Abstract
Regulatory T (Treg) cells play an important role in implantation of the embryo and maintenance of pregnancy after allogeneic mating. Implantation failure, miscarriage, and preeclampsia are associated with decreased numbers of Treg cells or with dysfunctional Treg cells. Treg cells are classified into naturally occurring Treg (nTreg) cells or thymus-derived Treg (tTreg) cells that differentiate in the thymus and induce tolerance to self-antigens, while induced Treg (iTreg) or peripheral Treg (pTreg) cells differentiate in the periphery and induce transient tolerance to foreign antigens. Memory nTreg or iTreg cells were recently reported to accumulate in the uterus during early pregnancy and contribute to the establishment of pregnancy. Miscarriage is characterized by the downregulation of the total numbers of Treg cells rather than a downregulation of the numbers of paternal/fetal antigen-specific Treg cells. In addition to the volume of paternal/fetal antigen-specific CD8<sup>+</sup> T cells, the number of paternal/fetal antigen-specific Treg cells, which protect the fetus/placenta against maternal immune cell attack, increases after the second trimester of pregnancy. Clonal Treg cells which are surrogate markers of paternal/fetal antigen-specific Treg cells in humans may be involved in the development of preeclampsia during the mid- to late pregnancy stage, as evidenced by their downregulation in the decidua of preeclamptic cases. This review summarizes recent findings on Treg cells and discusses the roles, in the maintenance of pregnancy, of different types of Treg cells such as paternal/fetal antigen-specific Treg, pregnancy-associated memory Treg, nTreg (or tTreg), and iTreg (or pTreg cells).
Keywords: Miscarriage, Paternal antigen-specific regulatory T cells, Peripheral regulatory T cells, Preeclampsia, Thymus-derived regulatory T cells
Highlights of the Study
Fetal antigen-specific regulatory T (Treg) cells are a minor population in the early pregnancy period.
In miscarriage, the total numbers of Treg cells rather than the numbers of paternal/fetal antigen-specific Treg cells are downregulated.
Decidual PD-1− clonal CD8+ T cell numbers are upregulated in miscarriage.
In preeclampsia, decidual effector and clonal Treg cell numbers are downregulated.
Decidual PD-1− clonal CD8+ T cell numbers are upregulated in preeclampsia.
Introduction
As the fetus is a semi-allograft to the mother, decidual CD4+ and CD8+ T cells recognize paternal antigens, express activated antigens such as CD69 and HLA-DR [1], and produce various cytokines [2, 3]. Decidual CD4+ and CD8+ T cells mainly comprise effector memory T cells [3, 4, 5], suggesting that they recognize fetal/placental antigens locally at the maternal-fetal interface and subsequently undergo activation. In contrast, most CD4+ and CD8+ T cells in the peripheral blood are naïve T cells although maternal blood contains large amounts of syncytiotrophoblast-derived extracellular vesicles. These findings indicate that immune activation is limited to the maternal-fetal interface and is mediated by local regulatory mechanisms without the activation of systemic immunity.
Several mechanisms have been proposed to be involved in minimizing fetal rejection [6, 7, 8, 9, 10, 11, 12, 13, 14]. (1) Paternal/fetal antigens have been shown to not be presented directly to maternal T cells by fetal antigen-presenting cells (APCs), such as dendritic cells (DCs) but are instead presented indirectly to maternal T cells by maternal APCs, which decreases the probability of acute transplant rejection. (2) Maternal DCs are proposed to be trapped in the uterus, which minimizes fetal antigen presentation by uterine DCs in the uterine draining lymph nodes. (3) It has been hypothesized that maternal T cells recognize paternal/fetal antigens and undergo clonal depletion or exhaustion. (4) Paternal/fetal antigen-specific and paternal/fetal antigen-nonspecific regulatory T (Treg) cells have been demonstrated to suppress the activation of maternal T cells and natural killer (NK) cells. (5) Chemokine-encoding genes in the decidua have been proposed to prevent the migration of immune cells to the uterus. (6) The trophoblast expresses indoleamine 2,3-dioxygenase 1 (which inhibits immune activation) and secretes IL-10 and TGF-β (which suppress immune activation). (7) Progesterone, whose levels are upregulated during pregnancy, suppresses the production of Th1-type cytokines and upregulated the production of Th2 cytokines, thus supporting pregnancy; it also induces the production of progesterone-induced blocking factor which induces Th2-type cytokine production [15]. (8) HLA-G expressed on extravillous trophoblast (EVT) reduces the cytotoxic activity of CD8+T cells and NK cells.
These mechanisms protect the fetus and placenta from maternal T and NK cell-mediated immune attacks. Treg cells play a central role in inducing immunological tolerance to fetal/placental antigens [10, 11, 12, 13, 16, 17]. Recent studies have demonstrated that Treg cells regulate the cytotoxic activity of paternal/fetal antigen-specific effector T cell and also prevent the activation of paternal/fetal antigen-nonspecific T cells and NK cells. Treg cells are involved in placentation, especially in remodeling uterine spiral arteries [10, 18, 19, 20]. Thymus-derived naturally occurring Treg cells (nTreg cells or tTreg cells) suppress immune responses to self-antigens, and periphery-induced Treg cells (pTreg cells or iTreg cells) suppress immune responses to foreign antigens. This review focuses on the role of paternal/fetal antigen-specific and nonspecific Treg cells and the roles of nTreg (or tTreg) and pTreg (or iTreg) cells in pregnancy maintenance. It also discusses the downregulation of total numbers of Treg cell in miscarriage and numbers of paternal/fetal antigen-specific Treg cell in preeclampsia (relative to those in healthy pregnancies), which is suggested to contribute to the breakdown of tolerance between the mother and fetus.
The expression, by CD8+ cells, of programmed cell death protein 1 (PD-1), an immune check point molecule, induces immunosuppression. CD8+ cytotoxic T cells, which recognize the fetus and placenta, do not attack the fetus and placenta when PD-1 is expressed. Conversely, CD8+ cytotoxic T cells attack the fetus and placenta in the absence of PD-1 expression. The balance between these Treg cells and PD-1− CD8+ T cells in miscarriage and preeclampsia will be discussed based on recent studies.
Role of Paternal Antigen-Specific Treg Cells in the Maintenance of Pregnancy
Paternal antigens in the semen are phagocytosed and degraded into peptides by maternal APCs, and subsequently presented to maternal T cells in the uterine draining lymph nodes during implantation and the early pregnancy period. However, paternal antigens expressed on trophoblast-derived extracellular vesicles are systemically distributed upon spiral artery remodeling, which involves the replacement of vascular endothelial cells with trophoblasts in the uterine spiral artery (at day 10.5 post-coitus in mice and 16–18 weeks of gestation in humans). Subsequently, the paternal antigens are present in lymph nodes throughout the periphery [7, 10, 21] (Fig. 1).
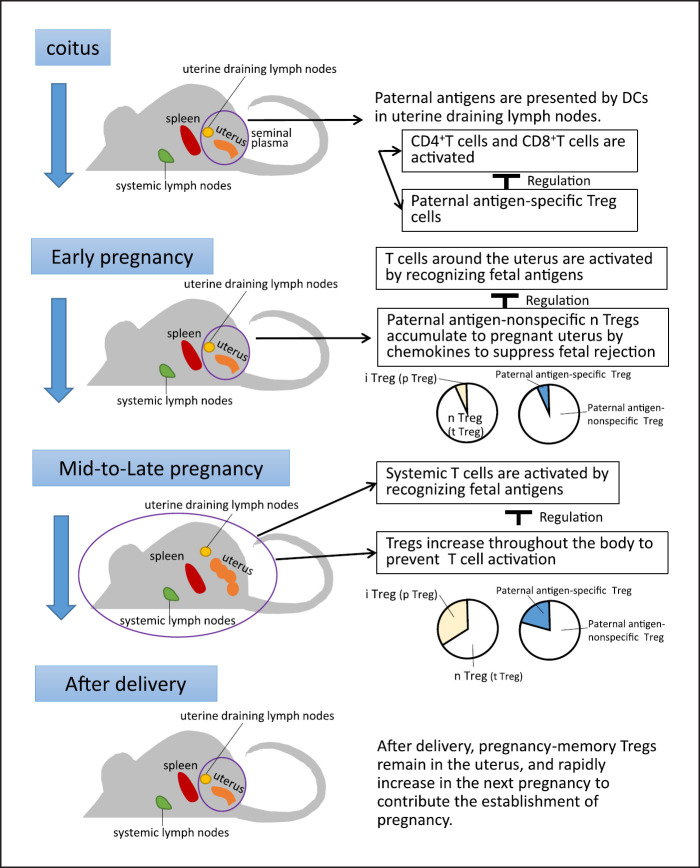
Fig. 1.
Role of regulatory T (Treg) cells in pregnancy (from sexual intercourse to the early and mid-pregnancy periods and postpartum). During sexual intercourse, paternal antigens in the seminal plasma induce paternal antigen-specific Treg cells and regulate T cell activation. In the early pregnancy period, maternal T cells are activated in the uterus and uterine regional lymph nodes. However, paternal antigen-nonspecific naturally occurring Treg (nTreg) cells, which suppress T cell and natural killer (NK) cell activation, are recruited to the uterus mainly by chemokines and the chemokine receptor system. From the mid-pregnancy period onward, fetal antigens spread throughout the body, which may lead to systemic T cell activation. To prevent this, the number of induced Treg (iTreg) cells is systemically increased to suppress the activation of T and NK cells. After parturition, the number of Treg cells decreases. However, some pregnancy-associated memory Treg cells remain in the uterus and rapidly increase in number during the next pregnancy, contributing to the maintenance of pregnancy.
The number of maternal ovalbumin (OVA)-specific CD4+ and CD8+ T cells systemically increases during the mid-pregnancy period in female mice that do not express OVA and are mated with male mice expressing OVA [6, 7, 21, 22]. Meanwhile, the number of CD4+ T cells that respond to the male antigen H-Y increased during this period when the fetus was male [23]. The proliferation of T cells in response to paternal antigens is limited to the uterine regional lymph nodes during implantation and the early pregnancy period [22] (Fig. 1). However, maternal T cells against paternal antigens are activated in the lymph nodes throughout the body from the mid-pregnancy period in mice [6, 7, 22] (Fig. 1). This indicates that the response of maternal T cells to paternal antigens is limited to the uterine draining lymph nodes during early pregnancy, but systemic immune regulation is necessary from the mid-pregnancy period because T cells throughout the body can recognize paternal antigens. Remodeling of the spiral arteries in humans begins at approximately week 10 of gestation and ends at approximately weeks 16–18 of gestation [24]. Hence, systemic presentation of paternal/fetal antigens may occur after the mid-pregnancy period. The activation of effector T cells that recognize paternal/fetal antigens is prevented by Treg cells in the uterus, which is the site of contact between the mother and fetus. The administration of anti-CD25 antibodies that reduces CD4+CD25+Treg cells to mice before implantation in allogeneic mating or the transient depletion of Foxp3+ Treg cells using the Foxp3 diphtheria toxin receptor increases the number of activated CD4+ and CD8+ T cells in the uterine draining lymph nodes, resulting in impaired implantation [25, 26, 27, 28]. Additionally, the number of Treg cells during the implantation period in allogeneic pregnancies is higher than that in syngeneic pregnancies [28]. This suggests that increased Treg cell numbers in allogeneic pregnancies play an important role in establishing implantation in mice [25, 26, 27].
In mice, it has been demonstrated that seminal plasma plays an important role in inducing paternal antigen-specific Treg cells. Seminal plasma priming is reported to be the mechanism underlying the accumulation of Treg cells in the para-aortic lymph nodes, which are uterine draining lymph nodes [11, 22, 29, 30, 31, 32]. The number of paternal antigen-specific and proliferating Treg cells is upregulated in the uterine draining lymph nodes by day 3.5 post-coitus and 1 day before implantation, peaking at day 11.5 post-coitus (mid-gestation period) but decreases to the levels observed in nonpregnant animals at day 18.5 post-coitus (late gestation) [29]. The number of paternal antigen-specific Treg cells in the uterine draining lymph nodes did not increase 1 day before implantation after mating with seminal vesicle-resected male mice (that lack seminal plasma but not sperm) [29]. In contrast, when female mice mated with males with ligated spermatic ducts (that lack sperm but not seminal plasma), the number of proliferating paternal antigen-specific Treg cells in the uterine draining lymph nodes 1 day before implantation was similar to that in females mated with nonsurgically modified males. This indicates that seminal plasma priming increases the number of paternal antigen-specific Treg cells in the uterine regional lymph nodes before implantation [29, 31]. In the uterus, the number of proliferating paternal antigen-specific Treg cells does not increase before implantation, but increases slightly on day 5.5 post-coitus and 1 day after implantation and markedly increases during the mid- to late pregnancy periods [29]. The number of proliferating paternal antigen-specific Treg cells in the uterine draining lymph nodes decreases during the late gestation period. This suggests that paternal/fetal antigen-specific Treg cells migrate from the uterine draining lymph nodes to the pregnant uterus during the mid- to late pregnancy periods.
Chemokine-mediated mechanisms are important for the accumulation of Treg in pregnant uteri. In mice, the expression of CCR7 on Treg cells and CCL19 on uterine tissue is critical for the semen-mediated accumulation of Treg cells [30]. However, one study reported that CCR7 expression was not upregulated in Treg cells from human endometrial samples obtained from the peri-implantation stage of the menstrual cycle [33]. Another study on mice showed that CCL4 was upregulated in the pregnant uterus, resulting in increased numbers of CCR5+ Treg cells with strong immunosuppressive activity [34]. A previous study reported that CCR4 and CCR5 are upregulated on proliferating paternal antigen-specific Treg cells and that the expression of CCR7 on proliferating paternal antigen-specific Treg cells was almost absent in mice [29]. CCR5 is expressed in both Ki67+ proliferative and Ki67− (nonactivated) paternal antigen-specific Treg cells, whereas CCR4 is highly expressed only in Ki67+ paternal antigen-specific Treg cells within the pregnant uterus. CCR4 may be expressed on Treg cells after the recognition of paternal antigens in mice [29]. Additionally, CCR8 expression is upregulated on Treg cells in the endometrium during the peri-implantation period in humans. The levels of expression of CCR1, CCR2, CCR5, CXCR3, and CXCR6 in Treg cells in the pregnant uterus are higher than those in Treg cells in the peripheral blood in human [33, 35]. These chemokine receptors may play an important role in the uterine accumulation of Treg cells from the periphery. As CCR5+ and CXCR3+ Treg cells are critical for suppressing Th1 immunity in mice and humans [36, 37], the accumulation of Treg cells in the pregnant uterus may prevent fetal rejection. Thus, despite some differences in chemokine receptors expressed on Treg cells in mice and humans, chemokines play an important role in accumulation of Treg cells in the gestational uterus in both species.
Treg cells in the placental bed share transcriptional signals with tumor-infiltrating Treg cells, suggesting that Treg cells may accumulate at the implantation site through a mechanism common to both pregnancy and tumors in mice and humans [13, 35, 38]. Tumor antigen-nonspecific Treg cells reach the tumor microenvironment before CD8+ T cells; this prevents tumor cells from being attacked by host immune cells. The paternal/fetal antigen-specific Treg cells are hypothesized to be unimportant for the early stages of pregnancy maintenance, whereas memory Treg cells that react with self-antigens are important. This hypothesis explains the nature and properties of Treg cells in the early pregnancy period; however, further studies are needed to validate this hypothesis.
Rowe et al. [21] demonstrated that the number of paternal/fetal antigen-specific Treg cells increases from the mid-pregnancy period to approximately 100-fold by the end of gestation and decreases slightly after parturition but is approximately 10-fold higher at day 100 postpartum in mice (Fig. 1). Additionally, paternal/fetal antigen-specific Treg cells express Ki67 during pregnancy and exhibit a Ki67-negative phenotype after parturition in mice [21]. Thus, these Treg cells recognize paternal/fetal antigens, and their numbers increase during pregnancy, but these Treg cells stop proliferating after parturition owing to the lack of fetal antigens. During the second pregnancy, fetal antigen-specific Treg cells that remain after delivery rapidly increase in number after conception and promote pregnancy maintenance in mice [21]. Granne et al. [33] performed transcriptome analysis of Treg cells collected from the peri-implantation endometrium of multiparous and nulliparous women and reported a marked difference in gene expression in humans. Treg cells remember past pregnancies and may remain in the uterus after delivery in mice and humans. During the next pregnancy with the same partner, the number of Treg cells increases rapidly, which contributes to the establishment of tolerance to paternal/fetal antigens (Fig. 1). NKG2C+ (CD159C+) and leukocyte immunoglobulin-like receptor 1+ NK cells in the uterus also remember past pregnancies and produce enhanced levels of vascular endothelial growth factor α and interferon γ during the subsequent pregnancy, which is beneficial for placentation in humans [39]. The cooperation between Treg and uterine NK cells may aid in establishing a second or subsequent pregnancy.
We will proceed to review paternal/fetal antigen-specific Treg cells in humans. Decidual Treg cells exhibit the capacity to mediate fetal antigen-specific suppression in mixed lymphocyte reaction between mother lymphocytes and fetus-derived lymphocytes [40]. This indicates that fetal antigen-specific Treg cells are present in utero in humans. It is difficult to identify paternal-antigen-specific Treg cells in humans because models that express specific antigens like transgenic mice cannot be applied to humans. My research group hypothesized that human maternal Treg cells with T cell receptors (TCRs) clonally proliferate if they recognize fetal antigens. Therefore, effector Treg cells from the decidua and peripheral blood were subjected to single-cell sorting. Subsequently, the TCRs of each Treg cell were sequenced using reverse transcription polymerase chain reaction. Treg cells with the same TCR sequence were defined as clonal Treg cells. The peripheral blood did not contain clonal Treg cells during both early and late pregnancy periods, while clonal Treg cells were detected in the decidua. The average proportion of clonal Treg cells in the early and late pregnancy periods were 9.3% (4.4–14.5%) and 20.9% (15.4–28.1%), respectively. The number of clonal Treg cells increased with the progression of pregnancy [41]. The same clonotype decidual Treg cells were detected in previous and subsequent pregnancies, suggesting that these clonotype decidual Treg cells recognize the same paternal/fetal antigens [41]. Additionally, peripheral blood and decidual Treg cells rarely shared a common sequence [41] indicating that Treg cells that recognize paternal/fetal antigens are localized and remain in the pregnant uterus.
Wienke et al. [35] demonstrated that Treg cells in the placental bed exhibit a tissue-resident phenotype with high expression of CD69, an important tissue-residency molecule, and a gene expression pattern similar to that of tissue-resident CD4+ T cells in the lungs and skin in humans. Treg cells in the placental bed express higher levels of Treg markers such as FOXP3, cytotoxic T-lymphocyte-associated protein 4, and T cell immunoreceptor with Ig and ITIM domains (TIGIT) compared to those in the nonplacental bed area of the uterus. Additionally, Treg cells in the placental bed exhibit upregulated levels of immune checkpoint molecules, such as glucocorticoid-induced tumor necrosis factor receptor family-related gene (GITR), tumor necrosis factor (TNF) receptor superfamily member 1B, TNF receptor superfamily member 4, 4-1BB (CD137), IL10, hepatitis A virus cellular receptor 2, Layilin, and programmed cell death 1, all of which are associated with immunosuppression. This suggests that the gene profile of Treg cells in the placental bed is similar to that of tumor-infiltrating Treg cells [35]. Although Weinke et al. [35] did not examine paternal/fetal antigen-specific Treg cells, clonally proliferated Treg cells have similar characteristics and may not be detectable in peripheral blood because they are localized to the uterus.
Roles of nTreg and iTreg Cells in Maintaining Pregnancy
Treg cells can be classified into nTreg cells (or tTreg cells) which differentiate in the thymus in response to self-antigens and pTreg cells (or iTreg cells) which differentiate in the periphery in response to antigens in peripheral tissues and cytokines such as TGF-β [42]. nTreg cells express FOXP3 for prolonged durations and inhibit T cell activation through cell contact [42, 43, 44, 45, 46]. In contrast, pTreg cells express FOXP3 only for a short duration and exert transient immunosuppressive effects. Markers of nTreg cells include Helios, neuropilin 1 (NRP1), and Ig superfamily protein glycoprotein A 33, while pTreg cells exhibit an Helios−, NRP1−, and glycoprotein A 33− phenotype although the specificity of these markers is controversial [47, 48, 49].
The conserved CNS1, an enhancer of FOXP3, is essential for the differentiation of pTreg cells. Samstein et al. [19] proposed that pTreg cells play a central role in the immunological acceptance of the placenta of a semi-allograft fetus in mammals. The abortion rate in allogenic CNS1 knock-out mouse pregnancies is approximately 10%, which is slightly higher than that in wild-type mouse pregnancies (approximately 5%) but not higher than that in syngeneic pregnancies [19]. Rowe et al. [21] reported that fetal antigen-specific Treg cells mainly comprise nTreg cells with upregulated expression of Helios during early pregnancy in mice, while the number of fetal antigen-specific pTreg cells with a Helios-negative phenotype increases in the late pregnancy period. The analysis of the expression pattern of NRP1 (a marker of nTreg cells) and the nTreg-specific demethylation pattern of the FOXP-encoding gene region revealed that the number of nTreg cells (tTreg cells) increases after immune stimulation by semen in the uterine draining lymph nodes of mice [50]. These Treg cells are Ki67+ dividing cells. The expression levels of cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and GITR, which are involved in immunosuppression, are upregulated in NRP1+ nTreg cells, which are strongly immunosuppressive [50]. Treg cells are reported to expand in the thymus during the early pregnancy period in mice [51]. Thus, nTreg cells may have a critical role during early pregnancy [21, 50, 51, 52].
Several fetal/placental antigens access the thymus via the bloodstream and are presented by thymic medullary epithelial cells and induced to differentiate [53]. Large amounts of fetal antigens are transported via extracellular vesicles in the post-mid-term stage of pregnancy. Autoimmune regulator (Aire)-deficient mice lack nTreg cells and exhibit implantation failure and embryonic loss [54]. This indicates that nTreg cells are essential for implantation and success of pregnancy during the early stages. The miscarriage rate when all Treg cells are depleted is higher (approximately 50%) than that when pTreg cells are absent (approximately 10%) [16, 21, 27]. In humans, Helios+ nTreg cells account for more than 90% of the decidual cells during early pregnancy [52]. Additionally, the number of Helios− iTreg cells increases toward the end of gestation [55], which is consistent with findings in mice. Furthermore, the efflux of Treg cells from the thymus decreases in the late gestation phase [56]. The activity of progesterone during pregnancy results in thymic involution. Therefore, the pool of nTreg cells decreases during the late pregnancy period in mice [57]. In humans, the number of PD-1hi Helios+ FOXP3+ nTreg cells increases via cell contact in co-cultures of EVTs and CD4+ T cells [58]. PD-1 regulates the immune system and promotes self-tolerance by suppressing inflammatory T cell activity. The administration of anti-HLA-C antibodies decreases the induction of PD-1hi Treg cells, suggesting that the immunosuppressive function of nTreg cells is enhanced via HLA-C expressed on EVT [58]. These findings indicate that the nTreg cells are abundant in the uterus during early pregnancy, the number of iTreg cells increases in the late pregnancy period, and each type of Treg cell plays a different role in the early and late pregnancy periods to sustain pregnancy in mice and humans (Fig. 1).
Pathophysiological Aspects of Miscarriage and Involvement of Treg Cells
The uterine abundance of Treg cells in allogeneic pregnancies is higher than that in syngeneic pregnancies [28]. Depletion of Treg cells results in an increased frequency of abortions in allogeneic pregnancies in mice. Meanwhile, abortions of some male fetuses but not those of female fetuses are frequent in syngeneic pregnancies in mice [16, 21, 23, 27]. The decidua of women with miscarriages and normal physiological fetal chromosomal content exhibited decreased numbers of effector Treg cells with strong immunosuppressive activity and increased numbers of effector T cells that can attack the fetus [52, 59]. The expression of sphingosine-1 phosphate receptor 1 (s1pr1), which is involved in lymphocyte activation and migration, is upregulated in uterine Treg cells during the peri-implantation period in cases of recurrent pregnancy loss (RPL) in humans. However, the expression of TIGIT, which is involved in immunosuppression of T cells via the TIGIT-CD155 pathway, and the function of uterine Treg cells are downregulated in RPL cases before conception in humans [32, 52, 59]. Thus, the function of Treg cells in the uterus is impaired in RPL even before conception.
These results demonstrate that Treg cells are necessary for establishing pregnancy during the peri-implantation period. However, the role of paternal/fetal antigen-specific Treg cells in establishing pregnancy during the peri-implantation period is unclear. Currently, experimental systems using mice have been used to eliminate Treg cells, but the specific elimination of paternal/fetal antigen-specific Treg cells during gestation has not been achieved yet.
A previous study hypothesized that immunological aberrations are the etiological factors for miscarriage in human pregnancies with normal fetal chromosomal content [12]. Although the number of effector Treg cells in the decidua was downregulated in cases of miscarriage with normal fetal chromosomes, the proportion of clonal Treg cells among Treg cells (a surrogate marker for paternal/fetal antigens-specific Treg cells) was unchanged in humans [41]. In contrast, in miscarriage with a normal fetal chromosomal content, effector memory CD8+ T cell populations comprised increased proportions of immune checkpoint molecule PD-1− clonal CD8+ T cells in humans [5]. This suggests that increased maternal clonal PD-1− CD8+ T cells that recognize paternal/fetal antigens and clonally expanded, a surrogate marker for paternal/fetal antigens-specific CD8+ T cells, may attack the fetus leading to miscarriage (Fig. 2). In early pregnancy, memory effector Treg cells, which are paternal/fetal antigen-nonspecific Treg cells, suppress the widespread activation of T cells and NK cells (Fig. 2). Chen et al. demonstrated that, in early murine pregnancy, pre-existing CD44high CD62low activated/memory Treg cells accumulate at implantation sites in allogeneic pregnancies and play a role in the maintenance of pregnancy [38]. The number of these activated/memory Treg cells increases from day 4 post-coitus, which corresponds to the implantation phase, especially in the uterine regional lymph nodes of allogeneic pregnancies, and plateaus at day 7 post-coitus. These Treg cells are similar to those found in tumors [13, 38]. Recently, Wienke et al. [35] demonstrated similarities between Treg cells that accumulate in the placental bed and those at tumor sites in humans. The duration for the differentiation of progenitor CD4+ T cells into paternal/fetal antigen-specific iTreg cells is approximately 1 week in vitro [31]. Hence, activated/memory nTreg cells are recruited to the uterus during the implantation period and suppress the activation of antigen-nonspecific T cells and NK cells, resulting in successful pregnancy. As the number of paternal/fetal antigen-specific CD8+ T cells systemically increases after the mid-pregnancy period in mice and humans [5, 6, 9, 22], the number of paternal/fetal antigen-specific Treg cells may also increase after the mid-pregnancy period and suppress rejection (Fig. 2). In cases of HLA-C mismatch between mother and fetus, the numbers of activated CD4+ T cells and Treg cells are reported to increase in the decidua in term pregnancy [60], suggesting that paternal HLA-C antigen-specific Treg cells are involved in maintaining pregnancy after the mid-pregnancy period in humans. Future studies should clarify the role of the downregulation of HLA-C-specific Treg cells in miscarriage.
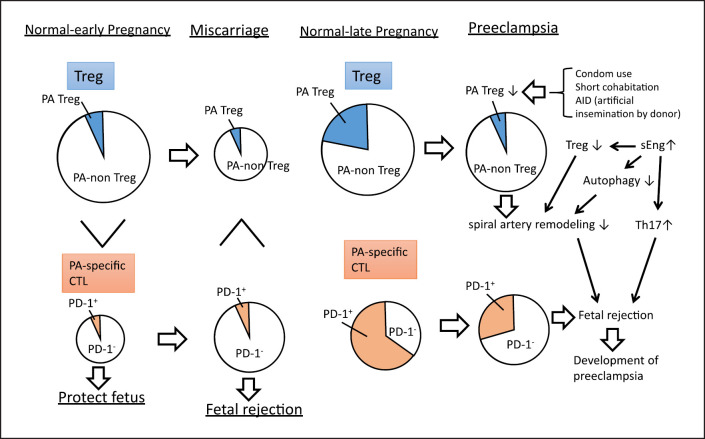
Fig. 2.
Changes in regulatory T (Treg) cells in miscarriage and preeclampsia. The size of the circle represents the cell numbers. The frequencies of paternal antigen-specific Treg (PA-Treg) and PA-nonspecific Treg (PA-non-Treg) among Treg cells, and PD-1+ cytotoxic T (PD-1+CTL) and PD-1−CTL among CTL are shown. The total number of Treg cells in miscarriage is lower than that in healthy early pregnancy, while the percentage of paternal antigen-specific Treg cells remains unchanged. Compared with that in healthy early pregnancies, the number of paternal antigen-specific cytotoxic T cells is higher in miscarriage. In the early pregnancy period, PD-1− paternal antigen-specific cytotoxic T cells are predominant, resulting in fetal rejection. In late normal pregnancy, the total numbers of Treg cells and paternal antigen-specific Treg cells are upregulated. In preeclampsia, there is not only a decrease in Treg volume, but also a decrease in PA-Treg cell rate. As shown in the upper right of the figure, in cases of inadequate semen exposure, such as condom use, short cohabitation, and sperm donor pregnancies, mothers are at risk for preeclampsia due to insufficient induction of paternal antigen-specific Treg cells. In addition to the decrease in Treg cell volume and ratio of paternal antigen-specific Treg cells to nonpaternal antigen-specific Treg cells, increased secretion of soluble endoglin (sEng) may suppress the autophagy of endovascular trophoblasts (EVTs) in preeclampsia (middle right panel). This results in impaired remodeling of spiral arteries. In addition, sEng neutralizes TGF-β, thus inhibiting the differentiation into Treg cells and promoting differentiation into inflammatory Th17 cells (middle right panel). Additionally, PD-1 expression in paternal antigen-specific CTLs is downregulated, and the fetus may be attacked by maternal cytotoxic T cells, contributing to the development of preeclampsia (lower right).
Involvement of Treg Cells in the Pathophysiology of Preeclampsia
Epidemiological studies have revealed that preeclampsia is more common in the first pregnancy than in subsequent pregnancies and also in the first pregnancy with a new partner (even in multiparous women). Women are at an increased risk for developing preeclampsia if more than 10 years have elapsed after the last pregnancy (even when the risk is corrected for age). This indicates that the inadequate induction of maternal tolerance to the fetus is involved in the pathogenesis of preeclampsia [61, 62, 63]. Other epidemiological findings, such as an increased risk of developing preeclampsia in couples who use condoms [64], couples with a short duration of cohabitation [64], and sperm donation pregnancies [65], suggest that inadequate induction of paternal antigen-specific Treg cells by seminal plasma is a pathological factor for the development of preeclampsia. Seminal plasma induces paternal-specific Treg cells. The epidemiological risk factors observed in preeclampsia are absent in RPL, suggesting that preeclampsia and RPL are both associated with decreased numbers of Treg cells but differ in the type of Treg cells.
Reduced uterine arterial perfusion pressure (RUPP) is an animal model for preeclampsia [66]. However, RUPP does not cause hypertension in nude rats, which lack the thymus and T cells [67]. This indicates that T cells are important in the pathogenesis of preeclampsia. The infusion of Th17 cells collected from rats subjected to RUPP into wild-type pregnant rats increased the blood pressure and decreased the pup weight, indicating that Th17 cells are involved in preeclampsia pathogenesis [68]. Meanwhile, the infusion of Treg cells from wild-type pregnant rats into female rats subjected to RUPP resulted in decreased blood pressure and reduced serum levels of IL-17 and TNFα [69]. CD4+ T cells differentiate into Treg cells in an environment with high levels of TGF but differentiate into Th17 cells upon downregulation of TGF-β and upregulation of inflammatory cytokines [70]. Levels of soluble endoglin (sEng) are elevated in patients with preeclampsia even before the onset of the disease [71, 72]. This may explain the decrease in numbers of Treg cells and the increase in numbers of Th17 cells [70] as sEng binds to TGF-β and neutralizes its effects. The number of decidual IL-17-producing Treg cells in preeclamptic pregnancies is higher than that in healthy pregnancy [73].
Several clinical studies have demonstrated that in addition to the decreased number of Treg cells in the decidua and peripheral blood, the immunosuppressive function of Treg cells is downregulated in preeclamptic women [74, 75]. In preeclampsia, Treg cells are prone to apoptosis owing to the downregulation of the anti-apoptotic B-cell CLL/lymphoma 2 (Bcl-2) and the upregulation of the pro-apoptotic B-cell CLL/lymphoma 2-associated X (BAX) protein [76]. sEng inhibits autophagy and neutralizes TGTβ, resulting in the suppression of Treg differentiation [70]. Furthermore, autophagy is required for Treg function. Impaired autophagy promotes Treg cell dysfunction and apoptosis [77] (Fig. 2 middle right panel). In patients with preeclampsia, Treg cell differentiation is aberrant, and the life span of Treg cells is low due to apoptotic cell death.
The mechanism through which decreased numbers of Treg cells contribute to preeclampsia deserves attention. Trophoblast invasion in women with preeclampsia, especially in the early-onset type, is shallower than that in healthy pregnancies. Additionally, placentas of preeclamptic patients exhibit impaired remodeling of the spiral arteries, resulting in insufficient blood flow to the placenta [78, 79] (stage 1). After 20 weeks of gestation, maternal environmental factors, genetic factors, and substances produced by the hypoxic placenta (including sFlt-1 and sEng) cause systemic vascular endothelial damage (stage 2), leading to hypertension, proteinuria, and fetal growth restriction.
Autophagy deficiency is involved in the mechanism of insufficient spiral artery remodeling during stage 1 [80]. Increased levels of serum sEng in preeclampsia are attributed to placental hypoxic stress. Transient depletion of Treg cells in mice by administering anti-CD25 antibodies in early pregnancy increases uterine artery vascular resistance owing to impaired spiral artery remodeling in the mid-pregnancy period [20]. Additionally, levels of the inflammatory cytokines/chemokines interferon γ (IFN-γ), TNFα, IL-1, IL-17, and Ccl2 are elevated in these mice. These phenotypes are similar to those observed in preeclampsia although the mice did not develop hypertension. However, the blood pressure in anti-CD25 antibody-treated (i.e., Treg-depleted) mice administered with NG-nitro-L-arginine methyl ester hydrochloride (L-NAME), an inhibitor of NO synthesis, was higher than that in mice treated administered with L-NAME alone. The depletion of Treg cells in the late pregnancy period did not exacerbate hypertension or proteinuria [20]. Thus, the downregulation of Treg cell numbers is associated with the development of preeclampsia although this is not the sole cause of preeclampsia. Redman et al. [81] hypothesized that in addition to shallow placentation, excessive inflammation may cause preeclampsia. Shallow placentation due to decreased numbers of Treg cells and excessive inflammation due to increased numbers of Th17 cells may contribute to the development of preeclampsia. Future studies should focus on the involvement of the immune system, metabolism, and genetic predisposition in preeclampsia.
Early-onset preeclampsia, which occurs before 34 weeks of gestation, may be associated with an impairment of the immune system and inadequate induction of tolerance, resulting in shallow placentation. In contrast, late-onset preeclampsia, which occurs after 34 weeks of gestation, may be associated with metabolic syndromes such as obesity, gestational diabetes, and hyperlipidemia as the main pathogenic factors. The early-onset and late-onset forms of preeclampsia are reported to exhibit distinct pathophysiology [63]. This hypothesis is supported by the fact that the number of Treg cells in early-onset preeclampsia is markedly decreased when compared with that in late-onset preeclampsia [82]. Additionally, late-onset preeclampsia increases rapidly after 34 weeks of gestation in obese patients [83, 84].
The number of Helios-positive nTreg cells is high in the early pregnancy period, while the number of iTreg cells is high in the late pregnancy period. However, the number of iTreg cells does not increase in preeclampsia, resulting in a decrease in total Treg volume (nTreg cells and iTreg cells combined) [55] (Fig. 2). In healthy pregnancy, CD14+ DC-SIGN (CD209)+ APCs in the decidua express high levels of HLA-G and ILT4 that induce pTreg cells. However, in preeclampsia, CD14+ CD209DC-SIGN+ APCs in the decidua do not exhibit sufficient expression of HLA-G and ILT4. Hsu et al. [55] proposed that the production of iTreg cells is inadequate in preeclampsia. However, another study [74] reported that the ratio of iTreg cells to nTreg cells did not change in preeclampsia, suggesting that further studies are needed.
A previous study examined decidual Treg cells that recognize paternal/fetal antigens and clonally proliferate and reported that the number of clonal Treg cells in preeclampsia decreases by 50% when compared with that in healthy pregnancy [41] (Fig. 2). The number of clonal Treg cells does not decrease in women with miscarriage. Semen exposure induces paternal antigen-specific Treg cells [22, 30, 31, 32]. The risk of developing preeclampsia is high in couples who use condom contraception [64], where exposure to semen is for short periods [85], and when the recipient is not exposed to semen in sperm donation pregnancies [65], which is consistent with the above results. However, these epidemiologic risk factors are not associated with miscarriage, suggesting that decreased paternal/fetal antigen-specific Treg cells are closely related to the pathogenesis of preeclampsia. Paternal antigen-specific Treg cells are present after the first delivery [21, 33] and rapidly increase in number during the second pregnancy, inducing paternal antigen-specific tolerance [21]. Future studies should determine whether the number of clonal Treg cells changes between first and second pregnancies with the same partner.
The cytotoxic activity against paternal antigens is high in preeclampsia [86]. CD8+ T cells that recognize paternal antigens are present during healthy pregnancy but paternal antigen-specific CD8+ T cells are in an exhausted state [87, 88] and cannot attack EVT presenting paternal antigens or cells presenting fetal antigens. Although paternal antigen-specific CD8+ T cells are present after delivery, they exhibit an exhausted phenotype during the second pregnancy and do not attack EVT presenting fetal/paternal antigens [87]. Stimulation by PD-1 and lymphocyte-activation gene 3 (LAG3) plays an important role in the exhausted phenotype of CD8+ T cells against paternal antigens [87]. The level of expression of PD-1 on clonal CD8+ T cells in the decidua of preeclamptic pregnancies is less than 40% when compared with that in healthy pregnancies, indicating that the fetus/placenta is vulnerable to attack by maternal CD8+ T cells [5]. Fetal rejection can result from a decrease in the number of clonal Treg cells in preeclampsia and an increase in the number of clonal CD8+ T cells that recognize the fetus (Fig. 2). This suggests that the fetus/placenta is attacked by the maternal immune system during preeclampsia in humans.
Conclusion
According to recent studies, memory Treg cells, similar to those present at tumor sites, are present before conception and play an important role in implantation and the early pregnancy period. Memory Treg cells are potentially fetal antigen-nonspecific Treg cells. Fetal antigen-specific nTreg and iTreg cells constitute a minor population of Treg cells during early pregnancy. iTreg cells play an ancillary role in maintaining pregnancy during the early pregnancy period. In human miscarriage, the number of effector Treg cells in the decidua decreases while the number of clonal Treg cells does not. However, the number of PD-1− clonal CD8+ T cells increases in the decidua of women with miscarriage, suggesting that the fetus/placenta can be attacked. The number of Treg cells that recognize paternal/fetal antigens increases from the mid-pregnancy period. As the number of iTreg cells also increases, paternal/fetal antigen-specific Treg cells after the mid-pregnancy period comprise nTreg and iTreg cells. In preeclampsia, the number of effector and clonal Treg cells decreases in the decidua, which can be interpreted as a decrease in the number of paternal/fetal antigen-specific Treg cells. The number of clonal PD-1− CD8+ T cells increases in preeclampsia cases, suggesting the induction of paternal/fetal antigen-specific cytotoxicity in preeclampsia.
Therefore, strategies to increase memory nTreg cells that react with self-antigens need to be developed to treat implantation failure and miscarriages. Meanwhile, strategies must be developed to increase the number of paternal/fetal antigen-specific Treg cells to treat preeclampsia. Further studies will aid in the development of Treg cell-based therapies for implantation failure, unexplained miscarriages, and preeclampsia.
Conflict of Interest Statement
The author has no conflict of interest to declare.
Funding Sources
This study was supported by JST Moonshot R&D (Grant No. JPMJMS2021).
Author Contributions
Shigeru Saito prepared and edited the manuscript.
Funding Statement
This study was supported by JST Moonshot R&D (Grant No. JPMJMS2021).
References
- 1.Saito S, Nishikawa K, Morii T, Narita N, Enomoto M, Ichijo M. Expression of activation antigens CD69 interleukin-2 receptor-alpha (IL-2R alpha) and IL-2R beta on T cells of human decidua at an early stage of pregnancy. Immunology. 1992;75((4)):710–2. [PMC free article] [PubMed] [Google Scholar]
- 2.Saito S, Nakashima A, Shima T, Tsuda S. Pregnancy depends on a delicate balance of immune activation and regulation. Explor Immunol. 2021;1:461–78. [Google Scholar]
- 3.Powell RM, Lissauer D, Tamblyn J, Beggs A, Cox P, Moss P, et al. Decidual T cells exhibit a highly differentiated phenotype and demonstrate potential fetal specificity and a strong transcriptional response to IFN. J Immunol. 2017;199((10)):3406–17. doi: 10.4049/jimmunol.1700114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tilburgs T, Schonkeren D, Eikmans M, Nagtzaam NM, Datema G, Swings GM. Human decidual tissue contains differentiated CD8+ effector-memory T cells with unique properties. J Immunol. 2010;185((7)):4470–7. doi: 10.4049/jimmunol.0903597. [DOI] [PubMed] [Google Scholar]
- 5.Morita K, Tsuda S, Kobayashi E, Hamana H, Tsuda K, Shima T, et al. Analysis of TCR repertoire and PD-1 expression in decidual and peripheral CD8+ T cells reveals distinct immune mechanisms in miscarriage and preeclampsia. Front Immunol. 2020;11:1082. doi: 10.3389/fimmu.2020.01082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erlebacher A, Vencato D, Price KA, Zhang D, Glimcher LH. Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. J Clin Invest. 2007;117((5)):1399–411. doi: 10.1172/JCI28214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erlebacher A. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat Rev Immunol. 2013;13((1)):23–33. doi: 10.1038/nri3361. [DOI] [PubMed] [Google Scholar]
- 8.Erlebacher A. Immunology of the maternal-fetal interface. Annu Rev Immunol. 2013;31((1)):387–411. doi: 10.1146/annurev-immunol-032712-100003. [DOI] [PubMed] [Google Scholar]
- 9.Moldenhauer LM, Hayball JD, Robertson SA. Utilising T cell receptor transgenic mice to define mechanisms of maternal T cell tolerance in pregnancy. J Reprod Immunol. 2010;87((1–2)):1–13. doi: 10.1016/j.jri.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Robertson SA, Care AS, Moldenhauer LM. Regulatory T cells in embryo implantation and the immune response to pregnancy. J Clin Invest. 2018;128((10)):4224–35. doi: 10.1172/JCI122182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guerin LR, Prins JR, Robertson SA. Regulatory T-cells and immune tolerance in pregnancy a new target for infertility treatment? Hum Reprod Update. 2009;15((5)):517–35. doi: 10.1093/humupd/dmp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsuda S, Nakashima A, Shima T, Saito S. New paradigm in the role of regulatory T cells during pregnancy. Front Immunol. 2019;10:573. doi: 10.3389/fimmu.2019.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jørgensen N, Persson G, Hviid TVF. The tolerogenic function of regulatory T cells in pregnancy and cancer. Front Immunol. 2019;10:911. doi: 10.3389/fimmu.2019.00911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kieffer TEC, Laskewitz A, Scherjon SA, Faas MM, Prins JR. Memory T cells in pregnancy. Front Immunol. 2019;10:625. doi: 10.3389/fimmu.2019.00625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piccinni MP, Raghupathy R, Saito S, Szekeres-Bartho J. Cytokines, hormones and cellular regulatory mechanisms favoring successful reproduction. Front Immunol. 2021;12:717808. doi: 10.3389/fimmu.2021.717808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5((3)):266–71. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 17.Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod. 2004;10((5)):347–53. doi: 10.1093/molehr/gah044. [DOI] [PubMed] [Google Scholar]
- 18.Yamashita T, Sasaki N, Kasahara K, Hirata KI. Anti-inflammatory and immune-modulatory therapies for preventing atherosclerotic cardiovascular disease. J Cardiol. 2015;66:1–8. doi: 10.1016/j.jjcc.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. 2012;150((1)):29–38. doi: 10.1016/j.cell.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Care AS, Bourque SL, Morton JS, Hjartarson EP, Robertson SA, Davidge ST. Reduction in regulatory T cells in early pregnancy causes uterine artery dysfunction in mice. Hypertension. 2018;72((1)):177–87. doi: 10.1161/HYPERTENSIONAHA.118.10858. [DOI] [PubMed] [Google Scholar]
- 21.Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490((7418)):102–6. doi: 10.1038/nature11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moldenhauer LM, Diener KR, Thring DM, Brown MP, Hayball JD, Robertson SA. Cross-presentation of male seminal fluid antigens elicits T cell activation to initiate the female immune response to pregnancy. J Immunol. 2009;182((12)):8080–93. doi: 10.4049/jimmunol.0804018. [DOI] [PubMed] [Google Scholar]
- 23.Kahn DA, Baltimore D. Pregnancy induces a fetal antigen-specific maternal T regulatory cell response that contributes to tolerance. Proc Natl Acad Sci U S A. 2010;107((20)):9299–304. doi: 10.1073/pnas.1003909107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pijnenborg R, Dixon G, Robertson WB, Brosens I. Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta. 1980;1:3–19. doi: 10.1016/s0143-4004(80)80012-9. [DOI] [PubMed] [Google Scholar]
- 25.Darrasse-Jèze G, Darasse-Jèze G, Klatzmann D, Charlotte F, Salomon BL, Cohen JL. CD4+CD25+ regulatory/suppressor T cells prevent allogeneic fetus rejection in mice. Immunol Lett. 2006;102((1)):106–9. doi: 10.1016/j.imlet.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Zenclussen AC, Gerlof K, Zenclussen ML, Sollwedel A, Bertoja AZ, Ritter T, et al. Abnormal T-cell reactivity against paternal antigens in spontaneous abortion adoptive transfer of pregnancy-induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am J Pathol. 2005;166((3)):811–22. doi: 10.1016/S0002-9440(10)62302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shima T, Sasaki Y, Itoh M, Nakashima A, Ishii N, Sugamura K, et al. Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J Reprod Immunol. 2010;85((2)):121–9. doi: 10.1016/j.jri.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Zhao JX, Zeng YY, Liu Y. Fetal alloantigen is responsible for the expansion of the CD4(+)CD25(+) regulatory T cell pool during pregnancy. J Reprod Immunol. 2007;75((2)):71–81. doi: 10.1016/j.jri.2007.06.052. [DOI] [PubMed] [Google Scholar]
- 29.Shima T, Inada K, Nakashima A, Ushijima A, Ito M, Yoshino O, et al. Paternal antigen-specific proliferating regulatory T cells are increased in uterine-draining lymph nodes just before implantation and in pregnant uterus just after implantation by seminal plasma-priming in allogeneic mouse pregnancy. J Reprod Immunol. 2015;108:72–82. doi: 10.1016/j.jri.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Guerin LR, Moldenhauer LM, Prins JR, Bromfield JJ, Hayball JD, Robertson SA. Seminal fluid regulates accumulation of FOXP3+ regulatory T cells in the preimplantation mouse uterus through expanding the FOXP3+ cell pool and CCL19-mediated recruitment. Biol Reprod. 2011;85((2)):397–408. doi: 10.1095/biolreprod.110.088591. [DOI] [PubMed] [Google Scholar]
- 31.Shima T, Nakashima A, Yasuda I, Ushijima A, Inada K, Tsuda S, et al. Uterine CD11c+ cells induce the development of paternal antigen-specific Tregs via seminal plasma priming. J Reprod Immunol. 2020;141:103165. doi: 10.1016/j.jri.2020.103165. [DOI] [PubMed] [Google Scholar]
- 32.Schjenken JE, Sharkey DJ, Green ES, Chan HY, Matias RA, Moldenhauer LM, et al. Sperm modulate uterine immune parameters relevant to embryo implantation and reproductive success in mice. Commun Biol. 2021;4((1)):572. doi: 10.1038/s42003-021-02038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Granne I, Shen M, Rodriguez-Caro H, Chadha G, O'Donnell E, Brosens JJ, et al. Characterisation of peri-implantation endometrial Treg and identification of an altered phenotype in recurrent pregnancy loss. Mucosal Immunol. 2022;15((1)):120–9. doi: 10.1038/s41385-021-00451-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kallikourdis M, Andersen KG, Welch KA, Betz AG. Alloantigen-enhanced accumulation of CCR5+ ‘effector’ regulatory T cells in the gravid uterus. Proc Natl Acad Sci U S A. 2007;104((2)):594–9. doi: 10.1073/pnas.0604268104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wienke J, Brouwers L, van der Burg LM, Mokry M, Scholman RC, Nikkels PG, et al. Human Tregs at the materno-fetal interface show site-specific adaptation reminiscent of tumor Tregs. JCI Insight. 2020;5((18)):e137926. doi: 10.1172/jci.insight.137926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Littringer K, Moresi C, Rakebrandt N, Zhou X, Schorer M, Dolowschiak T. Common features of regulatory T cell specialization during Th1 responses. Front Immunol. 2018;9:1344. doi: 10.3389/fimmu.2018.01344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang N, Schröppel B, Lal G, Jakubzick C, Mao X, Chen D, et al. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity. 2009;30((3)):458–69. doi: 10.1016/j.immuni.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen T, Darrasse-Jèze G, Bergot AS, Courau T, Churlaud G, Valdivia K, et al. Self-specific memory regulatory T cells protect embryos at implantation in mice. J Immunol. 2013;191((5)):2273–81. doi: 10.4049/jimmunol.1202413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gamliel M, Goldman-Wohl D, Isaacson B, Gur C, Stein N, Yamin R, et al. Trained memory of human uterine NK cells enhances their function in subsequent pregnancies. Immunity. 2018;48((5)):951.e5–962.e5. doi: 10.1016/j.immuni.2018.03.030. [DOI] [PubMed] [Google Scholar]
- 40.Tilburgs T, Roelen DL, van der Mast BJ, de Groot-Swings GM, Kleijburg C, Scherjon SA, et al. Evidence for a selective migration of fetus-specific CD4+CD25bright regulatory T cells from the peripheral blood to the decidua in human pregnancy. J Immunol. 2008;180((8)):5737–45. doi: 10.4049/jimmunol.180.8.5737. [DOI] [PubMed] [Google Scholar]
- 41.Tsuda S, Zhang X, Hamana H, Shima T, Ushijima A, Tsuda K, et al. Clonally expanded decidual effector regulatory T cells increase in late gestation of normal pregnancy, but not in preeclampsia, in humans. Front Immunol. 2018;9:1934. doi: 10.3389/fimmu.2018.01934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–7. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 43.Lal G, Zhang N, van der Touw W, Ding Y, Ju W, Bottinger EP, et al. Epigenetic regulation of Foxp3 expression in regulatory T cells by DNA methylation. J Immunol. 2009;182((1)):259–73. doi: 10.4049/jimmunol.182.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kitagawa Y, Ohkura N, Sakaguchi S. Molecular determinants of regulatory T cell development the essential roles of epigenetic changes. Front Immunol. 2013;4:106. doi: 10.3389/fimmu.2013.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463((7282)):808–12. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toker A, Engelbert D, Garg G, Polansky JK, Floess S, Miyao T. Active demethylation of the Foxp3 locus leads to the generation of stable regulatory T cells within the thymus. J Immunol. 2013;190((7)):3180–8. doi: 10.4049/jimmunol.1203473. [DOI] [PubMed] [Google Scholar]
- 47.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, et al. Expression of Helios an Ikaros transcription factor family member differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184((7)):3433–41. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiss JM, Bilate AM, Gobert M, Ding Y, Curotto de Lafaille MA, Parkhurst CN. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells but not mucosa-generated induced Foxp3+ Treg cells. J Exp Med. 2012;209((10)):1723–42. doi: 10.1084/jem.20120914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szurek E, Cebula A, Wojciech L, Pietrzak M, Rempala G, Kisielow P, et al. Differences in expression level of Helios and neuropilin-1 do not distinguish thymus-derived from extrathymically-induced CD4+Foxp3+ regulatory T cells. PLoS One. 2015;10:e0141161. doi: 10.1371/journal.pone.0141161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moldenhauer LM, Schjenken JE, Hope CM, Green ES, Zhang B, Eldi P, et al. Thymus-derived regulatory T cells exhibit Foxp3 epigenetic modification and phenotype attenuation after mating in mice. J Immunol. 2019;203((3)):647–57. doi: 10.4049/jimmunol.1900084. [DOI] [PubMed] [Google Scholar]
- 51.Thuere C, Zenclussen ML, Schumacher A, Langwisch S, Schulte-Wrede U, Teles A, et al. Kinetics of regulatory T cells during murine pregnancy. Am J Reprod Immunol. 2007;58((6)):514–23. doi: 10.1111/j.1600-0897.2007.00538.x. [DOI] [PubMed] [Google Scholar]
- 52.Inada K, Shima T, Ito M, Ushijima A, Saito S. Helios-positive functional regulatory T cells are decreased in decidua of miscarriage cases with normal fetal chromosomal content. J Reprod Immunol. 2015;107:10–9. doi: 10.1016/j.jri.2014.09.053. [DOI] [PubMed] [Google Scholar]
- 53.Ahn SH, Nguyen SL, Petroff MG. Exploring the origin and antigenic specificity of maternal regulatory T cells in pregnancy. Front Immunol. 2020;11:1302. doi: 10.3389/fimmu.2020.01302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Warren BD, Ahn SH, McGinnis LK, Grzesiak G, Su RW, Fazleabas AT, et al. Autoimmune regulator is required in female mice for optimal embryonic development and implantation. Biol Reprod. 2019;100((6)):1492–504. doi: 10.1093/biolre/ioz023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hsu P, Santner-Nanan B, Dahlstrom JE, Fadia M, Chandra A, Peek M, et al. Altered decidual DC-SIGN+ antigen-presenting cells and impaired regulatory T-cell induction in preeclampsia. Am J Pathol. 2012;181((6)):2149–60. doi: 10.1016/j.ajpath.2012.08.032. [DOI] [PubMed] [Google Scholar]
- 56.Wagner MI, Jöst M, Spratte J, Schaier M, Mahnke K, Meuer S, et al. Differentiation of ICOS+ and ICOS− recent thymic emigrant regulatory T cells (RTE T regs) during normal pregnancy pre-eclampsia and HELLP syndrome. Clin Exp Immunol. 2015;183((1)):129–42. doi: 10.1111/cei.12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tibbetts TA, DeMayo F, Rich S, Conneely OM, O'Malley BW. Progesterone receptors in the thymus are required for thymic involution during pregnancy and for normal fertility. Proc Natl Acad Sci U S A. 1999;96((21)):12021–6. doi: 10.1073/pnas.96.21.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salvany-Celades M, van der Zwan A, Benner M, Setrajcic-Dragos V, Bougleux Gomes HA, Iyer V, et al. Three types of functional regulatory T cells control T cell responses at the human maternal-fetal interface. Cell Rep. 2019;27((9)):2537.e5–2547.e5. doi: 10.1016/j.celrep.2019.04.109. [DOI] [PubMed] [Google Scholar]
- 59.Keller CC, Eikmans M, van der Hoorn MP, Lashley LEELO. Recurrent miscarriages and the association with regulatory T cells a systematic review. J Reprod Immunol. 2020;139:103105. doi: 10.1016/j.jri.2020.103105. [DOI] [PubMed] [Google Scholar]
- 60.Tilburgs T, Scherjon SA, van der Mast BJ, Haasnoot GW, Versteeg-vdVoort-Maarschalk M, Roelen DL, et al. Fetal-maternal HLA-C mismatch is associated with decidual T cell activation and induction of functional T regulatory cells. J Reprod Immunol. 2009;82((2)):148–57. doi: 10.1016/j.jri.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 61.Saito S, Sakai M, Sasaki Y, Nakashima A, Shiozaki A. Inadequate tolerance induction may induce pre-eclampsia. J Reprod Immunol. 2007;76((1–2)):30–9. doi: 10.1016/j.jri.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 62.Kenny LC, Kell DB. Immunological tolerance, and preeclampsia. the roles of semen microbes and the father. Front Med. 2017;4:239. doi: 10.3389/fmed.2017.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robillard PY, Dekker G, Scioscia M, Saito S. Progress in the understanding of the pathophysiology of immunologic maladaptation related to early-onset preeclampsia and metabolic syndrome-related to late-onset preeclampsia. Am J Obstet Gynecol. 2022;226((2)):S867–S875. doi: 10.1016/j.ajog.2021.11.019. [DOI] [PubMed] [Google Scholar]
- 64.Klonoff-Cohen HS, Savitz DA, Cefalo RC, McCann MF. An epidemiologic study of contraception and preeclampsia. JAMA. 1989;262((22)):3143–7. [PubMed] [Google Scholar]
- 65.González-Comadran M, Avila JU, Tascón AS, Jimenéz R, Solà I, Brassesco M, et al. The impact of donor insemination on the risk of preeclampsia a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol X. 2014;182:160–166. doi: 10.1016/j.ejogrb.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 66.Gilbert J, Dukes M, LaMarca B, Cockrell K, Babcock S, Granger J. Effects of reduced uterine perfusion pressure on blood pressure and metabolic factors in pregnant rats. Am J Hypertens. 2007;20((6)):686–91. doi: 10.1016/j.amjhyper.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 67.Novotny S, Wallace K, Herse F, Moseley J, Darby M, Heath J, et al. CD4+ T cells play a critical role in mediating hypertension in response to placental ischemia. J Hypertens. 2013;2((02)):14873. doi: 10.4172/2167-1095.1000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cornelius DC, Amaral LM, Wallace K, Campbell N, Thomas AJ, Scott J, et al. Reduced uterine perfusion pressure T-helper 17 cells cause pathophysiology associated with preeclampsia during pregnancy. Am J Physiol Regul Integr Comp Physiol. 2016;311((6)):R1192–R1199. doi: 10.1152/ajpregu.00117.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cornelius DC, Amaral LM, Harmon A, Wallace K, Thomas AJ, Campbell N. An increased population of regulatory T cells improves the pathophysiology of placental ischemia in a rat model of preeclampsia. Am J Physiol Regul Integr Comp Physiol. 2015;309((8)):R884–R891. doi: 10.1152/ajpregu.00154.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saito S. Th17 cells and regulatory T cells new light on pathophysiology of preeclampsia. Immunol Cell Biol. 2010;88((6)):615–7. doi: 10.1038/icb.2010.68. [DOI] [PubMed] [Google Scholar]
- 71.Venkatesha S, Toporsian M, Lam C, Hanai JI, Mammoto T, Kim YM, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12((6)):642–9. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 72.Farina A, Sekizawa A, De Sanctis P, Purwosunu Y, Okai T, Cha DH, et al. Gene expression in chorionic villous samples at 11 weeks gestation from women destined to develop preeclampsia. Prenat Diagn. 2008;28((10)):956–61. doi: 10.1002/pd.2109. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Y, Liu Z, Tian M, Hu X, Wang L, Ji J, et al. The altered PD-1/PD-L1 pathway delivers the ‘one-two punch’ effects to promote the Treg/Th17 imbalance in pre-eclampsia. Cell Mol Immunol. 2018;15((7)):710–23. doi: 10.1038/cmi.2017.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Toldi G, Vásárhelyi ZE, Rigó J, Jr, Orbán C, Tamássy Z, Bajnok A, et al. Prevalence of regulatory T-cell subtypes in preeclampsia. Am J Reprod Immunol. 2015;74((2)):110–5. doi: 10.1111/aji.12380. [DOI] [PubMed] [Google Scholar]
- 75.Green S, Politis M, Rallis KS, Saenz de Villaverde Cortabarria A, Efthymiou A, Mureanu N. Regulatory T cells in pregnancy adverse outcomes a systematic review and meta-analysis. Front Immunol. 2021;12:737862. doi: 10.3389/fimmu.2021.737862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Darmochwal-Kolarz D, Saito S, Tabarkiewicz J, Kolarz B, Rolinski J, Leszczynska-Gorzelak B, et al. Apoptosis signaling is altered in CD4+CD25+FoxP3+ T regulatory lymphocytes in pre-eclampsia. Int J Mol Sci. 2012;13((6)):6548–60. doi: 10.3390/ijms13066548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wei J, Long L, Yang K, Guy C, Shrestha S, Chen Z. Autophagy enforces functional integrity of regulatory T cells by coupling environmental cues and metabolic homeostasis. Nat Immunol. 2016;17((3)):277–85. doi: 10.1038/ni.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roberts JM, Hubel CA. The two stage model of preeclampsia variations on the theme. Placenta. 2009;30((Suppl l)):S32–S37. doi: 10.1016/j.placenta.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Staff AC. The two-stage placental model of preeclampsia an update. J Reprod Immunol. 2019:134–135. doi: 10.1016/j.jri.2019.07.004. 1–10. [DOI] [PubMed] [Google Scholar]
- 80.Nakashima A, Yamanaka-Tatematsu M, Fujita N, Koizumi K, Shima T, Yoshida T. Impaired autophagy by soluble endoglin under physiological hypoxia in early pregnant period is involved in poor placentation in preeclampsia. Autophagy. 2013;9((3)):303–16. doi: 10.4161/auto.22927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Redman CW, Sacks GP, Sargent IL. Preeclampsia an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180((2)):499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 82.Quinn KH, Lacoursiere DY, Cui L, Bui J, Parast MM. The unique pathophysiology of early-onset severe preeclampsia role of decidual T regulatory cells. J Reprod Immunol. 2011;91((1–2)):76–82. doi: 10.1016/j.jri.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 83.Bicocca MJ, Mendez-Figueroa H, Chauhan SP, Sibai BM. Maternal obesity and the risk of early-onset and late-onset hypertensive disorders of pregnancy. Obstet Gynecol. 2020;136((1)):118–27. doi: 10.1097/AOG.0000000000003901. [DOI] [PubMed] [Google Scholar]
- 84.Robillard PY, Dekker G, Scioscia M, Bonsante F, Iacobelli S, Boukerrou M, et al. Increased BMI has a linear association with late-onset preeclampsia a population-based study. PLoS One. 2019;14((10)):e0223888. doi: 10.1371/journal.pone.0223888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Robillard PY, Périanin J, Miri E, Janky E, Hulsey T, Papiernik E. Association of pregnancy-induced hypertension with duration of sexual cohabitation before conception. Lancet. 1994;344((8928)):973–5. doi: 10.1016/s0140-6736(94)91638-1. [DOI] [PubMed] [Google Scholar]
- 86.de Groot CJ, van der Mast BJ, Visser W, De Kuiper P, Weimar W, Van Besouw NM. Preeclampsia is associated with increased cytotoxic T-cell capacity to paternal antigens. Am J Obstet Gynecol. 2010;203((5)):496.e1–496.e6. doi: 10.1016/j.ajog.2010.06.047. [DOI] [PubMed] [Google Scholar]
- 87.Kinder JM, Turner LH, Stelzer IA, Miller-Handley H, Burg A, Shao TY, et al. CD8+ T cell functional exhaustion overrides pregnancy-induced fetal antigen alloimmunization. Cell Rep. 2020;31((12)):107784. doi: 10.1016/j.celrep.2020.107784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lewis EL, Xu R, Beltra JC, Ngiow SF, Cohen J, Telange R. NFAT-dependent and -independent exhaustion circuits program maternal CD8 T cell hypofunction in pregnancy. J Exp Med. 2022;219((1)):e20201599. doi: 10.1084/jem.20201599. [DOI] [PMC free article] [PubMed] [Google Scholar]