Abstract
Objective
The objective of this study was to evaluate utilization and perioperative outcomes of video-assisted thoracoscopic surgery (VATS) or robotic-assisted thoracoscopic surgery (RATS) for lung cancer in the United States using a nationally representative database.
Methods
Hospital admissions for lobectomy or sublobar resection (segmentectomy or wedge resection) using VATS or RATS in patients with nonmetastatic lung cancer from October 2015 through December 2018 in the National Inpatient Sample were studied. Patient and hospital characteristics, perioperative complications and mortality, length of stay (LOS), and total hospital cost were compared. Logistic regression was used to assess whether the surgical approach was independently associated with adverse outcomes.
Results
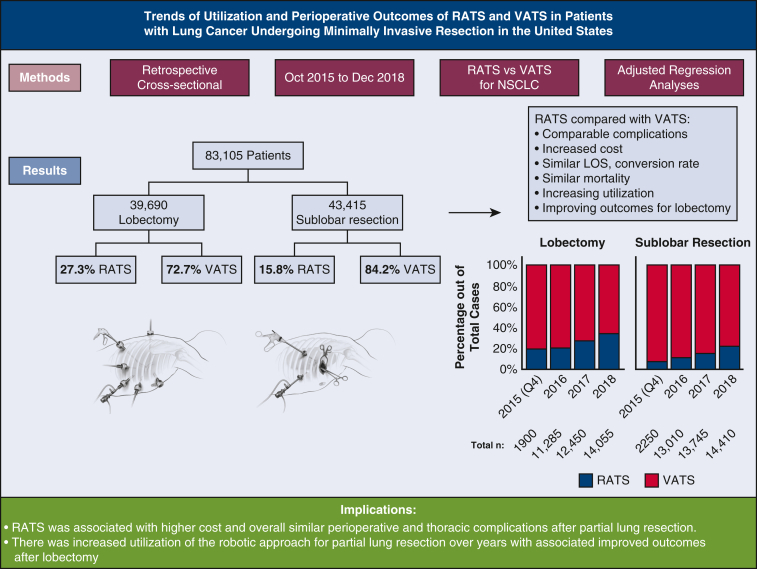
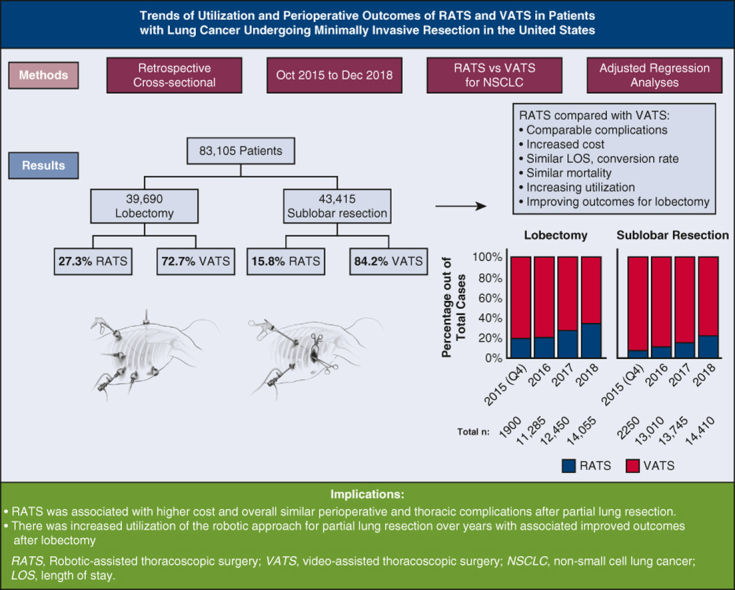
There were 83,105 patients who had VATS (n = 65,375) or RATS (n = 17,710) for lobectomy (72.7% VATS) or sublobar resection (84.2% VATS). Utilization of RATS for lobectomy and sublobar resection increased from 19.2% to 34% and 7.3% to 22%, respectively. Mortality, LOS, and conversion rates were comparable. The cost was higher for RATS (P <.01). Multivariate analyses showed comparable RATS and VATS complications with no independent association between the minimally invasive surgery approach used and adverse surgical outcomes, except for a decreased risk of pneumonia with RATS, relative to VATS sublobar resection (P <.01). Thoracic complication rates and LOS decreased after RATS lobectomy in 2018, compared with previous years (P <.005).
Conclusions
The utilization of robotic-assisted lung resection for cancer has increased in the United States between 2015 and 2018 for sublobar resection and lobectomy. In adjusted regression analysis, compared with VATS, patients who underwent RATS had similar complication rates and LOS. The robotic approach was associated with increased total hospital cost. LOS and thoracic complication rates trended down after RATS lobectomy.
Key Words: lung cancer, robotic, video-assisted thoracoscopic surgery
Abbreviations and Acronyms: HCUP, Healthcare Cost and Utilization Project; ICD-10, International Classification of Diseases, 10th Revision; ICD-10-CM, International Classification of Diseases, 10th Revision, Clinical Modification; ICD-10-PCS, International Classification of Diseases, 10th Revision Procedure Coding System; LOS, length of stay; MIS, minimally invasive surgery; NIS, National Inpatient Sample; Q4, fourth quarter; RATS, robotic-assisted thoracoscopic surgery; VATS, video-assisted thoracoscopic surgery
Video Abstract
Graphical abstract
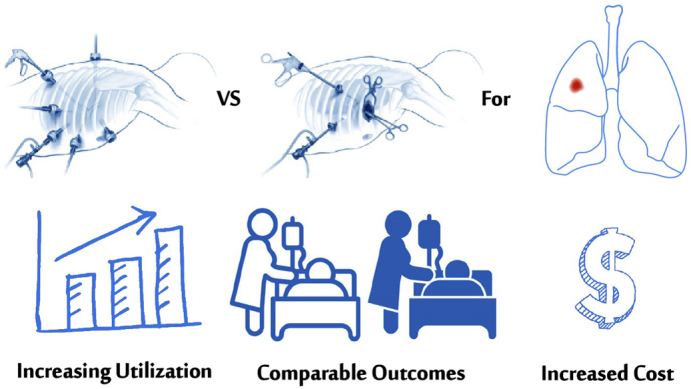
Robotic versus video-assisted thoracoscopic partial resection in patients with lung cancer.
Central Message.
The utilization of RATS lung resection for cancer has increased in the United States between 2015 and 2018. Compared with VATS, RATS was associated with comparable risk of perioperative complications and higher cost.
Perspective.
One-third of lobectomies and one-fifth of sublobar resections for lung cancer were performed with a robotic-assisted approach in the United States in 2018. Compared with VATS, RATS had 15% added cost for lobectomy and 24% for sublobar resection. RATS was associated with comparable risks of perioperative complications and a similar LOS. Thoracic complications after RATS lobectomy improved compared with earlier years.
Lung cancer is one of the most common cancers worldwide and it leads to the highest rate of cancer-related deaths in men and is second only to breast cancer in women.1,2 The International Agency for Research on Cancer reports that the highest rates of lung cancer in the world are observed in Northern America3 with an estimated incidence and mortality per 100,000 persons being 58.4 and 38.5, respectively, in the United States, compared with 22.5 and 18.6 worldwide.4 Lung cancer survival continues to be among the lowest compared with other kinds of cancer.5
For early-stage non-small cell lung cancer, surgical resection is the standard of care and can be performed through a thoracotomy or minimally invasive surgery (MIS) using video-assisted thoracoscopic surgery (VATS) or robotic-assisted thoracoscopic surgery (RATS) approaches.6 Advantages of VATS and RATS over the traditional open thoracotomy include fewer perioperative complications, reduced postoperative pain, and shorter length of stay (LOS).7, 8, 9, 10, 11 However, despite the increased utilization of the RATS approach in thoracic surgery, level 1 evidence of perioperative or long-term advantages of RATS compared with VATS resection is still lacking.12
In large database studies and meta-analyses, lower conversion rates to open thoracotomy and more frequent home discharges have been observed in RATS compared with VATS lobectomy, with similar 30-day readmission rates.13, 14, 15, 16, 17 Comparable mortality rates between approaches have been shown in several reports14, 15, 16,18, 19, 20, 21, 22, 23; however, decreased odds of 90-day mortality24,25 and increased 1-year mortality risk have also been reported after RATS lobectomy.13 Lower perioperative or 30-day complication rates have been reported with RATS lobectomy; however, others have shown no differences19,23,26,27 or even higher rates of overall or selected types of perioperative complications like pulmonary complications or intraoperative injuries.16,18,28 Similarly, a potential favorable effect on the LOS after RATS compared with VATS lobectomy or segmentectomy for lung cancer has been shown in a few studies14,19,25 yet disproven in others.8,16,20,24,26,27
The question remains whether or not RATS lung resection provides surgical outcome benefits for patients with lung cancer and whether the conflicting data reported in the past few years might partially be related to variations in learning curve effects in single- or multicenter studies8,25,26 and differences in sampling design, nature of the data, and study years included in administrative or large databases.16,18,20,24,27,28 Furthermore, nationally representative data on VATS and RATS utilization and outcomes in recent years are lacking. We aimed to evaluate utilization trends and perioperative outcomes (complications, LOS, and cost) of lung resection including lobectomy and sublobar resection for cancer with VATS or RATS in the United States using a nationally representative database.
Methods
This study was a cross-sectional retrospective analysis of hospital admissions for lung resection using VATS or RATS in patients with nonmetastatic lung cancer. Patients who underwent surgery from October 2015 through December 2018 in the National Inpatient Sample (NIS; Figure 1; Video Abstract) were included.
Figure 1.
Cross-sectional retrospective study of hospital admissions for lung resection using video-assisted thoracoscopic surgery (VATS) or robotic-assisted thoracoscopic surgery (RATS) in patients with non-metastatic lung cancer in the National Inpatient-Sample (NIS). NSCLC, Non-small cell lung cancer; LOS, length of stay.
Patient Population and Database Description
Starting in October of 2015, the United States has transitioned to the 10th revision of the International Classification of Diseases (ICD-10), Clinical Modification (ICD-10-CM)/ICD-10 Procedure Coding System (ICD-10-PCS) for medical billing. We included inpatient hospital admissions of patients who underwent lobectomy or sublobar resection (segmentectomy or wedge resection) using VATS or RATS approaches (see Table E1 for the ICD-10-PCS) and had the following ICD-10-CM diagnosis codes for lung tumor: C34XX for malignant neoplasms of the bronchus and the lung, D14.3X for benign neoplasms, D38.1 for neoplasms of uncertain behavior, D3A.090 for carcinoid tumors, D02.21 and D02.22 for carcinoma in situ, D49.1 for other neoplasms of unspecified behavior of the respiratory system, and R91.1 for solitary pulmonary nodules. The ICD-10-PCS does not accurately distinguish between a wedge resection or segmentectomy and they were thus combined as sublobar resection.29 Patients with metastatic primary lung cancer or secondary metastatic disease to the lung and those who underwent lung resection for other pathologies (including pulmonary abscess, mycobacterial infection, coccidioidomycosis, and mycoses) or trauma were excluded from the study. This was because of the known technical difficulties and heterogeneity in outcomes in these cases. Because of the deidentified nature of the data, this study was deemed exempt by our institutional review board.
The NIS is part of the Healthcare Cost and Utilization Project (HCUP) sponsored by the US Agency for Healthcare Research and Quality. The NIS is the best available data source for analyses of national estimates for different inpatient hospitalization measures and outcomes.30, 31, 32 It includes a stratified sample of hospital admissions to US community hospitals (excluding rehabilitation and long-term acute care hospitals) and contains more than 7 million hospital stays each year, estimating more than 35 million weighted hospitalizations. The NIS sampling frame currently includes data from 47 states and the District of Columbia, which covers more than 97% of the US population. The NIS provides information on patient demographic characteristics, diagnosis and procedure codes, expected source of payment, hospital characteristics, LOS, and total charges.33
Patient and Hospital Characteristics and Study Outcomes
The following patient characteristics were studied: age, sex, race/ethnicity (White, Black, Hispanic, and others), expected payer (private insurance, Medicare, Medicaid, and other), and comorbidities. HCUP Elixhauser Comorbidity Software for ICD-10-CM34 was used to identify the following conditions: diabetes, hypertension, congestive heart failure, chronic pulmonary disease, renal failure, cerebrovascular disease, peripheral vascular disease, obesity, and history of smoking. Hospital characteristics included location and teaching status (rural, urban nonteaching, urban teaching), bed size (small, medium, large), and geographic region (Northeast, Midwest, South, West).
Study outcomes included annual estimates of the number of inpatient lung resections for lung cancer using either RATS or VATS, adverse perioperative outcomes (conversion to open approach, complications, and mortality) occurring during admission, and extended LOS (>7 days), and total hospital cost. Complications were on the basis of the ICD-10-CM codes shown in Table E2, and were grouped into the following categories: thoracic, cardiovascular and thromboembolic, bleeding, and other complications. Of note, unlike the International Classification of Diseases Ninth Revision coding system, the ICD-10-CM more accurately differentiates between diagnoses present at admission (ie, comorbidities) and perioperative complications occurring during hospitalization. Total hospital cost in US dollars was calculated from the individual hospitalization total charges multiplied by the individual hospital's cost-to-charge ratio that is provided by HCUP for a subset of participating hospitals.35 To account for inflation, costs were adjusted to the 2018 dollar value using the annual consumer price index.36 Admissions with missing cost-to-charge ratio information were excluded from the cost analysis.
Statistical Analyses
Statistical analyses were performed using the survey procedures in SAS version 9.4 (SAS Institute Inc) to account for stratification and clustering in the sampling design of the NIS. Categorical variables are presented as the weighted number and percentage and were compared using the Rao–Scott χ2 test in the SURVEYFREQ procedure. Continuous variables are presented as the mean and standard errors of the mean and were compared using the SURVEYREG procedure. Patient and hospital characteristics and study outcomes were compared between patients who had RATS versus VATS approaches and separate analyses were done for patients who had a lobectomy and those who had sublobar resection (including segmentectomy and wedge resection). Additionally, to study trends in outcomes over time, LOS and complication rates after RATS resection were compared between 2018 and previous study years (2015 fourth quarter [Q4], 2016, and 2017).
Logistic regression analyses were done using the SURVEYLOGISTIC procedure to assess if the surgical approach was independently associated with adverse perioperative outcomes (any complication, thoracic complications, bleeding, extended LOS, conversion to open, and mortality). Factors included in the model were: patient age, sex, race/ethnicity, comorbid conditions (diabetes, hypertension, peripheral vascular disease, chronic lung disease, renal failure, and obesity), smoking, surgical approach (RATS relative to VATS), insurance, hospital characteristics, and location. The Holm–Bonferroni multiple comparisons method was used to adjust the P values.
Results
Utilization of VATS and RATS
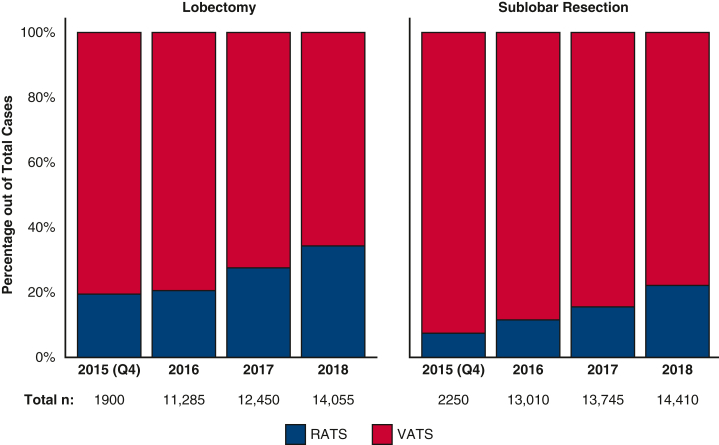
From October 2015 through December 2018, an estimated 83,082 patients had an inpatient sublobar lung resection (n = 43,415) or lobectomy (n = 39,670) for nonmetastatic lung cancer in the United States using VATS (n = 65,375) or RATS (n = 17,710). Figure 2 shows the estimated annual utilization (percentage of RATS and VATS approaches for lobectomy and sublobar resection) during the study years. Between 2015 and 2018, the proportion of patients who had the RATS approach increased from 19.2% to 34% for lobectomy and from 7% to 22% for sublobar resection (P < .001).
Figure 2.
Utilization trends of robotic-assisted lobectomy and sublobar resection in patients with lung cancer in the National Inpatient Sample from October 2015 through December 2018. VATS, Video-assisted thoracoscopic surgery; RATS, robotic-assisted thoracoscopic surgery.
Patient and Hospital Characteristics
Patient and hospital characteristics of patients who had a lobectomy and sublobar resection are presented in Tables 1 and 2, respectively. Of all patients who underwent a lobectomy, 28,850 (72.7%) had VATS and 10,840 (27.3%) had RATS. Patients' age, sex, insurance, hospital bed size, location, or teaching status were comparable (P >.05). A higher percentage of Hispanic patients had RATS (5.2% vs 3.3%; P < .001). The utilization of RATS versus VATS lobectomies varied in different regions of the country. Patients who had RATS had higher rates of hypertension, chronic pulmonary disease, and obesity, compared with VATS (P < .0.3; Table 1). Of all patients who underwent a sublobar resection, 36,545 (84.2%) had VATS and 6870 (15.8%) had RATS (Table 2). Patients who had RATS were older and had higher rates of diabetes, hypertension, and a history of smoking (P < .04). There were differences between the 2 approach groups in patients' race/ethnicity, hospital location and teaching status, and region of the country (P < .03).
Table 1.
Patient and hospital characteristics of patients who had lobectomy for lung cancer using VATS or RATS in the National Inpatient Sample database from October 2015 through December 2018
| Patient and hospital characteristics | All (N = 39,690) | VATS (n = 28,850; 72.7%) | RATS (n = 10,840; 27.3%) | P value |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Mean age ± SEM, y | 67.62 ± 0.10 | 67.50 ± 0.12 | 67.97 ± .18 | .14 |
| Female sex | 22,810 (57.50) | 16,675 (57.84) | 6135 (56.60) | .30 |
| Insurance: | ||||
| Private | 10,955 (27.63) | 8050 (27.93) | 2905 (26.85) | .14 |
| Medicare | 25,375 (64.01) | 18,415 (63.89) | 6960 (64.33) | |
| Medicaid | 2275 (5.74) | 1670 (5.79) | 605 (5.59) | |
| Race | ||||
| White | 31,605 (79.63) | 23,045 (79.88) | 8560 (78.97) | <.01 |
| Black | 3000 (7.56) | 2135 (7.40) | 865 (7.98) | |
| Hispanic | 1595 (4.02) | 965 (3.34) | 630 (5.81) | |
| Missing | 1165 (2.94) | 980 (3.40) | 185 (1.71) | |
| Other | 2325 (5.86) | 1725 (5.98) | 600 (5.54) | |
| Comorbidities | ||||
| Diabetes | 7780 (19.60) | 5525 (19.15) | 2255 (2.80) | .13 |
| Hypertension | 24,740 (62.33) | 17,635 (61.13) | 7105 (65.54) | <.01 |
| Congestive heart failure | † | † | 0 (.00) | ∗ |
| Chronic pulmonary disease | 17,640 (44.44) | 12,570 (43.57) | 5070 (46.77) | .02 |
| Renal failure | 215 (0.54) | 160 (0.55) | 55 (.51) | .79 |
| Cerebrovascular disease | 345 (0.87) | 260 (0.90) | 85 (.78) | .62 |
| Peripheral vascular disease | 3465 (8.73) | 2475 (8.58) | 990 (9.13) | .47 |
| Obesity | 4990 (12.57) | 3455 (11.98) | 1535 (14.16) | .02 |
| History of smoking | 26,270 (66.18) | 18,925 (65.59) | 7345 (67.75) | .08 |
| Hospital characteristics | ||||
| Hospital Bed Size | ||||
| Large | 27,270 (68.71) | 20,080 (69.60) | 7190 (66.33) | .37 |
| Medium | 8870 (22.35) | 6280 (21.77) | 2590 (23.89) | |
| Small | 3550 (8.94) | 2490 (8.63) | 1060 (9.78) | |
| Location and teaching status | ||||
| Urban teaching | 35,335 (89.03) | 25,640 (88.87) | 9695 (89.44) | .59 |
| Urban nonteaching | 3575 (9.01) | 2600 (9.01) | 975 (8.99) | |
| Rural | 780 (1.97) | 610 (2.11) | 170 (1.57) | |
| Region of the country | ||||
| South | 14,490 (36.51) | 10,315 (35.75) | 4175 (38.51) | <.01 |
| Midwest | 7795 (19.64) | 5100 (17.68) | 2695 (24.86) | |
| West | 6575 (16.57) | 5080 (17.61) | 1495 (13.79) | |
| Northeast | 10,830 (27.29) | 8355 (28.96) | 2475 (22.83) | |
Data are presented as n (%) except where otherwise noted. VATS, Video-assisted thoracoscopic surgery; RATS, robotic-assisted thoracoscopic surgery; SEM, standard error of the mean.
The Rao-Scott χ2 test does not provide a P value when one of the cells has a value of 0.
The HCUP National Inpatient Sample does not permit reporting of observations smaller than 10 due to privacy concerns.
Table 2.
Patient and hospital characteristics of patients who had a sublobar resection (segmentectomy or wedge resection) for lung cancer using VATS or RATS in the National Inpatient Sample database from October 2015 through December 2018
| Patient and hospital characteristics | All (N = 43,415) | VATS (n = 36,545; 84.2%) | RATS (n = 6870; 15.8%) | P value |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Mean age ± SEM, y | 65.65 ± 0.12 | 65.39 ± 0.13 | 67.15 ± 0.26 | <.01 |
| Female sex | 23,715 (54.67) | 19,840 (54.34) | 3875 (56.40) | .16 |
| Insurance | ||||
| Private | 13,575 (31.30) | 11,530 (31.58) | 2045 (29.83) | .79 |
| Medicare | 25,535 (58.88) | 21,380 (58.55) | 4155 (60.61) | |
| Medicaid | 2920 (6.73) | 2470 (6.76) | 450 (6.56) | |
| Race | ||||
| White | 33,985 (78.28) | 28,720 (78.59) | 5265 (76.64) | <.01 |
| Black | 3405 (7.84) | 2910 (7.96) | 495 (7.21) | |
| Hispanic | 1885 (4.34) | 1395 (3.82) | 490 (7.13) | |
| Missing | 1410 (3.25) | 1320 (3.61) | 90 (1.31) | |
| Other | 2730 (6.29) | 2200 (6.02) | 530 (7.71) | |
| Comorbidities | ||||
| Diabetes | 8715 (20.07) | 7190 (19.67) | 1525 (22.20) | .03 |
| Hypertension | 25,095 (57.80) | 20,945 (57.31) | 4150 (60.41) | .04 |
| Congestive heart failure | 5 (0.01) | 5 (0.01) | 0 (0.00) | .75 |
| Chronic pulmonary disease | 17,655 (40.67) | 14,685 (40.18) | 2970 (43.23) | .05 |
| Renal failure | 315 (0.73) | 260 (0.71) | 55 (0.80) | .72 |
| Cerebrovascular disease | 315 (0.72) | 255 (0.69) | 60 (0.87) | .47 |
| Peripheral vascular disease | 3395 (7.81) | 2825 (7.73) | 570 (8.29) | .49 |
| Obesity | 5695 (13.12) | 4810 (13.16) | 885 (12.88) | .79 |
| History of smoking | 25,600 (58.96) | 21,245 (58.13) | 4355 (63.39) | <.01 |
| Hospital characteristics | ||||
| Hospital bed size | ||||
| Large | 30,600 (70.48) | 25,880 (70.82) | 4720 (68.70) | .66 |
| Medium | 9050 (20.85) | 7530 (20.60) | 1520 (22.13) | |
| Small | 3765 (8.67) | 3135 (8.58) | 630 (9.17) | |
| Location and teaching status | ||||
| Urban teaching | 38,550 (88.79) | 32,605 (89.22) | 5945 (86.54) | .03 |
| Urban nonteaching | 4275 (9.85) | 3410 (9.33) | 865 (12.59) | |
| Rural | 590 (1.36) | 530 (1.45) | 60 (0.87) | |
| Region of the Country | ||||
| South | 14,805 (34.10) | 12,090 (33.08) | 2715 (39.52) | .03 |
| Midwest | 7840 (18.06) | 6570 (17.98) | 1270 (18.49) | |
| West | 6795 (15.65) | 5980 (16.36) | 815 (11.86) | |
| Northeast | 13,975 (32.19) | 11,905 (32.58) | 2070 (30.13) | |
Data are presented as n (%) except where otherwise noted. VATS, Video-assisted thoracoscopic surgery; RATS, robotic-assisted thoracoscopic surgery; SEM, standard error of the mean.
Adverse Perioperative Outcomes
Adverse perioperative outcomes, LOS, and total hospital cost of patients who had a lobectomy and sublobar resection are presented in Tables 3 and 4, respectively. Of all patients who had a lobectomy, 33.5% experienced a complication during admission for surgery. Compared with VATS, patients who had a RATS lobectomy had a higher rate of any complication (VATS: 32.7% vs RATS: 35.6%; P = .022). However, there were no differences in cardiovascular and thromboembolic complications, bleeding complications, conversion to open, or mortality (Table 3).
Table 3.
Adverse perioperative outcomes, length of stay, and total hospital cost of patients who had a lobectomy for lung cancer using VATS or RATS in the National Inpatient Sample database from October 2015 through December 2018
| Perioperative complication | All (N = 39,690) | VATS (n = 28,850) | RATS (n = 10,840) | P value (unadjusted) |
|---|---|---|---|---|
| Any | 13,285 (33.5) | 9425 (32.7) | 3860 (35.6) | .02 |
| Thoracic | 10,320 (26) | 7325 (25.4) | 2995 (27.6) | .06 |
| Pneumonia | 1530 (3.9) | 1090 (3.8) | 440 (4.1) | .58 |
| Pneumothorax | 2910 (7.3) | 2040 (7.1) | 870 (8) | .16 |
| Air leak | 4345 (11) | 3130 (10.9) | 1215 (11.2) | .68 |
| Acute respiratory failure | 295 (0.7) | 190 (0.7) | 105 (1) | .20 |
| Pulmonary collapse or atelectasis | 3330 (8.4) | 2375 (8.2) | 955 (8.8) | .45 |
| Other complications of respiratory system | 630 (1.6) | 390 (1.4) | 240 (2.2) | <.01 |
| Cardiovascular and thromboembolic | 3250 (8.2) | 2275 (7.9) | 975 (9) | .11 |
| Cardiac arrest | 185 (0.5) | 125 (0.4) | 60 (0.6) | .50 |
| Atrial fibrillation | 2760 (7) | 1945 (6.7) | 815 (7.5) | .24 |
| Periprocedural shock or other cardiovascular complications | 225 (0.6) | 165 (0.6) | 60 (0.6) | .92 |
| Deep venous thrombosis | 850 (2.1) | 585 (2) | 265 (2.4) | .26 |
| Pulmonary embolism | 140 (0.4) | 100 (0.4) | 40 (0.4) | .88 |
| Bleeding | 405 (1) | 285 (1) | 120 (1.1) | .64 |
| Blood transfusion | 700 (1.8) | 525 (1.8) | 175 (1.6) | .53 |
| Hemoperitoneum, postoperative hematoma or hemothorax | 330 (0.8) | 240 (0.8) | 90 (0.8) | .99 |
| Other | ||||
| Device-related complications | 140 (0.4) | 90 (0.3) | 50 (0.5) | .32 |
| Injuries to other organs | 165 (0.4) | 100 (0.4) | 65 (0.6) | .12 |
| Renal failure or other urinary complications | 135 (0.3) | 90 (0.3) | 45 (0.4) | .51 |
| Paralytic ileus | 110 (0.3) | 60 (0.2) | 50 (0.5) | .04 |
| Other or unspecified | 3235 (8.2) | 2310 (8) | 925 (8.5) | .52 |
| Other outcomes | ||||
| Mortality | 225 (0.6) | 160 (0.6) | 65 (0.6) | .81 |
| Conversion to open | 500 (1.3) | 400 (1.4) | 100 (0.9) | .60 |
| Extended length of stay (>7 d) | 5655 (14.3) | 4155 (14.4) | 1500 (13.8) | .56 |
| Mean length of stay ± SEM, d | 5 ± 0.1 | 5.1 ± 0.1 | 4.8 ± 0.1 | .13 |
| Median length of stay (IQR), d | 3.4 (2.1-5.4) | 3.4 (2.2-5.5) | 3.2 (2.1-5.3) | |
| Cost | (N∗ = 39,330; 99.1%) | (n∗ = 28,520; 98.9%) | (n∗ = 10,810; 99.7%) | |
| Mean total hospital cost ± SEM, $ | 24,866 ± 278 | 23,922 ± 304 | 27,356 ± 547 | <.01 |
| Median (IQR) | 21,438 (16,218-28,841) | 20,487 (15,555-27,689) | 23,805 (18,317-31,672) | |
Data are presented as n (%) unless otherwise specified. SAS survey procedures do not include comparative analyses of medians. VATS, Video-assisted thoracoscopic surgery; RATS, robotic-assisted thoracoscopic surgery; SEM, standard error of the mean; IQR, interquartile range.
Total hospital cost was only calculated for hospitals that have submitted their distinct cost-to-charge ratio in the Healthcare Cost and Utilization Project.
Table 4.
Adverse perioperative outcomes, length of stay, and total hospital cost of patients who had a sublobar resection (segmentectomy or wedge resection) for lung cancer using VATS or RATS in the National Inpatient Sample database from October 2015 through December 2018
| Perioperative complication | All (N = 43,415) | VATS (n = 36,545) | RATS (n = 6870) | P value |
|---|---|---|---|---|
| Any | 10,480 (24.1) | 8650 (23.7) | 1830 (26.6) | .03 |
| Thoracic | 7790 (17.9) | 6495 (17.8) | 1295 (18.9) | .39 |
| Pneumonia | 1385 (3.2) | 1275 (3.5) | 110 (1.6) | <.01 |
| Pneumothorax | 2260 (5.2) | 1905 (5.2) | 355 (5.2) | .94 |
| Air leak | 2355 (5.4) | 1870 (5.1) | 485 (7.1) | <.01 |
| Acute respiratory failure | 255 (0.6) | 180 (0.5) | 75 (1.1) | <.01 |
| Pulmonary collapse or atelectasis | 2805 (6.5) | 2355 (6.4) | 450 (6.6) | .89 |
| Other complications of respiratory system | 485 (1.1) | 400 (1.1) | 85 (1.2) | .67 |
| Cardiovascular and thromboembolic | 2515 (5.8) | 2080 (5.7) | 435 (6.3) | .33 |
| Cardiac arrest | 55 (0.1) | 45 (0.1) | 10 (0.2) | .83 |
| Atrial fibrillation | 2050 (4.7) | 1695 (4.6) | 355 (5.2) | .40 |
| Periprocedural shock or other cardiovascular complication | 125 (0.3) | 110 (0.3) | 15 (0.2) | .60 |
| Deep venous thrombosis | 670 (1.5) | 515 (1.4) | 155 (2.3) | .02 |
| Pulmonary embolism | 170 (0.4) | 145 (0.4) | 25 (0.4) | .86 |
| Bleeding | 380 (0.9) | 310 (0.8) | 70 (1) | .55 |
| Blood transfusion | 665 (1.5) | 590 (1.6) | 75 (1.1) | .14 |
| Hemoperitoneum, postoperative hematoma or hemothorax | 390 (0.9) | 320 (0.9) | 70 (1) | .62 |
| Other | ||||
| Device-related complications | 55 (0.1) | 35 (0.1) | 20 (0.3) | .05 |
| Injuries to other organs | 135 (0.3) | 100 (0.3) | 35 (0.5) | .15 |
| Renal failure or other urinary complications | 165 (0.4) | 115 (0.3) | 50 (0.7) | .02 |
| Paralytic ileus | 65 (0.2) | 40 (0.1) | 25 (0.4) | .03 |
| Other or unspecified | 2240 (5.1) | 1730 (4.7) | 510 (7.4) | <.01 |
| Other outcomes | ||||
| Mortality | 310 (0.7) | 290 (0.8) | 20 (0.3) | .05 |
| Conversion to open | 405 (0.9) | 335 (0.9) | 70 (1) | .72 |
| Extended length of stay (>7 d) | 4500 (10.4) | 3840 (10.5) | 660 (9.6) | .35 |
| Mean length of stay ± SEM, d | 4.1 ± 0.1 | 4.2 ± 0.1 | 4.1 ± 0.2 | .78 |
| Median (IQR) | 2.3 (1.2-4.2) | 2.3 (1.1-4.2) | 2.3 (1.3-4.1) | |
| Cost | (N∗ = 43,140) | (n∗ = 36,285) | (n∗ = 6855) | |
| Mean total hospital cost ± SEM, $ | 19,278 ± 282 | 18,574 ± 267 | 23,008 ± 868 | <.01 |
| Median (IQR) | 15,629 (11,361-22,210) | 14,990 (10,923-21,372) | 19,150 (14,648-26,462) | |
Data are presented as n (%) except where otherwise specified. SAS survey procedures do not include comparative analyses of medians. VATS, Video-assisted thoracoscopic surgery; RATS, robotic-assisted thoracoscopic surgery; SEM, standard error of the mean; IQR, interquartile range.
Total hospital cost is calculated for the subset of hospitals that have submitted their distinct cost-to-charge ratio in the Healthcare Cost and Utilization Project.
Of all patients who had a sublobar resection, 24.1% experienced a complication during admission. Compared with VATS, patients who had a RATS sublobar resection had a higher rate of any complication (VATS: 23.7% vs RATS: 26.6%; P = .03). The rate of any thoracic complication was not different between approaches; however, patients in the VATS group had a higher rate of pneumonia (VATS: 3.5% vs RATS: 1.6%; P < .01) whereas those in the RATS group had higher rates of air leak (VATS: 5.1% vs RATS: 7.1%; P < .01) and acute respiratory failure (VATS: 0.5% vs RATS: 1.1%; P < .01). The rates of cardiovascular and thromboembolic complications, bleeding complications, mortality, and conversion to open were comparable; however, individual complications varied among the 2 approaches (Table 4).
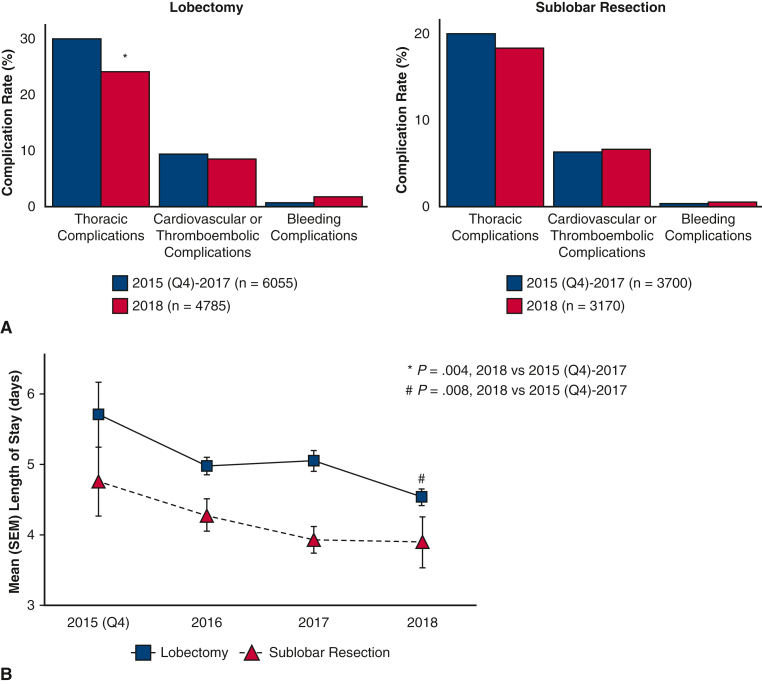
Figure 3 shows a comparison of thoracic complications, cardiovascular or thromboembolic complications, and bleeding complications after RATS resection between 2018 (lobectomy: n = 4785, sublobar resection: n = 3170) and 2015 (Q4) to 2017 (lobectomy: n = 6055; sublobar resection: n = 3700) study years. The rate of thoracic complications decreased after RATS lobectomy (2015 [Q4]-2017: 30.2% vs 2018: 24.4%; P = .004) but not after sublobar resection (2015 [Q4]-2017: 19.6% vs 2018: 18%; P = .49). There was no difference in the rate of cardiovascular or thromboembolic complications between study years. Bleeding complications did not differ between 2018 and previous years after RATS sublobar resection or lobectomy (2015 [Q4]-2017: 1.6% vs 2018: 0.7%; P = .065; Figure 3, panel A).
Figure 3.
Trends in complication rates (panel A) and length of stay (panel B) after robotic-assisted lobectomy and sublobar resection in patients with lung cancer in the National Inpatient Sample from October 2015 through December 2018. SEM, Standard error of the mean.
Results of the logistic regression analyses of the independent association of surgical approach with adverse perioperative outcomes are shown in Table 5. Only data for the surgical approach (RATS relative to VATS) are presented. In patients who had a lobectomy, RATS was not independently associated with adverse surgical outcomes (Table 5, Lobectomy section). In patients who had a sublobar resection, RATS (relative to VATS) was associated with a decreased risk of pneumonia (odds ratio, 0.43; 95% CI, 0.27-0.67; adjusted P < .01); however, the surgical approach was not independently associated with any complication, thoracic complications, bleeding, mortality, conversion to open, or extended LOS (Table 5, Sublobar resection section).
Table 5.
Logistic regression analyses of independent association of surgical approach with adverse perioperative outcomes in patients who had a lobectomy and those who had sublobar resection for lung cancer in the National Inpatient Sample database from October 2015 through December 2018
| Adverse perioperative outcomes | Lobectomy; RATS (relative to VATS) |
Sublobar resection; RATS (relative to VATS) |
||||
|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | Unadjusted P value | Holm-adjusted P value | Odds ratio (95% CI) | Unadjusted P value | Holm-adjusted P value | |
| Any complication | 1.1 (1.01-1.27) | .03 | .33 | 1.1 (.96-1.30) | .12 | .84 |
| Thoracic complications | 1.1 (1.01-1.28) | .04 | .40 | 1.04 (.88-1.23) | .64 | 1.00 |
| Pneumonia | 1.1 (0.82-1.40) | .62 | 1.00 | .4 (.27-.67) | <.01 | <.01 |
| Pneumothorax | 1.2 (0.98-1.45) | .08 | .72 | .97 (.75-1.25) | .79 | 1.00 |
| Air leak | 1.1 (0.89-1.27) | .51 | 1.00 | 1.4 (1.08-1.78) | .01 | .09 |
| Acute respiratory failure | 1.4 (0.72-2.55) | .35 | 1.00 | 2.2 (1.24-3.85) | <.01 | .07 |
| Pulmonary collapse or atelectasis | 1.1 (0.89-1.31) | .43 | 1.00 | 1 (.77-1.29) | .99 | 1.00 |
| Bleeding requiring transfusion | 2.2 (0.77-6.43) | .14 | 1.00 | 2.3 (.78-6.73) | .13 | .84 |
| Other outcomes | ||||||
| Mortality | 1.01 (0.51-1.97) | .99 | 1.00 | .4 (.13-.98) | .05 | .40 |
| Conversion to open, n (%) | 0.5 (0.12-2.44) | .42 | 1.00 | .7 (.29-1.47) | .30 | 1.00 |
| Extended length of stay (>7 d) | 0.95 (0.81-1.11) | .50 | 1.00 | .9 (.72-1.10) | .27 | 1.00 |
Other factors included in the model were: patient age, sex, race/ethnicity, comorbid conditions, smoking, insurance, hospital characteristics and location. RATS, Robotic-assisted thoracoscopic surgery; VATS, video-assisted thoracoscopic surgery; CI, confidence interval.
LOS and Total Hospital Cost
For patients who had a lobectomy (Table 3) and those who had a sublobar resection (Table 4), LOS and the proportion of patients who had an extended LOS were similar after VATS and RATS but the mean total hospital cost was higher for RATS admissions (P < .001). Figure 3, panel B shows trends of LOS over time during the study years. From 2015 (Q4) to 2018, mean LOS decreased from 5.7 ± 0.5 to 4.5 ± 0.1 after lobectomy and from 4.8 ± 0.5 to 3.9 ± 0.4 after sublobar resection. When LOS in 2018 was compared with previous years combined, there was a decrease in mean LOS after RATS lobectomy (2015 [Q4]-2017 [n = 6055]: 4.1 ± 0.1 days vs 2018 [n = 4785]: 3.9 ± 0.4 days; P = .008) but not after sublobar resection (2015 [Q4]-2017 (n = 3700): 5.1 ± 0.1 days vs 2018 (n = 3170): 4.5 ± 0.1 days; P = .67).
Discussion
In this study, we report estimates of the numbers and perioperative outcomes of subtotal pulmonary resections for nonmetastatic lung cancer that were performed using MIS techniques in the United States. Previous estimates of RATS utilization using the Nationwide Readmission Database show that between 2010 and 2014, 12.5% of MIS lobectomies were done with the RATS approach.16 Using the most recent nationally representative data from the NIS, we found that the proportion of the robotic-assisted approach increased, between 2015 and 2018, from 19.2% to 34% of all MIS lobectomies and from 7% to 22% of sublobar resections. With this growth in adoption, the question remains whether the RATS provides any additional perioperative outcomes benefits compared with the VATS approach.
Our findings indicate that at the national level, RATS was associated with a comparable risk of perioperative complications compared with VATS during the study period, at a higher hospital cost and similar LOS. After adjusting for patient and hospital characteristics, RATS was associated with a similar risk of overall perioperative complications and thoracic complications after lobectomy and sublobar resection and a higher likelihood of air leak and acute respiratory failure after a sublobar resection. We also found no independent association between the MIS approach (RATS vs VATS) and mortality, conversion rates, and extended LOS. In agreement with our findings, a recent randomized trial showed no difference in perioperative complications or LOS between RATS and VATS lobectomy at a higher cost.12 However, a recent meta-analysis of retrospective studies suggests potential advantages of RATS compared with VATS lobectomy/segmentectomy including less blood loss, lower conversion rate, shorter LOS, and lower overall complication rate in addition to a higher lymph node yield.37 Other studies, however, showed no difference in number of retrieved lymph nodes, perioperative complications, or LOS between the 2 MIS approaches.17 In a meta-analysis from Ma and colleagues,37 pooled results showed a lower conversion rate in RATS (relative to VATS) lobectomy but not segmentectomy. Furthermore, the sensitivity analysis of high-quality articles (n = 7) showed no differences between the 2 approaches in conversion rates when lobectomy and segmentectomies were included. Other meta-analyses, however, showed no difference in number of retrieved lymph nodes, perioperative complications, or LOS between the 2 MIS approaches.17
Among several factors, the improvement in robotic approach outcomes can partially be related to the surgeon's learning curve. The existing literature regarding the robotic lung resection learning curve is incongruent with some studies showing the need for 20 to 30 robotic cases38 and others estimating the learning curve to be upward of 100.39 Su and colleagues40 reported an association between surgeon experience and postrobotic lobectomy prolonged air leak, emphasizing the importance of the learning curve and its potential effect on patient outcomes. This learning curve might be present independent of the surgeon's previous VATS experience.41 Our study showed a decrease in the number of thoracic complications associated with robotic lobectomy compared with earlier years. Likewise, a decrease in length of hospitalization after robotic lung resection over time was noted. The NIS database does not provide surgeon-level data to assess the effect of the learning curve on outcomes or provide direct insight into the relationship between the surgeon learning curve and outcomes. This needs to be assessed in a prospectively designed multicenter study. Although our multivariate analyses accounted for some hospital characteristics such as hospital bed volume, location, and teaching status, the study was not designed to compare the 2 MIS approaches performed by surgeons and centers of equivalent expertise; rather, we report an update on the current status of surgical outcomes with MIS lung resection at the national level. We found that 66% and 89% of RATS resections were done at large and urban teaching hospitals, respectively, with no differences in hospital size, location, or teaching status between the VATS and RATS groups.
Our study is subject to multiple limitations, intrinsic to the nature of data in national administrative databases. First, our findings are primarily on the basis of medical coding with possible errors related to miscoding or missing data. Importantly, the ICD-10-PCS does not allow researchers to discern segmentectomy from wedge resections.30 Thus, our findings should not be interpreted as outcomes of 1 of the 2 procedures separately from the other, but rather outcomes of sublobar resections in general. It is not possible with using ICD-10 coding to determine reliably the ratio of segmentectomy to wedge resections per surgical approach in our study. Second, the NIS only includes diagnoses and procedures documented during in-patient hospital admission; therefore, we could not report morbidity or mortality from any complications that might have occurred after discharge from the index procedure hospitalization. Similarly, diagnoses and procedures/treatments done before admission were not captured. This includes any preoperative neoadjuvant therapy. Third, although our multivariate analyses were adjusted for some patient and hospital characteristics available in the NIS, we were unable to account for possible differences in cancer staging, American Society of Anesthesiologists Classification, hospital case volume, and individual surgeon's experiences with MIS approaches or other factors that might have influenced outcomes. Fourth, hospital costs in our study were estimated from hospital charges data using hospital-wide cost-to-charge ratios.42 This approach has been widely used in the literature including in studies that have compared robotic and laparoscopic surgical approaches.43 The methods used did not account for specific potential variations among the cohorts; however, reliable estimates can generally be obtained for hospital admissions with similar diagnosis-related groups, type of hospital services and departments,44 and similar insurance coverage45, 46 as was the case in our study. Finally, similar to most administrative databases, the nature of the NIS data do not allow an accurate assessment of the severity of the perioperative outcomes.
Conclusions
This national-level analysis showed an increase in the utilization of the robotic-assisted approach for subtotal pulmonary resection in patients with lung cancer in the United States between 2015 and 2018. The RATS approach was associated with a comparable risk of perioperative complications and LOS and a higher hospital cost than the VATS approach.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Footnotes
Read at the 102nd Annual Meeting of The American Association for Thoracic Surgery, Boston, Massachusetts, May 14-17, 2022.
Appendix E1
Table E1.
International Classification of Diseases, 10th Revision (ICD-10) procedural codes for VATS and robotic partial lung resections
| ICD-10 procedural codes |
|---|
| Thoracoscopic lobectomy, percutaneous endoscopic |
| 0BTC4ZZ Upper lung lobe, right |
| 0BTD4ZZ Middle lung lobe, right |
| 0BTF4ZZ Lower lung lobe, right |
| 0BTG4ZZ Upper lung lobe, left |
| 0BTH4ZZ Lung lingula |
| 0BTJ4ZZ Lower lung lobe, left |
| Thoracoscopic sublobar resection |
| 0BBC4ZX Upper lung lobe, right |
| 0BBC4ZZ Upper lung lobe, right |
| 0BBD4ZX Middle lung lobe, right |
| 0BBD4ZZ Middle lung lobe, right |
| 0BBF4ZX Lower lung lobe, right |
| 0BBF4ZZ Lower lung lobe, right |
| 0BBG4ZX Upper lung lobe, left |
| 0BBG4ZZ Upper lung lobe, left |
| 0BBH4ZX Lung lingula |
| 0BBH4ZZ Lung lingula |
| 0BBJ4ZX Lower lung lobe, left |
| 0BBJ4ZZ Lower lung lobe, left |
| 0BBK4ZX Lung, right |
| 0BBK4ZZ Lung, right |
| 0BBL4ZX Lung, left |
| 0BBL4ZZ Lung, left |
| 0BBM4ZX Lungs, bilateral |
| 0BBM4ZZ Lungs, bilateral |
| Robotic-assisted codes |
| 8E0WXCZ Robotic-assisted procedure of trunk region |
| 8E0W3CZ Robotic-assisted procedure of trunk region, percutaneous approach |
| 8E0W4CZ Robotic-assisted procedure of trunk region, percutaneous endoscopic approach |
| 8E0W7CZ Robotic-assisted procedure of trunk region, via natural or artificial opening |
| 8E0W8CZ Robotic-assisted procedure of trunk region, via natural or artificial opening, endoscopic |
Table E2.
ICD-10 codes for perioperative complications
| Complication | Code |
|---|---|
| Thoracic | |
| Pneumonia | J13, J14, J15, J16, J17, J18, J69.0, J95.4, J95.851 |
| Postprocedural pneumothorax | J95.811 |
| Postprocedural air leak | J95.812 |
| Acte respiratory failure | J95.1, J95.82 |
| Pulmonary collapse (atelectasis) | J98.11 |
| Other complications of respiratory system | J95.88, J95.89, J95.0, J98.0 |
| Cardiovascular | |
| Cardiac arrest, insufficiency | I97.7, I97.1 |
| Atrial fibrillation | I97.89, I48.0 |
| Periprocedural shock, hypertension, or other cardiovascular complications | T81.1, I97.3, I97.88, T81.7 |
| Thromboembolic | |
| Pulmonary embolism | I26 |
| Deep venous or other venous thromboembolisms | I82, I97.89 |
| Bleeding | |
| Postoperative blood transfusion | 30233N1 |
| Hemoperitoneum, postoperative hematoma, hemothorax | J95.83, J94.2, J95.86 |
| Other | |
| Device-related | Y81, Y82 |
| Accidental injuries to other organs | J95.7, S27.3, D78.1, E36.1, G97.4, I97.5, K91.72, N99.7 |
| Renal failure or other urinary | N99.0, N99.81, N99.89 |
| Pneumoperitoneum and other peritoneal | K66.8, K66.9 |
| Paralytic ileus | K91.89, K56.0 |
| Other or unspecified | T81.89, T81.9, T88.8, Y83, Y84, Y69, Y65 |
| Conversion to open | Z53.32 |
Supplementary Data
Trends of utilization and perioperative outcomes of robotic and video-assisted thoracoscopic surgery in patients with lung cancer undergoing minimally invasive resection in the United States. Video available at: https://www.jtcvs.org/article/S2666-2736(22)00316-3/fulltext.
References
- 1.Mao Y., Yang D., He J., Krasna M.J. Epidemiology of lung cancer. Surg Oncol Clin N Am. 2016;25:439–445. doi: 10.1016/j.soc.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Dela Cruz C.S., Tanoue L.T., Matthay R.A. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med. 2011;32:605–644. doi: 10.1016/j.ccm.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferlay J., Shin H.R., Bray F., Forman D., Mathers C., Parkin D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 4.Ferlay J., Colombet M., Soerjomataram I., Mathers C., Parkin D.M., Pineros M., et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 5.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 6.Howington J.A., Blum M.G., Chang A.C., Balekian A.A., Murthy S.C. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e278S–e313S. doi: 10.1378/chest.12-2359. [DOI] [PubMed] [Google Scholar]
- 7.Ghaly G., Kamel M., Nasar A., Paul S., Lee P.C., Port J.L., et al. Video-assisted thoracoscopic surgery is a safe and effective alternative to thoracotomy for anatomical segmentectomy in patients with clinical stage I non-small cell lung cancer. Ann Thorac Surg. 2016;101:465–472. doi: 10.1016/j.athoracsur.2015.06.112. discussion: 472. [DOI] [PubMed] [Google Scholar]
- 8.Kwon S.T., Zhao L., Reddy R.M., Chang A.C., Orringer M.B., Brummett C.M., et al. Evaluation of acute and chronic pain outcomes after robotic, video-assisted thoracoscopic surgery, or open anatomic pulmonary resection. J Thorac Cardiovasc Surg. 2017;154:652–659.e1. doi: 10.1016/j.jtcvs.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Nwogu C.E., D'Cunha J., Pang H., Gu L., Wang X., Richards W.G., et al. VATS lobectomy has better perioperative outcomes than open lobectomy: CALGB 31001, an ancillary analysis of CALGB 140202 (Alliance) Ann Thorac Surg. 2015;99:399–405. doi: 10.1016/j.athoracsur.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Sullivan K.E., Kreaden U.S., Hebert A.E., Eaton D., Redmond K.C. A systematic review and meta-analysis of robotic versus open and video-assisted thoracoscopic surgery approaches for lobectomy. Interact Cardiovasc Thorac Surg. 2019;28:526–534. doi: 10.1093/icvts/ivy315. [DOI] [PubMed] [Google Scholar]
- 11.Long H., Tan Q., Luo Q., Wang Z., Jiang G., Situ D., et al. Thoracoscopic surgery versus thoracotomy for lung cancer: short-term outcomes of a randomized trial. Ann Thorac Surg. 2018;105:386–392. doi: 10.1016/j.athoracsur.2017.08.045. [DOI] [PubMed] [Google Scholar]
- 12.Jin R., Zheng Y., Yuan Y., Han D., Cao Y., Zhang Y., et al. Robotic-assisted versus video-assisted thoracoscopic lobectomy: short-term results of a randomized clinical trial (RVlob Trial) Ann Surg. 2022;275:295–302. doi: 10.1097/SLA.0000000000004922. [DOI] [PubMed] [Google Scholar]
- 13.Cui Y., Grogan E.L., Deppen S.A., Wang F., Massion P.P., Bailey C.E., et al. Mortality for robotic- vs video-assisted lobectomy-treated stage I non-small cell lung cancer patients. JNCI Cancer Spectr. 2020;4:pkaa028. doi: 10.1093/jncics/pkaa028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oh D.S., Reddy R.M., Gorrepati M.L., Mehendale S., Reed M.F. Robotic-assisted, video-assisted thoracoscopic and open lobectomy: propensity-matched analysis of recent premier data. Ann Thorac Surg. 2017;104:1733–1740. doi: 10.1016/j.athoracsur.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 15.Reddy R.M., Gorrepati M.L., Oh D.S., Mehendale S., Reed M.F. Robotic-assisted versus thoracoscopic lobectomy outcomes from high-volume thoracic surgeons. Ann Thorac Surg. 2018;106:902–908. doi: 10.1016/j.athoracsur.2018.03.048. [DOI] [PubMed] [Google Scholar]
- 16.Bailey K.L., Merchant N., Seo Y.J., Elashoff D., Benharash P., Yanagawa J. Short-term readmissions after open, thoracoscopic, and robotic lobectomy for lung cancer based on the Nationwide Readmissions database. World J Surg. 2019;43:1377–1384. doi: 10.1007/s00268-018-04900-0. [DOI] [PubMed] [Google Scholar]
- 17.Liang H., Liang W., Zhao L., Chen D., Zhang J., Zhang Y., et al. Robotic versus video-assisted lobectomy/segmentectomy for lung cancer: a meta-analysis. Ann Surg. 2018;268:254–259. doi: 10.1097/SLA.0000000000002346. [DOI] [PubMed] [Google Scholar]
- 18.Paul S., Jalbert J., Isaacs A.J., Altorki N.K., Isom O.W., Sedrakyan A. Comparative effectiveness of robotic-assisted versus thoracoscopic lobectomy. Chest. 2014;146:1505–1512. doi: 10.1378/chest.13-3032. [DOI] [PubMed] [Google Scholar]
- 19.Louie B.E., Wilson J.L., Kim S., Cerfolio R.J., Park B.J., Farivar A.S., et al. Comparison of video-assisted thoracoscopic surgery and robotic approaches for clinical stage I and stage II non-small cell lung cancer using the Society of Thoracic Surgeons database. Ann Thorac Surg. 2016;102:917–924. doi: 10.1016/j.athoracsur.2016.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kent M., Wang T., Whyte R., Curran T., Flores R., Gangadharan S. Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann Thorac Surg. 2014;97:236–242. doi: 10.1016/j.athoracsur.2013.07.117. discussion: 242-4. [DOI] [PubMed] [Google Scholar]
- 21.Kodia K., Razi S.S., Alnajar A., Nguyen D.M., Villamizar N. Comparative analysis of robotic segmentectomy for non-small cell lung cancer: a National Cancer database study. Innovations (Phila) 2021;16:280–287. doi: 10.1177/1556984521997805. [DOI] [PubMed] [Google Scholar]
- 22.Sesti J., Langan R.C., Bell J., Nguyen A., Turner A.L., Hilden P., et al. A comparative analysis of long-term survival of robotic versus thoracoscopic lobectomy. Ann Thorac Surg. 2020;110:1139–1146. doi: 10.1016/j.athoracsur.2020.03.085. [DOI] [PubMed] [Google Scholar]
- 23.Veluswamy R.R., Whittaker Brown S.A., Mhango G., Sigel K., Nicastri D.G., Smith C.B., et al. Comparative effectiveness of robotic-assisted surgery for resectable lung cancer in older patients. Chest. 2020;157:1313–1321. doi: 10.1016/j.chest.2019.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herb J.N., Kindell D.G., Strassle P.D., Stitzenberg K.B., Haithcock B.E., Mody G.N., et al. Trends and outcomes in minimally invasive surgery for locally advanced non-small-cell lung cancer with N2 disease. Semin Thorac Cardiovasc Surg. 2021;33:547–555. doi: 10.1053/j.semtcvs.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J.T., Liu P.Y., Huang J., Lu P.J., Lin H., Zhou Q.J., et al. Perioperative outcomes of radical lobectomies using robotic-assisted thoracoscopic technique versus video-assisted thoracoscopic technique: retrospective study of 1,075 consecutive p-stage I non-small cell lung cancer cases. J Thorac Dis. 2019;11:882–891. doi: 10.21037/jtd.2019.01.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y., Chen C., Hu J., Han Y., Huang M., Xiang J., et al. Early outcomes of robotic versus thoracoscopic segmentectomy for early-stage lung cancer: a multi-institutional propensity score-matched analysis. J Thorac Cardiovasc Surg. 2020;160:1363–1372. doi: 10.1016/j.jtcvs.2019.12.112. [DOI] [PubMed] [Google Scholar]
- 27.Swanson S.J., Miller D.L., McKenna R.J., Jr., Howington J., Marshall M.B., Yoo A.C., et al. Comparing robot-assisted thoracic surgical lobectomy with conventional video-assisted thoracic surgical lobectomy and wedge resection: results from a multihospital database (Premier) J Thorac Cardiovasc Surg. 2014;147:929–937. doi: 10.1016/j.jtcvs.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 28.Glenn Z.F., Zubair M., Hussain L., Grannan K. Comparison of pulmonary lobectomies using robotic and video-assisted thoracoscopic approaches: results from 2010-2013 National Inpatient Sample. J Cardiovasc Surg (Torino) 2019;60:526–531. doi: 10.23736/S0021-9509.19.10744-6. [DOI] [PubMed] [Google Scholar]
- 29.Clark J.M., Utter G.H., Nuno M., Romano P.S., Brown L.M., Cooke D.T. ICD-10-CM/PCS: potential methodologic strengths and challenges for thoracic surgery researchers and reviewers. J Thorac Dis. 2019;11(Suppl 4):S585–S595. doi: 10.21037/jtd.2019.01.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project Overview of the National (Nationwide) Inpatient Sample (NIS) 2021. https://www.hcup-us.ahrq.gov/nisoverview.jsp
- 31.Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project Introduction to the HCUP National Inpatient Sample (NIS) 2018. https://www.hcup-us.ahrq.gov/db/nation/nis/NISIntroduction2018.pdf
- 32.Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project Producing national HCUP estimates. https://hcup-us.ahrq.gov/tech_assist/nationalestimates/508_course/508course_2018.jsp
- 33.Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project NIS description of data elements. https://hcup-us.ahrq.gov/db/nation/nis/nisdde.jsp
- 34.Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project Elixhauser comorbidity software refined for ICD-10-CM. https://www.hcup-us.ahrq.gov/toolssoftware/comorbidityicd10/comorbidity_icd10.jsp
- 35.Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project Cost-to-charge ratio files. https://www.hcup-us.ahrq.gov/db/ccr/costtocharge.jsp
- 36.US Bureau of Labor Statistics Databases, tables & calculators by subject. CPI for all urban consumers (CPI-U) https://data.bls.gov/timeseries/CUUR0000SAM?output_view=pct_12mths
- 37.Ma J., Li X., Zhao S., Wang J., Zhang W., Sun G. Robot-assisted thoracic surgery versus video-assisted thoracic surgery for lung lobectomy or segmentectomy in patients with non-small cell lung cancer: a meta-analysis. BMC Cancer. 2021;21:498. doi: 10.1186/s12885-021-08241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song G., Sun X., Miao S., Li S., Zhao Y., Xuan Y., et al. Learning curve for robot-assisted lobectomy of lung cancer. J Thorac Dis. 2019;11:2431–2437. doi: 10.21037/jtd.2019.05.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li X., Wang J., Ferguson M.K. Competence versus mastery: the time course for developing proficiency in video-assisted thoracoscopic lobectomy. J Thorac Cardiovasc Surg. 2014;147:1150–1154. doi: 10.1016/j.jtcvs.2013.11.036. [DOI] [PubMed] [Google Scholar]
- 40.Su L., Ho H., Stock C.T., Quadri S.M., Williamson C., Servais E.L. Surgeon experience is associated with prolonged air leak after robotic-assisted pulmonary lobectomy. Ann Thorac Surg. 2022;114:434–441. doi: 10.1016/j.athoracsur.2021.07.033. [DOI] [PubMed] [Google Scholar]
- 41.Andersson S.E., Ilonen I.K., Pälli O.H., Salo J.A., Räsänen J.V. Learning curve in robotic-assisted lobectomy for non-small cell lung cancer is not steep after experience in video-assisted lobectomy; single-surgeon experience using cumulative sum analysis. Cancer Treat Res Commun. 2021;27:100362. doi: 10.1016/j.ctarc.2021.100362. [DOI] [PubMed] [Google Scholar]
- 42.Campos G.M., Khoraki J., Browning M.G., Pessoa B.M., Mazzini G.S., Wolfe L. Changes in utilization of bariatric surgery in the United States from 1993 to 2016. Ann Surg. 2020;271:201–209. doi: 10.1097/SLA.0000000000003554. [DOI] [PubMed] [Google Scholar]
- 43.Andiman S.E., Bui A.H., Ascher-Walsh C., Wright J.D., Xu X. Surgical complications and hospital costs in robot-assisted versus conventional laparoscopic hysterectomy with concurrent sacrocolpopexy: analysis of the nationwide readmissions database. Female Pelvic Med Reconstr Surg. 2022;28:e142–e148. doi: 10.1097/SPV.0000000000001133. [DOI] [PubMed] [Google Scholar]
- 44.Healthcare Cost and Utilization Project (HCUP), Agency for Healthcare Research and Quality Tools for more accurate inpatient cost estimates with HCUP databases. Accessed September 10, 2022. https://www.hcup-us.ahrq.gov/reports/methods/methods.jsp
- 45.Smith M.W., Friedman B., Karaca Z., Wong H.S. Predicting inpatient hospital payments in the United States: a retrospective analysis. BMC Health Serv Res. 2015;15:372. doi: 10.1186/s12913-015-1040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khoraki J., Campos G.M., Alwatari Y., Mazzini G.S., Mangino M.J., Wolfe L.G. Perioperative outcomes of inpatient laparoscopic Heller myotomy and per-oral endoscopic myotomy in the United States. Surgery. 2022;171:1263–1272. doi: 10.1016/j.surg.2021.10.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trends of utilization and perioperative outcomes of robotic and video-assisted thoracoscopic surgery in patients with lung cancer undergoing minimally invasive resection in the United States. Video available at: https://www.jtcvs.org/article/S2666-2736(22)00316-3/fulltext.