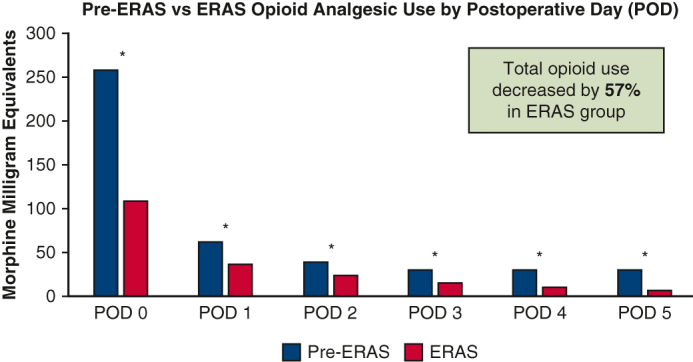
Perioperative daily opioid analgesic use was significantly reduced in the ERAS group. In the first 24 hours of care, which included the intraoperative phase of case, median opioid use was 113 MME in ERAS versus 259 MME in pre-ERAS (P < .0001). Over the ensuing 5 postoperative days, median MME in the ERAS versus pre-ERAS groups, respectively, were 48 versus 63 MME (P = .002), 30 versus 40 MME (P = .007), 20 versus 30 MME (P = .004), 15 versus 30 MME (P < .0001), and 10 versus 30 MME (P < .0001). In the multivariable quantile (median) regression, we found a similar pattern of MME by postoperative day (Figure E1, Tables E2 and E4). During the entire hospital length of stay, ERAS patients had a 57% reduction in total opioid use (459 vs 261 MME, P < .0001) (Figure 3).
Figure E1.
Repeated-measures analysis. ERAS, Enhanced Recovery After Surgery; CI, confidence interval.
Figure 3.
Pre-ERAS versus ERAS opioid analgesic use among all patients by postoperative day. ERAS, Enhanced Recovery After Surgery; POD, postoperative day.
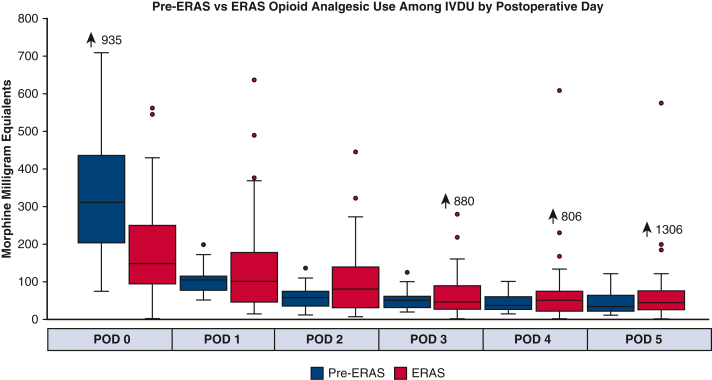
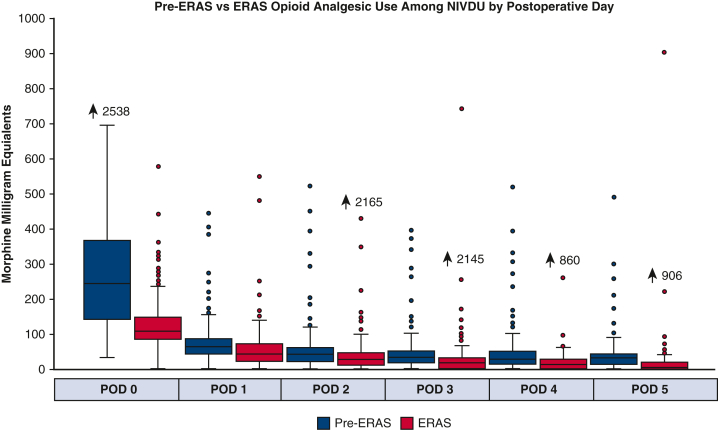
Because a significant portion of the ERAS group (n = 56, 22%) had a history of IVDU, a subgroup analysis was performed to see if this group also benefited from the multimodal pain management protocol. In general, the patients with a history of IVDU were younger (44 vs 64 years, P < .0001), with less comorbidities aside from endocarditis (n = 40 [54.8%] vs n = 32 [8.1%], P < .0001) and liver disease (n = 28 [38.4%] vs n = 8 [2.0%]) when compared with nonintravenous drug use (NIVDU) (Table E5). For patients with a history of IVDU, those who received the ERAS protocol had a significant reduction in opioid analgesic use on day of surgery (postoperative day zero) compared with pre-ERAS patients (318 MME vs 148 MME, P < .001), but there was no difference in the subsequent postoperative days (Figure 4). In contrast, in patients without IVDU history, ERAS patients demonstrated significant reduction in opioid analgesic use compared with pre-ERAS patients throughout the entire length of stay starting with day of surgery to postoperative day 5, respectively: 247 versus 106 MME, 62 versus 40 MME, 40 versus 25 MME, 30 versus 15 MME, 25 versus 10 MME, and 30 versus 5 MME (all days P < .0001) (Figure 5).
Figure 4.
Pre-ERAS versus ERAS opioid analgesic use among IVDU by postoperative day. ERAS, Enhanced Recovery After Surgery; IVDU, intravenous drug use; POD, postoperative day.
Figure 5.
Pre-ERAS versus ERAS opioid analgesic use among NIVDU by postoperative day. ERAS, Enhanced Recovery After Surgery; NIVDU, nonintravenous drug use; POD, postoperative day.
Other Secondary Outcomes
In addition to reduction in postoperative opioid use, we found that the ERAS group had chest tubes removed earlier (postoperative day 3 vs 4; P < .0001) than the pre-ERAS group. There was no significant difference in the following secondary outcomes between pre-ERAS and ERAS groups, respectively: total hospital length of stay (6 vs 6.5 days, P = .505), total intensive care unit length of stay (3.3 vs 3.1 days, P = .302), initial ventilation duration (4.7 vs 4.9 hours, P = .540), 30-day mortality (n = 5 [2.3%] vs n = 8 [3.2%], P = .779), and 30-day readmission (n = 17 [7.9%] vs n = 32 [12.8%], P = .084). Additionally, there was no difference in postoperative complications, including surgical site infection (n = 1 [0.5%] vs n = 4 [1.6%], P = .379), pneumonia (n = 14 [6.5%] vs n = 12 [4.8], P = .430), renal failure (n = 6 [2.8%] vs n = 8 [3.2%], P = .790), atrial fibrillation (n = 62, [28.7%] vs n = 69 [27.6%], P = .792), gastrointestinal side effects (n = 10 [4.6%] vs n = 13 [5.2%], P = .777), and stroke (n = 1 [0.5%] vs n = 2 [0.8%], P > .999).
Patient Satisfaction
In response to the predischarge survey, 79% of patients stated that they were “satisfied” or “very satisfied” with their pain control after surgery; 2.5% of patients reported being “dissatisfied” with their level of postoperative pain control.
Discussion
This study represents the effects after the implementation of a novel ERAS protocol and its impact on perioperative analgesic use in cardiac surgery patients at our institution.8 Our objective in developing an ERAS protocol was to create a perioperative management strategy that would optimize patient recovery. Although analgesia is but one component of the ERAS program, we elected to focus on this aspect for purposes of this study because of its immediate and notable impact on the care pathway.
Overall, the ERAS group had a 57% reduction in total opioid requirements during admission when compared with the control group. The primary intent of ERAS was not opioid use reduction per se; rather, the goal was to provide the patient sufficient analgesia that, in turn, could facilitate other aspects of their postoperative recovery such as early mobility and reduction of gastrointestinal and medication adverse effects. Reduction in opioid use has been proposed to enhance patient recovery my minimizing side effects including nausea, constipation, urinary retention, respiratory depression, pruritis, and delirium.4 Our results demonstrate that the ERAS analgesic protocol successfully achieves this while also reducing opioid consumption.
The preoperative administration of acetaminophen and gabapentinoids (gabapentin, pregabalin) is common among ERAS protocols. Administering acetaminophen before surgery has been associated with reduced pain scores and opioid analgesic use in the immediate postoperative period in noncardiac surgical patients.9 Likewise, in a randomized study among coronary artery bypass grafting patients, Menda and colleagues10 found that patients who received gabapentin preoperatively had significant reduction in postoperative morphine requirements. However, the postoperative use of gabapentinoids has been associated with oversedation, dizziness, and visual disturbances.11,12 The use of liposomal bupivacaine at incision and drain sites has also been shown to decrease opioid requirements after laparoscopic abdominal surgeries.13 However, this analgesic benefit typically diminishes within 24 hours.11 Dexmedetomidine is a centrally acting α-2 agonist that inhibits the release of norepinephrine and activation of pain signals.14 This analgesic effect has been linked to decreased opioid requirements in the first 24 hours after surgery, but places patients at risk for bradycardia and potentially hemodynamic instability.3,6,9 By combining all of these different analgesic modalities, an ERAS protocol aims to combat surgical pain even before the patient arouses from general anesthesia. We also suspect that this regimen contributes to the large reduction in opioid use seen on day of surgery (postoperative day zero). This time period includes the intraoperative phase as well as the first few immediate postoperative hours, during which the patient is often still under effects of general anesthetic. Our protocol also included encouragement of reduced intraoperative reduction of opioid anesthetics and increased use of agents such as dexmedetomidine at the conclusion of surgery rather than agents such as fentanyl infusion.
Over the subsequent 5 postoperative days, the ERAS group consistently used less opioid analgesic than the control group. This continued reduction is likely due to the postoperative multimodal regimen, which included scheduled acetaminophen, gabapentin, lidocaine topical patches, and ketorolac (in patients without contraindication) in addition to as needed tramadol, oxycodone, and hydromorphone. This regimen allowed opioid analgesics to serve as a rescue medication rather than the primary pain regimen.2 The postoperative administration of scheduled acetaminophen is ubiquitous in ERAS protocols and has been associated with a 20% to 30% decrease in morphine consumption.13,15 The postoperative use of gabapentinoids has not only been shown to decrease postoperative pain and opioid requirements, but also has the added benefit of reducing anxiety, nausea, and vomiting.3,16 In cardiac surgery patients, lidocaine patches can be applied to the back or chest to help with pain caused by intraoperative chest wall retraction and chest tube placement. Although the efficacy of lidocaine patches is not well described, they are generally well tolerated by patients and pose limited adverse effect risk.11,17 The use of tramadol has the benefit of a dual mechanism to address pain, working through both opioid and nonopioid pain pathways and has been associated with a 25% decrease in opioid analgesic requirement in cardiac surgical patients.3 Furthermore, tramadol has a favorable safety profile in comparison with other opioid analgesics because it causes less cardiovascular and respiratory depression, is less addictive, and has a lower rate of constipation.11 Patients should be monitored for onset of postoperative delirium when receiving tramadol because this is one of its most common side effects.3
ERAS protocols developed by other surgical subspecialties often emphasize the use of NSAIDs, because evidence suggests that their use in combination with acetaminophen can provide pain control equivalent to opioid analgesics.17 However, the use of NSAIDs is often limited in cardiac surgery due to the risk of acute kidney injury, bleeding, thromboembolic events, and gastrointestinal complications.3,12,15,18 In our series, ERAS patients were administered ketorolac followed by ibuprofen starting postoperative day 1. These medications could be withheld at the discretion of the surgeon for renal insufficiency or concerns of platelet dysfunction. In our study, there was no increase in acute kidney injury rates or bleeding seen in those receiving these agents.
Williams and colleagues19 conducted a retrospective cohort review on the implementation of ERAS protocol in cardiac surgery patients. Their postoperative protocol included scheduled acetaminophen and gabapentin. Oxycodone and fentanyl were provided as needed for pain. The ERAS group had a 30% reduction in IV opioid analgesic use on postoperative day zero. Likewise, in a prospective, observational study of cardiac surgery patients, Fleming and colleagues20 used a multimodal pain regimen which included the preoperative administration of gabapentin along with postoperative administration of acetaminophen, codeine, and as needed morphine. They demonstrated significantly lower pain scores on postoperative days 1 to 3 in the ERAS group and shorter duration of IV opioid infusion. Our results are similar to these other studies and demonstrate that multimodal pain management is associated with improved pain control and consequently reduces opioid analgesic use after cardiac surgery. It is important to note that our results were not achieved by sacrificing patient perception of pain control. In fact, approximately 80% of our ERAS patients reported being satisfied or very satisfied with their analgesic regimen. Because of the retrospective nature of this study, we were unable to ascertain patient perceptions of the control group. Nonetheless, our results indicate that the pain control achieved with ERAS is well received by patients.
Within our ERAS group, a significant portion of patients (22%) had a history of illicit IVDU. We hypothesized that the nonopioid components of an ERAS protocol would prove beneficial for patients who likely have a tolerance to opioid medications. Although subgroup analysis revealed that although these patients had reduction in opioid analgesic use on postoperative day zero, there was no difference between ERAS and control groups in the subsequent days. Unfortunately, our results reveal that ERAS may not have a notable effect with opioid use in these patients. We continue to use ERAS in IVDU patients, however, because there is some evidence that suggests appropriate treatment of pain and addiction in these patients decreases opioid withdrawal symptoms and the number of patients who leave against medical advice.21 Similar to ERAS principles, the authors suggest a perioperative strategy to adequately treat postoperative pain and addiction, which begins with consultation of specialists in pain management and addiction medicine on admission.21 These findings present an opportunity to improve outcomes in patients with a history of addiction and substance use.21
Study Limitations
Our study represents a single-center experience and therefore may not be generalizable to other institutions. The retrospective design reduces our ability to understand adjustments or deviations from the ERAS protocol made in real-time by bedside providers. For example, the decision on whether to start, continue, or discontinue ketorolac varied among different surgeons and critical care physicians. In addition, with the current analysis, it is difficult to discern how much of the other ERAS protocol components contributed to the results; the protocol encouraged early chest tube removal, for instance, the final decision on timing of drain removal was left to the discretion of the attending surgeon. Because chest tube removal likely has a beneficial effect on pain control, it is possible that this aspect contributed to differing degrees of opioid use. Although we primarily attribute the reduction in opioid requirements to the multimodal pain protocol, it is possible that a Hawthorne Effect impacted provider's prescribing patterns during the postoperative period. Finally, the predischarge patient surveys were not collected in the control group, and thus we are unable to directly compare patient perception between the ERAS and pre-ERAS groups. Nonetheless, our results demonstrate that an ERAS protocol is associated with a significant reduction in overall opioid use in the perioperative period without compromising patient perception of pain control.
Conclusions
ERAS protocols with an emphasis on multimodal pain management throughout perioperative care are associated with a significant reduction in the postoperative use of opioid analgesics. Patients with a history of IVDU may not have as pronounced a reduction in opioid use but likely benefit from the nonopioid analgesic regimen. Patients may benefit from future studies analyzing the effects of other ERAS protocol components beyond pain management aspects.
Webcast
You can watch a Webcast of this AATS meeting presentation by going to: https://aats.blob.core.windows.net/media/21%20AM/AM21_P02/AM21_P02_05.mp4.

Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Footnotes
Institutional Review Board Approval: The Institutional Review Board of Indiana University approved this retrospective study on 9/25/2020 (IRB #2009811102). Informed written consent was obtained by all patients who were included in this study.
Appendix E1
Table E1.
Morphine milligram equivalent conversion factors for common opioid medications
| Opioid | Conversion factor |
|---|---|
| Codeine | 0.15 |
| Fentanyl (IV) | 0.1 |
| Hydrocodone | 1 |
| Hydromorphone | 4 |
| Morphine | 1 |
| Oxycodone | 1.5 |
IV, Intravenous.
Table E2.
Pre–Enhanced Recovery After Surgery versus Enhanced Recovery After Surgery multivariable quantile (median) regression analysis by postoperative day
| Morphine milligram equivalents by POD |
||||||
|---|---|---|---|---|---|---|
| POD 0 | POD 1 | POD 2 | POD 3 | POD 4 | POD 5 | |
| Pre- (Ref) vs Post-ERAS, (95% CI, P value) | –148.8 (–183.8 to –113.8), <.0001 | –25.6 (–36.7 to –14.6), <.0001 | –17.6 (–25.6 to –9.6), <.0001 | –16 (–23.1 to –8.9), <.0001 | –15.9 (–23 to –8.9), <.0001 | –21.6 (–28.4 to –14.9), <.0001 |
| Patient characteristics (95% CI, P value) | ||||||
| Procedure type | ||||||
| Ascending aortic | Ref | Ref | Ref | Ref | Ref | Ref |
| CABG | –18.1 (–85.4-49.1), .597 | 0.3 (–21-21.6), .977 | –7.5 (–22.9-7.8), .336 | –3.8 (–17.5-9.9), .584 | –2.6 (–16.5-11.3), .714 | –5.1 (–18.2-7.9), .438 |
| Valve | –11.6 (–68.2-45), .687 | –6.4 (–24.2-11.4), .483 | –13.3 (–26.2-0.4), .043 | –15.6 (–27- –4.1), .008 | –5.2 (–16.6-6.1), .362 | –3.7 (–14.2-6.8), .487 |
| Valve + CABG | –27.0 (–108-54.0), .512 | 8.7 (–17-34.5), .505 | 5.9 (–12.7-24.6), .531 | 3.6 (–13.3-20.5), .674 | –9.1 (–25.6-7.3), .277 | –5.4 (–21.2-10.4), .504 |
| Other | –21.4 (–89.2-46.4), .535 | 4.3 (–17.2-25.8), .697 | 8.7 (–6.8-24.1), .272 | 11 (–2.7-24.7), .117 | 14.3 (0.8-27.7), .038 | 7.3 (–4.7-19.3), .232 |
| No endocarditis (Ref) vs endocarditis | 3.2 (–54.2-60.7), .912 | 6.8 (–11.4-4.9), .464 | 28.8 (15.7-42), <.0001 | 7.6 (–4-19.3), .199 | 7.8 (–3.5-19.1), .175 | 6.6 (–3.5-16.7), .199 |
| No hypertension (Ref) vs hypertension | 17.7 (–24.1-59.6), .405 | –10.4 (–23.9-3), .127 | –5.1 (–14.7-.5), .300 | –7.7 (–16.3-0.8), .075 | –7.9 (–16.3-0.4), .063 | –5.8 (–13.6-2.1), .152 |
| NIVDU (Ref) vs IVDU | 53.7 (–0.4-107.7), .052 | 34.9 (17.9-51.9), <.0001 | 12.1 (–0.4-24.5), .057 | 15.5 (4.3-26.8), .007 | 15.6 (4.7-26.5), .005 | 20.2 (10.2-30.1), <.0001 |
| No liver disease (Ref) vs Liver disease | –6.0 (–76.6-64.6), .868 | 17.4 (–4.8-39.5), .125 | 5.8 (–10.3-21.8), .483 | 4.9 (–9.4-19.2), .505 | 1.0 (–13.7-13.8), .992 | –0.9 (–13.2-11.4), .886 |
| Cardiac presentation | ||||||
| Anginal equivalent | Ref | Ref | Ref | Ref | Ref | Ref |
| Stable angina | 52.0 (–106.5-210.5), .519 | –14.9 (–65.2-35.4), .561 | –4.2 (–40.3-32), .821 | –11.7 (–43.8-20.3), .472 | 13.6 (–27.1-54.2), .513 | 27.1 (–8.4-62.7), .134 |
| Unstable angina | 39.6 (–105.5-184.8), .592 | –7 (–52.5-38.6), .763 | 2.8 (–30.3-35.8), .869 | –0.3 (–29.6-29), .986 | 22.7 (–15.3-60.7), .242 | 20 (–12.5-52.6), .227 |
| Non-ST elevation | 49.6 (–120.2-219.5), .566 | 6.4 (–46.9-59.7), .813 | 6 (–32.6-44.6), .760 | 2.6 (–31.6-36.8), .882 | 23.5 (–18.4-65.5), .271 | 28.8 (–7.4-65), .119 |
| ST-elevation MI | 71.4 (–169.3-312.1), .560 | –4.2 (–79.6-71.3), .913 | –0.8 (–55.5-53.9), .978 | –10.7 (–59.2-37.8), .665 | 13.6 (–44.3-71.5), .645 | 22.5 (–39-84.1), .472 |
| Asymptomatic | 31.5 (–117.4-180.5), .677 | –14.3 (–61-32.3), .546 | –5.7 (–39.6-28.2), .741 | –11.5 (–41.5-18.6), .453 | 17.3 (–20.7-55.3), .372 | 15.4 (–17.6-48.4), .359 |
| Other | 39.4 (–100.5-179.4), .580 | –12 (-55.9-31.9), .591 | –2.9 (–34.8-28.9), .856 | 0.1 (–28.1-28.4), .993 | 20.2 (–16.1-56.5), .274 | 17.3 (–13.6-48.1), .271 |
| Intraoperative times (min) | ||||||
| CPB | –0.2 (–0.6-0.3), .487 | –0.02 (–0.2-0.1), .775 | –0.03 (–0.1-0.1), .672 | 0.01 (–0.1-0.1), .862 | 0.01 (–0.1-0.1), .875 | 0.03 (–0.1-0.1), .545 |
| Crossclamp | 0.1 (–0.5-0.8), .655 | 0.04 (–0.2-0.2), .719 | –0.03 (–0.2-0.1), .721 | –0.03 (–0.2-0.1), .620 | –0.01 (–0.1-0.1), .863 | –0.1 (–0.2-0.1), .347 |
POD, Postoperative day; ERAS, Enhanced Recovery After Surgery; CI, confidence interval; CABG, coronary artery bypass grafting; NIVDU, nonintravenous drug use; IVDU, intravenous drug use; MI, myocardial infarction; CPB, cardiopulmonary bypass.
Table E3.
Enhanced Recovery After Surgery multimodal pain medication patient compliance
| Medications | Compliance, n (%) |
|---|---|
| Preoperative | |
| Acetaminophen | 152 (60.8) |
| Gabapentin | 152 (60.8) |
| Intraoperative | |
| Liposomal bupivacaine | 214 (85.6) |
| Postoperative | |
| Dexmedetomidine | 176 (70.4) |
| Acetaminophen | 248 (99.2) |
| Lidocaine patches | 248 (99.2) |
| Gabapentin | 228 (91.2) |
| Tramadol | 235 (94.0) |
| Oxycodone | 245 (98.0) |
| IV fentanyl/hydromorphone | 246 (98.4) |
IV, Intravenous.
Table E4.
Patient characteristics in intravenous drug user (IVDU) versus nonintravenous drug user (NIVDU)
| Characteristics, n (%) | NIVDU (393) | IVDU (73) | P value |
|---|---|---|---|
| Procedure type | <.0001 | ||
| Ascending aortic | 75 (19.08) | 8 (10.96) | |
| CABG | 153 (38.93) | 17 (23.29) | |
| Valve | 107 (27.23) | 12 (16.44) | |
| Valve + CABG | 23 (5.85) | 1 (1.37) | |
| Other | 35 (8.91) | 35 (47.95) | |
| Age, median (Q1, Q3) | 64 (57, 71) | 44 (34, 60) | <.0001 |
| Gender | .008 | ||
| Male | 277 (70.48) | 40 (54.79) | |
| Female | 116 (29.52) | 33 (45.21) | |
| Race | .16 | ||
| White | 342 (87.02) | 59 (80.82) | |
| Non-White | 51 (12.98) | 14 (19.18) | |
| Risk factors | |||
| BMI, median (Q1, Q3) | 29.85 (26.02, 34.27) | 25.77 (22.77, 30.94) | .0001 |
| Diabetes | 161 (40.97) | 13 (17.81) | <.0001 |
| Endocarditis | 32 (8.14) | 40 (54.79) | <.0001 |
| Cerebrovascular disease | 67 (17.05) | 16 (21.92) | .318 |
| Chronic lung disease | 102 (25.95) | 15 (20.55) | .328 |
| Family history of CAD | 11 (2.80) | 1 (1.37) | .701 |
| Hypertension | 326 (82.95) | 31 (42.47) | <.0001 |
| Last HbA1c level, median (Q1, Q3) | 6 (5.5, 7) | 5.7 (5.4, 6.5) | .0282 |
| Liver disease | 8 (2.04) | 28 (38.36) | <.0001 |
| Peripheral artery disease | 57 (14.50) | 6 (8.22) | .149 |
| Previous cardiac interventions | |||
| Any | 145 (36.90) | 28 (38.36) | .813 |
| CABG | 6 (1.53) | 1 (1.37) | >.999 |
| Valve | 36 (9.16) | 14 (19.18) | .011 |
| Other cardiac surgery | 52 (13.23) | 12 (16.44) | .465 |
| PCI | 83 (21.12) | 11 (15.07) | .237 |
| Preoperative cardiac status | |||
| Prior MI | 78 (19.85) | 12 (16.44) | .687 |
| Heart failure | 121 (30.79) | 13 (17.81) | .024 |
| Cardiogenic shock | 2 (0.51) | 1 (1.37) | .401 |
| Cardiac arrhythmia | 89 (22.65) | 12 (16.44) | .237 |
| Operative times (min) | |||
| CPB, median (Q1, Q3) | 138 (105, 184) | 117 (86, 155) | .0043 |
| Crossclamp, median (Q1, Q3) | 97 (74, 136) | 82 (61, 117) | .0027 |
CABG, Coronary artery bypass grafting; BMI, body mass index; CAD, coronary artery disease; HbA1c, hemoglobin A1c; PCI, percutaneous coronary intervention; MI, myocardial infarction; CPB, cardiopulmonary bypass.
Table E5.
Pre–Enhanced Recovery After Surgery versus Enhanced Recovery After Surgery (ERAS) opioid analgesic use by repeated measures multivariable design
| MME over time (95% CI, P value) | |
|---|---|
| Pre-ERAS (Reference) vs Post-ERAS | –24.4 (–29.2-19.5), <.0001 |
| Morphine equivalents (0-5 d) | |
| Day 0 | Ref |
| Day 1 | –98 (–112 to –84), <.0001 |
| Day 2 | –118.9 (–132.6 to –105.2), <.0001 |
| Day 3 | –128.4 (–142.2 to –114.7), <.0001 |
| Day 4 | –133.3 (–147 to –119.7), <.0001 |
| Day 5 | –133.7 (-147.3 to –120.1), <.0001 |
| Procedure type | |
| Ascending aortic | Ref |
| CABG | –4.3 (–13.8-5.2), 0.377 |
| Valve | –4.7 (–11.6-2.2), 0.180 |
| Valve + CABG | 1.6 (–14.6-17.7), 0.851 |
| Other | 7.3 (–4.6-19.2), 0.227 |
| No endocarditis (Ref) vs ENDOCARDITIS | 8 (–1.3-17.2), 0.091 |
| No hypertension (Ref) vs Hypertension | –7 (–13.9 to –0.03), 0.049 |
| NIVDU (Ref) vs IVDU | 22.9 (11.8-34.1), <.0001 |
| No liver disease (Ref) vs liver disease | 4.8 (–12.3-21.9), 0.584 |
| Cardiac presentation | |
| Anginal equivalent | Ref |
| Stable angina | –2.6 (–22.5-17.3), 0.796 |
| Unstable angina | 1.9 (–18.2-22), 0.854 |
| Non-ST elevation MI | 6.1 (–19.3-31.5), 0.636 |
| ST-elevation MI | 2.3 (–28.7-33.3), 0.886 |
| Asymptomatic | –6.7 (–27.3-14), 0.526 |
| Other | –3.1 (–23.1-17), 0.765 |
| Intraoperative times (min) | |
| CPB | 0.01 (–0.1-0.1), 0.804 |
| Crossclamp | –0.03 (–0.1-0.1), 0.551 |
MME, Morphine milligram equivalent; CI, confidence interval; CABG, coronary artery bypass grafting; NIVDU, nonintravenous drug user; IVDU, intravenous drug user; MI, myocardial infarction; CPB, cardiopulmonary bypass.
References
- 1.Ljungqvist O., Scott M., Fearon K.C. Enhanced recovery after surgery: a review. JAMA Surg. 2017;152:292–298. doi: 10.1001/jamasurg.2016.4952. [DOI] [PubMed] [Google Scholar]
- 2.Brown J.K., Singh K., Dumitru R., Chan E., Kim M.P. The benefits of enhanced recovery after surgery programs and their application in cardiothoracic surgery. Methodist Debakey Cardiovasc J. 2018;14:77–88. doi: 10.14797/mdcj-14-2-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engelman D.T., Ben Ali W., Williams J.B., Perrault L.P., Reddy V.S., Arora R.C., et al. Guidelines for perioperative care in cardiac surgery: enhanced recovery after surgery society recommendations. JAMA Surg. 2019;154:755–766. doi: 10.1001/jamasurg.2019.1153. [DOI] [PubMed] [Google Scholar]
- 4.Barr L.F., Boss M.J., Mazzeffi M.A., Taylor B.S., Salenger R. Postoperative multimodal analgesia in cardiac surgery. Crit Care Clin. 2020;36:631–651. doi: 10.1016/j.ccc.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention Calculating total daily dose of opioid for safer dosage [Internet] https://www.cdc.gov/drugoverdose/pdf/calculating_total_daily_dose-a.pdf
- 6.StataCorp . StataCorp LLC; 2019. Stata Statistical Software: Release 16. [Google Scholar]
- 7.Kadri A.N., Wilner B., Hernandez A.V., Nakhoul G., Chahine J., Griffin B., et al. Geographic trends, patient characteristics, and outcomes of infective endocarditis associated with drug abuse in the United States from 2002 to 2016. J Am Heart Assoc. 2019;8:e012969. doi: 10.1161/JAHA.119.012969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu S.Y., Lai Y., Dalia A.A. Implementing a cardiac enhanced recovery after surgery protocol: nuts and bolts. J Cardiothorac Vasc Anesth. 2020;34:3104–3112. doi: 10.1053/j.jvca.2019.12.022. [DOI] [PubMed] [Google Scholar]
- 9.Echeverria-Villalobos M., Stoicea N., Todeschini A.B., Fiorda-Diaz J., Uribe A.A., Weaver T., et al. Enhanced recovery after surgery (ERAS): a perspective review of postoperative pain management under ERAS pathways and its role on opioid crisis in the United States. Clin J Pain. 2020;36:219–226. doi: 10.1097/AJP.0000000000000792. [DOI] [PubMed] [Google Scholar]
- 10.Menda F., Köner O., Sayın M., Ergenoğlu M., Küçükaksu S., Aykaç B. Effects of single-dose gabapentin on postoperative pain and morphine consumption after cardiac surgery. J Cardiothorac Vasc Anesth. 2010;24:808–813. doi: 10.1053/j.jvca.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 11.Wick E.C., Grant M.C., Wu C.L. Postoperative multimodal analgesia pain management with nonopioid analgesics and techniques: a review. JAMA Surg. 2017;152:691–697. doi: 10.1001/jamasurg.2017.0898. [DOI] [PubMed] [Google Scholar]
- 12.McConnell G., Woltz P., Bradford W.T., Ledford J.E., Williams J.B. Enhanced recovery after cardiac surgery program to improve patient outcomes. Nursing. 2018;48:24–31. doi: 10.1097/01.NURSE.0000546453.18005.3f. [DOI] [PubMed] [Google Scholar]
- 13.Tan M., Law L.S., Gan T.J. Optimizing pain management to facilitate enhanced recovery after surgery pathways. Can J Anaesth. 2015;62:203–218. doi: 10.1007/s12630-014-0275-x. [DOI] [PubMed] [Google Scholar]
- 14.Simpson J.C., Bao X., Agarwala A. Pain management in enhanced recovery after surgery (ERAS) protocols. Clin Colon Rectal Surg. 2019;32:121–128. doi: 10.1055/s-0038-1676477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Batchelor T.J.P., Rasburn N.J., Abdelnour-Berchtold E., Brunelli A., Cerfolio R.J., Gonzalez M., et al. Guidelines for enhanced recovery after lung surgery: recommendations of the enhanced recovery after surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS) Eur J Cardiothorac Surg. 2019;55:91–115. doi: 10.1093/ejcts/ezy301. [DOI] [PubMed] [Google Scholar]
- 16.Kołodziej T., Maciejewski T., Mendrala K., Darocha T., Węglarzy A., Budziarz B., et al. Enhanced recovery after cardiac surgery. Kardiochir Torakochirurgia Pol. 2019;16:32–36. doi: 10.5114/kitp.2019.83943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harvin J.A., Green C.E., Vincent L.E., Motley K.L., Podbielski J., Miller C.C., et al. Multi-modal analgesic strategies for trauma (MAST): protocol for a pragmatic randomized trial. Trauma Surg Acute Care Open. 2018;3:e000192. doi: 10.1136/tsaco-2018-000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gregory A.J., Grant M.C., Manning M.W., Cheung A.T., Ender J., Sander M., et al. Enhanced recovery after cardiac surgery (ERAS cardiac) recommendations: an important first step-but there is much work to be done. J Cardiothorac Vasc Anesth. 2020;34:39–47. doi: 10.1053/j.jvca.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Williams J.B., McConnell G., Allender J.E., Woltz P., Kane K., Smith P.K., et al. One-year results from the first US-based enhanced recovery after cardiac surgery (ERAS cardiac) program. J Thorac Cardiovasc Surg. 2019;157:1881–1888. doi: 10.1016/j.jtcvs.2018.10.164. [DOI] [PubMed] [Google Scholar]
- 20.Fleming I.O., Garratt C., Guha R., Desai J., Chaubey S., Wang Y., et al. Aggregation of marginal gains in cardiac surgery: feasibility of a perioperative care bundle for enhanced recovery in cardiac surgical patients. J Cardiothorac Vasc Anesth. 2016;30:665–670. doi: 10.1053/j.jvca.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 21.Ray V., Waite M.R., Spexarth F.C., Korman S., Berget S., Kodali S., et al. Addiction management in hospitalized patients with intravenous drug use-associated infective endocarditis. Psychosomatics. 2020;61:678–687. doi: 10.1016/j.psym.2020.06.019. [DOI] [PubMed] [Google Scholar]