Bacterial pathogens must execute a prodigious array of complex functions in order to survive, multiply, and disseminate within mammalian hosts. Virulence determinants are usually proteinaceous in nature and are often either secreted to the bacterial cell surface or released into the external environment. Although secreted virulence proteins serve many divergent functions, remarkably, gram-negative bacteria have developed only a small number of secretion systems by which such proteins pass through their outer membranes. In this review, we discuss the virulence functions assigned or suspected for proteins secreted via the type V autotransporter pathway (Table 1).
TABLE 1.
Functions of the autotransporter passenger domains
| Organism | Protein | Function(s)a | Reference(s) |
|---|---|---|---|
| Bordetella spp. | Pertactin | Adhesin | 22, 94, 95 |
| BrkA | Serum resistance | 41 | |
| TcfA | Adhesin | 46 | |
| Vag8 | Adhesin? | 45 | |
| Dichelobacter nodosus | BprV | Elastase? | 131 |
| BprB | Elastase? | 96 | |
| AprV2 | Elastase? | 149 | |
| BprX | Elastase? | 96 | |
| Escherichia coli | EspP | Proteolytic toxin | 16, 32 |
| Pet | Proteolytic toxin | 37 | |
| Sat | Proteolytic toxin | 61a | |
| Tsh | Hemagglutinin/hemoglobin binding | 114, 126 | |
| Pic | Mucinase | 69 | |
| AIDA-I | Adhesin | 9 | |
| TibA | Adhesin | 98 | |
| Ag43 | Biofilm formation/ adhesin | 74 | |
| Haemophilus influenzae | IgA1 protease | Cleavage of IgA1 | 125 |
| Hap | Adhesin/protease | 144 | |
| Hia | Adhesin | 5 | |
| Hsf | Adhesin | 143 | |
| Helicobacter mustelae | Hsr | S layer? | 113 |
| Helicobacter pylori | VacA | Toxin | 130 |
| BabA | Adhesin | 81 | |
| Moraxella catarrhalis | UspA1 | Adhesin | 2 |
| UspA2 | Serum resistance | 2 | |
| UspA2h | Adhesin | 90 | |
| Neisseria spp. | IgA1 protease | Cleavage of IgA1 | 123 |
| Pasteurella haemolytica | Ssa1 | Protease | 60, 99 |
| Pseudomonas aeruginosa | EstA | Esterase | 155 |
| Pseudomonas fluorescens | PspA | Protease | 84 |
| PspB | Protease | 84 | |
| Rickettsiales | rOmpA | Adhesin | 29 |
| rOmpB | S layer/adhesin | 52 | |
| Salmonella enterica serovar Typhimurium | ApeE | Esterase | 19 |
| Serratia marcesens | PrtS | Protease | 103 |
| PrtT | Protease | 103 | |
| Ssp-H1 | Protease | 110 | |
| Ssp-H2 | Protease | 110 | |
| Shigella flexneri | SepA | Protease/inflammation/invasion | 7 |
| Pic | Mucinase | 69 | |
| SigA | Proteolytic toxin | 3 | |
| IcsA | Mediator of intracellular motility | 58 | |
| Xenorhabdus luminescens | PlaA | Lipase | 152 |
A list with items separated by a shill(s) indicates a multiplicity of functions.
GRAM-NEGATIVE SECRETION MECHANISMS
Five major protein secretion pathways (respectively numbered, for better or worse, from I to V) have been characterized for gram-negative bacteria. Type I secretion is exemplified by the secretion pathway defined for Escherichia coli hemolysin (HlyA). Secretion requires three accessory proteins which comprise a channel spanning both the inner and outer membranes (40, 145). The type II protein secretion system is exemplified by the pullulanase (PulA) system of Klebsiella oxytoca. PulA secretion requires the action of approximately 14 additional accessory proteins, which are encoded on a single continuous operon (127). The type II system also features a macromolecular, multicomponent structure that most probably spans both inner and outer membranes (109). Notably, unlike the type I and type III secretion systems and some members of the type IV secretion system, secretion of pullulanase across the inner membrane is sec-dependent.
The type III secretion pathway is an area of intensive research. Secretion of type III effector molecules requires a complex apparatus of proteins which assemble into a tightly regulated oligomeric structure spanning the inner and outer membranes. Although the sec system is not required for secretion of the effector molecules, the sec machinery is required for translocation of some of the component proteins of the secretion apparatus across the inner membrane (50, 80).
It is worth noting here that for a variety of reasons the terminology for two secretion systems (type IV and type V) has been confused within the literature for quite some time. Recently, a consensus opinion on the terminology was published, and the nomenclature we use here reflects that consensus (68, 136). Thus, type IV secretion involves the coordinate action of at least nine proteins that are variously associated with the inner and outer membranes and are localized within the periplasm and cytoplasm. The best-characterized systems are those utilized by Bordetella pertussis to secrete pertussis toxin and by Agrobacterium tumefaciens to deliver T-DNA (17, 157). The export process is perhaps the least understood of the gram-negative bacterial secretion mechanisms.
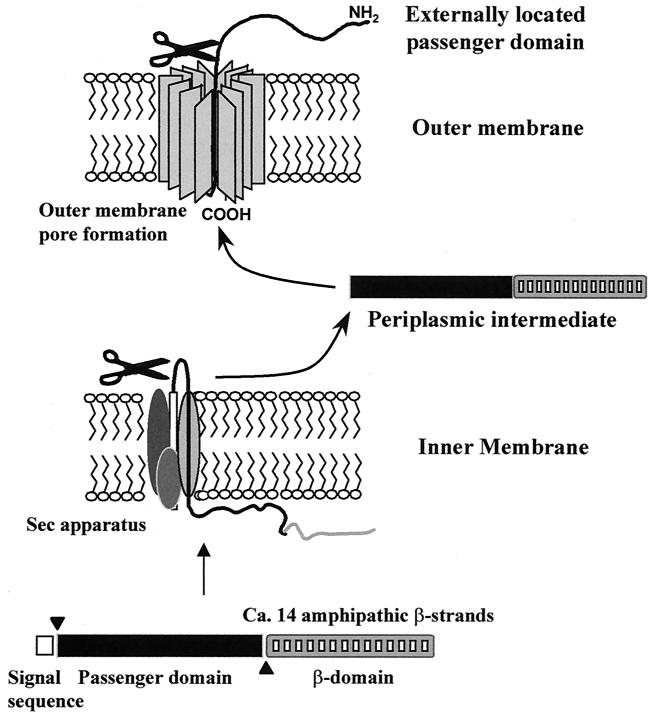
Given the complex nature of the other secretion systems described above, the simplicity of the autotransporter (type V [see references 68 and 136]) secretion mechanism is remarkable. All proteins that are secreted by this mechanism possess an overall unifying structure, comprising (i) an amino-terminal leader peptide (for secretion across the inner membrane), (ii) the secreted mature protein (or passenger domain), and (iii) a dedicated C-terminal domain, which forms a pore in the outer membrane through which the passenger domain passes to the cell surface (Fig. 1). It is assumed that all the requirements for secretion across the outer membrane are contained within a single molecule and that secretion is an energy-independent process. Although this represents the simplest form of secretion, there are considerable biophysical constraints on secretion; since the protein must be secreted through a relatively small pore, the structural properties of the proteins are confined by the size of the pore (73).
FIG. 1.
Model of autotransporter (type V) secretion mechanism. Proteins exported by the autotransporter secretion mechanism are translated as a polyprotein possessing three domains. The three domains of the polyprotein (the leader sequence, the passenger domain, and the C-terminal β-domain) are indicated. The leader sequence directs secretion via the sec apparatus and is cleaved at the inner membrane by a signal peptidase releasing the remaining portion of the molecule into the periplasm. Once in the periplasm the β-domain assumes a biophysically favored state characterized by a β-barrel shaped structure which inserts itself into the outer membrane to form a pore. After insertion into the outer membrane the passenger domain is translocated to the bacterial cell surface where it may remain intact or undergo processing. A processed protein may be released into the extracellular milieu or remain associated with the bacterial cell surface.
Once at the cell surface the destinies of autotransporter passenger domains diverge. The passenger domains may remain uncleaved and protrude from the bacterial surface, as a large polyprotein, or instead the protein may be cleaved from the β-domain and then either remain loosely associated with the β-domain or become released into the external milieu (73). Evidence from the study of other systems suggests that the proteolytic pathway followed by each passenger domain is integrally associated with the physiological function of the protein. Although a sizeable amount of evidence supports the above descriptions, they are still largely hypothetical and many questions relating to the basic mechanism of autotransporter secretion remain. The secretion mechanism and hypotheses surrounding secretion of the autotransporters have been adequately reviewed elsewhere (73) and will not be considered further here.
PHYLOGENY OF AUTOTRANSPORTER PROTEINS
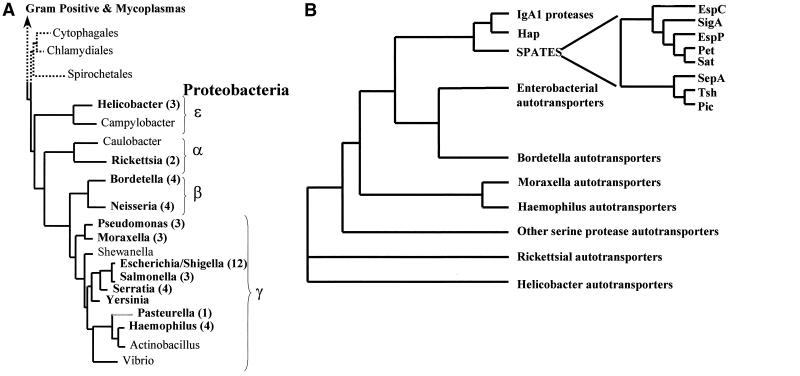
The autotransporters are a growing family of proteins, members of which have been identified across the breadth of the gram-negative evolutionary tree (Fig. 2A); as expected, the phylogeny of the proteins is complex. Although the β-domains generally display a degree of conservation consistent with their highly conserved function, the passenger domains are widely divergent, reflecting their remarkably disparate roles (73). However, despite this complexity, several inferences can be made from the phylogenetic study of these proteins. The influence of horizontal gene transfer on autotransporter distribution within the gram-negative bacteria is illustrated by the immunoglobulin A1 (IgA1) proteases; despite the fact that Neisseria and Haemophilus are located on distinct branches of the evolutionary tree (Fig. 2), members of the IgA1 proteases can be found in both species. Evidence for divergent evolution among the autotransporters is provided by examination of the serine protease autotransporters of the Enterobacteriaceae (SPATEs). Members of this closely related group of proteins have evolved specific and distinct functions which are adaptive for the particular niche occupied by the pathogen even though they possess significant homology (40 to 100% identity) through the complete protein. In this review, we will discuss known functions (Table 1) of the best-characterized autotransporter passenger domains within the phylogenetic context. Our ability to identify distinct subfamilies allows us to infer evolutionary histories for many of these proteins and serves as a platform for a discussion of their functions (Fig. 2B).
FIG. 2.
Phylogenetic distribution of the autotransporters. (A) Phylogenetic tree of the eubacteria based on 16S rRNA sequences. The tree contains a detailed list of the gram-negative proteobacteria and their subdivisons (identified by Greek symbols) and was adapted from information appearing elsewhere (137) and at the Bergey's Manual Trust website (http://www.cme.msu.edu/Bergeys/btcomments/bt9.pdf). Genera for which autotransporters have been identified and characterized are indicated by boldface. The number of autotransporters identified per genus is given in parentheses after the genus name. (B) Phylogenetic tree of the autotransporter proteins inferred from comparison of the complete amino acid sequences of the polyproteins. The sequences were aligned using the multisequence alignment program CLUSTALX. Phylogenetic relationships and evolutionary distances were calculated, and a dendrogram was constructed using the neighbor-joining method. The positions of the autotransporter proteins within the tree are indicated by families such that the IgA1 proteases from Neisseria meningitidis and N. gonorrhoeae and H. influenzae cluster in the IgA1 protease family, etc.
SERINE PROTEASE AUTOTRANSPORTERS OF THE TRYPSIN FAMILY
IgA1 proteases.
The IgA1 proteases of Neisseria and Haemophilus were the first proteins for which the autotransporter secretion strategy was identified (123, 125). These are proteases of the serine protease family whose outer membrane translocation is dependent on the activity of the trypsin-like active site (GDGSP, where S is the catalytic residue) (122). Interestingly, the proteins are processed after secretion to form the mature active IgA1 protease and separate γ- and α-peptides which appear to have distinct and separate functional roles (123, 124).
Although numerous functions have been ascribed to the IgA1 proteases, their roles in pathogenesis remain enigmatic. In vitro studies have determined clearly that these enzymes are capable of cleaving the hinge region of human secretory IgA1 (sIgA1). Initial studies focused on the potential of IgA1 proteases to promote bacterial colonization through cleavage of sIgA1 on the mucosal surface (85). However, IgA1 is purportedly a relatively minor host defense factor at the sites of infection (156). Indeed, in vitro studies suggest that Neisseria gonorrhoeae and Haemophilus influenzae IgA1 protease mutants are not impaired in their ability to colonize the mucosa. Moreover, in studies of challenge in humans an IgA1 protease mutant was not impaired in its ability to initiate an infection in the human male urethra (82). Similar conclusions were made in an examination of N. gonorrhoeae infection of the female lower genital tract (65).
Reexamination of the potential role(s) of the IgA1 proteases is extending beyond cleavage of sIgA1. Recent studies have suggested that IgA1 protease contributes to gonococcal transepithelial trafficking in T84 monolayers (79) and that more invasive gonococci display enhanced protease activity (151). In addition, IgA1 protease also cleaves LAMP1 (a major integral membrane glycoprotein of lysosomes with an IgA1-like hinge in its luminal domain), a property which contributes to the bacterium's ability to reproduce intracellularly (64, 97). Furthermore, IgA1 protease exhibits important immunostimulatory properties and may contribute substantially to the pathogenesis of neisserial infections by inducing the release of tumor necrosis factor alpha and other proinflammatory cytokines (83, 100).
Hap.
Nontypeable H. influenzae, a common commensal organism, is an important cause of localized respiratory tract disease. Infection is generally preceded by bacterial colonization of the nasopharynx; under certain circumstances, such as obstruction of the eustachian tube or sinus ostia, the organism can infect the middle ear or sinuses (129).
St. Geme and coworkers have characterized a H. influenzae protein called Hap, which is ubiquitous among H. influenzae and which promotes both bacterial attachment to and internalization into cultured epithelial cells (76, 144). This protein has significant homology with the IgA1 proteases (35% identity) and possesses a serine-protease catalytic site; like the IgA1 proteases, the majority of the Hap protein is cleaved and released from the bacterial cell surface (75). However, despite the high level of homology between Hap and the IgA1 proteases, Hap is unable to cleave purified sIgA1. Interestingly, it appears that only the uncleaved cell-associated form of Hap mediates adherence of H. influenzae to cultured epithelial cells and bacterial aggregation, features which lead to microcolony formation on the epithelial cell surface (76). Adherence is amplified further by secretory leukocyte protease inhibitor, a natural component of respiratory secretions whose primary function is to protect the host epithelium from bacterial infection but which augments Hap function by inhibiting Hap autoproteolysis, thereby causing accumulation of the Hap protein on the bacterial surface (76).
In spite of the above observations, extracellular release, a property which appears contrary to the demonstrated function of the protein, has been demonstrated for Hap (75, 144). Possibly, release of the protein serves to facilitate evasion of the local immune system by allowing the bacteria to disperse and migrate to other areas of the respiratory tract. Given the situation described above for the IgA1 proteases, other possible functions for Hap, such as degradation of complement components, immunoglobulins, and extracellular matrix proteins, may occur.
SPATEs.
Over the last 10 years, workers at several laboratories have described serine protease autotransporters secreted by members of the Enterobacteriaceae. Members of this family, accordingly termed the SPATEs, have been found in several pathogens, although their full contributions to pathogenesis are as yet unknown (73). One of the prominent features of these proteins, however, is the fact that each is among the predominant secreted proteins of their respective pathogens and also that no SPATE has been identified in a nonpathogenic organism (I. R. Henderson and J. P. Nataro, unpublished observations).
The first SPATE to be described was the temperature-sensitive hemagglutinin (Tsh) (126). This protein is secreted by strains of avian pathogenic E. coli, an organism that causes disseminated infections in birds. Although the protein was initially described as a hemagglutinin (126, 146), more-recent studies have suggested that the protease also cleaves hemoglobin, which may be adaptive for the systemic phase of infection by promoting the release of complexed ferric ions (114).
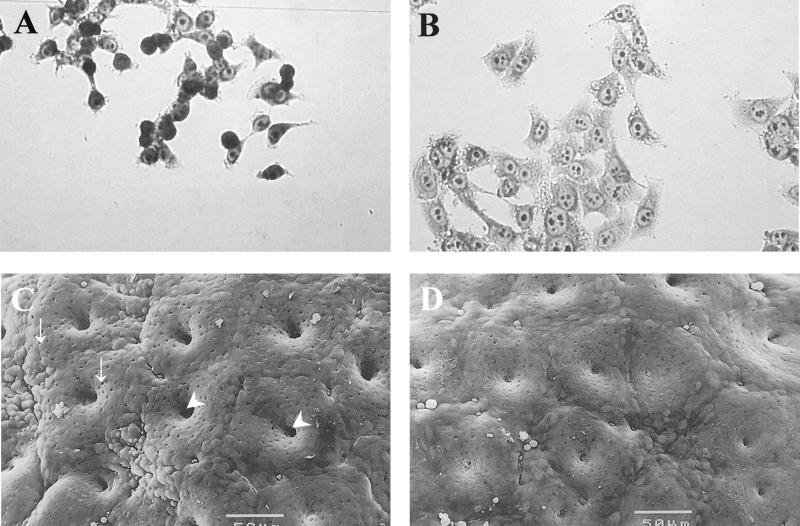
Several SPATE proteins have been identified in human pathogenic E. coli strains (73). The prototype of these is Pet (plasmid-encoded toxin) of enteroaggregative E. coli (EAEC) (37). This pathogen adheres to human colonic tissue in an explant model and elicits rounding of colonic absorptive epithelial cells, followed by sloughing of these cells from the mucosal surface (77, 78, 105). Several lines of evidence have implicated Pet in this effect and have partially characterized the cellular events that underlie this phenomenon. Henderson et al. (70) constructed a null mutation in the pet gene and demonstrated that this mutant was able to adhere to colonic tissue but was unable to elicit the characteristic mucosal changes (Fig. 3). The toxic effects were restored by trans-complementation of the pet gene. Navarro-Garcia et al. (106) showed that Pet induced damage to the mucosa of rat intestinal sections and that these changes were accompanied by rises in short circuit current in the Ussing chamber (Fig. 3).
FIG. 3.
Toxic effects of Pet. Both treated (A) and untreated (B) HEp-2 epithelial cells were incubated for 6 h at 37°C in culture medium without antibiotics and serum. Release of cellular focal contacts from the glass substratum, rounding of the cells, and cell detachment from the glass substratum are features consistently observed after treatment with Pet. Remnant cells after almost complete detachment of cells from the glass substratum are visible in panel A. Scanning electron photomicrographs of in vitro-cultured human colonic tissues infected with wild-type enteroaggregative E. coli expressing Pet (C) and an insertional mutant strain not expressing the protein (D) are also shown. The surface of the colon specimen shown in panel C is abnormal compared to that shown in panel D, as manifested by increased crypt aperture (arrowheads) and goblet cell pitting (arrows) when compared Bars, 50 μm.
When applied to HEp-2 or HT-29 cells in culture, Pet causes the cells to round and to be sloughed from the glass substratum (Fig. 3); this effect is preceded by loss of the actin stress fibers within the affected cells (107). Interestingly, Navarro-Garcia et al. (107) have recently shown that Pet is internalized by epithelial cells and that the effects of Pet are blocked by the simultaneous addition of brefeldin A. These experiments suggest that Pet has an intracellular site of action. While the mode of action of Pet is not fully elucidated, Villaseca et al. (150a) have shown that Pet is capable of cleaving fodrin (nonerythrocytic spectrin) in vitro and that fodrin is cleaved in Pet-intoxicated HEp-2 cells. Immunofluorescence microscopy demonstrates that the fodrin submembrane network undergoes condensation and peripheral redistribution before loss of actin stress fibers, which are known to be anchored to the fodrin network (31). These data suggest that fodrin is involved at an early step in Pet intoxication. Indeed, fodrin cleavage as the primary mode of action could account for the cellular changes induced by Pet toxin. Several membrane channels have been shown to be linked to the fodrin network (31), and therefore cleavage of fodrin could explain the enterotoxic effects of Pet as well.
Several related SPATE proteins may elicit similar effects. The EspP protein of Shiga toxin-producing E. coli has also been shown to elicit cytoskeletal effects on epithelial cells (16, 32), as has the recently described Sat toxin of uropathogenic E. coli (61a). The latter causes rounding of bladder epithelial cells and may contribute to epithelial damage in urinary tract infection.
Several SPATE proteins have been described for Shigella species: SepA (7) SigA (3), and Pic (69). SigA induces epithelial cytopathic effects similar to those induced by Pet. The function of SepA is unknown; however, a sepA mutant had attenuated histopathology in a rabbit ligated-loop model (7). A more distantly related protein, Pic, is present in the majority of Shigella flexneri 2a strains (108) and in most strains of EAEC (30, 69). This protein has not been shown to induce cytopathic effects but is able to cleave mucin and human complement. Pic mutants in EAEC are less adept at colonizing the mouse intestine, whereas a Shigella Pic mutant elicited less intense intestinal inflammation in the rabbit ligated-loop model (Henderson and Nataro, unpublished). The complete role of Pic in pathogenesis has yet to be characterized. Interestingly, the pic gene (GenBank accession no. U3556) encodes the Shigella enterotoxin 1 (ShET1) oligomeric toxin on the antisense strand in an overlapping fashion (39, 69) and the presence of antisense genes may not be exclusive to this autotransporter protein.
Whereas SPATE proteins share some effects, Dutta et al. (P. R. Dutta, I. R. Henderson, and J. P. Nataro, Abstr. 100th Gen. Meet. Am. Soc. Microbiol. 2000, abstr. B-31, p. 47, 2000) have demonstrated differences in their protease cleavage profiles and end effects as well. EspP has been reported to be cytopathic and cleave coagulation factor V, whereas the related EspC protein from enteropathogenic E. coli does neither but is an enterotoxin. Thus, related SPATE proteins may have been adapted to fulfill specific roles in the pathogenesis of their host organisms by virtue of subtle modification of their active sites as well as other critical toxin domains.
NONPROTEASE AUTOTRANSPORTERS OF ENTEROBACTERIACEAE
Ag43, AIDA-I, and TibA.
Antigen 43 (Ag43), AIDA-I, and TibA appear to belong to an E. coli autotransporter subfamily which is defined on the basis of amino acid homology and the presence of repetitive amino acid motifs (as shown in the phylogram of Fig. 2). In this respect, these proteins resemble pertactin of Bordetella spp., which forms the largest β-helix known to date (see below). Although the full roles of these repetitive elements has not been investigated, repetitive amino acid sequence motifs are often characteristic of proteins with adherence functions. Indeed, adhesive phenotypes have been attributed to all three of these proteins.
Ag43, a species-specific antigen of E. coli, was one of the first autotransporters discovered (116–118). However, it was not until recently, upon sequence determination of the agn43 locus, that Ag43 was identified as a member of the autotransporter family (74). Ag43 is thus far the only autotransporter known to undergo independent phase variation (72, 74, 119). The biological function of Ag43 is controversial. In addition to its phase variability, Ag43 displays several properties common to adhesins, as follows: (i) Ag43 possesses two RGD motifs of the type implicated in binding to human integrins, (ii) like many fimbrial subunits the passenger domain (α43) can be released from E. coli outer membranes by brief heating to 60°C, (iii) Ag43 displays sequence homology with several known adhesins, (iv) Ag43 mediates bacterial aggregation, and (v) Ag43 confers a low level of adhesion to certain mammalian cells (18, 71, 74, 115). The levels of adhesion to mammalian cells may not be biologically significant (119), but Ag43-mediated autoaggregation apparently contributes to biofilm formation and may enhance colonization of the mammalian intestine. Indeed, the autoaggregation phenomenon and coupled reversible on-off switching of Ag43 expression are reminiscent of the natural pattern of programmed detachment and reattachment observed for bacteria in biofilms: bacteria are released from the biofilm when Ag43 expression is switched off and reattach when expression is resumed.
AIDA-I is an outer membrane protein adhesin of some diffusely adherent E. coli strains. Genotypic analyses of the gene encoding AIDA-I have revealed that, like Ag43, AIDA-I is synthesized as a precursor molecule; the passenger domain possesses repetitive stretches and is located on the bacterial surface (8, 11). Upon cleavage, the protein is stabilized by noncovalent interaction with the β-domain. Electron microscopy studies suggest that AIDA-I is distributed evenly around the cell surface and does not form a filamentous pilus-like structure (9). Direct evidence for the adhesive properties of AIDA-I was derived from the saturable, specific binding of the purified protein to HeLa cells (10, 12). The diffuse cell adherent phenotype was lost in AIDA-I mutant strains and expression of AIDA-I by recombinant methods conferred the diffuse adherence phenotype.
The pathogenesis of another E. coli pathotype, enterotoxigenic E. coli (ETEC), has classically been described as comprising small bowel colonization via plasmid-encoded colonization factor antigens and subsequent elaboration of heat-labile or heat-stable enterotoxins. However, recent investigations have identified two unlinked chromosomal loci, tia and tib, which are each capable of directing recombinant laboratory E. coli strains to adhere to and invade intestinal epithelial cells (34, 47, 98). Although there is currently no direct evidence to support a role for ETEC invasion in human pathogenesis, intestinal biopsies from ETEC-infected piglets exhibit intracellular bacteria.
The tib locus comprises several open reading frames (ORFs) (tibA to -D). TibA encodes an autotransporter with homology to AIDA-I and Ag43 and possesses homologous amino acid repeats. However, TibA contains a second repetitive region of proline-rich 5-amino-acid motifs that is located near the C terminus of the passenger domain. Notably, unlike any other autotransporter described to date, the TibA protein is glycosylated and is the first surface-localized glycoprotein described for E. coli. Production of the mature, glycosylated TibA protein requires additional sequences within the tib locus. Upstream of the tibA gene resides an (ORF) designated tibC, which is thought to encode a glycosyltransferase. The carbohydrates on TibA are surface localized and are associated with the protein's adherence phenotypes. Nonglycosylated forms of TibA do not confer these effects (98).
IcsA.
S. flexneri causes diarrhea by directly invading the colonic epithelium and eliciting a localized inflammatory response. Once inside the cell, S. flexneri lyses the phagocytic vacuole and is released into the cytoplasm. Here the bacterium subverts the host cell cytoskeleton to move from one cell to the next. IcsA (also known as VirG), a 120-kDa autotransporter, mediates this intracellular motility by assembling actin into bundled filaments at one pole of the organism (57, 59, 147). The continuous polymerization of actin in this fashion propels the organism through the cytoplasm, eventually allowing the bacterium to spread to adjacent host cells. This autotransporter has been reviewed in detail elsewhere and will not be considered further here (56, 58, 133, 134).
AUTOTRANSPORTERS OF BORDETELLA
The autotransporters of Bordetella form a homologous family defined on the basis of their conserved C termini and the possession of RGD motifs of the type implicated in integrin binding. However, despite the similarity and possession of identical functional motifs the apparent physiological roles of these proteins are remarkably divergent.
Pertactin.
B. pertussis and Bordetella parapertussis are closely related organisms that are responsible for human pertussis. Bordetella bronchiseptica is normally an animal pathogen, but can cause respiratory disease in compromised human hosts. These three species produce a number of well-characterized virulence factors, including filamentous hemagglutinin (FHA), adenylate cyclase, pili, and a species-specific protein termed pertactin; only B. pertussis produces pertussis toxin (121). The passenger domains of the pertactin molecules are represented in B. pertussis (22), B. parapertussis (95), and B. bronchiseptica (94) as proteins of 69 kDa (P.69), 70 kDa (P.70), and 68 kDa (P.68), respectively. The processed mature passenger domain remains noncovalently associated with the 30-kDa β-domain in a manner reminiscent of Ag43 and AIDA-I, described above (21). The difference in size between P.68, P.69, and P.70 can be accounted for by the number of internal repeats contained within the passenger domain; P.68 possesses the fewest and P.70 the most.
A function has not been defined definitively for any of the pertactins, and the role of these proteins in virulence remains controversial despite over 15 years of investigation. All three forms of pertactin possess two RGD motifs of the type implicated in cell binding (33), one localized in the passenger domain and the other in the β-domain. However, a receptor has not been identified for pertactin. Furthermore, evidence of a role for pertactin as an adhesin, as judged by in vitro adhesion assays, is contradictory. Pertactin has been shown to confer bacterial adhesion to CHO and HeLa cells (38, 91, 92), yet no evidence was found for the contribution of pertactin in B. pertussis adhesion to either bronchial or laryngeal cells (148). Indeed, in one study pertactin was found to increase the level of Salmonella enterica serovar Typhimurium invasion when expressed heterologously in a weakly invasive strain, whereas the same heterologous construct in E. coli HB101 did not affect the level of invasion (although it did increase the level of adhesion). However, expression of the same construct in a bvg-negative strain of B. pertussis had no effect on the ability of this organism to adhere or invade (38). Pertactin was also found to increase the level of Staphylococcus aureus invasion in a manner that was inhibited by the presence of the pertactin RGD peptide. In contrast, pertactin was found to inhibit bacterial uptake in human tracheal epithelial cells (6). Investigation of the role of pertactin in B. brochiseptica pathogenesis revealed that pertactin was involved in the cytotoxicity of this organism for mononuclear phagocytic cells (47). The investigators suggested that the effect was due to the fact that pertactin promoted stable adhesion of the organism to the macrophages, though they acknowledged other possible explanations. Pertactin is produced at an intermediate time in vitro during B. pertussis growth, later than FHA and earlier than pertussis toxin (86), supporting the notion that in vivo FHA acts as an initial adhesin and pertactin promotes more intimate adhesion to mammalian cells at a subsequent step and prior to toxin release.
P.69 is the only autotransporter for which the crystal structure has been determined (resolution, 2.5 Å). The protein comprises a 16-stranded parallel β-helix with a V-shaped cross-section and is the largest β-helix known to date. Several between-strand weakly conserved amino acid repeats form internal and external ladders (35, 36). The structure appears as a helix from which several loops protrude; each helix contains sequence motifs associated with the biological activity of the protein. One particular (GGXXP)5 sequence is located directly after the RGD motif and may mediate interaction with epithelial cells (35). The C-terminal region of P.69 incorporates a (PQP)5 motif containing the major immunoprotective epitope (23). Given the level of homology to Ag43, AIDA-I, and TibA, in addition to the repetitive nature of these molecules, it is likely that these E. coli molecules possess a similar structure.
BrkA.
Not surprisingly, wild-type B. pertussis is relatively resistant to the classical pathway of complement-dependent killing by normal human serum compared with E. coli HB101. Such resistance in B. pertussis is bvg regulated. FHA has been reported to bind C4 binding protein (13), an inhibitor of complement, but this activity does not contribute to serum resistance, as evidenced by the resistant phenotype of FHA mutants. Further studies have found that neither pertactin, pertussis toxin, adenylate cyclase toxin, dermonecrotic toxin, tracheal colonization factor, nor Vag8 mutants were more sensitive to serum killing than the wild type (42). Fernandez and Weiss identified an insertional mutant of B. pertussis which was at least 10-fold more susceptible to serum killing than the wild type and was less virulent in mice (41). The locus encoding this serum resistance function encodes two divergently transcribed open reading frames, termed BrkA and BrkB. Both ORFs are necessary for serum resistance. Within the 300 bases which separate the two ORFs are putative sites for BvgA binding. BrkA shows 29% identity to pertactin and has two RGD motifs in addition to a conserved proteolytic processing site and an outer membrane-targeting signal. Like pertactin, BrkA is involved in adherence and invasion. Despite the similarities, a pertactin mutant was not as sensitive to serum killing as the BrkA or BrkB mutants. BrkB is similar to ORFs of unknown function in E. coli and Mycobacterium leprae and is predicted to be a cytoplasmic membrane protein (41).
In Southern blot analysis, brkAB sequences were found in B. bronchiseptica and B. parapertussis but not in Bordetella avium. Clinical isolates of B. bronchiseptica and B. parapertussis were serum resistant, and wild-type strains possessing additional copies of the brk locus were two- to fivefold more resistant to serum killing (42). Although BrkA confers resistance to killing by complement in B. pertussis, interestingly, loss of BrkA in B. bronchiseptica did not confer sensitivity to complement-mediated killing. This may be explained by the fact that B. bronchiseptica is inherently more resistant to complement than B. pertussis (128).
TcfA and Vag8.
TcfA is produced by strains of B. pertussis but not B. bronchiseptica or B. parapertussis. The predicted amino acid sequence of tcfA suggests a 68-kDa RGD-containing, proline-rich protein, whose β-domain displays 50% identity to the pertactin β-domain (46). The 34-kDa passenger domain contains the RGD motif implicated in integrin binding. Polyclonal antiserum raised against the unique N-terminal portion of TcfA recognizes 90- and 60-kDa bands in immunoblots of B. pertussis whole-cell lysates. However, culture supernatants of B. pertussis contain only the 60-kDa form, indicating that outer-membrane-associated and secreted versions of the passenger domain exist. When mice are challenged with a strain of B. pertussis lacking TcfA, the number of bacteria isolated from the tracheas is decreased 10-fold compared to that isolated from tracheas of mice challenged with the wild-type organism (46). The mechanism contributing to this difference has yet to be elucidated.
B. pertussis expresses a bvg-regulated 95-kDa protein called Vag8. In contrast to results for TcfA, Southern blot analysis indicates that strains of B. bronchiseptica and B. parapertussis carry vag8-homologous DNA, yet expression of the protein has not been detected in B. parapertussis (45). A Vag8-negative derivative of B. pertussis colonized mice as efficiently as the parent B. pertussis strain in a mouse aerosol model of pertussis. However, previous experiments to evaluate the effect of adhesin-defective B. pertussis strains in the aerosol model have shown conflicting results. Thus, authors Finn and Amsbaugh (45) suggest that B. pertussis expresses an array of virulence determinants, some of which may have similar functions such that the loss of one may be compensated for by the others.
MORAXELLA AUTOTRANSPORTERS
Moraxella catarrhalis, an unencapsulated, gram-negative bacterium, is an important pathogen of the upper respiratory tract in children and adults. Efforts to identify potential vaccine candidates among M. catarrhalis surface antigens have focused on outer membrane proteins, in particular the UspA antigen (ubiquitous surface protein A) (66). Initial investigations of UspA indicated it was a high-molecular-weight protein (>250 kDa) responsible for serum resistance and attachment to human epithelial cells. It was therefore surprising when detailed investigations by Hansen and coworkers revealed that this UspA antigen was in fact composed of two proteins, UspA1 and UspA2, with different characteristics (2, 27). In M. catarrhalis 035E, the uspA1 and uspA2 genes encode predicted proteins of 88 and 62 kDa, the native forms of which form oligomers or aggregates (2). The amino acid sequences of UspA1 and UspA2 are 43% identical, but each possesses an internal immunoreactive region displaying 93% identity (102). This region, which is present in all disease-associated isolates of M. catarrhalis tested to date, induces the synthesis of antibodies that enhance the elimination of M. catarrhalis in a mouse pulmonary model (66).
Although structurally related, UspA1 and UspA2 appear to mediate different biological functions. Antiserum directed against the original purified UspA inhibited attachment of M. catarrhalis to epithelial cells (D. Chen, J. McMichael, K. Vandermeid, D. Hahn, R. Smith, J. Eldridge, and J. Cowell, Abstr. 95th Gen. Meet. Am. Soc. Microbiol. 1995, abstr. E-53, p. 290, 1995). In contrast, Verduin et al. (150; C. M. Verduin, H. J. Bootsma, C. Hol, A. Fleer, M. Jansze, K. L. Klingman, T. F. Murphy, and H. Var Dijk, Abstr. 95th Gen. Meet. Am. Soc. Microbiol. 1995, abstr. B-137, p. 189, 1995) suggested that UspA was a serum resistance factor produced by disease isolates of M. catarrhalis. Both studies were published before it was known that UspA was composed of two separate proteins. To dissect these properties Aebi et al. (1) constructed a set of isogenic mutants that lacked the ability to express UspA1, UspA2, or both of these proteins. Investigations using these mutants revealed that both the uspA1 and uspA1 uspA2 mutants were significantly handicapped in their ability to adhere to cells in vitro compared to the wild type. In contrast, the uspA2 mutant was unaffected. When the set of three isogenic M. catarrhalis mutants was incubated in normal human serum, the uspA2 mutant and the uspA1 uspA2 mutant were both readily killed. In contrast, the uspA1 mutant resisted killing as effectively as the wild-type parent strain did (1). However, the available data do not reveal whether UspA2 exerts a direct or indirect effect on serum resistance of M. catarrhalis; this is particularly important in view of the fact that a previous study had shown that lack of expression of the CopB outer membrane protein resulted in loss of serum resistance by M. catarrhalis (67).
Interestingly, analysis of other M. catarrhalis strains led to the discovery of a second type of UspA2 protein (UspA2h) that is comprised of amino acid segments from both UspA1 and UspA2 (90). Investigations suggested that like UspA1, this second type of UspA2 protein acts as an adhesin when expressed in H. influenzae and that it provides no protection against serum killing. This is not surprising given that the greatest degree of conservation between UspA2 and UspA2h lies in the C-terminal β-barrel domains, whereas the greatest degree of conservation between UspA1 and UspA2h is found in the putative passenger or functional domain.
NONSERINE PROTEASE AUTOTRANSPORTERS OF HAEMOPHILUS
In addition to the Hap and IgA1 proteases, H. influenzae possesses two other autotransporters, Hsf and Hia (5, 143). These proteins share significant homology (72% identity), predominately at the N- and C-terminal ends. However, Hsf is larger than Hia (244 kDa versus 114 kDa), a feature mediated in part by the presence of a repetitive domain in Hsf which is only present as a single copy in Hia. Interestingly, in experiments with human epithelial cell lines, both Hia and Hsf were found to confer similar adhesive properties. This phenotypic similarity in conjunction with the amino acid similarity suggests that Hia and Hsf are alleles of the same locus (142). Expression of Hsf has been observed in H. influenzae type b and is associated with the production of surface appendages termed fibrils (143). Analysis of a collection of nontypeable H. influenzae demonstrated the presence of Hia in approximately 20% of strains. Expression of Hia was only detected in those strains which were deficient in expression of the high-molecular-weight proteins HMW1 and HMW2, proteins which were secreted by a mechanism analogous to that of FHA from B. pertussis (89). The importance of Hia as a virulence determinant was suggested by the uniform presence of Hia in nontypeable H. influenzae lacking HMW1- and HMW2-like proteins or hemagglutinating pili (142).
OTHER SERINE PROTEASE AUTOTRANSPORTERS
Aside from the trypsin-like serine proteases described above, the autotransporter family possesses serine proteases belonging to two other enzymatic families. Recently, a family of lipolytic autotransporter enzymes has been characterized which possess an active site defined by the residues GDSL, where the serine has been defined as the catalytic residue. The other group of autotransporter serine proteases belongs to an extensive family whose catalytic activity is provided by a charge relay system similar to that employed by the trypsin family but which apparently evolved convergently. The sequence surrounding the residues involved in the catalytic triad (D, S, and H residues) is different from the analogous residues in the trypsin-like serine proteases described above. Interestingly, this family of proteases is best characterized for Bacillus, and as a result its members are referred to as the subtilases.
Several autotransporter subtilases from different species have been identified, including PrtT and PrtS (both previously designated Ssp) (103). Ssp-H1 and Ssp-H2 from Serratia marcesens (110), PspA and PspB from Pseudomonas fluorescens (85), Ssa1 from Pasteurella haemolytica (60, 99), and the putative autotransporters BprV, BprB, AprV2, and BprX from Dichelobacter nodosus (96, 131, 149). Despite the identification of PrtT over 15 years ago, the subsequent identification of multiple homologs in different strains (110), and the extensive investigation on the secretion of this autotransporter (103, 111, 112, 138–140), little has been done to elucidate a role for this protein. PspA and PspB were only recently described and appear to be present on a chromosomal island with genes encoding the P. fluorescens lipase and alkaline protease. A role has not been demonstrated for either of these proteins (84). P. haemolytica strains have been grouped into 15 serotypes on the basis of standardized typing antisera raised in rabbits. In contrast to S. marcesens and P. fluorescens, P. haemolytica serotype 1 has been established as the primary agent responsible for acute lobar fibrinonecrotizing pleuropneumonia in cattle. Serotype 1 is rarely found in healthy cattle, but serotype 2 is cultured frequently from the upper respiratory tracts of healthy animals (60, 99). Interestingly, both serotypes possess the gene encoding serotype-specific antigen Ssa1 but P. haemolytica serotype 2 strains do not express serologically detectable levels of the Ssa1 protein. In addition, cattle showing resistance to pneumonic pasteurellosis have been shown to possess antibodies to Ssa1 which appear to be protective (153). Despite this promising early work on Ssa1, a distinct role for this autotransporter was never defined. D. nodosus is an obligate anaerobe and the causative agent of ovine foot rot (14). Virulent strains secrete at least three proteases into the extracellular milieu. Based on the levels of homology these putative serine protease autotransporters from D. nodosus appear to represent a different lineage of the subtilase family. The proteins have been implicated in tissue destruction, and hence virulence, by virtue of their ability to act as elastases in in vitro assays (14, 96, 131, 162).
Pseudomonas aeruginosa is a soil bacterium that is an important opportunistic pathogen of humans. Strains isolated from cystic fibrosis patients secrete a variety of proteins including lipase and two extracellular phopholipases which are involved in hydrolyzing long- and short-chain fatty acids and a variety of phosphoric monoester substrates. Recent investigations with lipase-negative mutants of P. aeruginosa identified residual lipolytic activity associated with the surface of the bacterium. This activity was attributed to a novel autotransporter esterase, EstA, which is a member of the GDSL family of serine proteases (155). Such lipases are important in the pathogenesis of cystic fibrosis, mediating complete hydrolysis of the major lung surfactant lipid, dipalmitoylphosphatidylcholine (155). Moreover, such enzymes have been shown to induce the release of the inflammatory mediator 12-hydroxyeicosatetraenoic acid from human platelets (87, 88). However, a specific substrate for EstA, and its relevance to pathogenicity, have yet to be defined in detail. Similar enzymes ApeE and PlaA, have been identified in Salmonella serovar Typhimurium (19, 26) and the insect pathogen Xenorhabdus luminescens (152), respectively. The data presented in the initial descriptions of these enzymes provide few clues to the physiological function of these proteins. They are not required for growth, although it is conceivable that these enzymes are required for the catabolic breakdown of fatty acid esters.
RICKETTSIAL AUTOTRANSPORTERS
The Rickettsiales are fastidious bacteria which form a distinct monophylectic group within the α-Proteobacteriaceae. Based on a variety of clinical and molecular factors Rickettsiales is normally divided into the typhus and the spotted fever groups. Two homologous autotransporter proteins, rOmpA and rOmpB, have been identified among the Rickettsiales (25, 29, 52, 54, 55). The rOmpB proteins appear to form an S layer on the surface of the bacteria (20, 62, 63), whereas a surface structure for rOmpA has not been described. No function has been clearly assigned to these proteins. The sequence variations in the rOmpA proteins have been used extensively in an effort to determine the evolutionary status of the spotted fever group of Rickettsiales (49, 132, 154), however, such analysis may be misleading, since rOmpA exhibits a mosaic structure (53). Nevertheless, rOmpA has been proposed to act as a mediator of adhesion and intracellular motility. In the latter respect, Rickettsia spp. have been shown to recruit actin and move through the cellular cytoplasm in a manner similar to that described for Shigella (see IcsA above) (61). Indeed, a common rOmpA-IcsA domain has been described, although mutagenesis of this domain had no apparent affect on actin-mediated motility (24). Furthermore, given the remarkable repetitive structure of the protein, in addition to the homology displayed with other known adhesins, a role as an adhesion factor seems more likely. Moreover, Li and Walker (93) have demonstrated that recombinant rOmpA protein inhibited rickettsial adhesion to host cells in a dose-dependent fashion. Similarly, antibodies directed to rOmpA competitively inhibited rickettsial attachment to eukaryotic cells. Alternatively, rOmpA may play a role in the induction of phagocytosis of Rickettsia spp.
The rOmpB protein is the most abundant surface protein on Rickettsia spp. anti-rOmpB antibodies protect mice against otherwise lethal rickettsial challenge. Ultrastructural analysis of the outer membrane reveals that rOmpB is responsible for the presence of a regularly arrayed surface structure, or S layer (20). Although information about the physical and chemical structure and the mode of assembly of S layers has been accumulating, the functions of the S layers remain unclear. As S layers exist as an interface between the cell and its environment, they have been purported to function as protective coats, molecular sieves, and ion traps and to be instrumental in cell adhesion (25, 135, 141).
HELICOBACTER AUTOTRANSPORTERS
Helicobacter pylori is a gram-negative human pathogen which colonizes the human stomach in childhood and persists within the gastric mucus layer (104). Epidemiological investigations reveal a strong association between H. pylori disease and the ability of the bacterium to produce the vacuolating cytotoxin (VacA), the cytotoxin associated antigen (CagA), and the blood-group antigen binding adhesin (BabA) (43, 51). Interestingly, two of these proteins, VacA and BabA, are autotransporters.
VacA.
VacA is a major virulence factor of H. pylori; several recent reviews are available on this well-studied toxin (4, 28, 44, 101, 120, 130). VacA is produced as a 140-kDa precursor, which is processed to a 95-kDa mature protein upon secretion into the extracellular milieu; here, it undergoes further processing into 37- and 58-kDa fragments that remain associated through noncovalent interaction. Acting at an intracellular site, mature VacA induces vacuolization of target cells by a mechanism that is still not completely understood. Transfection of recombinant VacA toxin into the cytosol of cultured cells has shown that the toxic domain resides within the 37-kDa polypeptide.
BabA.
Recent studies have identified H. pylori adherence factors that mediate its tropism for the gastric epithelium. H. pylori bound to gastric surface mucous cells expressing the Lewisb (α-1,3/4-difucosylated) blood group antigen and colonized stomachs of transgenic mice expressing the Lewisb antigen in gastric epithelial cells. A 78-kDa H. pylori outer membrane protein adhesin termed BabA was identified by cross-linking to the Lewisb antigen (81). Moreover, additional investigations demonstrated that all members of a subset of strains expressing BabA bound to Lewisb-coated microtiter dishes, whereas those organisms which did not express the protein did not adhere (51).
The BabA sequence features a repeat motif which undergoes frequent deletion, leading to phase variation. Furthermore, babA belongs to a family of approximately 30 closely related H. pylori autotransporter genes, suggesting possibilities for recombination. Such recombination may allow adhesin synthesis to be switched on and off or to undergo antigenic variation. The greatest sequence divergence within this family appears in the passenger domains of these proteins in a manner reminiscent of the highly homologous SPATE proteins described above (51, 81).
Epidemiological studies suggest that BabA is a virulence factor of H. pylori. BabA was found to be highly associated with gastric ulcers. Further investigations revealed that the simultaneous expression of CagA, VacA, and BabA among clinical isolates of diverse origin yields a highly significant association with ulcer disease and distal gastric adenocarcinoma (51).
Hsr.
Helicobacter mustelae is a spiral microaerophilic bacterium linked to gastritis and gastric ulcers in ferrets. The cell surface of H. mustelae differs markedly from other members of the genus Helicobacter by exhibiting a laterally extensive array of 8.5-nm-diameter rings. Genotypic, phenotypic, and immunological analyses of H. mustelae reveal that Hsr, a 150-kDa protein associated with the outer membrane of the organism, is responsible for the formation of these ring structures (113). Such analyses also reveal that Hsr is a member of the autotransporter family, is not cleaved into separate passenger and β-domains, and remains anchored in the outer membrane as a single proteinaceous molecule. Cross-reactive proteins were identified in three H. mustelae strains, but not in H. pylori or Helicobacter felis (113). Hsr surface arrays are reminiscent of S layers described for many species of bacteria and bearing functional homology to the autotransporters of Rickettsia spp. Indeed, rOmpA and rOmpB display the greatest homology with the passenger domain of Hsr. In contrast, the C-terminal autotransporter domain displays the closest homology with the SPATEs described above (Henderson and Nataro, unpublished). It is possible that the presence of Hsr contributes to H. mustelae colonization by conferring serum resistance, antiphagocytic activity, or antigenic variation, roles which have been described for other S-layer proteins.
CONCLUSIONS
The autotransporter, or type V, secretion system is a dedicated protein translocation mechanism which allows the organism to secrete proteins to and beyond the bacterial surface. The characterization of the IgA1 protease secretion mechanism commenced the study of this now rapidly expanding family of proteins and it is likely that future investigations will reveal an even more complex array of proteins secreted in this fashion. The simplicity of the secretion mechanism and the ability to develop a new autotransporter protein simply by a single recombination event have presented bacteria with abundant opportunities to increase the efficiency of secretion of proteins that were probably already developed as periplasmic or exported virulence factors. In addition, phylogenetic data suggest that several autotransporter-secreted proteins were embued by evolution with new or expanded functions, often upon introduction into new pathogens occupying different niches (Henderson and Nataro, unpublished).
In addition to the autotransporter proteins listed above, several others have been described, e.g., MisL of Salmonella serovar Typhimurium (15), but are not yet characterized. Indeed, examination of the sequence databases reveals a plethora of new autotransporter candidates that await postgenomic studies (Henderson and Nataro, unpublished). Moreover, we feel that reexamination of already identified proteins is likely to expand the size and perhaps the definition of the autotransporter family. For example, Ssa1 of P. haemolytica possesses the characteristics of autotransporters but has not previously been recognized as a member of this family. A great deal of research is required to understand both the roles of these proteins and the fundamental features of autotransporter secretion and processing.
ACKNOWLEDGMENTS
Work in the lab of J.P.N. is supported by U. S. Public Health Service grants AI33096 and AI43615. Work in the lab of I.R.H. is supported by the Royal Society grant 21784/SM.
We thank Harry Mobley for his helpful comments concerning the manuscript and Brian Long and Derek Hoelz for their helpful discussions about autotransporter proteins. We also extend our gratitude to Pinaki Dutta and Renato Cappello for aid in generating figures used in the paper.
REFERENCES
- 1.Aebi C, Lafontaine E R, Cope L D, Latimer J L, Lumbley S L, McCracken G H, Jr, Hansen E J. Phenotypic effect of isogenic uspA1 and uspA2 mutations on Moraxella catarrhalis 035E. Infect Immun. 1998;66:3113–3119. doi: 10.1128/iai.66.7.3113-3119.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aebi C, Maciver I, Latimer J L, Cope L D, Stevens M K, Thomas S E, McCracken G H, Jr, Hansen E J. A protective epitope of Moraxella catarrhalis is encoded by two different genes. Infect Immun. 1997;65:4367–4377. doi: 10.1128/iai.65.11.4367-4377.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Hasani K, Henderson I, Sakellaris H, Rajakumar K, Grant T, Nataro J, Robins-Browne R, Adler B. The sigA gene borne on the she pathogenicity island of Shigella flexneri 2a encodes an exported cytopathic protease involved in intestinal fluid accumulation. Infect Immun. 2000;68:2457–2463. doi: 10.1128/iai.68.5.2457-2463.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atherton J C. H. pylori virulence factors. Br Med Bull. 1998;54:105–120. doi: 10.1093/oxfordjournals.bmb.a011662. [DOI] [PubMed] [Google Scholar]
- 5.Barenkamp S J, St. Geme J W., III Identification of a second family of high-molecular-weight adhesion proteins expressed by non-typeable Haemophilus influenzae. Mol Microbiol. 1996;19:1215–1223. doi: 10.1111/j.1365-2958.1996.tb02467.x. [DOI] [PubMed] [Google Scholar]
- 6.Bassinet L, Gueirard P, Maitre B, Housset B, Gounon P, Guiso N. Role of adhesins and toxins in invasion of human tracheal epithelial cells by Bordetella pertussis. Infect Immun. 2000;68:1934–1941. doi: 10.1128/iai.68.4.1934-1941.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benjelloun-Touimi Z, Sansonetti P J, Parsot C. SepA, the major extracellular protein of Shigella flexneri: autonomous secretion and involvement in tissue invasion. Mol Microbiol. 1995;17:123–135. doi: 10.1111/j.1365-2958.1995.mmi_17010123.x. [DOI] [PubMed] [Google Scholar]
- 8.Benz I, Schmidt M A. AIDA-I, the adhesin involved in diffuse adherence of the diarrhoeagenic Escherichia coli strain 2787 (O126:H27), is synthesized via a precursor molecule. Mol Microbiol. 1992;6:1539–1546. doi: 10.1111/j.1365-2958.1992.tb00875.x. [DOI] [PubMed] [Google Scholar]
- 9.Benz I, Schmidt M A. Cloning and expression of an adhesin (AIDA-I) involved in diffuse adherence of enteropathogenic Escherichia coli. Infect Immun. 1989;57:1506–1511. doi: 10.1128/iai.57.5.1506-1511.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benz I, Schmidt M A. Diffuse adherence of enteropathogenic Escherichia coli strains. Res Microbiol. 1990;141:785–786. doi: 10.1016/0923-2508(90)90111-3. [DOI] [PubMed] [Google Scholar]
- 11.Benz I, Schmidt M A. Diffuse adherence of enteropathogenic Escherichia coli strains—processing of AIDA-I. Int J Med Microbiol Virol Parasitol Infect Dis. 1993;278:197–208. doi: 10.1016/s0934-8840(11)80837-6. [DOI] [PubMed] [Google Scholar]
- 12.Benz I, Schmidt M A. Isolation and serologic characterization of AIDA-I, the adhesin mediating the diffuse adherence phenotype of the diarrhea-associated Escherichia coli strain 2787 (O126:H27) Infect Immun. 1992;60:13–18. doi: 10.1128/iai.60.1.13-18.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berggard K, Johnsson E, Mooi F R, Lindahl G. Bordetella pertussis binds the human complement regulator C4BP: role of filamentous hemagglutinin. Infect Immun. 1997;65:3638–3643. doi: 10.1128/iai.65.9.3638-3643.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Billington S J, Johnston J L, Rood J I. Virulence regions and virulence factors of the ovine foot rot pathogen, Dichelobacter nodosus. FEMS Microbiol Lett. 1996;145:147–156. doi: 10.1111/j.1574-6968.1996.tb08570.x. [DOI] [PubMed] [Google Scholar]
- 15.Blanc-Potard A B, Solomon F, Kayser J, Groisman E A. The SPI-3 pathogenicity island of Salmonella enterica. J Bacteriol. 1999;181:998–1004. doi: 10.1128/jb.181.3.998-1004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brunder W, Schmidt H, Karch H. EspP, a novel extracellular serine protease of enterohaemorrhagic Escherichia coli O157:H7 cleaves human coagulation factor V. Mol Microbiol. 1997;24:767–778. doi: 10.1046/j.1365-2958.1997.3871751.x. [DOI] [PubMed] [Google Scholar]
- 17.Burns D L. Biochemistry of type IV secretion. Curr Opin Microbiol. 1999;2:25–29. doi: 10.1016/s1369-5274(99)80004-6. [DOI] [PubMed] [Google Scholar]
- 18.Caffrey P, Owen P. Purification and N-terminal sequence of the alpha subunit of antigen 43, a unique protein complex associated with the outer membrane of Escherichia coli. J Bacteriol. 1989;171:3634–3640. doi: 10.1128/jb.171.7.3634-3640.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carinato M E, Collin-Osdoby P, Yang X, Knox T M, Conlin C A, Miller C G. The apeE gene of Salmonella typhimurium encodes an outer membrane esterase not present in Escherichia coli. J Bacteriol. 1998;180:3517–3521. doi: 10.1128/jb.180.14.3517-3521.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carl M, Dobson M E, Ching W M, Dasch G A. Characterization of the gene encoding the protective paracrystalline-surface-layer protein of Rickettsia prowazekii: presence of a truncated identical homolog in Rickettsia typhi. Proc Natl Acad Sci USA. 1990;87:8237–8241. doi: 10.1073/pnas.87.21.8237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charles I, Fairweather N, Pickard D, Beesley J, Anderson R, Dougan G, Roberts M. Expression of the Bordetella pertussis P.69 pertactin adhesin in Escherichia coli: fate of the carboxy-terminal domain. Microbiology. 1994;140:3301–3308. doi: 10.1099/13500872-140-12-3301. [DOI] [PubMed] [Google Scholar]
- 22.Charles I G, Dougan G, Pickard D, Chatfield S, Smith M, Novotny P, Morrissey P, Fairweather N F. Molecular cloning and characterization of protective outer membrane protein P.69 from Bordetella pertussis. Proc Natl Acad Sci USA. 1989;86:3554–3558. doi: 10.1073/pnas.86.10.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charles I G, Li J L, Roberts M, Beesley K, Romanos M, Pickard D J, Francis M, Campbell D, Dougan G, Brennan M J, et al. Identification and characterization of a protective immunodominant B cell epitope of pertactin (P.69) from Bordetella pertussis. Eur J Immunol. 1991;21:1147–1153. doi: 10.1002/eji.1830210509. [DOI] [PubMed] [Google Scholar]
- 24.Charles M, Magdalena J, Theriot J A, Goldberg M B. Functional analysis of a rickettsial OmpA homology domain of Shigella flexneri IcsA. J Bacteriol. 1999;181:869–878. doi: 10.1128/jb.181.3.869-878.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ching W M, Dasch G A, Carl M, Dobson M E. Structural analyses of the 120-kDa serotype protein antigens of typhus group rickettsiae. Comparison with other S-layer proteins. Ann NY Acad Sci. 1990;590:334–351. doi: 10.1111/j.1749-6632.1990.tb42241.x. [DOI] [PubMed] [Google Scholar]
- 26.Collin-Osdoby P, Miller C G. Mutations affecting a regulated, membrane-associated esterase in Salmonella typhimurium LT2. Mol Gen Genet. 1994;243:674–680. doi: 10.1007/BF00279577. [DOI] [PubMed] [Google Scholar]
- 27.Cope L D, Lafontaine E R, Slaughter C A, Hasemann C A, Jr, Aebi C, Henderson F W, McCracken G H, Jr, Hansen E J. Characterization of the Moraxella catarrhalis uspA1 and uspA2 genes and their encoded products. J Bacteriol. 1999;181:4026–4034. doi: 10.1128/jb.181.13.4026-4034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cover T L. The vacuolating cytotoxin of Helicobacter pylori. Mol Microbiol. 1996;20:241–246. doi: 10.1111/j.1365-2958.1996.tb02612.x. [DOI] [PubMed] [Google Scholar]
- 29.Crocquet-Valdes P A, Weiss K, Walker D H. Sequence analysis of the 190-kDa antigen-encoding gene of Rickettsia conorii (Malish 7 strain) Gene. 1994;140:115–119. doi: 10.1016/0378-1119(94)90740-4. [DOI] [PubMed] [Google Scholar]
- 30.Czeczulin J R, Whittam T S, Henderson I R, Nataro J P. Phylogenetic analysis of enteroaggregative and diffusely adherent Escherichia coli. Infect Immun. 1999;67:2692–2699. doi: 10.1128/iai.67.6.2692-2699.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhermy D. The spectrin super-family. Biol Cell. 1991;71:249–254. [PubMed] [Google Scholar]
- 32.Djafari S, Ebel F, Deibel C, Kramer S, Hudel M, Chakraborty T. Characterization of an exported protease from Shiga toxin-producing Escherichia coli. Mol Microbiol. 1997;25:771–784. doi: 10.1046/j.1365-2958.1997.5141874.x. [DOI] [PubMed] [Google Scholar]
- 33.D'Souza S E, Ginsberg M H, Burke T A, Lam S C, Plow E F. Localization of an Arg-Gly-Asp recognition site within an integrin adhesion receptor. Science. 1988;242:91–93. doi: 10.1126/science.3262922. [DOI] [PubMed] [Google Scholar]
- 34.Elsinghorst E A, Weitz J A. Epithelial cell invasion and adherence directed by the enterotoxigenic Escherichia coli tib locus is associated with a 104-kilodalton outer membrane protein. Infect Immun. 1994;62:3463–3471. doi: 10.1128/iai.62.8.3463-3471.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Emsley P, Charles I G, Fairweather N F, Isaacs N W. Structure of Bordetella pertussis virulence factor P.69 pertactin. Nature. 1996;381:90–92. doi: 10.1038/381090a0. [DOI] [PubMed] [Google Scholar]
- 36.Emsley P, McDermott G, Charles I G, Fairweather N F, Isaacs N W. Crystallographic characterization of pertactin, a membrane-associated protein from Bordetella pertussis. J Mol Biol. 1994;235:772–773. doi: 10.1006/jmbi.1994.1029. [DOI] [PubMed] [Google Scholar]
- 37.Eslava C, Navarro-Garcia F, Czeczulin J R, Henderson I R, Cravioto A, Nataro J P. Pet, an autotransporter enterotoxin from enteroaggregative Escherichia coli. Infect Immun. 1998;66:3155–3163. doi: 10.1128/iai.66.7.3155-3163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Everest P, Li J, Douce G, Charles I, De Azavedo J, Chatfield S, Dougan G, Roberts M. Role of the Bordetella pertussis P. 69/pertactin protein and the P. 69/pertactin RGD motif in the adherence to and invasion of mammalian cells. Microbiology. 1996;142:3261–3268. doi: 10.1099/13500872-142-11-3261. [DOI] [PubMed] [Google Scholar]
- 39.Fasano A, Noriega F R, Liao F M, Wang W, Levine M M. Effect of shigella enterotoxin 1 (ShET1) on rabbit intestine in vitro and in vivo. Gut. 1997;40:505–511. doi: 10.1136/gut.40.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fath M J, Kolter R. ABC transporters: bacterial exporters. Microbiol Rev. 1993;57:995–1017. doi: 10.1128/mr.57.4.995-1017.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernandez R C, Weiss A A. Cloning and sequencing of a Bordetella pertussis serum resistance locus. Infect Immun. 1994;62:4727–4738. doi: 10.1128/iai.62.11.4727-4738.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernandez R C, Weiss A A. Serum resistance in bvg-regulated mutants of Bordetella pertussis. FEMS Microbiol Lett. 1998;163:57–63. doi: 10.1111/j.1574-6968.1998.tb13026.x. [DOI] [PubMed] [Google Scholar]
- 43.Figura N. Helicobacter pylori factors involved in the development of gastroduodenal mucosal damage and ulceration. J Clin Gastroenterol. 1997;25:S149–S163. doi: 10.1097/00004836-199700001-00025. [DOI] [PubMed] [Google Scholar]
- 44.Figura N, Valassina M. Helicobacter pylori determinants of pathogenicity. J Chemother. 1999;11:591–600. doi: 10.1179/joc.1999.11.6.591. [DOI] [PubMed] [Google Scholar]
- 45.Finn T M, Amsbaugh D F. Vag8, a Bordetella pertussis bvg-regulated protein. Infect Immun. 1998;66:3985–3989. doi: 10.1128/iai.66.8.3985-3989.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Finn T M, Stevens L A. Tracheal colonization factor: a Bordetella pertussis secreted virulence determinant. Mol Microbiol. 1995;16:625–634. doi: 10.1111/j.1365-2958.1995.tb02425.x. [DOI] [PubMed] [Google Scholar]
- 47.Fleckenstein J M, Kopecko D J, Warren R L, Elsinghorst E A. Molecular characterization of the tia invasion locus from enterotoxigenic Escherichia coli. Infect Immun. 1996;64:2256–2265. doi: 10.1128/iai.64.6.2256-2265.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Forde C B, Shi X, Li J, Roberts M. Bordetella bronchiseptica-mediated cytotoxicity to macrophages is dependent on bvg-regulated factors, including pertactin. Infect Immun. 1999;67:5972–5978. doi: 10.1128/iai.67.11.5972-5978.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fournier P E, Roux V, Raoult D. Phylogenetic analysis of spotted fever group rickettsiae by study of the outer surface protein rOmpA. Int J Syst Bacteriol. 1998;48:839–849. doi: 10.1099/00207713-48-3-839. [DOI] [PubMed] [Google Scholar]
- 50.Galan J E, Collmer A. Type III secretion machines: bacterial devices for protein delivery into host cells. Science. 1999;284:1322–1328. doi: 10.1126/science.284.5418.1322. [DOI] [PubMed] [Google Scholar]
- 51.Gerhard M, Lehn N, Neumayer N, Boren T, Rad R, Schepp W, Miehlke S, Classen M, Prinz C. Clinical relevance of the Helicobacter pylori gene for blood-group antigen-binding adhesin. Proc Natl Acad Sci USA. 1999;96:12778–12783. doi: 10.1073/pnas.96.22.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gilmore R D., Jr Characterization of the 120-kDa surface protein gene of Rickettsia rickettsii (R strain) Ann NY Acad Sci. 1990;590:459–467. doi: 10.1111/j.1749-6632.1990.tb42254.x. [DOI] [PubMed] [Google Scholar]
- 53.Gilmore R D., Jr Comparison of the rompA gene repeat regions of Rickettsiae reveals species-specific arrangements of individual repeating units. Gene. 1993;125:97–102. doi: 10.1016/0378-1119(93)90752-o. [DOI] [PubMed] [Google Scholar]
- 54.Gilmore R D, Jr, Cieplak W, Jr, Policastro P F, Hackstadt T. The 120 kilodalton outer membrane protein (rOmp B) of Rickettsia rickettsii is encoded by an unusually long open reading frame: evidence for protein processing from a large precursor. Mol Microbiol. 1991;5:2361–2370. doi: 10.1111/j.1365-2958.1991.tb02082.x. [DOI] [PubMed] [Google Scholar]
- 55.Gilmore R D, Jr, Joste N, McDonald G A. Cloning, expression and sequence analysis of the gene encoding the 120 kD surface-exposed protein of Rickettsia rickettsii. Mol Microbiol. 1989;3:1579–1586. doi: 10.1111/j.1365-2958.1989.tb00143.x. [DOI] [PubMed] [Google Scholar]
- 56.Goldberg M B. Cleavage of the Shigella surface protein IcsA (VirG): intention or accident? Trends Microbiol. 1994;2:35–37. doi: 10.1016/0966-842x(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 57.Goldberg M B, Barzu O, Parsot C, Sansonetti P J. Unipolar localization and ATPase activity of IcsA, a Shigella flexneri protein involved in intracellular movement. Infect Agents Dis. 1993;2:210–211. [PubMed] [Google Scholar]
- 58.Goldberg M B, Sansonetti P J. Shigella subversion of the cellular cytoskeleton: a strategy for epithelial colonization. Infect Immun. 1993;61:4941–4946. doi: 10.1128/iai.61.12.4941-4946.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goldberg M B, Theriot J A. Shigella flexneri surface protein IcsA is sufficient to direct actin-based motility. Proc Natl Acad Sci USA. 1995;92:6572–6576. doi: 10.1073/pnas.92.14.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gonzalez C T, Maheswaran S K, Murtaugh M P. Pasteurella haemolytica serotype 2 contains the gene for a noncapsular serotype 1-specific antigen. Infect Immun. 1995;63:1340–1348. doi: 10.1128/iai.63.4.1340-1348.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gouin E, Gantelet H, Egile C, Lasa I, Ohayon H, Villiers V, Gounon P, Sansonetti P J, Cossart P. A comparative study of the actin-based motilities of the pathogenic bacteria Listeria monocytogenes, Shigella flexneri and Rickettsia conorii. J Cell Sci. 1999;112:1697–1708. doi: 10.1242/jcs.112.11.1697. [DOI] [PubMed] [Google Scholar]
- 61a.Guyer D M, Henderson I R, Nataro J P, Mobley H L. Identification of SAT, an autotransporter toxin produced by uropathogenic Escherichia coli. Mol Microbiol. 2000;38:53–66. doi: 10.1046/j.1365-2958.2000.02110.x. [DOI] [PubMed] [Google Scholar]
- 62.Hahn M J, Chang W H. Expression and purification of the crystalline surface layer protein of Rickettsia typhi. Microbiol Immunol. 1996;40:233–236. doi: 10.1111/j.1348-0421.1996.tb03339.x. [DOI] [PubMed] [Google Scholar]
- 63.Hahn M J, Kim K K, Kim I, Chang W H. Cloning and sequence analysis of the gene encoding the crystalline surface layer protein of Rickettsia typhi. Gene. 1993;133:129–133. doi: 10.1016/0378-1119(93)90237-w. [DOI] [PubMed] [Google Scholar]
- 64.Hauck C R, Meyer T F. The lysosomal/phagosomal membrane protein h-lamp-1 is a target of the IgA1 protease of Neisseria gonorrhoeae. FEBS Lett. 1997;405:86–90. doi: 10.1016/s0014-5793(97)00163-4. [DOI] [PubMed] [Google Scholar]
- 65.Hedges S R, Mayo M S, Kallman L, Mestecky J, Hook III E W, Russell M W. Evaluation of immunoglobulin A1 (IgA1) protease and IgA1 protease-inhibitory activity in human female genital infection with Neisseria gonorrhoeae. Infect Immun. 1998;66:5826–5832. doi: 10.1128/iai.66.12.5826-5832.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Helminen M E, Maciver I, Latimer J L, Klesney-Tait J, Cope L D, Paris M, McCracken G H, Jr, Hansen E J. A large, antigenically conserved protein on the surface of Moraxella catarrhalis is a target for protective antibodies. J Infect Dis. 1994;170:867–872. doi: 10.1093/infdis/170.4.867. [DOI] [PubMed] [Google Scholar]
- 67.Helminen M E, Maciver I, Paris M, Latimer J L, Lumbley S L, Cope L D, McCracken G H, Jr, Hansen E J. A mutation affecting expression of a major outer membrane protein of Moraxella catarrhalis alters serum resistance and survival in vivo. J Infect Dis. 1993;168:1194–1201. doi: 10.1093/infdis/168.5.1194. [DOI] [PubMed] [Google Scholar]
- 68.Henderson I, Nataro J, Kaper J, Meyer T, Farrand S, Burns D, Finlay B, St. Geme J., III Redefining the protein secretion nomenclature. Trends Microbiol. 2000;8:352. [PubMed] [Google Scholar]
- 69.Henderson I R, Czeczulin J, Eslava C, Noriega F, Nataro J P. Characterization of Pic, a secreted protease of Shigella flexneri and enteroaggregative Escherichia coli. Infect Immun. 1999;67:5587–5596. doi: 10.1128/iai.67.11.5587-5596.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Henderson I R, Hicks S, Navarro-Garcia F, Elias W P, Philips A D, Nataro J P. Involvement of the enteroaggregative Escherichia coli plasmid-encoded toxin in causing human intestinal damage. Infect Immun. 1999;67:5338–5344. doi: 10.1128/iai.67.10.5338-5344.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Henderson I R, Meehan M, Owen P. Antigen 43, a phase-variable bipartite outer membrane protein, determines colony morphology and autoaggregation in Escherichia coli K- 12. FEMS Microbiol Lett. 1997;149:115–120. doi: 10.1111/j.1574-6968.1997.tb10317.x. [DOI] [PubMed] [Google Scholar]
- 72.Henderson I R, Mechan M, Owen P. A novel regulatory mechanism for a novel phase-variable outer membrane protein of Escherichia coli. Adv Exp Med Biol. 1997;412:349–355. doi: 10.1007/978-1-4899-1828-4_56. [DOI] [PubMed] [Google Scholar]
- 73.Henderson I R, Navarro-Garcia F, Nataro J P. The great escape: structure and function of the autotransporter proteins. Trends Microbiol. 1998;6:370–378. doi: 10.1016/s0966-842x(98)01318-3. [DOI] [PubMed] [Google Scholar]
- 74.Henderson I R, Owen P. The major phase-variable outer membrane protein of Escherichia coli structurally resembles the immunoglobulin A1 protease class of exported protein and is regulated by a novel mechanism involving Dam and OxyR. J Bacteriol. 1999;181:2132–2141. doi: 10.1128/jb.181.7.2132-2141.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hendrixson D R, de la Morena M L, Stathopoulos C, St Geme J W., III Structural determinants of processing and secretion of the Haemophilus influenzae Hap protein. Mol Microbiol. 1997;26:505–518. doi: 10.1046/j.1365-2958.1997.5921965.x. [DOI] [PubMed] [Google Scholar]
- 76.Hendrixson D R, St Geme J W., III The Haemophilus influenzae Hap serine protease promotes adherence and microcolony formation, potentiated by a soluble host protein. Mol Cell. 1998;2:841–850. doi: 10.1016/s1097-2765(00)80298-1. [DOI] [PubMed] [Google Scholar]
- 77.Hicks S, Candy D C, Phillips A D. Adhesion of enteroaggregative Escherichia coli to formalin-fixed intestinal and ureteric epithelia from children. J Med Microbiol. 1996;44:362–371. doi: 10.1099/00222615-44-5-362. [DOI] [PubMed] [Google Scholar]
- 78.Hicks S, Candy D C, Phillips A D. Adhesion of enteroaggregative Escherichia coli to pediatric intestinal mucosa in vitro. Infect Immun. 1996;64:4751–4760. doi: 10.1128/iai.64.11.4751-4760.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hopper S, Vasquez B, Merz A, Clary S, Wilbur J S, So M. Effects of the immunoglobulin A1 protease on Neisseria gonorrhoeae trafficking across polarized T84 epithelial monolayers. Infect Immun. 2000;68:906–911. doi: 10.1128/iai.68.2.906-911.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hueck C J. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev. 1998;62:379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ilver D, Arnqvist A, Ogren J, Frick I M, Kersulyte D, Incecik E T, Berg D E, Covacci A, Engstrand L, Boren T. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science. 1998;279:373–377. doi: 10.1126/science.279.5349.373. [DOI] [PubMed] [Google Scholar]
- 82.Johannsen D B, Johnston D M, Koymen H O, Cohen M S, Cannon J G. A Neisseria gonorrhoeae immunoglobulin A1 protease mutant is infectious in the human challenge model of urethral infection. Infect Immun. 1999;67:3009–3013. doi: 10.1128/iai.67.6.3009-3013.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jose J, Wolk U, Lorenzen D, Wenschuh H, Meyer T F. Human T-cell response to meningococcal immunoglobulin A1 protease associated alpha-proteins. Scand J Immunol. 2000;51:176–185. doi: 10.1046/j.1365-3083.2000.00670.x. [DOI] [PubMed] [Google Scholar]
- 84.Kawai E, Idei A, Kumura H, Shimazaki K, Akatsuka H, Omori K. The ABC-exporter genes involved in the lipase secretion are clustered with the genes for lipase, alkaline protease, and serine protease homologues in Pseudomonas fluorescens no. 33. Biochim Biophys Acta. 1999;1446:377–382. doi: 10.1016/s0167-4781(99)00094-9. [DOI] [PubMed] [Google Scholar]
- 85.Kilian M, Reinholdt J, Lomholt H, Poulsen K, Frandsen E V. Biological significance of IgA1 proteases in bacterial colonization and pathogenesis: critical evaluation of experimental evidence. APMIS. 1996;104:321–338. doi: 10.1111/j.1699-0463.1996.tb00724.x. [DOI] [PubMed] [Google Scholar]
- 86.Kinnear S M, Boucher P E, Stibitz S, Carbonetti N H. Analysis of BvgA activation of the pertactin gene promoter in Bordetella pertussis. J Bacteriol. 1999;181:5234–5241. doi: 10.1128/jb.181.17.5234-5241.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Konig B, Jaeger K E, Konig W. Induction of inflammatory mediator release (12-hydroxyeicosatetraenoic acid) from human platelets by Pseudomonas aeruginosa. Int Arch Allergy Immunol. 1994;104:33–41. doi: 10.1159/000236706. [DOI] [PubMed] [Google Scholar]
- 88.König B, Jaeger K-E, Sage A E, Vasil M L, König W. Role of Pseudomonas aeruginosa lipase in inflammatory mediator release from human inflammatory effector cells (platelets, granulocytes, and monocytes) Infect Immun. 1996;64:3252–3258. doi: 10.1128/iai.64.8.3252-3258.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Krasan G P, Cutter D, Block S L, St Geme J W., III Adhesin expression in matched nasopharyngeal and middle ear isolates of nontypeable Haemophilus influenzae from children with acute otitis media. Infect Immun. 1999;67:449–454. doi: 10.1128/iai.67.1.449-454.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lafontaine E R, Cope L D, Aebi C, Latimer J L, McCracken G H, Jr, Hansen E J. The UspA1 protein and a second type of UspA2 protein mediate adherence of Moraxella catarrhalis to human epithelial cells in vitro. J Bacteriol. 2000;182:1364–1373. doi: 10.1128/jb.182.5.1364-1373.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leininger E, Ewanowich C A, Bhargava A, Peppler M S, Kenimer J G, Brennan M J. Comparative roles of the Arg-Gly-Asp sequence present in the Bordetella pertussis adhesins pertactin and filamentous hemagglutinin. Infect Immun. 1992;60:2380–2385. doi: 10.1128/iai.60.6.2380-2385.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Leininger E, Roberts M, Kenimer J G, Charles I G, Fairweather N, Novotny P, Brennan M J. Pertactin, an Arg-Gly-Asp-containing Bordetella pertussis surface protein that promotes adherence of mammalian cells. Proc Natl Acad Sci USA. 1991;88:345–349. doi: 10.1073/pnas.88.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li H, Walker D H. rOmpA is a critical protein for the adhesion of Rickettsia rickettsii to host cells. Microb Pathog. 1998;24:289–298. doi: 10.1006/mpat.1997.0197. [DOI] [PubMed] [Google Scholar]
- 94.Li J, Fairweather N F, Novotny P, Dougan G, Charles I G. Cloning, nucleotide sequence and heterologous expression of the protective outer-membrane protein P.68 pertactin from Bordetella bronchiseptica. J Gen Microbiol. 1992;138:1697–1705. doi: 10.1099/00221287-138-8-1697. [DOI] [PubMed] [Google Scholar]
- 95.Li L J, Dougan G, Novotny P, Charles I G. P.70 pertactin, an outer-membrane protein from Bordetella parapertussis: cloning, nucleotide sequence and surface expression in Escherichia coli. Mol Microbiol. 1991;5:409–417. doi: 10.1111/j.1365-2958.1991.tb02123.x. [DOI] [PubMed] [Google Scholar]
- 96.Lilley G G, Riffkin M C, Stewart D J, Kortt A A. Nucleotide and deduced protein sequence of the extracellular, serine basic protease gene (bprB) from Dichelobacter nodosus strain 305: comparison with the basic protease gene (bprV) from virulent strain 198. Biochem Mol Biol Int. 1995;36:101–111. [PubMed] [Google Scholar]
- 97.Lin L, Ayala P, Larson J, Mulks M, Fukuda M, Carlsson S R, Enns C, So M. The Neisseria type 2 IgA1 protease cleaves LAMP1 and promotes survival of bacteria within epithelial cells. Mol Microbiol. 1997;24:1083–1094. doi: 10.1046/j.1365-2958.1997.4191776.x. [DOI] [PubMed] [Google Scholar]
- 98.Lindenthal C, Elsinghorst E A. Identification of a glycoprotein produced by enterotoxigenic Escherichia coli. Infect Immun. 1999;67:4084–4091. doi: 10.1128/iai.67.8.4084-4091.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lo R Y, Strathdee C A, Shewen P E, Cooney B J. Molecular studies of Ssal, a serotype-specific antigen of Pasteurella haemolytica A1. Infect Immun. 1991;59:3398–3406. doi: 10.1128/iai.59.10.3398-3406.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lorenzen D R, Dux F, Wolk U, Tsirpouchtsidis A, Haas G, Meyer T F. Immunoglobulin A1 protease, an exoenzyme of pathogenic Neisseriae, is a potent inducer of proinflammatory cytokines. J Exp Med. 1999;190:1049–1058. doi: 10.1084/jem.190.8.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McGee D J, Mobley H L. Mechanisms of Helicobacter pylori infection: bacterial factors. Curr Top Microbiol Immunol. 1999;241:155–180. doi: 10.1007/978-3-642-60013-5_9. [DOI] [PubMed] [Google Scholar]
- 102.McMichael J C, Fiske M J, Fredenburg R A, Chakravarti D N, VanDerMeid K R, Barniak V, Caplan J, Bortell E, Baker S, Arumugham R, Chen D. Isolation and characterization of two proteins from Moraxella catarrhalis that bear a common epitope. Infect Immun. 1998;66:4374–4381. doi: 10.1128/iai.66.9.4374-4381.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Miyazaki H, Yanagida N, Horinouchi S, Beppu T. Characterization of the precursor of Serratia marcescens serine protease and COOH-terminal processing of the precursor during its excretion through the outer membrane of Escherichia coli. J Bacteriol. 1989;171:6566–6572. doi: 10.1128/jb.171.12.6566-6572.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nabwera H M, Logan R P. Epidemiology of Helicobacter pylori: transmission, translocation and extragastric reservoirs. J Physiol Pharmacol. 1999;50:711–722. [PubMed] [Google Scholar]
- 105.Nataro J P, Hicks S, Phillips A D, Vial P A, Sears C L. T84 cells in culture as a model for enteroaggregative Escherichia coli pathogenesis. Infect Immun. 1996;64:4761–4768. doi: 10.1128/iai.64.11.4761-4768.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Navarro-Garcia F, Eslava C, Villaseca J M, Lopez-Revilla R, Czeczulin J R, Srinivas S, Nataro J P, Cravioto A. In vitro effects of a high-molecular-weight heat-labile enterotoxin from enteroaggregative Escherichia coli. Infect Immun. 1998;66:3149–3154. doi: 10.1128/iai.66.7.3149-3154.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Navarro-Garcia F, Sears C, Eslava C, Craviotot A, Nataro J. Cytoskeletal effects induced by Pet, the serine protease enterotoxin of enteroaggregative Escherichia coli. Infect Immun. 1999;67:2184–2192. doi: 10.1128/iai.67.5.2184-2192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Noriega F R, Liao F M, Formal S B, Fasano A, Levine M M. Prevalence of Shigella enterotoxin 1 among Shigella clinical isolates of diverse serotypes. J Infect Dis. 1995;172:1408–10. doi: 10.1093/infdis/172.5.1408. [DOI] [PubMed] [Google Scholar]
- 109.Nunn D. Bacterial type II protein export and pilus biogenesis: more than just homologies? Trends Cell Biol. 1999;9:402–408. doi: 10.1016/s0962-8924(99)01634-7. [DOI] [PubMed] [Google Scholar]
- 110.Ohnishi Y, Beppu T, Horinouchi S. Two genes encoding serine protease homologues in Serratia marcescens and characterization of their products in Escherichia coli. J Biochem (Tokyo) 1997;121:902–913. doi: 10.1093/oxfordjournals.jbchem.a021672. [DOI] [PubMed] [Google Scholar]
- 111.Ohnishi Y, Horinouchi S. Extracellular production of a Serratia marcescens serine protease in Escherichia coli. Biosci Biotechnol Biochem. 1996;60:1551–1558. doi: 10.1271/bbb.60.1551. [DOI] [PubMed] [Google Scholar]
- 112.Ohnishi Y, Nishiyama M, Horinouchi S, Beppu T. Involvement of the COOH-terminal pro-sequence of Serratia marcescens serine protease in the folding of the mature enzyme. J Biol Chem. 1994;269:32800–32806. [PubMed] [Google Scholar]
- 113.O'Toole P W, Austin J W, Trust T J. Identification and molecular characterization of a major ring-forming surface protein from the gastric pathogen Helicobacter mustelae. Mol Microbiol. 1994;11:349–361. doi: 10.1111/j.1365-2958.1994.tb00315.x. [DOI] [PubMed] [Google Scholar]
- 114.Otto B R, van Dooren S J M, Nuijens J H, Luirink J, Oudega B. Characterization of a hemoglobin protease secreted by the pathogenic Escherichia coli strain EB1. J Exp Med. 1998;188:1091–1103. doi: 10.1084/jem.188.6.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Owen P, Caffrey P, Josefsson L G. Identification and partial characterization of a novel bipartite protein antigen associated with the outer membrane of Escherichia coli. J Bacteriol. 1987;169:3770–3777. doi: 10.1128/jb.169.8.3770-3777.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Owen P, Kaback H R. Antigenic architecture of membrane vesicles from Escherichia coli. Biochemistry. 1979;18:1422–1426. doi: 10.1021/bi00575a005. [DOI] [PubMed] [Google Scholar]
- 117.Owen P, Kaback H R. Immunochemical analysis of membrane vesicles from Escherichia coli. Biochemistry. 1979;18:1413–1422. doi: 10.1021/bi00575a004. [DOI] [PubMed] [Google Scholar]
- 118.Owen P, Kaback H R. Molecular structure of membrane vesicles from Escherichia coli. Proc Natl Acad Sci USA. 1978;75:3148–3152. doi: 10.1073/pnas.75.7.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Owen P, Mechan M, de Loughry-Doherty H, Henderson I. Phase-variable outer membrane proteins in Escherichia coli. FEMS Immunol Med Microbiol. 1996;16:63–76. doi: 10.1111/j.1574-695X.1996.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 120.Papini E, Satin B, de Bernard M, Molinari M, Arico B, Galli C, Telford J R, Rappuoli R, Montecucco C. Action site and cellular effects of cytotoxin VacA produced by Helicobacter pylori. Folia Microbiol. 1998;43:279–284. doi: 10.1007/BF02818613. [DOI] [PubMed] [Google Scholar]
- 121.Parton R. Review of the biology of Bordetella pertussis. Biologicals. 1999;27:71–76. doi: 10.1006/biol.1999.0182. [DOI] [PubMed] [Google Scholar]
- 122.Plaut A G, Bachovchin W W. IgA-specific prolyl endopeptidases: serine type. Methods Enzymol. 1994;244:137–151. doi: 10.1016/0076-6879(94)44012-3. [DOI] [PubMed] [Google Scholar]
- 123.Pohlner J, Halter R, Beyreuther K, Meyer T F. Gene structure and extracellular secretion of Neisseria gonorrhoeae IgA protease. Nature. 1987;325:458–462. doi: 10.1038/325458a0. [DOI] [PubMed] [Google Scholar]
- 124.Pohlner J, Langenberg U, Wolk U, Beck S C, Meyer T F. Uptake and nuclear transport of Neisseria IgA1 protease-associated alpha-proteins in human cells. Mol Microbiol. 1995;17:1073–1083. doi: 10.1111/j.1365-2958.1995.mmi_17061073.x. [DOI] [PubMed] [Google Scholar]
- 125.Poulsen K, Brandt J, Hjorth J P, Thogersen H C, Kilian M. Cloning and sequencing of the immunoglobulin A1 protease gene (iga) of Haemophilus influenzae serotype b. Infect Immun. 1989;57:3097–3105. doi: 10.1128/iai.57.10.3097-3105.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Provence D L, Curtiss R., III Isolation and characterization of a gene involved in hemagglutination by an avian pathogenic Escherichia coli strain. Infect Immun. 1994;62:1369–1380. doi: 10.1128/iai.62.4.1369-1380.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pugsley A P, Francetic O, Hardie K, Possot O M, Sauvonnet N, Seydel A. Pullulanase: model protein substrate for the general secretory pathway of gram-negative bacteria. Folia Microbiol. 1997;42:184–192. doi: 10.1007/BF02818976. [DOI] [PubMed] [Google Scholar]
- 128.Rambow A A, Fernandez R C, Weiss A A. Characterization of BrkA expression in Bordetella bronchiseptica. Infect Immun. 1998;66:3978–3980. doi: 10.1128/iai.66.8.3978-3980.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rao V K, Krasan G P, Hendrixson D R, Dawid S, St Geme J W., III Molecular determinants of the pathogenesis of disease due to non-typeable Haemophilus influenzae. FEMS Microbiol Rev. 1999;23:99–129. doi: 10.1111/j.1574-6976.1999.tb00393.x. [DOI] [PubMed] [Google Scholar]
- 130.Reyrat J M, Pelicic V, Papini E, Montecucco C, Rappuoli R, Telford J L. Towards deciphering the Helicobacter pylori cytotoxin. Mol Microbiol. 1999;34:197–204. doi: 10.1046/j.1365-2958.1999.01592.x. [DOI] [PubMed] [Google Scholar]
- 131.Riffkin M C, Wang L F, Kortt A A, Stewart D J. A single amino-acid change between the antigenically different extracellular serine proteases V2 and B2 from Dichelobacter nodosus. Gene. 1995;167:279–283. doi: 10.1016/0378-1119(95)00664-8. [DOI] [PubMed] [Google Scholar]
- 132.Roux V, Fournier P E, Raoult D. Differentiation of spotted fever group rickettsiae by sequencing and analysis of restriction fragment length polymorphism of PCR-amplified DNA of the gene encoding the protein rOmpA. J Clin Microbiol. 1996;34:2058–2065. doi: 10.1128/jcm.34.9.2058-2065.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sansonetti P J. Genetic and molecular basis of epithelial cell invasion by Shigella species. Rev Infect Dis. 1991;13(Suppl)4:S285–S292. doi: 10.1093/clinids/13.supplement_4.s285. [DOI] [PubMed] [Google Scholar]
- 134.Sansonetti P J, Egile C. Molecular bases of epithelial cell invasion by Shigella flexneri. Antonie Leeuwenhoek. 1998;74:191–197. doi: 10.1023/a:1001519806727. [DOI] [PubMed] [Google Scholar]
- 135.Sara M, Sleytr U B. S-layer proteins. J Bacteriol. 2000;182:859–868. doi: 10.1128/jb.182.4.859-868.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schesser K, Francis M, Forsberg Å, Wolf-Watz H. Type III secretion systems in animal- and plant-interacting bacteria. In: Cossart P, Boquet P, Normark S, Rappuoli R, editors. Cellular microbiology. Washington, D.C.: ASM Press; 2000. pp. 239–263. [Google Scholar]
- 137.Sharples G J, Ingleston S M, Lloyd R G. Holliday junction processing in bacteria: insights from the evolutionary conservation of RuvABC, RecG, and RusA. J Bacteriol. 1999;181:5543–5550. doi: 10.1128/jb.181.18.5543-5550.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shikata S, Shimada K, Kataoka H, Horinouchi S, Beppu T. Detection of large COOH-terminal domains processed from the precursor of Serratia marcescens serine protease in the outer membrane of Escherichia coli. J Biochem (Tokyo) 1992;111:627–632. doi: 10.1093/oxfordjournals.jbchem.a123809. [DOI] [PubMed] [Google Scholar]
- 139.Shikata S, Shimada K, Ohnishi Y, Horinouchi S, Beppu T. Characterization of secretory intermediates of Serratia marcescens serine protease produced during its extracellular secretion from Escherichia coli cells. J Biochem (Tokyo) 1993;114:723–731. doi: 10.1093/oxfordjournals.jbchem.a124244. [DOI] [PubMed] [Google Scholar]
- 140.Shimada K, Ohnishi Y, Horinouchi S, Beppu T. Extracellular transport of pseudoazurin of Alcaligenes faecalis in Escherichia coli using the COOH-terminal domain of Serratia marcescens serine protease. J Biochem (Tokyo) 1994;116:327–334. doi: 10.1093/oxfordjournals.jbchem.a124527. [DOI] [PubMed] [Google Scholar]
- 141.Sleytr U B, Beveridge T J. Bacterial S-layers. Trends Microbiol. 1999;7:253–260. doi: 10.1016/s0966-842x(99)01513-9. [DOI] [PubMed] [Google Scholar]
- 142.St. Geme J W., III Insights into the mechanism of respiratory tract colonization by nontypeable Haemophilus influenzae. Pediatr Infect Dis J. 1997;16:931–935. doi: 10.1097/00006454-199710000-00005. [DOI] [PubMed] [Google Scholar]
- 143.St. Geme J W, III, Cutter D, Barenkamp S J. Characterization of the genetic locus encoding Haemophilus influenzae type b surface fibrils. J Bacteriol. 1996;178:6281–6287. doi: 10.1128/jb.178.21.6281-6287.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.St. Geme J W, III, de la Morena M L, Falkow S. A Haemophilus influenzae IgA protease-like protein promotes intimate interaction with human epithelial cells. Mol Microbiol. 1994;14:217–233. doi: 10.1111/j.1365-2958.1994.tb01283.x. [DOI] [PubMed] [Google Scholar]
- 145.Stanley P, Koronakis V, Hughes C. Acylation of Escherichia coli hemolysin: a unique protein lipidation mechanism underlying toxin function. Microbiol Mol Biol Rev. 1998;62:309–333. doi: 10.1128/mmbr.62.2.309-333.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Stathopoulos C, Provence D L, Curtiss R., III Characterization of the avian pathogenic Escherichia coli hemagglutinin Tsh, a member of the immunoglobulin A protease-type family of autotransporters. Infect Immun. 1999;67:772–781. doi: 10.1128/iai.67.2.772-781.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Suzuki T, Saga S, Sasakawa C. Functional analysis of Shigella VirG domains essential for interaction with vinculin and actin-based motility. J Biol Chem. 1996;271:21878–21885. doi: 10.1074/jbc.271.36.21878. [DOI] [PubMed] [Google Scholar]
- 148.van den Berg B M, Beekhuizen H, Willems R J, Mooi F R, van Furth R. Role of Bordetella pertussis virulence factors in adherence to epithelial cell lines derived from the human respiratory tract. Infect Immun. 1999;67:1056–1062. doi: 10.1128/iai.67.3.1056-1062.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vaughan P R, Wang L F, Stewart D J, Lilley G G, Kortt A A. Expression in Escherichia coli of the extracellular basic protease from Dichelobacter nodosus. Microbiology. 1994;140:2093–2100. doi: 10.1099/13500872-140-8-2093. [DOI] [PubMed] [Google Scholar]
- 150.Verduin C M, Jansze M, Hol C, Mollnes T E, Verhoef J, van Dijk H. Differences in complement activation between complement-resistant and complement-sensitive Moraxella (Branhamella) catarrhalis strains occur at the level of membrane attack complex formation. Infect Immun. 1994;62:589–595. doi: 10.1128/iai.62.2.589-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150a.Villaseca J M, Navarro-García F, Mendoza-Hernández G M, Nataro J P, Cravioto A, Eslava C. Pet toxin from enteroaggregative Escherichia coli produces cellular damage associated with fodrin disruption. Infect Immun. 2000;68:5920–5927. doi: 10.1128/iai.68.10.5920-5927.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Vitovski S, Read R C, Sayers J R. Invasive isolates of Neisseria meningitidis possess enhanced immunoglobulin A1 protease activity compared to colonizing strains. FASEB J. 1999;13:331–337. doi: 10.1096/fasebj.13.2.331. [DOI] [PubMed] [Google Scholar]
- 152.Wang H, Dowds B C. Phase variation in Xenorhabdus luminescens: cloning and sequencing of the lipase gene and analysis of its expression in primary and secondary phases of the bacterium. J Bacteriol. 1993;175:1665–1673. doi: 10.1128/jb.175.6.1665-1673.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Weldon S K, Mosier D A, Simons K R, Craven R C, Confer A W. Identification of a potentially important antigen of Pasteurella haemolytica. Vet Microbiol. 1994;40:283–291. doi: 10.1016/0378-1135(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 154.Weller S J, Baldridge G D, Munderloh U G, Noda H, Simser J, Kurtti T J. Phylogenetic placement of rickettsiae from the ticks Amblyomma americanum and Ixodes scapularis. J Clin Microbiol. 1998;36:1305–1317. doi: 10.1128/jcm.36.5.1305-1317.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wilhelm S, Tommassen J, Jaeger K E. A novel lipolytic enzyme located in the outer membrane of Pseudomonas aeruginosa. J Bacteriol. 1999;181:6977–6986. doi: 10.1128/jb.181.22.6977-6986.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zakrzewski J, Bechert T, Guggenbichler J P. IgA1 protease production by bacteria colonizing the upper respiratory tract. Infection. 1998;26:116–119. doi: 10.1007/BF02767773. [DOI] [PubMed] [Google Scholar]
- 157.Zupan J R, Ward D, Zambryski P. Assembly of the VirB transport complex for DNA transfer from Agrobacterium tumefaciens to plant cells. Curr Opin Microbiol. 1998;1:649–655. doi: 10.1016/s1369-5274(98)80110-0. [DOI] [PubMed] [Google Scholar]