Abstract
Introduction
Classic galactosemia is a disorder of the galactose metabolism and is inherited as an autosomal recessive disease. It is caused by a complete or severe deficiency of galactose-1-phosphate uridyltransferase (GALT), and in rare cases, atypical galactosemia can manifest at older ages. Wilson disease (WD) is a disorder of the copper metabolism that, like galactosemia, is inherited as an autosomal recessive disease. Hepatic, neurological, or psychiatric symptoms can be seen, independently or in combination, and symptoms vary from family to family. We present here a patient diagnosed with both WD and galactosemia.
Case Presentation
A 6-year-old girl was referred to our center with elevated transaminase levels and hepatosplenomegaly. The child, birthweight of 2,200 g, was born to first-degree consanguineous parents after a full-term uneventful pregnancy and was hospitalized in the neonatal period due to indirect hyperbilirubinemia, gastrointestinal bleeding, diarrhea lasting 2 weeks, and elevated liver enzymes. Hepatosplenomegaly was evident at the time of admission, a cataract was detected, and a neuropsychiatric evaluation revealed borderline mental capacity, as well as cognitive and speech retardation. Metabolic investigations revealed no specific findings other than trace positivity of reducing substances in the urine. A liver biopsy revealed copper accumulation in hepatocytes and low ceruloplasmin levels. Although WD was suspected in the patient, this diagnosis did not explain the intellectual disability, behavioral disorder, or cataract findings. A genetic analysis revealed homozygous mutations in the ATP7B and GALT genes. The galactose-1-phosphate uridyltransferase enzyme level was found to be low, and the patient was diagnosed with coexisting WD and galactosemia.
Conclusion
Coexistences of rare genetically transmitted diseases can be seen in countries where consanguineous marriages are common (Saudi Arabia, Iran, Pakistan, etc.), as in our country, Turkey.
Keywords: Galactosemia, Wilson disease, Atypical manifestation, Coexistence, Expanded genetic analysis
Established Facts
Atypical late onset manifestations of galactosemia are rarely reported.
The coexistence of rare genetically transmitted diseases may be seen in countries where consanguineous marriages are common.
It should be kept in mind that blood transfusions can lead to false-normal GALT enzyme activity measurements.
Novel Insights
To the best of our knowledge, this is the first case of coexisting galactosemia and Wilson disease to be reported in the literature.
We report on an atypical late onset manifestation of galactosemia as a rarely reported condition.
Our case suggests that in the presence of findings that cannot be explained by a single disease, the presence of other diseases should be investigated.
Introduction
Galactosemia is a disorder of the galactose metabolism and is inherited as an autosomal recessive disease. Classic galactosemia (CG) (OMIM 230400) is caused by a complete or severe deficiency of the galactokinase (GALT) enzyme.
Affected babies are born healthy, with symptoms usually developing in the early days of life upon breastfeeding. Most galactosemia patients present in the neonatal period with clinical findings of feeding intolerance, vomiting, diarrhea, jaundice, weight loss, and lethargy, while hepatomegaly, excessive bleeding, cataract, and E. coli septicemia may also develop [Anderson, 2018; Cerone and Rios, 2019]. Atypical manifestations of galactosemia in children and adults have been reported previously [Weits-Binnerts et al., 1993; Kisa et al., 2019; Simpson et al., 2019]. Diagnosis is confirmed from measurements of galactose-1-phosphate levels and GALT enzyme activity and through a molecular genetic analysis [Saudubray et al., 2012; Welling et al., 2017; Anderson, 2018].
Wilson disease (WD) is an inherited autosomal recessive disorder of the copper metabolism that is caused by an overload of copper in the organs, particularly the liver and brain. Hepatic, neurological, or psychiatric symptoms, individually or in combination, can be seen but may vary from family to family. Studies have shown that patients presenting primarily with liver involvement findings are younger than those presenting with neurological symptoms, although both may occur at an early or late age [Weiss, 2016; Członkowska et al., 2018].
Herein, we present atypical manifestation of galactosemia in a 6-year-old patient diagnosed with WD.
Case Report
A 6-year-old Turkish girl was referred to our center with elevated transaminase levels and hepatosplenomegaly.
The child was born to first-degree consanguineous parents after a full-term uneventful pregnancy. Her birth weight was 2,200 g (<3rd percentile), body length was 44.5 cm (between the 25th and 50th percentile), and occipital-frontal circumference (OFC) was 33.5 cm (50th percentile; percentiles according to growth charts of Fenton [2013]). She had been hospitalized in the neonatal period due to indirect hyperbilirubinemia (total bilirubin: 18.6 mg/dL; direct bilirubin: 1.1 mg/dL), gastrointestinal bleeding, diarrhea lasting 2 weeks, and elevated liver enzymes. She received an erythrocyte transfusion in the neonatal period due to a reduced hemoglobin level (9.6 g/dL). At that time, urinary reducing substances, urine sugar chromatography, and GALT enzyme activity were found to be normal. She had been fed via a nasogastric tube for 2 years due to feeding intolerance and failure to thrive when she was 2 years old. She was followed-up until the age of 4, but no result could be reached, and the family discontinued the follow-up.
Upon physical examination, her weight was 18 kg (10–25th percentile for age), and height was 120 cm (3–10th percentile for age). Hepatosplenomegaly was evident, a cataract was detected, and a neuropsychiatric evaluation revealed borderline mental capacity and cognitive and speech retardation. Other system examinations were unremarkable.
Laboratory investigations revealed elevated transaminases (aspartate aminotransferase [AST] 142 U/L [RR:0–35], alanine aminotransferase [ALT] 194 U/L [RR:0–35]) and low ceruloplasmin (0.03/0.04 g/L; RR:0.23–0.48) levels. No cholestasis was detected, and blood glucose was normal. Metabolic investigations revealed no specific finding other than trace positivity for reducing substances in the urine.
An abdominal ultrasonography revealed no pathological findings in the liver or spleen, and cranial magnetic resonance imaging was normal. The 24-h urine copper level was normal, while a liver biopsy revealed a copper accumulation in hepatocytes (hepatic copper concentration: 380 μg/g dry weight), a cytoplasmic enlargement of hepatocytes, and occasional glycogenated nuclei. Based on the finding of low ceruloplasmin levels and liver biopsy, WD was suspected in the patient. Whole ATP7B gene sequencing was performed with a pre-diagnosis of WD using an Illumina MiSeq next-generation sequencing (NGS) system, and the results were evaluated using Qiagen Clinical Insight (QCI) interpretation software. The sequence analysis of the ATP7B gene revealed a homozygous NM_000053.4:c.2293G>A (p.Asp765Asn) variant in the ATP7B gene that had previously been reported as pathogenic according to the American College of Medical Genetics and Genomics (ACMG) criteria. Penicillamine treatment was initiated, and she was followed-up with the diagnosis of WD.
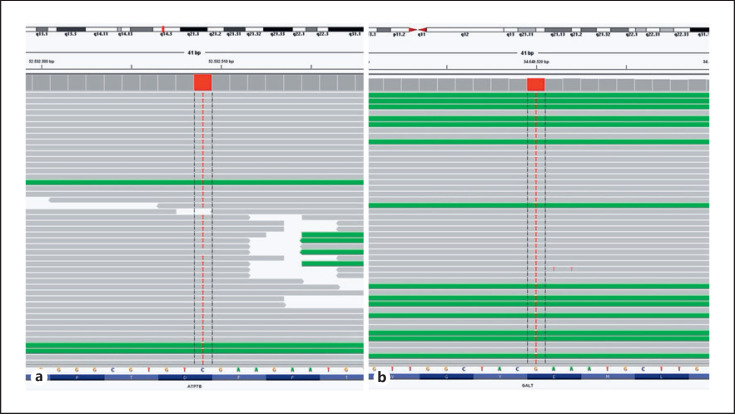
In the clinical follow-up of the patient, significant mental retardation, behavioral disorder, and cataract findings that were incompatible with the WD diagnosis were detected. No Kayser-Fleischer rings were observed. An expanded genetic analysis was performed to evaluate the etiology, and a homozygous NM_000155.4:c.1018G>T (p.Glu340Ter) nonsense pathogenic mutation in the GALT gene was detected (Fig. 1b). Despite GALT enzyme activity being found to be normal in the neonatal period, the GALT enzyme level was reassessed and was found to be low at 0.07 μmol/mL.h (RR:4–12). The patient was diagnosed with coexisting WD and galactosemia and was started on a galactose-free diet and appropriate doses of calcium and vitamin D supplements.
Fig. 1.
a Genetic analysis revealed a homozygous c.2293G>A (p.Asp765Asn) variant in the ATP7B gene. b A homozygous c.1018G>T (p.Glu340Ter) variant in the GALT gene was also detected.
Expanded Genetic Analysis
DNA was extracted from the peripheral leukocytes of the patient using an IprepTM PureLink® gDNA Blood Kit (Invitrogen, Carlsbad, CA, USA), in accordance with the manufacturer's protocol.
The patient underwent targeted NGS panel sequencing for 450 different inherited metabolic diseases (online suppl. Table 1; for online suppl. material, see www.karger.com/doi/10.1159/524004) using Ion AmpliSeqTM Library Kit Plus (Life Technologies, Guilford, CT, South San Francisco, CA, USA) kits − ready-to-go analysis kits that include 1,269 primer pairs for gene sequencing, for which we used an Ion GeneStudio S5 platform (Life Technologies). An Ion Torrent 540 Chip (Life Technologies) was used for the analyses, which were successful with an overall 100% reads on target, 100× coverage of 99.99%, and 20× coverage of 99.99%. The results were investigated with an Integrated Genomic Viewer [Robinson et al., 2011], with the human genome 19 used as the reference. An ingenuity variant analysis was used to filter the variants. The ACMG guidelines were followed for variant interpretation [Richards et al., 2015]. PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/), SIFT (https://sift.bii.a-star.edu.sg/), and MutationTaster (https://www.mutationtaster.org/) were used for in silico analysis, revealing probably damaging, damaging, and disease causing, respectively. The population frequency of the variant was evaluated based on data from the Genome Aggregation Database (https://gnomad.broadinstitute.org/), and while this variant has no gnomAD exomes entry, its locus is covered under gnomAD exomes.
Results and Discussion
We report here on a patient with an atypical late presentation of galactosemia with coexisting WD. Although the patient had a history of hospitalization in the neonatal period with findings compatible with galactosemia, her normal GALT activity and urine sugar chromatography ruled out the differential diagnosis of galactosemia. However, in cases where patients receive blood transfusions upon the development of an acute condition, as in our patient, GALT activity can be normal up to 4 months post-transfusion [Sokol et al., 1989; Pasquali et al., 2018]. Another interesting finding in the presented case suggests that an expanded genetic analysis should be considered if the findings of a patient do not precisely explain the clinical features of the disease, especially in countries where consanguineous marriages are common.
Classic galactosemia can be identified by newborn screening (NBS) through the measurement of GALT enzyme activity [Pyhtila et al., 2014; Lak et al., 2020], although it has been reported that NBS can overlook some patients with galactosemia. A definitive diagnosis is made based on the measurement of galactose-1-phosphate levels and GALT enzyme activity and through a molecular genetic analysis [Anderson, 2018; Cerone and Rios, 2019]. In countries where NBS is not implemented for galactosemia (Egypt, Iran, Iraq, Pakistan, Tunisia, etc.), like in Turkey, clinical findings and disease suspicion represent the first stage of a diagnosis [Romeo and Bittles, 2014; Kotb et al., 2019]. Galactosemia patients usually present in the first days of life with the commencement of milk feeding, when feeding difficulties, excessive weight loss, vomiting, diarrhea, prolonged jaundice, liver failure, cataract, and E. coli sepsis can be seen [Saudubray et al., 2012; Anderson, 2018; Cerone and Rios, 2019]. In our patient, while clinical findings of galactosemia such as feeding difficulties, excessive weight loss, cataract, and elevated transaminase levels were all detected, the normal GALT activity and urine sugar chromatography recorded in the neonatal period led to the diagnosis of galactosemia being delayed. Our case suggests that blood transfusion can lead to false-normal GALT enzyme activity. As the homozygous c.1018G>T (p.Glu340Ter) variant identified in the GALT gene is located at the end of the C-terminus of the protein, affecting the last 36 amino acids, the resultant protein may have partial catalytic activity, leading GALT enzyme activity to be read as normal [Gathof et al., 1995]. Kisa et al. [2019] reported 2 siblings with the homozygous c.1018G>T (p.Glu340Ter) variant in the GALT gene who presented with severe phenotype on the 5th and 27th day of life. We believe that the reporting of the phenotype of these patients led us to better understand the genotype-phenotype relationship.
Although most CG patients present with severe clinical manifestation in early infancy, rare cases of late-onset atypical CG have been reported. Simpson et al. [2019] reported a case of atypical classic galactosemia diagnosed at the age of 7 years with findings of poor academic performance at school and vision problems due to bilateral cataracts. The patient had a family history of galactosemia, and the CG diagnosis was confirmed through a genetic analysis. Kisa et al. [2019] evaluated 76 galactosemia- diagnosed patients in Turkey and reported a maximum age at the time of diagnosis of 2 years, supporting the implementation of NBS for galactosemia, and drawing attention to the late diagnosis of galactosemia patients in our country, where inherited metabolic diseases are common. Finally, Weits-Binnerts et al. [1993] reported a patient diagnosed with galactosemia at the age of 22 years.
WD is an inherited disorder that is linked to the copper metabolism in which an accumulation of copper in the organs, particularly the liver, is seen. Liver involvement is the most common manifestation of the disease, and most patients present with hepatic and/or neurological symptoms. In laboratory investigations, low ceruloplasmin and serum copper levels and elevations in urinary copper and increased liver copper are detected. Since each of these parameters may be considered abnormal in other situations, they should be evaluated together. Urinary copper excretion over 24 h is increased in symptomatic patients but may be normal or slightly elevated in pre-symptomatic patients [Mulligan and Bronstein, 2020]. In our patient, elevated transaminases, low ceruloplasmin levels, as well as copper accumulation in the hepatocytes suggested WD, although the patient's 24-h urinary copper excretion was normal. A genetic analysis in which a homozygous mutation in the ATP7B gene was revealed led to a WD diagnosis. WD can appear with a wide range of psychiatric and cognitive symptoms, such as cognitive impairment, executive function impairment, mood disorder, and psychosis. The symptoms can develop at any stage of the illness. Movement disorders (tremors, poor coordination, loss of fine-motor control, chorea, choreoathetosis) and rigid dystonia (mask-like facial expressions, rigidity, gait disturbance, pseudobulbar involvement) are common neurological presentations, along with depression, neurotic behavior, and personality disorganization, while intellectual deterioration is a rarer manifestation [Członkowska et al., 2018]. In the present case, the cataracts and the significant mental retardation, behavioral disorder, and cognitive and speech retardation were incompatible with WD.
Particularly in countries where consanguineous marriages are common, simultaneous rare diseases are seen together in the same patient [Zlotogora, 2007]. Although the co-occurrence of 2 rare diseases is uncommon, recent years have seen an increase in reports of such cases with the development of genetic analysis methods, such as whole-exome sequencing and expanded genetic analysis. Posey et al. [2017] reported that 2 or more molecular diagnoses can be detected in 4.9% of patients with whole-exome sequencing. Hannah-Shmouni et al. [2021] reported on 8 dual diagnoses of 2 hereditary disorders, in which all the patients were found to have phenotypes that were incompatible with the first illness, prompting the search for a second genetic diagnosis. Dual molecular diagnostics entail the unique or overlapping clinical diagnosis of more than one genetic locus that segregates independently, and that is important in the resolution of complex cases. Our patient's clinical presentation, mental retardation, behavioral disorder, and cataract were incompatible with a diagnosis of WD, which led us to look for a co-occurring disease. Galactosemia was subsequently diagnosed following an expanded genetic analysis.
In conclusion, our case suggests that the coexistence of rare genetically transmitted diseases may be seen in countries where consanguineous marriages are common, such as in our country. In the presence of findings that cannot be explained by a single disease, the presence of other diseases should be investigated.
Statement of Ethics
This study was exempt from ethical approval procedures, being a case report of a single patient, whose parents provided verbal consent for their child's participation in the study, and written consent for the case to be published. Informed consent for the genetic analysis and the publication of clinical reports and photographs were obtained from the patient's parent/guardian, in compliance with the national ethics regulation. There is no name or number indicating the patient's identity. Ethical approval was not required for this study in accordance with local/national guidelines.
Conflict of Interest Statement
The authors declare no conflicts of interest.
Funding Sources
This report has not benefited from any specific grant from any funding agency in the public, commercial, or not-for-profit sectors. No funding from any source was provided for this study.
Author Contributions
N.D., E.K., C.T.K., F.S.E., Z.K., A.K., and F.T.E. contributed equally to the study. N.D., E.K., C.T.K., F.S.E., Z.K., A.K., and F.T.E. accept responsibility for the entire content of this submitted manuscript and its approved submission.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Supplementary Material
Supplementary data
Acknowledgement
We would like to express our gratitude to the patient's parents for their understanding and cooperation in this study.
Funding Statement
This report has not benefited from any specific grant from any funding agency in the public, commercial, or not-for-profit sectors. No funding from any source was provided for this study.
References
- 1.Anderson S. GALT Deficiency Galactosemia. MCN Am J Matern Child Nurs. 2018;43((1)):44–51. doi: 10.1097/NMC.0000000000000388. [DOI] [PubMed] [Google Scholar]
- 2.Cerone J, Rios A. Galactosemia. Pediatr Rev. 2019;40((Suppl 1)):24–7. doi: 10.1542/pir.2018-0150. [DOI] [PubMed] [Google Scholar]
- 3.Członkowska A, Litwin T, Dusek P, Ferenci P, Lutsenko S, Medici V, et al. Wilson disease. Nat Rev Dis Primers. 2018;4((1)):21. doi: 10.1038/s41572-018-0018-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59. doi: 10.1186/1471-2431-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gathof BS, Sommer M, Podskarbi T, Reichardt J, Braun A, Gresser U, et al. Characterization of two stop codon mutations in the galactose-1-phosphate uridyltransferase gene of three male galactosemic patients with severe clinical manifestation. Hum Genet. 1995;96((6)):721–5. doi: 10.1007/BF00210306. [DOI] [PubMed] [Google Scholar]
- 6.Hannah-Shmouni F, Al-Shahoumi R, Brady LI, Wu L, Frei J, Tarnopolsky MA. Dual molecular diagnoses in a neurometabolic specialty clinic. Am J Med Genet A. 2021;185((3)):766–73. doi: 10.1002/ajmg.a.62034. [DOI] [PubMed] [Google Scholar]
- 7.Kisa PT, Kose M, Unal O, Er E, Hismi BO, Bulbul FS, et al. Clinical and molecular characteristics and time of diagnosis of patients with classical galactosemia in an unscreened population in Turkey. J Pediatr Endocrinol Metab. 2019;32((7)):675–81. doi: 10.1515/jpem-2018-0457. [DOI] [PubMed] [Google Scholar]
- 8.Kotb MA, Mansour L, Shamma RA. Screening for galactosemia: is there a place for it? Int J Gen Med. 2019;12:193–205. doi: 10.2147/IJGM.S180706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lak R, Yazdizadeh B, Davari M, Nouhi M, Kelishadi R. Newborn screening for galactosaemia. Cochrane Database Syst Rev. 2020;6((6)):Cd012272. doi: 10.1002/14651858.CD012272.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mulligan C, Bronstein JM. Wilson disease: an overview and approach to management. Neurol Clin. 2020;38((2)):417–32. doi: 10.1016/j.ncl.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Pasquali M, Yu C, Coffee B. Laboratory diagnosis of galactosemia: a technical standard and guideline of the American College of Medical Genetics and Genomics (ACMG) Genet Med. 2018;20((1)):3–11. doi: 10.1038/gim.2017.172. [DOI] [PubMed] [Google Scholar]
- 12.Posey JE, Harel T, Liu P, Rosenfeld JA, James RA, Coban Akdemir ZH, et al. Resolution of disease phenotypes resulting from multilocus genomic variation. N Engl J Med. 2017;376((1)):21–31. doi: 10.1056/NEJMoa1516767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pyhtila BM, Shaw KA, Neumann SE, Fridovich-Keil JL. Newborn screening for galactosemia in the United States: looking back, looking around, and looking ahead. JIMD Rep. 2014;15:79–93. doi: 10.1007/8904_2014_302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17((5)):405–24. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, Getz G, et al. Integrative genomics viewer. Nat Biotechnol. 2011;29((1)):24–6. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romeo G, Bittles AH. Consanguinity in the contemporary world. Hum Hered. 2014;77((1-4)):6–9. doi: 10.1159/000363352. [DOI] [PubMed] [Google Scholar]
- 17.Saudubray J-M, Berghe G, Walter JH. Inborn metabolic diseases. Berlin: Springer; 2012. [Google Scholar]
- 18.Simpson SM, MacKenzie JJ, Strube YNJ. Atypical late presentation of galactosemia. Can J Ophthalmol. 2019;54((4)):e194–6. doi: 10.1016/j.jcjo.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Sokol RJ, McCabe ER, Kotzer AM, Langendoerfer SI. Pitfalls in diagnosing galactosemia: false negative newborn screening following red blood cell transfusion. J Pediatr Gastroenterol Nutr. 1989;8((2)):266–8. [PubMed] [Google Scholar]
- 20.Weiss KH. Wilson Disease. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet] Seattle (WA): University of Washington, Seattle; 2016. [Google Scholar]
- 21.Weits-Binnerts JJ, Hordijk R, Smit GP, van der Veer E, Reijngoud DJ, Berger R. [Late diagnosis of classical galactosemia. An adult with special biochemistry] Tijdschr Kindergeneeskd. 1993;61((5)):188–90. [PubMed] [Google Scholar]
- 22.Welling L, Bernstein LE, Berry GT, Burlina AB, Eyskens F, Gautschi M, et al. International clinical guideline for the management of classical galactosemia: diagnosis, treatment, and follow-up. J Inherit Metab Dis. 2017;40((2)):171–6. doi: 10.1007/s10545-016-9990-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zlotogora J. Multiple mutations responsible for frequent genetic diseases in isolated populations. Eur J Hum Genet. 2007;15((3)):272–8. doi: 10.1038/sj.ejhg.5201760. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.