Abstract
Glaucoma is the most prevalent form of optic neuropathy where a progressive degeneration of retinal ganglion cells (RGCs) leads to irreversible loss of vision. The mechanism underlying glaucomatous degeneration remains poorly understood. However, evidence suggests that microglia, which regulate RGC numbers and synaptic integrity during development and provide homeostatic support in adults, may contribute to the disease process. Hence, microglia represent a valid cellular target for therapeutic approaches in glaucoma. Here, we provide an overview of the role of microglia in RGC development and degeneration in the backdrop of neurogenesis and neurodegeneration in the central nervous system and discuss how pathological recapitulation of microglia-mediated developmental mechanisms may help initiate or exacerbate glaucomatous degeneration.
Keywords: microglia, retinal ganglion cell, neurogenesis, degeneration, glaucoma
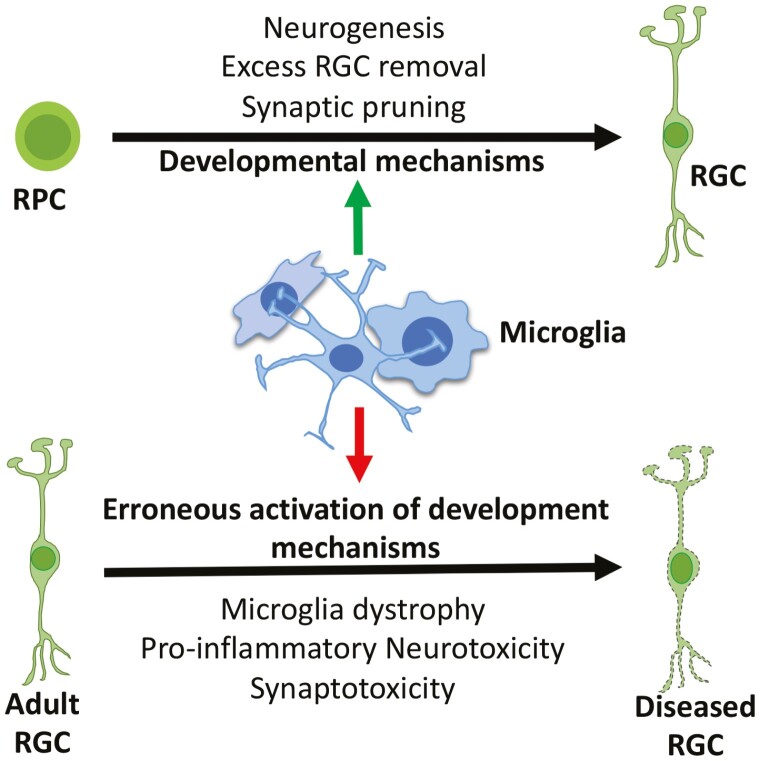
Graphical Abstract
Graphical Abstract.
Significance Statement.
Glaucoma, where retinal ganglion cells degenerate, is the leading cause of irreversible blindness. Evidence suggests that microglia, which play a homoeostatic role in the regulation of RGC development and maintenance, may determine the initiation and progression of RGC degeneration. Therefore, microglia represent a valid therapeutic target for glaucomatous degeneration.
Introduction
Glaucoma is a complex group of disorders with a common pathology of retinal ganglion cell (RGCs) degeneration.1 The progressive loss of these projection neurons that connect the retina to the brain for visual perception is the leading cause of irreversible blindness, estimated to affect 118 million people by 2040 worldwide.2 Advanced age, positive family history, and elevated intraocular pressure (IOP) are recognized risk factors for primary open angle glaucoma (POAG), the most common glaucoma subtypes.1 While these risk factors have helped in the diagnosis, monitoring, and management of the disease, their contributions to RGC degeneration are not well understood. These cells are generated and reside in close proximity of microglia, the resident immune cells in the CNS. In the adult retina, as elsewhere in the central nervous system (CNS), microglia surveil the environment to maintain the structural and functional homeostasis. They get activated in response to external pathogens/toxins or internal danger signal generated by neurons under duress and mount a swift inflammatory response to regain the structural and physiological balance.3 Their actions often consist of the redeployment of mechanisms, which help regulate the number of excess neurons generated and fine tune the wiring during development. However, the beneficial aspect of microglia becomes counterproductive to repair and remodeling in the degenerative diseases if their activation persisted for too long. Thus, the role of microglia is akin to that of a beat patrol, surveilling the neighborhood, talking to the community, and exchanging information to maintain normalcy. When there is a disturbance in the neighborhood the patrol gets into action, mediating and removing the cause of the chaos. The beneficial effects of the patrol are dependent upon whether or not the action is calibrated and its duration. When it is excessive and relentless it has an adverse effect on the community, which may become depressed and may not recover and regain the normalcy. The relationship outcome between microglia and glaucomatous RGCs may be equally contextual, which may play out all along the visual pathway, beginning with the proximal connections of RGCs within the retina and distally with the neurons in the superior colliculus (SC) and lateral geniculate nucleus (LGN), and axons in between. Since this relationship is traced back to RGC generation, in this review we will examine the role of microglia in RGC development and degeneration in the backdrop of neurogenesis and neurodegeneration in the CNS in general. The activation of the microglia-mediated developmental mechanisms during glaucoma may influence the initiation and or progression of the disease.
Development and Migration of Microglia
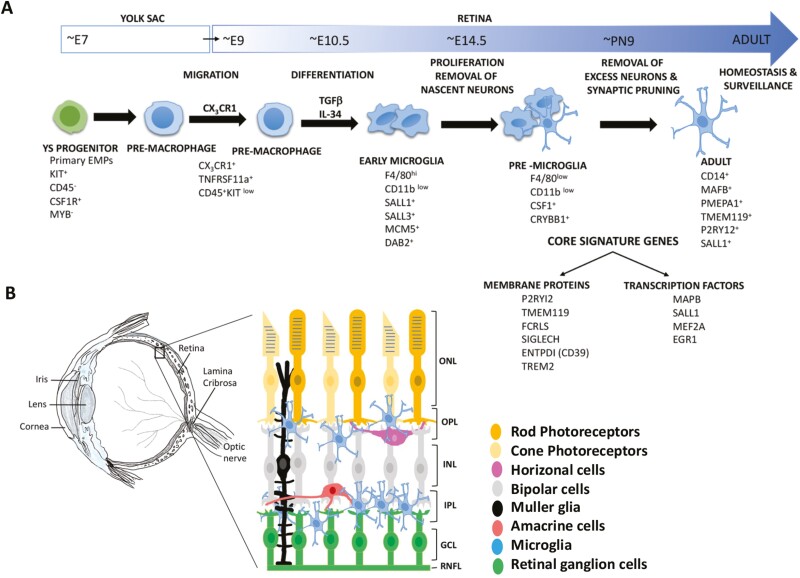
Lineage tracing studies have demonstrated that microglia are the immigrant cells from the embryonic yolk sac (YS) and begin to colonize the developing mouse brain, starting around embryonic day 9 (E9) till the formation of the blood brain barrier approximately at E14.4 These studies, along with the recent transcriptional profiling, have shed valuable light on different stages of development of the YS progenitors into mature microglia.5-7 The broadly defined stages, based on the stage-specific regulators and markers, are early microglia (recently immigrant microglia till ~E14; they share properties and markers with the YS macrophages, demonstrating their nascent stage and are enriched in genes associated with cell proliferation), pre-microglia (microglia, spanning ~E14 to early postanal weeks, displaying predominantly amoeboid morphology), and adult microglia (few weeks after birth into adulthood; these are predominantly ramified) (Fig. 1). These 3 stages correspond to the developmental function of microglia in supporting neurogenesis, refining the wiring in the developing brain (early- and pre-microglia), and maintaining the surveillance homeostasis in the adult brain (adult microglia). The differentiation of YS progenitors into migratory YS pre-macrophage is regulated by the transcription factors Runx1, PU.1, and interferon regulatory factors 8 (IRF8).8,9 These transitory cells begin to express the fractalkine (CX3CL1) receptor, CX3CR1 to facilitate their migration into the brain. Their differentiation into microglia once inside the embryonic brain is facilitated by transforming growth factor beta (TGFβ), colony stimulating factor 1 (CSF-1), and interleukin-34 (IL-34) mediated signaling.10,11 The retinal microglia are likely to go through similar developmental stages, identified by the corresponding markers, as the retina follows similar developmental trajectories like the rest of the CNS. Microglia precursors migrate into developing retina around the same time as in the developing brain because they are already present in the E11.5 mouse retina, the earliest stage studied.12 As they expand pre- and postnatally and the neuroblastic layer progressively differentiates into a laminar structure, microglia preferentially locate to the inner and outer plexiform layers (IPL, OPL), where synapses are formed and the ganglion-cell layer (GCL) and the inner nuclear layer (INL), where excess neurons undergoing apoptosis are removed in the first 2 postnatal weeks12,13 (Fig. 1). Given the temporally and spatially changing niche in the developing retina and microglia being highly responsive to their immediate environment, they may exist in different subtypes specific to the development and function of the retina. This premise has been temporally analyzed in the context of the mouse brain, where scRNA seq revealed 9 transcriptionally distinct clusters found across 5 different ages, at E14.5, PN4/5, PN30, PN100, and PN450.14 The diversity of the clusters was greatest at embryonic and early postnatal age versus adult. The transcriptional signature of these clusters reflected the functional state of microglia at that age. For example, the early postnatal microglia (PN4/5) were uniquely characterized by a cluster (cluster 4) that expressed high levels of osteopontin gene (Spp1) along with transcripts corresponding to insulin like growth factor 1 (Igf1), glycoprotein NMB (Gpnmb), immunomodulators of galectin family, galectin-1 (Lgals1) and galectin-3 (Lgals3), and a lysosomal glycoprotein, CD68 (Cd68), enriched in phagocytotic microglia and macrophages. They were associated with the pre-myelinated axon tracts and are thought to clear the debris before axons are myelinated14 and/or engulf oligodendrocytes.15 This subpopulation of amoeboid and phagocytotic brain microglia shares transcriptional signature with activated retinal microglia in the animal models of glaucoma16 and a subset of those exposed to apoptotic retinal neurons during development,17 suggesting the recruitment of developmentally regulated genes by microglia in response to degenerative changes in the retina.
Figure 1.
(A) A schematic representation of microglia development with associated markers. The scheme is adapted from Li and Barres6 and include information from Butovsky et al.10 (B) A schematic representation of the adult eye and the laminar localization of microglia in the adult retina.
Influence on Neurogenesis
The timing of the microglia precursors’ entry into the brain coincides with the early stage of neurogenesis. The experimental elimination of microglia from the developing brain, including retina, demonstrated their influence on the outcome of neurogenesis. The influence appeared contextual, depending upon when during retinal development microglia were eliminated. For example, when microglia precursors were prevented from migrating into the retina from the YS in Zebrafish by morpholino-based silencing of CSF-1R the manipulation caused microphthalmia, accompanied by the increased proliferation and reduced differentiation of the retinal progenitors.18 Similar observations were made for the cortical progenitors in the small brain phenotype when microglia were depleted in the developing brain by eliminating the Csf-1R gene,19,20 demonstrating that microglia may positively influence the neuronal differentiation. However, when microglia were eliminated after they had colonized the retina, the effect on the retina was rather subtle; the number of the newly born RGCs was increased, suggesting that microglia remove the nascent RGCs during early retinal neurogenesis,21 presumably regulating the neurogenic output by eliminating a subset of post-mitotic cells. This subset were tagged with C1q, the initiation component of the classic complement signaling pathway and removed by microglia by complement receptor3 (CR3)-mediated phagocytosis,21 the mechanism used in the postnatal pruning of RGC synapses.22 The eliminated cells were non-apoptotic like those removed by microglia from the developing cortex,23 and the hippocampal sub granular zone (SGZ) during adult neurogenesis.24 What makes the subset of cells destined for elimination during neurogenesis is currently not well understood. Evidence has emerged that microglia may target another subset of cells during development and eliminate them by causing apoptosis. For example, nascent neurons are removed in the developing cerebellum and hippocampus by microglia-mediated apoptosis, initiated by reactive oxygen species (ROS).25,26 Microglia-mediated apoptosis has also been observed in the developing retina.22 However, the apoptotic signal is different; it consists of microglia-derived NGF, which promotes programmed cell death through p75 neurotrophic receptor.27 Together, these observations point to an important role of microglia in homeostatic regulation of neurogenesis through active elimination of subsets of cells in different stages of development.
Regulation of RGC Number
About 50 percent of cells generated in the developing CNS are eliminated, the majority of them through apoptosis.28-30 The retina is no exception, and the chronology of generation and elimination of cells is well-documented. For example, cell loss in the postnatal retina is observed to be 5%, 50%, and 51% in ONL, INL, and GCL, respectively.31 From the viewpoint of glaucoma, the cells in the GCL are important. At birth almost all cells in the GCL are RGCs.32 These are the first-born neurons in the retina the majority of which are generated between ~E9 and ~E19 in rats and ~E7 and ~E16 in mice.33,34 RGC axons are detected in the optic nerve within 24-48 hours after their birth and reach SC and LGN by approximately 3 and 5 days, respectively, after their generation.35 However, axons of late born RGCs have to travel longer distance and thus take ~8 days to reach SC.36 Almost half the population of the RGCs are eliminated in the first postnatal week (the peak of elimination falling between PN2 and PN4) after they have reached their targets in the mouse retina.12,37,38 The space is taken over by the displaced amacrine cells (AC) such that in the adult, RGCs and ACs comprise 45% and 51% of cells in the GCL, respectively.32 Early evidence of microglia-mediated elimination of the apoptotic RGCs was provided by Hume et al, who demonstrated microglia, identified by F40/80 immunoreactivities, migrating to the GCL and in phagocytotic association with the pyknotic neurons in the first postnatal week.39 The mechanism by which microglia gravitate toward the dying neurons is not well understood, but evidence points toward chemoattraction through factors released by the dying cells, called the “find me” signals.40 These signals are exemplified by lisophosphatidylcholine (LPC) and ATP, recognized by G-protein-coupled receptor 132 (Gpr132) and purinergic receptor, P2Y12, respectively, on microglia.41,42 Once in the vicinity, microglia recognize the dying neurons for phagocytosis by the display on their surface phospholipid phosphatidylserine, the major “eat-me” signal.43
Modulation of Synaptic Structures and Activities
Microglia play an essential role in fine tuning the wiring in the developing brain by pruning inactive and/or immature synapses as a mean to remove inappropriate and excessive connections.6,44 They achieve this through the complement system, used systematically for the removal of cellular debris.6 This is best exemplified in the pruning of synapses between RGC axons and cells in the LGN in the first postnatal week in rodents.22,45 The strength of the retinogeniculate synapses is activity driven and relatively weak synapses, tagged with the complement components C1q and C3, are recognized by microglia and promptly phagocytosed through the interactions with the microglial complement receptor, CR3.22,45 Microglia also assist in the elimination of immature synapses in the developing hippocampus,46 supporting the notion that the sculpting of synapses is their essential function in the developing nervous system, including retina,47 and along the visual pathway.48 Therefore, a pathological activation of complement-mediated synapse pruning by microglia may lead to an abnormal or a loss of function. For example, there is early synapse loss in the hippocampus in the animal model of Alzheimer’s disease (AD) due to an inappropriate activation of microglia by the amyloid beta (Aβ) protein, suggesting that the loss of cognition in AD is due to a complement-mediated pathological pruning.49,50 Similarly, it is thought that the complement-mediated excess removal of synapses underlies the pathology of fewer synapses than normal in schizophrenic patients.51 Recent studies suggest that microglia can discriminate between the excitatory versus inhibitory synapses for the remodeling of connections, and their inappropriate removal has behavioral consequences.52 Evidence is emerging that abnormal complement-mediated pruning in the retina and along the visual pathway may also compromise normal function. For example, microglia have been observed to engulf postsynaptic elements in the OPL in a complement-dependent manner following rod photoreceptor degenerationin in the animal model of retinitis pigmentosa (RP).47 Conversely, under-pruning of synapses in the developing visual cortex due to the elimination of purinergic receptor P2Y12, which allows microglia to scan the parenchyma for pruning, results into immature synapses, abrogating the ocular dominance plasticity.48
Microglia and Glaucomatous Degeneration
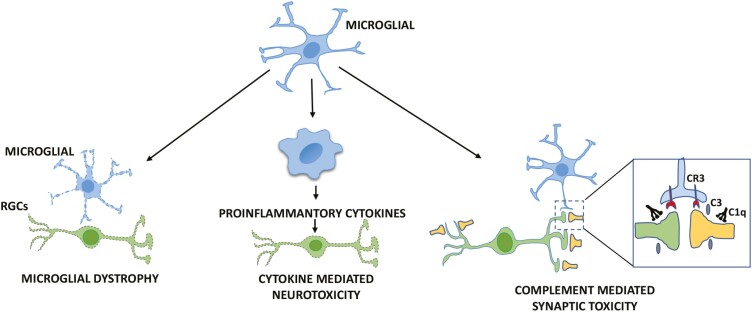
Given the role of microglia in maintaining homeostasis in the CNS and its macrophage lineage it is expected that these cells would react to degenerative changes in neurons and hence, the activation of microglia and proinflammatory responses are associative features in almost all neurodegenerative diseases53 including glaucoma.54 That glaucoma is associated with microglia activation was demonstrated by Neufeld55, who observed clusters of amoeboid microglia in the compressed lamina cribrosa (Fig. 1), encircling the blood vessels in the paraffin sections of the glaucomatous eyes with optic nerve damage. Later, these ameboid cells were shown to express the activation marker (HLA-DR), phagocytotic markers (TSP, CD68), inflammatory cytokine (TNFα), inflammatory mediators (NOS-2, COX-1), proliferation marker (PCNA), and several metalloproteases confirming their proliferative and activated status in glaucoma.56 Examination of microglia in the glaucomatous retina also revealed enhanced expression of Toll-like receptors, suggesting active signaling through these receptors, which may lead to pro-inflammatory changes.57 Although the association of microglia activation with glaucoma is well established, it is not clearly understood whether it precedes or follows the disease and therefore what is its effect on the onset and progression of the disease. The animal models of glaucoma have begun to shed light on these issues, with a caveat that some of the emerging information may not be clinically relevant given the species difference in ocular anatomy and genes/pathways involved. A detailed comparison of the relative merit of different rodent models of glaucoma is given in excellent reviews on the topic by Johnson and Tomarev and Pang and Clark.58,59 Microglia activation has been observed in both the ocular hypertension (OHT) models and a chronic model of pigmentary glaucoma, exemplified by the inbred DBA/2J (D2) mouse.58 For example, in an episcleral vein occlusion mediated OHT model, activation of microglia was observed coincidental with RGC degeneration.60 In the trans-limbal laser photocoagulation OHT model, activated microglia were observed in the retina, optic nerve, and in the optic tract, where the degree of activation of microglia was correlated with the axonal damage.61 In the D2 mice, where mutation in Gpnmb and Tyrp1 genes are associated with iris atrophy and pigment dispersion,58 RGC loss was detected maximally between 11 and 18 months of age, preceded by the elevation in the IOP that occurs approximately at 6-7 months of age.62,63 However, the degenerative changes in RGC dendrites in terms of synaptic loss and axonal dysfunction can be detected much earlier around 5 months of age.45,64 That microglia may have a causal role in the RGC degeneration was demonstrated by Bosco et al,65 when they observed microgliosis in D2 mice at an early age of 3 months, much before the onset of degenerative changes in RGCs. Activated microglia were specifically detected in the retina and unmyelinated regions of the optic nerve. The mechanism underlying the transition of homeostatic to proinflammatory or neurodegenerative microglial phenotype in glaucoma remains poorly understood. However, a recent study, carried out in both the OHT and D2 mouse models, demonstrated an important role of apoE-galectin3 signaling in conferring the neurodegenerative phenotype on microglia and that this phenotype may precede RGC degeneration.16 Based on the emerging role of activated microglia in animal models of neurodegeneration such as AD and microgliosis observed in the OHT models and D2 mice 3 broad pathways53 can be envisioned for microglia influencing the onset and progression of glaucomatous degeneration (Fig. 2).
Figure 2.
A schematic representation of 3 broad pathways for microglia influence on the onset and progression of glaucomatous degeneration. The dystrophic microglia represent those that are inherently abnormal due to mutations in genes expressed in microglia, exemplified by the TREM2 phenotype.66,67 C3 and C1q are complement proteins mediating complement-dependent synaptic toxicity via complement receptor, CR3.
Microglial Dystrophy
It is possible that the activation of microglia and associated inflammatory changes may not be secondary to neuronal degeneration, but due to the abnormalities inherent in microglia themselves, which may be manifested upon aging, contributing to neuronal degeneration. For example, Nasu-Hakola disease (NHD), which leads to a lethal presenile dementia is secondary to the dystrophic changes in microglia due to a mutation in TREM2, a gene that is exclusively expressed in microglia and involved in phagocytosis.66 Postmortem of NHD patients reveal demyelination of subcortical white matter. Examination of the TREM2 phenotype in Trem2-/- mice revealed glia with abnormal morphology which were impaired in responding to age-related myelin damage for the removal of the debris, thus presumably compromising the remyelination by oligodendrocytes.67 The non-cell autonomous influence of microglia on the progression of neuronal degeneration is also exemplified by the role of dystrophic microglia in amyotrophic lateral sclerosis (ALS), where there is a progressive loss of the motor neurons due to mutations in the ubiquitous super oxide dismutase 1 (SOD1) gene.68 As mentioned, the first evidence that microgliosis may be causal to glaucomatous degeneration emerged when the activation of microglia was observed prior to any overt neurodegenerative changes in the D2 mice,65 even before the onset of the IOP and aging, 2 of the prominent risk factors for glaucomatous degeneration. This early activation, preceding the disease, may reflect microglial dystrophy similar to that seen in NHD, which may be due to the mutation in genes that are exclusively or predominantly expressed in microglia. One such mutated gene in D2 mice is Gpnmb, which has an immune suppression role69 and thus may contribute to microglia dysfunction. Therefore, the emergence of the cell-autonomously activated microglia with hyperactive immune response in the retina and optic nerve may be the tipping point for the neurodegenerative changes. Another candidate gene underlying the microglial dystrophy preceding glaucoma could be Trem2, whose expression is decreased in the optic nerve head microglia in D2 mice.70 Given the essential role of this gene in the microglia-mediated homeostasis in the brain, a decrease in its expression will add to the dysfunction of the cells already burdened with the mutation in the immune suppressor gene (Gpnmb), making microglia proinflammatory to the detriment of the optic nerve and RGCs.
Pro-inflammatory Cytokines-mediated Neurotoxicity
Pro-inflammatory cytokines released by microglia could either be beneficial or harmful depending upon the context of time and space.71 In disease conditions, these cytokines create a neurotoxic environment, adding to the progression of the neurodegenerative changes. One of the molecular axes of the microglia-mediated homeostasis, whose dysregulation leads to the neurotoxic microglia activation, is the CX3CR1-fractlakine signaling pathway.72-74 Fractalkine, primarily secreted by neurons including RGCs,66 has been shown to be neuroprotective by suppressing the inflammatory response by binding to CX3CR1 on microglia.72,74 Thus, when the fractalkine-CX3CR1 axis was dysregulated by the homozygous deletion of Cx3cr1 gene neuronal loss was observed exacerbated in the animal models of PD and ALS.73 Disruption of the CX3CR1-fractalkine signaling has been observed to have an adverse effect on RGC survival and function as well. The extent of the effects of the disruption depends upon whether the signal is disrupted in acute or chronic model of glaucoma. When IOP was transiently elevated following ischemia reperfusion in the Cx3cr1-/- mouse, the microglia became amoeboid and expressed CD68, a lysosomal glycoprotein enriched in phagocytotic miroglia and macropghages, accompanied by significant degenerative changes in soma and dendrites of RGCs.75 The activated microglia were first observed in the central retina consistent with the observations of activated microglia recruitment in the optic nerve head in glaucomatous human eyes and animal models of glaucoma.60,61,65 These changes were alleviated in mice treated with minocycline-a tetracycline derivative that suppresses chronic microglial activation and neuroinflammation76 -linking RGC degeneration with Cx3cr1-/- microglia activation.75 However, in D2 mice the absence of Cx3cr1 degenerative changes in RGCs were confined to axon transport dysfunction,77 and similarly, minocycline-mediated suppression of microglia activation was neuroprotective of RGC axons.78 The association of the activated microglia with the dysfunction of the axons is informative in understanding the disease progression because the axonal transport is compromised before the overt cell loss in D2 mice.64,79 For example, the failure of the anterograde transport from RGCs to the superior colliculus (SC) is detected as early as 3-5 months of age with complete failure of the transport between 11 and 12 months and to all other central targets (ie, LGN) after 12 months.64 The axonal transport deficit may reflect an early state in the disease progression, as observed in the animal model of ALS, where it is inflammation induced.80 This suggests that a neuroinflammatory lesion is causing the early axonal dysfunction, which may also underlie the early transport deficits seen in glaucoma. However, it is important to keep in mind from a therapeutic viewpoint the observation that CX3CR1 deficiency is not always deleterious, but could be neuro protective, depending on the stage of degenerative changes. For example, during the early stage of AD when Aβ accumulates, blocking of the CX3CR1-fractalkine signaling reduces the Aβpathology, presumably through the phagocytosis of the extracellular Aβaggregates, whereas in the late stage the dysregulation of the signaling may add to the progression of the disease by the microglia-mediated aggregation of microtubule associated protein tau (MAPT).81
Complement-mediated Synaptotoxicity
As mentioned, the complement-mediated synapse pruning by microglia refines the wiring in the developing brain.44 A pathological re-activation of this mechanism of phagocytosis by microglia in response to the degenerative changes may initiate or add to the progression of the degenerative changes.44 This was demonstrated in the animal model of AD, where microglia, activated by Aβoligomers, phagocytosed synapses tagged with the fluorescent Homer protein.49 The phagocytosis was complement-mediated as the Aβ-induced synaptic loss was not observed when CR3 gene was deleted from the microglia.49 Of note, Aβ has been observed elevated in the animal models of glaucoma and glaucoma patients,82 raising the possibility of Aβ-mediated synaptotoxicity in the disease process. Further evidence of synaptotoxicity due to the aberrant activation of the complement in microglia was obtained in the animal model of autosomal dominant frontotemporal dementia (FTD) with the mutation in progranulin (Prgn) gene.83 The loss of the Prgn gene was associated with the age-dependent up regulation of lysosomal (Cd68) and complement (C1, C3) genes; this increased the complement production and synaptic phagocytosis by microglia, which preferentially eliminated the inhibitory synapses in the ventral thalamus, causing hyperexcitability in the thalamocortical circuits.83 A clear evidence of microglia in the aberrant synaptic pruning as observed in the animal models of AD and FTD has yet to emerge in glaucoma. However, the upregulation of the complement components has been observed in both acute and chronic models of glaucoma and associated with the early degenerative changes in RGCs. For example, the induction of the complement cascade genes is one of the earliest identifiable molecular changes in the glaucomatous D2 mouse retina, where the synaptic and dendritic atrophy precedes any detectable degenerative changes seen in the axon or soma of RGCs.44,84,85 That the complement cascade is involved in these degenerative changes was demonstrated when genetic ablation (D2 mice) or pharmacological inhibition (acute OHT mice) of C1q preserved the synaptic and dendritic integrity.85 In the postnatal LGN, where microglia refine the synaptic input by the complement-mediated pruning,22,45 optic nerve crush caused the activation of the complement cascade to remove the debris due to the Wallerian degeneration of the RGC axons.86 In both these cases however, the direct involvement of the activated microglia in the synaptic toxicity was not demonstrated, which may be attributed to the observations that the dysregulated complement-mediated synaptic pruning may be contextual, depending on the mode of activation, location, and time.83
Stem Cell Modeling of Human Microglia-RGC Interactions
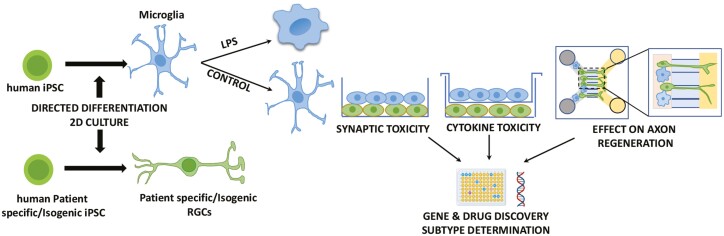
Animal models of glaucoma have shed valuable light on the association of activated microglia with the glaucomatous RGC dysfunction and degeneration.75,77,78 However, the premise that activated microglia make RGC vulnerable to the disease remains unexplored in humans because of the inaccessibility to the human microglia and RGCs. With the advent of the induced pluripotent stem cells (iPSCs) technology, fibroblast/blood cells obtained from patients and healthy donors can be reprogrammed into iPSCs. Neurons/glia can be generated from the patient-specific or isogenic control iPSCs in 2D or 3D culture to establish disease models in which the mechanism of RGC degeneration can be examined in controlled conditions87 (Fig. 3). Our lab developed a disease in a dish model of glaucomatous degeneration using iPSCs reprogrammed from the POAG patients88 and microfluidic model of optic nerve regeneration to identify genes and pathways as potential targets for axon regeneration.89 Both these models can be leveraged to examine the role of the human microglia in RGC dysfunction and degeneration in a co-culture paradigm. Several methods have been developed recently to generate microglia from the ES/iPSC cells and used to examine the role of microglia in neurodegeneration.90 From the disease modeling perspective, the ex vivo generated microglia display disease-specific phenotypes. For example, reduced phagocytosis is observed in the iPSC-derived microglia from PD patients with synuclein alpha triplication91 and those containing the TREM2 mutation.92 The human iPSC-derived microglia display the function specific-morphology (ameboid vs ramified), can be activated by the diseased neurons, mount inflammatory response, and induce synaptic loss.90,91,93 Hence, disease modeling using human iPSC-derived microglia and RGCs has the potential for determining the role microglia play in RGC dysfunction and degeneration from all 3 mechanistic possibilities, ie, microglia dystrophy, proinflammatory insults, and synaptotoxicity in controlled conditions. These mechanisms can be evaluated at the dendrite, soma, and axon levels and may reveal targets for diagnostics and therapeutic purposes.
Figure 3.
A schematic representation of stem cell modeling of microglia-RGC interactions. Both microglia and RGCs are generated from iPSCs by directed differentiation in 2D culture. Glaucoma patient-specific and isogenic control RGCs are co-cultured with LPS-activated/control microglia together across a membrane to examine synaptic and cytokine toxicity of microglia, respectively. The co-culture paradigm is adapted in a microfluidic model of optic nerve regeneration (Teotia et al.89) to examine the effects of microglia on axonal transport and regeneration. Each experimental paradigm is analyzed by population and single-cell RNA seq analysis for gene/drug discovery and subtype-based causes and effects, respectively.
Conclusion
The temporal and spatial relationship of microglia with RGCs suggests that they may not just be a bystander when the degeneration overtakes these output neurons in the retina. Whether the degeneration is due to the developmental susceptibility87,88 or an age-related degenerative process secondary to certain risks factors such as the high IOP, microglia are well positioned to affect the disease process. If the disease had a developmental origin, microglia could influence the process as they help calibrate the neurogenic output, determine the RGC number, and sculpt their post- and pre-synaptic connections within the retina and brain. In the adult retina, they maintain close surveillance of RGCs because of their proximity and expression of a battery of receptors whose ligands are expressed by RGCs.94 Therefore, microglia can read the disease state of RGCs effectively and, in initial state activate their developmental mechanism(s) of providing trophic support and removing toxic cellular debris to rescue cells under stress. Here, microglia execute the role of a friendly beat patrol, restoring normalcy through communicatiocations and calibrated actions. If microglia were inherently dysregulated because of a gene mutation (as observed in NHD) or they activated developmental mechanism (s) that were abberant and dysregulated (as observed in animal models of neurodegeneration), it would accelerate the degenerative changes, adding to the disease process. Here, microglia emerge as an overbearing beat patrol, adding to the disturbance by uncaliberated and relentless actions. Therefore, the key to microglia-dependent therapeutics in glaucoma may require a caliberated re-activation of their developmental mechanism(s) and/or suppression of their dysregulated functions toward restoring homeostasis.
Acknowledgment
We thank Drs. Matt Van Hook, Faraz Ahmad, Charles Klinetobe, and Ms. Mohanapriya Rajamoorthy for their helpful comments on the article.
Contributor Information
Iqbal Ahmad, Department of Ophthalmology and Visual Sciences, University of Nebraska Medical Center, Omaha, NE, USA.
Murali Subramani, Department of Ophthalmology and Visual Sciences, University of Nebraska Medical Center, Omaha, NE, USA.
Funding
This work was supported by R01EY029778, R01EY022051, Pearson Foundation, and Lincy Foundation.
Conflict of Interest
The authors indicated no financial relationships.
Author Contributions
I.A.: conceptualization and design, financial support, administrative support, provision of study material or patients, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript. M.S.: collection and/or assembly of data, data analysis and interpretation, manuscript writing.
Data Availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Weinreb RN, Aung T, Medeiros FA.. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311(18):1901-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081-2090. [DOI] [PubMed] [Google Scholar]
- 3. Prinz M, Jung S, Priller J.. Microglia biology: one century of evolving concepts. Cell. 2019;179:292-311. 10.1016/j.cell.2019.08.053 [DOI] [PubMed] [Google Scholar]
- 4. Ginhoux F, Guilliams M.. Tissue-resident macrophage ontogeny and homeostasis. Immunity. 2016;44:439-449. 10.1016/j.immuni.2016.02.024 [DOI] [PubMed] [Google Scholar]
- 5. Matcovitch-Natan O, Winter DR, Giladi A, et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science. 2016;353:aad8670. 10.1126/science.aad8670 [DOI] [PubMed] [Google Scholar]
- 6. Li Q, Barres BA.. Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol. 2018;18:225-242. [DOI] [PubMed] [Google Scholar]
- 7. Low D, Ginhoux F.. Recent advances in the understanding of microglial development and homeostasis. Cell Immunol. 2018;330:68-78. 10.1016/j.cellimm.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 8. Kierdorf K, Erny D, Goldmann T, et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat Neurosci. 2013;16:273-280. 10.1038/nn.3318 [DOI] [PubMed] [Google Scholar]
- 9. Ginhoux F, Greter M, Leboeuf M, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841-845. 10.1126/science.1194637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Butovsky O, Jedrychowski MP, Moore CS, et al. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat Neurosci. 2014;17:131-143. 10.1038/nn.3599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang Y, Szretter KJ, Vermi W, et al. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol. 2012;13:753-760. 10.1038/ni.2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Santos AM, et al. Embryonic and postnatal development of microglial cells in the mouse retina. J Comp Neurol. 2008;506:224-239. [DOI] [PubMed] [Google Scholar]
- 13. Pequignot MO, Provost AC, Sallé S, et al. Major role of BAX in apoptosis during retinal development and in establishment of a functional postnatal retina. Dev Dyn. 2003;228:231-238. 10.1002/dvdy.10376 [DOI] [PubMed] [Google Scholar]
- 14. Hammond TR, et al. Single-Cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity. 2019;50:253-271 e256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li Q, Cheng Z, Zhou L, et al. Developmental heterogeneity of microglia and brain myeloid cells revealed by deep single-cell RNA sequencing. Neuron. 2019;101:207-223.e10. 10.1016/j.neuron.2018.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Margeta MA, et al. Apolipoprotein E4 impairs the response of neurodegenerative retinal microglia and prevents neuronal loss in glaucoma. Immunity. 2022;55(9):1627-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anderson SR, et al. Neuronal apoptosis drives remodeling states of microglia and shifts in survival pathway dependence. Elife. 2022;11. https://www.ncbi.nlm.nih.gov/pubmed/35481836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang T, Cui J, Li L, Hitchcock PF, Li Y.. The role of microglia in the neurogenesis of zebrafish retina. Biochem Biophys Res Commun. 2012;421:214-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Erblich B, Zhu L, Etgen AM, Dobrenis K, Pollard JW.. Absence of colony stimulation factor-1 receptor results in loss of microglia, disrupted brain development and olfactory deficits. PLoS One. 2011;6:e26317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nandi S, Gokhan S, Dai X-M, et al. The CSF-1 receptor ligands IL-34 and CSF-1 exhibit distinct developmental brain expression patterns and regulate neural progenitor cell maintenance and maturation. Dev Biol. 2012;367:100-113. 10.1016/j.ydbio.2012.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anderson SR, et al. Complement targets newborn retinal ganglion cells for phagocytic elimination by microglia. J Neurosci. 2019;39:2025-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schafer DP, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cunningham CL, Martinez-Cerdeno V, Noctor SC.. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci. 2013;33:4216-4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luo C, Koyama R, Ikegaya Y.. Microglia engulf viable newborn cells in the epileptic dentate gyrus. Glia. 2016;64:1508-1517. 10.1002/glia.23018 [DOI] [PubMed] [Google Scholar]
- 25. Marin-Teva JL, et al. Microglia promote the death of developing Purkinje cells. Neuron. 2004;41:535-547. [DOI] [PubMed] [Google Scholar]
- 26. Wakselman S, Béchade C, Roumier A, et al. Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor. J Neurosci. 2008;28:8138-8143. 10.1523/JNEUROSCI.1006-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Frade JM, Barde YA.. Microglia-derived nerve growth factor causes cell death in the developing retina. Neuron. 1998;20:35-41. 10.1016/s0896-6273(00)80432-8 [DOI] [PubMed] [Google Scholar]
- 28. Burek MJ, Oppenheim RW.. Programmed cell death in the developing nervous system. Brain Pathol. 1996;6:427-446. 10.1111/j.1750-3639.1996.tb00874.x [DOI] [PubMed] [Google Scholar]
- 29. Jacobson MD, Weil M, Raff MC.. Programmed cell death in animal development. Cell. 1997;88:347-354. 10.1016/s0092-8674(00)81873-5 [DOI] [PubMed] [Google Scholar]
- 30. Yamaguchi Y, Miura M.. Programmed cell death in neurodevelopment. Dev Cell. 2015;32:478-490. 10.1016/j.devcel.2015.01.019 [DOI] [PubMed] [Google Scholar]
- 31. Voyvodic JT, Burne JF, Raff MC.. Quantification of normal cell death in the rat retina: implications for clone composition in cell lineage analysis. Eur J Neurosci. 1995;7:2469-2478. 10.1111/j.1460-9568.1995.tb01045.x [DOI] [PubMed] [Google Scholar]
- 32. Farah MH, Easter SS Jr. Cell birth and death in the mouse retinal ganglion cell layer. J Comp Neurol. 2005;489:120-134. [DOI] [PubMed] [Google Scholar]
- 33. Young RW. Cell differentiation in the retina of the mouse. Anat Rec. 1985;212:199-205. 10.1002/ar.1092120215 [DOI] [PubMed] [Google Scholar]
- 34. Rapaport DH, Wong LL, Wood ED, Yasumura D, LaVail MM.. Timing and topography of cell genesis in the rat retina. J Comp Neurol. 2004;474:304-324. [DOI] [PubMed] [Google Scholar]
- 35. Galli-Resta L, Ensini M.. An intrinsic time limit between genesis and death of individual neurons in the developing retinal ganglion cell layer. J Neurosci. 1996;16:2318-2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dallimore EJ, Cui Q, Beazley LD, Harvey AR.. Postnatal innervation of the rat superior colliculus by axons of late-born retinal ganglion cells. Eur J Neurosci. 2002;16:1295-1304. [DOI] [PubMed] [Google Scholar]
- 37. Young RW. Cell death during differentiation of the retina in the mouse. J Comp Neurol. 1984;229:362-373. 10.1002/cne.902290307 [DOI] [PubMed] [Google Scholar]
- 38. Perry VH, Henderson Z, Linden R.. Postnatal changes in retinal ganglion cell and optic axon populations in the pigmented rat. J Comp Neurol. 1983;219:356-368. 10.1002/cne.902190309 [DOI] [PubMed] [Google Scholar]
- 39. Hume DA, Perry VH, Gordon S.. Immunohistochemical localization of a macrophage-specific antigen in developing mouse retina: phagocytosis of dying neurons and differentiation of microglial cells to form a regular array in the plexiform layers. J Cell Biol. 1983;97:253-257. 10.1083/jcb.97.1.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Du XF, Du JL.. A death trap for microglia. Dev Cell. 2016;38:120-121. [DOI] [PubMed] [Google Scholar]
- 41. Xu J, Wang T, Wu Y, Jin W, Wen Z.. Microglia colonization of developing zebrafish midbrain is promoted by apoptotic neuron and lysophosphatidylcholine. Dev Cell. 2016;38:214-222. 10.1016/j.devcel.2016.06.018 [DOI] [PubMed] [Google Scholar]
- 42. Casano AM, Albert M, Peri F.. Developmental apoptosis mediates entry and positioning of microglia in the zebrafish brain. Cell Rep. 2016;16:897-906. [DOI] [PubMed] [Google Scholar]
- 43. Brown GC, Neher JJ.. Microglial phagocytosis of live neurons. Nat Rev Neurosci. 2014;15:209-216. [DOI] [PubMed] [Google Scholar]
- 44. Stephan AH, Barres BA, Stevens B.. The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci. 2012;35:369-389. [DOI] [PubMed] [Google Scholar]
- 45. Stevens B, Allen NJ, Vazquez LE, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164-1178. 10.1016/j.cell.2007.10.036 [DOI] [PubMed] [Google Scholar]
- 46. Paolicelli RC, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456-1458. [DOI] [PubMed] [Google Scholar]
- 47. He J, Zhao C, Dai J, et al. Microglia mediate synaptic material clearance at the early stage of rats with retinitis pigmentosa. Front Immunol. 2019;10:912. 10.3389/fimmu.2019.00912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sipe GO, Lowery RL, Tremblay M-E, et al. Microglial P2Y12 is necessary for synaptic plasticity in mouse visual cortex. Nat Commun. 2016;7:10905. 10.1038/ncomms10905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hong S, Beja-Glasser VF, Nfonoyim BM, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352:712-716. 10.1126/science.aad8373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Whalley K. Neurodegenerative disease: complement mediates pathological pruning. Nat Rev Neurosci. 2016;17:336. 10.1038/nrn.2016.52 [DOI] [PubMed] [Google Scholar]
- 51. Sellgren CM, et al. Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat Neurosci. 2019;22:374-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Favuzzi E, Huang S, Saldi GA, et al. GABA-receptive microglia selectively sculpt developing inhibitory circuits. Cell. 2021;184:4048-4063.e32. 10.1016/j.cell.2021.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ransohoff RM. How neuroinflammation contributes to neurodegeneration. Science. 2016;353:777-783. [DOI] [PubMed] [Google Scholar]
- 54. Silverman SM, Wong WT.. Microglia in the retina: roles in development, maturity, and disease. Annu Rev Vis Sci. 2018;4:45-77.m [DOI] [PubMed] [Google Scholar]
- 55. Neufeld AH. Microglia in the optic nerve head and the region of parapapillary chorioretinal atrophy in glaucoma. Arch Ophthalmol. 1999;117:1050-1056. 10.1001/archopht.117.8.1050 [DOI] [PubMed] [Google Scholar]
- 56. Yuan L, Neufeld AH.. Activated microglia in the human glaucomatous optic nerve head. J Neurosci Res. 2001;64:523-532. 10.1002/jnr.1104 [DOI] [PubMed] [Google Scholar]
- 57. Luo C, Yang X, Kain AD, et al. Glaucomatous tissue stress and the regulation of immune response through glial Toll-like receptor signaling. Invest Ophthalmol Vis Sci. 2010;51:5697-5707. 10.1167/iovs.10-5407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Johnson TV, Tomarev SI.. Rodent models of glaucoma. Brain Res Bull. 2010;81:349-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pang IH, Clark AF.. Inducible rodent models of glaucoma. Prog Retin Eye Res. 2020;75:100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Naskar R, Wissing M, Thanos S.. Detection of early neuron degeneration and accompanying microglial responses in the retina of a rat model of glaucoma. Invest Ophthalmol Vis Sci. 2002;43:2962-2968. [PubMed] [Google Scholar]
- 61. Ebneter A, Casson RJ, Wood JP, Chidlow G.. Microglial activation in the visual pathway in experimental glaucoma: spatiotemporal characterization and correlation with axonal injury. Invest Ophthalmol Vis Sci. 2010;51:6448-6460. [DOI] [PubMed] [Google Scholar]
- 62. Libby RT, et al. Inherited glaucoma in DBA/2J mice: pertinent disease features for studying the neurodegeneration. Vis Neurosci. 2005;22:637-648. [DOI] [PubMed] [Google Scholar]
- 63. Reichstein D, Ren L, Filippopoulos T, Mittag T, Danias J.. Apoptotic retinal ganglion cell death in the DBA/2 mouse model of glaucoma. Exp Eye Res. 2007;84:13-21. 10.1016/j.exer.2006.08.009 [DOI] [PubMed] [Google Scholar]
- 64. Crish SD, Sappington RM, Inman DM, Horner PJ, Calkins DJ.. Distal axonopathy with structural persistence in glaucomatous neurodegeneration. Proc Natl Acad Sci USA. 2010;107:5196-5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bosco A, Steele MR, Vetter ML.. Early microglia activation in a mouse model of chronic glaucoma. J Comp Neurol. 2011;519:599-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bianchin MM, Martin KC, de Souza AC, de Oliveira MA, Rieder CR.. Nasu-Hakola disease and primary microglial dysfunction. Nat Rev Neurol. 2010;6:2 p following 523. [DOI] [PubMed] [Google Scholar]
- 67. Poliani PL, et al.. , TREM2 sustains microglial expansion during aging and response to demyelination. J Clin Invest. 2015;125:2161-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Boillee S, Vande Velde C, Cleveland DW.. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39-59. [DOI] [PubMed] [Google Scholar]
- 69. Ripoll VM, Irvine KM, Ravasi T, Sweet MJ, Hume DA.. Gpnmb is induced in macrophages by IFN-gamma and lipopolysaccharide and acts as a feedback regulator of proinflammatory responses. J Immunol. 2007;178:6557-6566. [DOI] [PubMed] [Google Scholar]
- 70. Tribble JR, Harder JM, Williams PA, John SWM.. Ocular hypertension suppresses homeostatic gene expression in optic nerve head microglia of DBA/2 J mice. Mol Brain. 2020;13:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Smith JA, Das A, Ray SK, Banik NL.. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res Bull. 2012;87:10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Meucci O, Fatatis A, Simen AA, Miller RJ.. Expression of CX3CR1 chemokine receptors on neurons and their role in neuronal survival. Proc Natl Acad Sci USA. 2000;97:8075-8080. 10.1073/pnas.090017497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cardona AE, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917-924. [DOI] [PubMed] [Google Scholar]
- 74. Mizuno T, Kawanokuchi J, Numata K, Suzumura A.. Production and neuroprotective functions of fractalkine in the central nervous system. Brain Res. 2003;979:65-70. 10.1016/s0006-8993(03)02867-1 [DOI] [PubMed] [Google Scholar]
- 75. Wang K, Peng B, Lin B.. Fractalkine receptor regulates microglial neurotoxicity in an experimental mouse glaucoma model. Glia. 2014;62:1943-1954. 10.1002/glia.22715 [DOI] [PubMed] [Google Scholar]
- 76. Fan R, Xu F, Previti ML, et al. Minocycline reduces microglial activation and improves behavioral deficits in a transgenic model of cerebral microvascular amyloid. J Neurosci. 2007;27:3057-3063. 10.1523/JNEUROSCI.4371-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Breen KT, et al. Loss of fractalkine signaling exacerbates axon transport dysfunction in a chronic model of glaucoma. Front Neurosci. 2016;10:526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bosco A, Inman DM, Steele MR, et al. Reduced retina microglial activation and improved optic nerve integrity with minocycline treatment in the DBA/2J mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2008;49:1437-1446. 10.1167/iovs.07-1337 [DOI] [PubMed] [Google Scholar]
- 79. Buckingham BP, et al. Progressive ganglion cell degeneration precedes neuronal loss in a mouse model of glaucoma. J Neurosci. 2008;28:2735-2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sorbara CD, et al. Pervasive axonal transport deficits in multiple sclerosis models. Neuron. 2014;84:1183-1190. [DOI] [PubMed] [Google Scholar]
- 81. Bhaskar K, Konerth M, Kokiko-Cochran ON, et al. Regulation of tau pathology by the microglial fractalkine receptor. Neuron. 2010;68:19-31. 10.1016/j.neuron.2010.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ratnayaka JA, Serpell LC, Lotery AJ.. Dementia of the eye: the role of amyloid beta in retinal degeneration. Eye (Lond). 2015;29:1013-1026. 10.1038/eye.2015.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lui H, Zhang J, Makinson SR, et al. Progranulin deficiency promotes circuit-specific synaptic pruning by microglia via complement activation. Cell. 2016;165:921-935. 10.1016/j.cell.2016.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Howell GR, et al. Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. J Clin Invest. 2011;121:1429-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Williams PA, et al. Inhibition of the classical pathway of the complement cascade prevents early dendritic and synaptic degeneration in glaucoma. Mol Neurodegener. 2016;11:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Norris GT, et al. Neuronal integrity and complement control synaptic material clearance by microglia after CNS injury. J Exp Med. 2018;215:1789-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ahmad I, Teotia P, Erickson H, Xia X.. Recapitulating developmental mechanisms for retinal regeneration. Prog Retin Eye Res. 2020;76:100824. 10.1016/j.preteyeres.2019.100824 [DOI] [PubMed] [Google Scholar]
- 88. Teotia P, Van Hook MJ, Wichman CS, et al. Modeling glaucoma: retinal ganglion cells generated from induced pluripotent stem cells of patients with SIX6 risk allele show developmental abnormalities. Stem Cells. 2017;35:2239-2252. 10.1002/stem.2675 [DOI] [PubMed] [Google Scholar]
- 89. Teotia P, Van Hook MJ, Fischer D, Ahmad I.. Human retinal ganglion cell axon regeneration by recapitulating developmental mechanisms: effects of recruitment of the mTOR pathway. Development. 2019;146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Haenseler W, Rajendran L.. Concise review: modeling neurodegenerative diseases with human pluripotent stem cell-derived microglia. Stem Cells. 2019;37:724-730. 10.1002/stem.2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Haenseler W, Zambon F, Lee H, et al. Excess alpha-synuclein compromises phagocytosis in iPSC-derived macrophages. Sci Rep. 2017;7:9003. 10.1038/s41598-017-09362-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Garcia-Reitboeck P, Phillips A, Piers TM, et al. Human induced pluripotent stem cell-derived microglia-like cells harboring TREM2 missense mutations show specific deficits in phagocytosis. Cell Rep. 2018;24:2300-2311. 10.1016/j.celrep.2018.07.094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Park J, Wetzel I, Marriott I, et al. A 3D human triculture system modeling neurodegeneration and neuroinflammation in Alzheimer’s disease. Nat Neurosci. 2018;21:941-951. 10.1038/s41593-018-0175-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Chong RS, Martin KR.. Glial cell interactions and glaucoma. Curr Opin Ophthalmol. 2015;26:73-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.