Abstract
Introduction
Autosomal dominant pathogenic variations in the CSNK2A1 gene cause Okur-Chung neurodevelopmental syndrome (OCNDS).
Methods
The proband and her parents were examined thoroughly and observed for any issues related to OCNDS. Furthermore, peripheral blood samples were collected from each subject for further investigations. Whole-exome sequencing identified a pathogenic variant in CSNK2A1 (NM_001895: c.62G>A, p.R21Q; rs1402734448).
Results
The proband has global developmental delay, speech disorders, epilepsy, and behavioral issues. Despite the previously reported cases, she manifested both atonic and myoclonic seizures simultaneously. Lastly, we provide a review of the reported cases with OCNDS.
Discussion
p.R21Q causes OCNDS. Further studies are highly recommended concerning this mutation to validate the results of this study and expand the knowledge regarding CSNK2A1 and the phenotypic spectrum of OCNDS.
Keywords: Okur-Chung neurodevelopmental syndrome, CSNK2A1, Epilepsy, Whole-exome sequencing
Introduction
Okur-Chung neurodevelopmental syndrome (OCNDS, MIM #617062) is a rare genetic disorder caused by mutations in CSNK2A1. It was first described by Okur et al. [2016]. In the following years, multiple additional studies characterized more than 30 patients with a diverse range of symptoms. Among the previously reported cases, neurological disorders show the highest interindividual consistency. Nevertheless, patients show significant variability even in the neurological presentations of OCNDS. For example, some of the patients show abnormal brain architecture and abnormalities in brain MRI while others do not exhibit any abnormality in the brain architecture [Okur et al., 2016; Chiu et al., 2018; Owen et al., 2018].
The alpha subunit of casein kinase 2 (CK2) is encoded by CSNK2A1. CK2 comprises 2 catalytic (α and α′) and 2 regulatory subunits (β) (heterodimers: αββα, αββα′, or α′ββα′) [Niefind et al., 2001]. CK2 is notably expressed in the mammalian brain [Díaz-Nido et al., 1994]. Interestingly, the α subunit is disproportionately expressed in the brain in comparison to α′, suggesting that the α subunit has another role, at least in the development or the function of the brain beyond the involvement in CK2 [Ceglia et al., 2011]. Animal studies showed that the disruption of the alpha catalytic subunit of protein kinase CK2 in mice results in death of mouse embryos, neural tube defects, cardiac malformation, and hydrops fetalis [Lou et al., 2008].
Herein, we studied the clinical features and interindividual variability in the presentation of OCNDS in various cases. Moreover, we report the first case of OCNDS in the Middle Eastern population caused by a novel variant.
Materials and Methods
Subjects
The proband, an 8-year-old Iranian girl, and her parents were examined thoroughly and observed for any issues related to the topic of this study. Furthermore, peripheral blood samples were collected from each subject for further investigations.
DNA Sequencing and Analysis
As per the manufacturer's instructions, genomic DNA was extracted from white blood cells of the peripheral blood samples using a QIAamp DNA Blood Mini Kit. Whole-exome sequencing (WES) was performed on the genomic DNA obtained from the blood using a HiSeq 3000/4000 SBS Kit.
Data Analysis
The raw data, converted by HiSeq X, were filtered and aligned against the human reference genome (hg19) utilizing the Burrows-Wheeler aligner [Li and Durbin, 2010]. The single-nucleotide polymorphisms were called by the GATK (Genome Analysis Toolkit). Variant annotation was carried out using ANNOVAR [Wang et al., 2010]. All variants were classified as stated in the standards for the interpretation of sequence variations recommended by ACMG [Richards et al., 2015]. The variants were designated as pathogenic, likely pathogenic, variants of unknown clinical significance, likely benign, and benign. The associated phenotypic features of candidate genes were evaluated against the phenotype of the patient. Core phenotypes were extracted and used to acquire a gene list of the virtual panel by the OMIM database (OMIM #617062). Re-annotation was carried out according to the virtual panel.
Sanger Sequencing
Polymerase chain reaction was carried out to amplify the fragments covering the mutated region. Chromas Lite v2.01 was utilized to analyze the results of the Sanger sequencing. The primers were designed using Oligo Primer Designer [Rychlik, 2007].
Bioinformatics Analysis
Molecular graphics and analyses were performed with UCSF Chimera, developed by the Resource for Biocomputing, Visualization, and Informatics, the University of California, San Francisco [Pettersen et al., 2004]. The Rotamers and Find Clashes/contacts tools of the UCSF Chimera were applied to analyze the substitution of arginine by glutamine [Shapovalov and Dunbrack, 2011]. The pathogenicity of the mutation was assessed by bioinformatics pathogenicity prediction tools [Chun and Fay, 2009; Reva et al., 2011; Sim et al., 2012; Schwarz et al., 2014; Quang et al., 2015; Shihab et al., 2015; Pejaver et al., 2017; Rogers et al., 2017; Sundaram et al., 2018; Malhis et al., 2020]. The effect of the mutation on the protein stability and dynamics was assessed by DynaMut, iStable 2.0, AUTO-MUTE 2.0, and I-mutant 2.0 [Capriotti et al., 2005; Masso and Vaisman, 2014; Pires et al., 2014; Rodrigues et al., 2018; Chen et al., 2020]. Protein domains were retrieved from InterPro [Hunter et al., 2008]. PDB ID: 5CSV and PDB ID: 3WAR were utilized for bioinformatics structural analysis.
Case Presentation
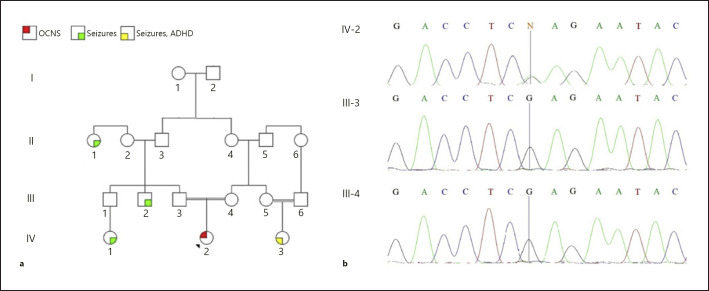
An 8-year-old Iranian girl presenting cognitive, motor, and language development delay was referred to our center. The proband is the product of an uneventful pregnancy and a normal vaginal delivery, born to consanguineous parents. Her birth weight, length, and head circumference were 2,950 g, 49.5 cm, and 33.0 cm, respectively. Hyperactive seizures accompanied by severe autistic features and isolated seizures are present in her family history (Fig. 1a).
Fig. 1.
Pedigree (a) and electropherogram (CSNK2A1) of the proband and her parents (b). IV-2, proband; III-3, her father; III-4, her mother.
The parents reported severe sleep disorders (as little as 2 h of sleep per 24 h) during her infancy. She has experienced several episodes of seizures between 4 and 6 years old. Her first report of seizures is an atonic seizure at the age of 4 marked by a sudden loss of the control of her head. The same kind of seizure happened again 1 month later. The second atonic seizure was followed by a myoclonic seizure on the following day. The myoclonic episode was marked by jerking spasms of the hands and global spasm for a minute, followed by atonia and fall of the head to the side. A week later, atonic seizures were repeated for 2 days, 6 to 8 times each day. A year later, 2 episodes of seizures were reported. The first seizure was described as spasms in the distal part of the upper extremities, clenching and jerking of the fists, and eye deviation to the side lasting about 4 min. Atonic seizures were observed before this episode. A month later, an isolated similar myoclonic seizure was repeated which lasted for at least 10 s.
At 6 years of age, urea cycle disorder (UCD) was diagnosed. At the time, free carnitine, butryl- and isobutyryl-L-carnitine, ammonia, citrulline, and histidine were abnormally high which are suggestive of inherited metabolic disorders. For the treatment of the UCD, she received sodium benzoate. Subsequently, she was referred for confirmation of the diagnosis and a liver transplantation. In due course, WES was carried out, which came up with surprising results. The only mutation that could justify the symptoms by attributing them to a metabolic disorder was a mutation in the MUT gene (NM_000255: c.1898T>G, p.V633G; rs200055428). Methylmalonyl-CoA mutase deficiency was ruled out because she was heterozygous for that mutation while the trait is an autosomal recessive one. To our surprise, another mutation in CSNK2A1 (NM_001895: c.62G>A, p.R21Q; rs1402734448) was detected, which could well justify the symptoms observed in the proband, raising doubts about the previous diagnosis. The metabolic tests were repeated, including ammonia, lactate, acylcarnitine profile, and amino acid profile, to elucidate the disease. All of the measurements were normal. For confirmation, ammonia was measured again 1 and 6 months later, coming up with normal results. The mutation in CSNK2A1 was not detected in her parents (Fig. 1b).
In physical examination, she presented several features suggestive of OCNDS. The behavioral assessment showed aggressive behavior and attention deficit hyperactivity disorder. She also speaks clumsily. She started to speak her very first words at the age of 5. She barely could speak at 6 years of age, which was improved later by speech therapy. She is not easy to interact with and has difficulty in interacting with her peers and communicating with others. Moreover, the parents reported that she used to show repetitive behaviors that have ceased recently. She also had difficulties in eye contact. These symptoms can be indicative of a mild autism spectrum disorder. As a result of the aforementioned issues, she has not attended school yet and her education is postponed. Her teeth show signs of dental compression accompanied by the mandibular protrusion. Five of her teeth are missing, and tooth decay is visible. There are no facial dysmorphic features.
On top of language development delay, she has intellectual disability. She also has a short stature, and her weight is low (121 cm and 21 kg, respectively; head circumference 48.5 cm). Her parents reported that she started sitting independently when she was 1 year old and walking when she was 2 years old. The parents stated that she was floppy and had poor head control until roughly 1 year of age and the classic “frog-leg” positioning when supine until 1.5 years of age. These features are pointing to hypotonia in infancy. Currently, she walks a bit clumsily and abnormally fast. Her walking disorder can be attributed to the short Achilles tendon, considering other similar reports in the literature and physical examinations. No other gross anatomical anomaly is recognizable in the lower extremities.
For further investigation, she went through a whole-body bone scan, liver and kidney ultrasonography, liver function test, blood chemistry, complete blood count, brain magnetic resonance imaging (MRI), and electroencephalography (EEG). The amino acid, acylcarnitine, organic acids, and acyl glycine profiles were also obtained. All of the mentioned workups were normal except for the EEG and brain MRI. EEG was performed on several occasions. The EEGs came up with rather conflicting results.
All of the EEGs showed abnormal background (diffusely slow; posterior dominant rhythm: 5–6 Hz) and architecture in sleep. They also showed that the photic stimulation and hyperventilation did not provoke an epileptic discharge. There were frequent and prolonged runs of 2–3 Hz bilateral spike waves, particularly in sleep. There also was excess polymorphic theta activity, which was especially frequent in the left mid-temporal, right occipital, and the motor cortex of the left hemisphere (any one of these findings was reported in separate EEGs). Concisely, the EEGs indicated that the findings are consistent with diffuse cerebral dysfunction and symptomatic focal epilepsy in the left temporal lobe.
In MRI, it was pointed out that there are nonspecific T2- and FLAIR hyperintense foci in periventricular white matter. In another MRI, evidence of 2 small high intensity foci in the white matter of the parietal lobe and a faint increase in the right trigon were present. Despite the findings of the 2 previous MRIs, another one was carried out 2 months later, which was completely normal.
The drugs and dosage used to treat the seizures were modified several times, according to the course of the seizures. Initially, she received sodium valproate. The medication was revised and replaced with Levetiracetam according to suboptimal response. Henceforth, Levetiracetam was quite successful in controlling the seizures. Since she was believed to have UCD, she received sodium benzoate until recently. Sodium benzoate was discontinued when the UCD diagnosis was called into question.
Bioinformatics Analysis
The R21Q mutation detected in our patient was predicted to be damaging and pathogenic by DANN, FATHMM-MKL, FATHMM-XF, LIST-S2, LRT, MutationTaster, and PrimateAI. Yet, several other tools suggested that the mutation is benign and tolerated including SIFT, MutPred, and Mutation Assessor.
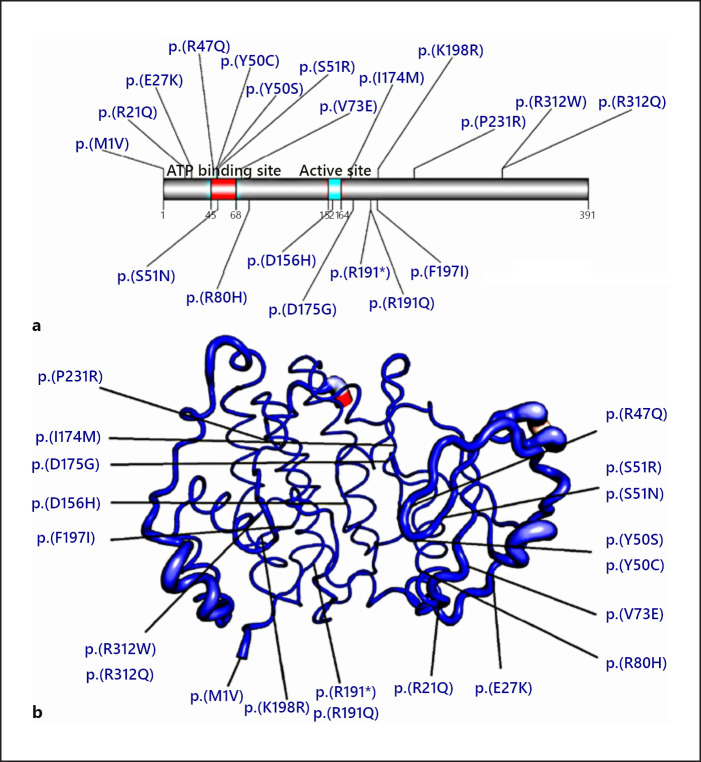
The stability of the protein, however, was predicted to be decreased. ΔΔG predicted by mCSM, Istable2.0, I-mutant 2.0, and Automute2.0 FR, respectively, were −0.191, −0.56474435, −0.57, and −0.84 kcal/mol. The mutated region is closely related to the E27, V73, and R80 amino acids. Mutations in the mentioned amino acids were previously reported to be pathogenic (Fig. 2; online suppl. Fig. 2; see www.karger.com/doi/10.1159/000522353 for all online suppl. material). Moreover, this mutation decreases the stability in the neighboring amino acids (online suppl. Fig. 1). The top 10 predicted positionings of the side chain of the glutamine amino acid, and the possible clash/es with the neighboring residues support the pathogenicity of the variant (online suppl. Fig. 3). The structural analysis is in line with the distortion and reduction of the stability in the region surrounding the mutation.
Fig. 2.
Representation of the mutations on the protein structure. a The previously reported pathogenic variants are shown in blue. b Atomic fluctuation. The magnitude of the fluctuation is represented by thin to thick tubes, colored blue (low), white (moderate), and red (high).
Discussion
In the present paper, we report an 8-year-old female born to consanguineous parents from Iran with a heterozygous novel variant (NM_001895: c.62G>A, p.R21Q; rs1402734448) in CSNK2A1. She presented with seizures and intellectual disability. The proband has speech disorder and does not communicate with her peers.
Here, we have reviewed 33 previously reported OCNDS cases (Table 1). The features are ranked from the most common to the rarest ones. Moreover, the prevalence of the traits among the OCNDS cases is assigned to each of them at the end of the table as a fraction of the whole. There are tens of different features associated with Okur-Chung syndrome; thus, we only included features observed in a large enough fraction of the cases.
Table 1.
A concise review of the phenotypes of the reported OCNDS patients
| ID | Sex | Age, years | Variants | DD | Language DD | Dysmorphic facial features | ID | Hypotonia | Behavioral issues | Eating disorders | Musculoskeletal disorders | Microcephaly | Sleep disorders | Abnormal brain MRI | Seizures | Herniation | GERD | History of recurrent infections | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subj. 1 | F | 8 | p.(R21Q) | + | + | + | + | + | + | – | + | – | + | – | + | – | – | – | Present case |
|
| |||||||||||||||||||
| Subj. 1 | F | 6 | p.(R47Q) | + | + | + | + | + | + | – | – | + | – | + | – | + | – | N/A | Okur et al. [2016] |
| Subj. 2 | F | 4.5 | p.(K198R) | + | + | + | + | + | + | + | + | + | – | + | – | – | + | N/A | |
| Subj. 3 | F | 4 | p.(D175G) | + | + | + | + | – | + | – | – | + | + | + | + | – | – | N/A | |
| Subj. 4 | F | 13 | c.824+2T>C | + | – | – | + | + | + | + | + | – | + | – | – | + | – | N/A | |
| Subj. 5 | F | 2 | p.(Y50S) | + | – | + | Ν/Α | + | – | + | + | + | – | – | + | – | – | + | |
|
| |||||||||||||||||||
| Subj. 1 | M | 5 | p.(K198R) | + | + | + | – | – | – | – | – | – | – | N/A | – | – | – | – | Chiu et al. [2018] |
| Subj. 2 | M | 4 | p.(E27K) | + | + | + | – | + | + | + | – | + | + | N/A | – | – | – | – | |
| Subj. 3 | M | 14 | p.(M1V) | + | – | – | + | + | + | + | + | – | + | – | – | + | – | + | |
| Subj. 4 | F | 2.5 | p.(P231R) | + | + | + | – | – | + | – | + | – | – | – | – | – | – | – | |
| Subj. 5 | M | 8 | p.(V73E) | + | + | – | – | – | – | + | – | + | + | – | – | – | + | – | |
| Subj. 6 | F | 8 | p.(R47Q) | + | + | + | – | + | – | + | + | + | + | + | + | – | – | – | |
| Subj. 7 | M | 14 | p.(R312Q) | + | + | + | – | – | – | – | – | – | – | N/A | – | – | – | – | |
| Subj. 8 | M | 4 | p.(S51R) | + | + | + | – | + | + | + | + | – | + | N/A | + | – | – | + | |
|
| |||||||||||||||||||
| Subj. 1 | M | 6.1 | p.(R47Q) | + | + | + | + | – | – | + | + | – | N/A | – | – | + | + | N/A | Owen et al. [2018] |
| Subj. 2 | F | 11.1 | p.(S51N) | + | + | + | + | – | – | – | + | – | N/A | N/A | – | – | – | N/A | |
| Subj. 3 | M | 10.7 | p.(R80H) | + | + | + | + | + | + | – | + | – | N/A | – | – | – | – | N/A | |
| Subj. 4 | M | 10.9 | p.(H74M) | + | + | – | + | + | + | + | – | – | N/A | N/A | – | – | – | N/A | |
| Subj. 5 | F | 10.0 | p.(R191Q) | + | + | – | + | + | + | + | – | + | N/A | N/A | – | – | – | N/A | |
| Subj. 6 | F | 8.0 | p.(F197l) | + | + | + | + | – | – | – | – | – | N/A | – | – | – | – | N/A | |
| Subj. 7 | F | 7.3 | p.(K198R) | + | + | + | + | + | + | – | – | – | N/A | – | + | – | – | N/A | |
| Subj. 8 | M | 10.9 | p.(R312W) | + | + | – | + | – | – | – | – | + | N/A | – | – | – | – | N/A | |
| Subj. 9 | M | 18.3 | p.(K198R) | + | N/A | + | + | + | – | – | – | – | N/A | N/A | + | – | – | N/A | |
| Subj. 10 | F | 18.7 | p.(K198R) | + | + | – | + | + | – | – | – | + | N/A | + | – | – | – | N/A | |
| Subj. 11 | F | 6.0 | p.(R312W) | + | – | + | + | + | + | – | + | – | N/A | N/A | – | – | – | N/A | |
|
| |||||||||||||||||||
| Subj. 1 | F | 15 | p.(K198R) | + | + | + | + | + | – | + | + | – | + | + | + | – | – | – | Nakashima et al. [2019] |
| Subj. 2 | M | 1.7 | p.(R191*) | + | – | – | Ν/Α | + | Ν/Α | Ν/Α | – | + | N/A | – | + | – | – | – | |
|
| |||||||||||||||||||
| Subj. 1 | M | 7 | p.(D156H) | + | + | + | + | – | + | – | – | + | + | + | – | – | – | – | Trinh et al. [2017] |
| Subj. 1 | M | 1 | c.1061-1G>C | + | – | – | – | + | + | – | – | – | + | + | – | – | – | – | Colavito et al. [2018] |
| Subj. 1 | M | 8 | p.(K198R) | + | + | + | + | + | + | – | – | – | – | + | – | – | – | – | Akahira-Azuma et al. [2018] |
| Subj. 1 | F | 5.8 | p.(Y50C) | + | + | + | – | – | + | + | – | – | – | + | – | – | – | – | Martinez-Monseny et al. [2020] |
|
| |||||||||||||||||||
| Total | 31/31 | 24/31 | 23/31 | 20/31 | 20/31 | 19/31 | 13/31 | 13/31 | 12/31 | 11/31 | 10/31 | 9/31 | 4/31 | 3/31 | 3/31 | ||||
|
| |||||||||||||||||||
| M | 6 | p.(K198R) | + | + | + | + | – | + | + | – | + | – | + | – | – | – | – | Xu et al. [2020]a | |
| M | 1 | p.(D175G) | + | – | + | – | + | – | + | + | – | + | – | – | – | – | – | Duan et al. [2019]b | |
| M | 8 | p.(Y50C) | + | Ν/Α | Ν/Α | + | Ν/Α | Ν/Α | Ν/Α | Ν/Α | Ν/Α | N/A | N/A | N/A | N/A | N/A | N/A | Wu et al. [2020]b | |
The patient was diagnosed with both tricho-rhino-phalangeal syndrome type I and OCNDS.
The full text is not available in English. Cases marked by a and b were not included in the evaluation of the total prevalence of the features. DD, developmental delay; ID, intellectual disability; OCNDS, Okur-Chung neurodevelopmental syndrome; Subj, subject; N/A, not available; GERD, gastroesophageal reflux disease.
The most prevalent symptom among the subjects is developmental delay, which has been universally reported (31/31). Despite the high prevalence of developmental delay, the reports show a wide range of variability in presentations, from very mild to the most severe cases.
A large fraction of the affected cases show speech disorders ranging from mild forms to aphasia (24/31). Namely, Chiu et al. [2018] reported that a case in their study had mild language developmental delay, while another one could not speak even a single word.
On a smaller scale, dysmorphic facial features are the third most common among the reported features (23/31). Typically, the cases show more than one facial feature, leaving out the case presented in our paper who merely has a mild mandibular protrusion.
Intellectual disability is also one of the most remarkable findings in the reports (20/31). Hypotonia, the state of low muscle tone, is also associated with OCNDS (20/31). The majority of the cases have distinct behavioral issues (19/31) including autism spectrum disorder features, anxiety, phonic and motor tics, and onychophagia.
A limited number of the cases show anomalies in the lower occipitofrontal circumference. Some of them develop microcephaly (12/31), while others are within a normal range. Interestingly, only a single patient has been described with macrocephaly [Owen et al., 2018].
Musculoskeletal disorders (12/31) observed in OCNDS include a wide range of anomalies such as joint hyperlaxity, hypermobility, kyphosis, butterfly vertebrae, scoliosis, hip dysplasia, congenital short Achilles tendon, etc.
Gastroesophageal reflux disease (GERD) and eating disorders are among the digestive disturbances. Although GERD is not significantly prevalent among eating patients (3/31), eating difficulties, in aggregate, are remarkable (13/31).
One of the relatively less common symptoms among the OCNDS cases is seizures (9/31). Various types of seizures occur among Okur-Chung patients, such as myoclonic, absence, tonic-clonic, and febrile seizures. Interestingly, our patient is the only reported case presenting 2 types of seizures, namely, absence and myoclonic seizures.
The data shown in Table 1 indicate that there is no strong correlation between MRI findings and OCNDS. Abnormal brain MRI findings were observed merely in 10 subjects. The majority of the findings are either nonspecific or not clinically significant. Roughly, both males and females are affected by OCNDS to a similar extent (18 males and 17 females). The full text of the reports by Duan et al. [2019] and Wu et al. [2020] was not available in English, and the patient described by Xu et al. [2020] had a dual molecular diagnosis of tricho-rhino-phalangeal syndrome type I and OCNDS. These reports were excluded and are reported separately at the bottom of Table 1.
According to the reports, this syndrome does not cause any direct fatality, except in one of the cases reported, who died from encephalitis. Those who suffer from OCNDS have to live with a crippling morbid condition.
Our patient presented severe developmental, behavioral, and speech disorders, which caused great difficulties for her and her family. Although some of the following symptoms were reported in previous cases, the subject of our study does not have any hyperflexibility in joints, herniation, GI-related symptoms, musculoskeletal, hepatic and renal (based on liver function test and ultrasonography), skin, or cardiovascular disorders.
Currently, there is no evidence available on the penetrance of the pathogenic mutations in the CSNK2A1 gene. As yet, all of the reported cases of OCNDS were caused by de novo mutations except for a case from China described by Xu et al. [2020], where the mutation was vertically transmitted from the proband's father. The authors report that the symptoms were present both in the father, carrying the defective mutation, and his son. Okur et al. [2016] suggested that because this trait is predominantly observed in females, X-chromosome inactivation might play a role in the pathophysiology of OCNDS. It was later refuted by Chiu et al. [2018] due to the fact that further studies described OCNDS male cases to the point that the male:female ratio reached just about 1:1. All variants reported thus far are de novo with no evidence of inheritance except the case described by Xu et al. [2020].
There are similarities between Okur-Chung syndrome and Kleefstra syndrome, Coffin-Siris syndrome, Rubinstein-Taybi syndrome, Gabriele-de Vries syndrome, epileptic encephalopathy 15, and a lot of other disorders [Akahira-Azuma et al., 2018; Khamirani et al., 2021, 2022]. All of the mentioned disorders present with a wide spectrum of symptoms including developmental delay, intellectual disability, and distinctive facial appearance. The majority of the symptoms are nonspecific, while the other symptoms are not definitive in differentiating between the different diagnoses. Hence, the necessity of either targeted sequencing with panels designed for these disorders or WES is pretty eminent. The correct diagnosis and detection of the causal mutation have a crucial impact on the management of these patients. Therefore, sequencing in patients presenting with symptoms mentioned above is highly desirable. In the current report, the meritoriousness of WES in diagnosing such disorders was clearly shown. Based on laboratory data, the patient was considered to have a metabolic disorder; however, using WES, it was further shown that the patient is a case of OCNDS.
Since the introduction and characterization of this syndrome by Okur et al. [2016], 33 patients have been identified, mainly in cohorts of cases with intellectual disability and related issues that were analyzed by various sequencing techniques. This suggests that OCNDS is relatively understudied and more prevalent than previously expected. It is quite plausible to assume that there are plenty of misdiagnosed patients, especially in underserved populations in underdeveloped regions. The current reports are probably only the tip of the iceberg which calls for further studies on the clinical and epidemiological aspects of this disease. Currently, WES seems to be the most suitable option for diagnosing this disease and similar disorders. The significance of appreciating this disease is emphasized when we take recent advances in prenatal diagnosis and therapeutic gene editing into account.
Statement of Ethics
Written informed consent was obtained from the patient's parents. The Ethics Committee of Shiraz University of Medical Sciences ethically approved this study (Ethics No.: IR.SUMS.MED.REC.1399.170).
Conflict of Interest Statement
The authors declare that there are no competing interests.
Funding Sources
Shiraz University of Medical Sciences financially supported this study. The funder was not involved in designing and writing of the manuscript or submission.
Author Contributions
H.J.K. and S.Z. designed the study. M.D., S.A.D., and S.M. conducted WES and Sanger sequencing. H.J.K. gathered the data. S.Z. and A.M. wrote the manuscript. A.M. and S.M.B.T. reviewed the literature. S.Z. and S.A.D. edited the final manuscript.
Data Availability Statement
All data generated or analyzed during this study are included in the final published article.
Supplementary Material
Supplementary data
Acknowledgement
The authors would like to thank Comprehensive Medical Genetics Center, Shiraz, Fars province, Iran for contributing to this study.
Funding Statement
Shiraz University of Medical Sciences financially supported this study. The funder was not involved in designing and writing of the manuscript or submission.
References
- 1.Akahira-Azuma M, Tsurusaki Y, Enomoto Y, Mitsui J, Kurosawa K. Refining the clinical phenotype of Okur-Chung neurodevelopmental syndrome. Hum Genome Var. 2018;5:18011. doi: 10.1038/hgv.2018.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Capriotti E, Fariselli P, Casadio R. I-Mutant2.0: predicting stability changes upon mutation from the protein sequence or structure. Nucleic Acids Res. 2005;33:W306–10. doi: 10.1093/nar/gki375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ceglia I, Flajolet M, Rebholz H. Predominance of CK2α over CK2α’ in the mammalian brain. Mol Cell Biochem. 2011;356:169–75. doi: 10.1007/s11010-011-0963-6. [DOI] [PubMed] [Google Scholar]
- 4.Chen CW, Lin MH, Liao CC, Chang HP, Chu YW. iStable 2.0: Predicting protein thermal stability changes by integrating various characteristic modules. Comput Struct Biotechnol J. 2020;18:622–30. doi: 10.1016/j.csbj.2020.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiu ATG, Pei SLC, Mak CCY, Leung GKC, Yu MHC, Lee SL, et al. Okur-Chung neurodevelopmental syndrome: Eight additional cases with implications on phenotype and genotype expansion. Clin Genet. 2018;93:880–90. doi: 10.1111/cge.13196. [DOI] [PubMed] [Google Scholar]
- 6.Chun S, Fay JC. Identification of deleterious mutations within three human genomes. Genome Res. 2009;19:1553–61. doi: 10.1101/gr.092619.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colavito D, Del Giudice E, Ceccato C, Dalle Carbonare M, Leon A, Suppiej A. Are CSNK2A1 gene mutations associated with retinal dystrophy? Report of a patient carrier of a novel de novo splice site mutation. J Hum Genet. 2018;63:779–81. doi: 10.1038/s10038-018-0434-y. [DOI] [PubMed] [Google Scholar]
- 8.Díaz-Nido J, Mizuno K, Nawa H, Marshak DR. Regulation of protein kinase CK2 isoform expression during rat brain development. Cell Mol Biol Res. 1994;40:581–5. [PubMed] [Google Scholar]
- 9.Duan HL, Peng J, Pang N, Chen SM, Xiong J, Guang SQ, et al. [A case of Okur-Chung syndrome caused by CSNK2A1 gene variation and review of literature] Zhonghua Er Ke Za Zhi. 2019;57:368–72. doi: 10.3760/cma.j.issn.0578-1310.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Hunter S, Apweiler R, Attwood T, Bairoch A, Bateman A, Binns D, et al. InterPro: The integrative protein signature database. Nucleic Acids Res. 2008;37:D211–5. doi: 10.1093/nar/gkn785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khamirani HJ, Zoghi S, Faghihi F, Dastgheib SA, Hassanipour H, Bagher Tabei SM, et al. Phenotype of ST3GAL3 deficient patients: A case and review of the literature. Eur J Med Genet. 2021;64:104250. doi: 10.1016/j.ejmg.2021.104250. [DOI] [PubMed] [Google Scholar]
- 12.Khamirani HJ, Zoghi S, Namdar ZM, Kamal N, Dianatpour M, Tabei SMB, et al. Clinical features of patients with Yin Yang 1 deficiency causing Gabriele‐de Vries syndrome: A new case and review of the literature. Ann Hum Genet. 2022;86:52–62. doi: 10.1111/ahg.12448. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics. 2010;26:589–95. doi: 10.1093/bioinformatics/btp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lou DY, Dominguez I, Toselli P, Landesman-Bollag E, O'Brien C, Seldin DC. The alpha catalytic subunit of protein kinase CK2 is required for mouse embryonic development. Mol Cell Biol. 2008;28:131–9. doi: 10.1128/MCB.01119-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malhis N, Jacobson M, Jones SJM, Gsponer J. LIST-S2: Taxonomy based sorting of deleterious missense mutations across species. Nucleic Acids Res. 2020;48:W154–61. doi: 10.1093/nar/gkaa288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez-Monseny AF, Casas-Alba D, Arjona C, Bolasell M, Casano P, Muchart J, et al. Okur-Chung neurodevelopmental syndrome in a patient from Spain. Am J Med Genet Part A. 2020;182:20–4. doi: 10.1002/ajmg.a.61405. [DOI] [PubMed] [Google Scholar]
- 17.Masso M, Vaisman II. AUTO-MUTE 2.0: A Portable Framework with Enhanced Capabilities for Predicting Protein Functional Consequences upon Mutation. Adv Bioinformatics. 2014;2014:278385. doi: 10.1155/2014/278385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakashima M, Tohyama J, Nakagawa E, Watanabe Y, Siew C, Kwong C, et al. Identification of de novo CSNK2A1 and CSNK2B variants in cases of global developmental delay with seizures. J Hum Genet. 2019;64:313–22. doi: 10.1038/s10038-018-0559-z. [DOI] [PubMed] [Google Scholar]
- 19.Niefind K, Guerra B, Ermakowa I, Issinger OG. Crystal structure of human protein kinase CK2: insights into basic properties of the CK2 holoenzyme. EMBO J. 2001;20:5320–31. doi: 10.1093/emboj/20.19.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okur V, Cho MT, Henderson L, Retterer K, Schneider M, Sattler S, et al. De novo mutations in CSNK2A1 are associated with neurodevelopmental abnormalities and dysmorphic features. Hum Genet. 2016;135:699–705. doi: 10.1007/s00439-016-1661-y. [DOI] [PubMed] [Google Scholar]
- 21.Owen CI, Bowden R, Parker MJ, Patterson J, Patterson J, Price S, et al. Extending the phenotype associated with the CSNK2A1-related Okur–Chung syndrome—A clinical study of 11 individuals. Am J Med Genet Part A. 2018;176:1108–14. doi: 10.1002/ajmg.a.38610. [DOI] [PubMed] [Google Scholar]
- 22.Pejaver V, Urresti J, Lugo-Martinez J, Pagel KA, Lin GN, Nam H-J, et al. MutPred2: inferring the molecular and phenotypic impact of amino acid variants. bioRxiv. 2017;134981 doi: 10.1038/s41467-020-19669-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, et al. UCSF Chimera—A visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–12. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 24.Pires DE, Ascher DB, Blundell TL. mCSM: predicting the effects of mutations in proteins using graph-based signatures. Bioinformatics. 2014;30:335–42. doi: 10.1093/bioinformatics/btt691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quang D, Chen Y, Xie X. DANN: a deep learning approach for annotating the pathogenicity of genetic variants. Bioinformatics. 2015;31:761–3. doi: 10.1093/bioinformatics/btu703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reva B, Antipin Y, Sander C. Predicting the functional impact of protein mutations: application to cancer genomics. Nucleic Acids Res. 2011;39:e118. doi: 10.1093/nar/gkr407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodrigues CH, Pires DE, Ascher DB. DynaMut: predicting the impact of mutations on protein conformation, flexibility and stability. Nucleic Acids Res. 2018;46:W350–5. doi: 10.1093/nar/gky300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogers MF, Shihab HA, Mort M, Cooper DN, Gaunt TR, Campbell C. FATHMM-XF: accurate prediction of pathogenic point mutations via extended features. Bioinformatics. 2017;34:511–3. doi: 10.1093/bioinformatics/btx536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rychlik W. OLIGO 7 primer analysis software. Methods Mol Biol. 2007;402:35–60. doi: 10.1007/978-1-59745-528-2_2. [DOI] [PubMed] [Google Scholar]
- 31.Schwarz JM, Cooper DN, Schuelke M, Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods. 2014;11:361–2. doi: 10.1038/nmeth.2890. [DOI] [PubMed] [Google Scholar]
- 32.Shapovalov MV, Dunbrack RL., Jr A smoothed backbone-dependent rotamer library for proteins derived from adaptive kernel density estimates and regressions. Structure. 2011;19:844–58. doi: 10.1016/j.str.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shihab HA, Rogers MF, Gough J, Mort M, Cooper DN, Day INM, et al. An integrative approach to predicting the functional effects of non-coding and coding sequence variation. Bioinformatics. 2015;31:1536–43. doi: 10.1093/bioinformatics/btv009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sim NL, Kumar P, Hu J, Henikoff S, Schneider G, Ng PC. SIFT web server: predicting effects of amino acid substitutions on proteins. Nucleic Acids Res. 2012;40:W452–7. doi: 10.1093/nar/gks539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sundaram L, Gao H, Padigepati SR, McRae JF, Li Y, Kosmicki JA, et al. Predicting the clinical impact of human mutation with deep neural networks. Nat Genet. 2018;50:1161–70. doi: 10.1038/s41588-018-0167-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trinh J, Hüning I, Budler N, Hingst V, Lohmann K, Gillessen-Kaesbach G. A novel de novo mutation in CSNK2A1: Reinforcing the link to neurodevelopmental abnormalities and dysmorphic features. J Hum Genet. 2017;62:1005–6. doi: 10.1038/jhg.2017.73. [DOI] [PubMed] [Google Scholar]
- 37.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu R, Tang W, Liang L, Li X, Ouyang N, Meng Z. [Identification of a novel de novo variant of CSNK2A1 gene in a boy with Okur-Chung neurodevelopmental syndrome] Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2020;37:641–4. doi: 10.3760/cma.j.issn.1003-9406.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 39.Xu S, Lian Q, Wu J, Li L, Song J. Dual molecular diagnosis of tricho-rhino-phalangeal syndrome type I and Okur-Chung neurodevelopmental syndrome in one Chinese patient: a case report. BMC Med Genet. 2020;21:158. doi: 10.1186/s12881-020-01096-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
All data generated or analyzed during this study are included in the final published article.