Abstract
Heart disease is one of the leading causes of death in the United States. Isolation and culture adult cardiomyocytes are important for studying cardiomyocyte contractility, heart hypertrophy, and cardiac failure. In contrast to neonatal cardiomyocyte isolation, adult mice cardiomyocytes isolation is challenging due to firm connections among cardiomyocytes through intercalated discs. The availability of newly generated genetically modified mouse lines requires to establish protocols to isolation and culture adult mouse cardiomyocyte for in vitro studies. In this manuscript, we described a straightforward method of isolating adult mouse cardiomyocytes using Langendorff perfusion apparatus. Briefly, the hearts were harvested from adult mice and the heart was mounted to Lagendorff apparatus. After perfusion with calcium depletion and collagenase digestion, the left ventricles were minced and filtered. Lastly, the separated cardiomyocytes were treated with CaCl2. The isolated cardiac myocytes can be utilized in a broad range of experiments including screening for drugs.
Keywords: Adult cardiomyocytes isolation, Trypsin, Langendorff
1. Introduction
The advances in genetically modified mice make it possible to investigate gene functions in cardiac development and diseases. However, the genetically modified mice models are not suitable for large-scale screening and studies on complex signal transduction pathways. The cultured adult cardiac myocytes can be utilized in a broad range of experiments, including cell electrical conduction, cell contractile activity, cell mechanics, and intracellular Ca2+ homeostasis, etc. With the recent advances in gene transfer technology, direct analysis of gene functions on isolated cardiomyocytes is possible. Therefore, establishing a straightforward protocol for isolation adult mouse cardiomyocyte will not only benefit in understanding cardiac physiology, but also employable to test the effect of gene therapy for clinical treatment of cardiovascular disorders.
Although techniques for isolating cardiomyocytes from other animals, such as rats and rabbits, have been established, successful isolation of adult cardiac myocytes in mice remains challenging. One possible reason is the complicated arrangement of the cardiac tissue. The cardiomyocytes exist as the fundamental contractile units of the heart. Within the intact cardiac tissue, cardiomyocytes are connected by intercalated discs through different junctional complexes [1]. These are closely associated with neighboring cells and the extracellular matrix [1]. Cardiomyocytes are also equipped with bundles of myofibrils (termed sarcomeres), deep invaginations of T-tubules, and a densely packed mitochondrial network [2, 3]. All these structures allow the heart to work as a single functional organ to respond to (hormonal and mechanical) signal regulations and environmental changes. Cardiomyocyte isolation requires disrupting these structures. In addition, cardiomyocytes are highly sensitive to mechanical stretching, enzymatic digestion, oxygen supply, ionic fluctuations, and metabolism changes [4]. All these characteristics make isolating functionally and morphologically intact cardiomyocytes very challenging because it is difficult to disrupt these structures without damaging the cardiomyocytes. Moreover, cardiomyocytes are terminally differentiated cells and have limited proliferative ability. They stop cell division after birth and thus do not multiply in culture. This means that cell numbers gradually diminish in culture. Therefore, establishing a protocol for isolating high-quality and quantity cardiomyocytes is critical for studying adult cardiac function.
Langendorff perfusion system has been the centerpiece for cardiomyocyte isolation for over 45 years [5]. However, the protocols used by different laboratories vary depending on the species. The goal of this chapter is to describe a straightforward method for isolating adult mouse cardiac myocytes. This protocol is a modification of a procedure used from prior work in the mouse [6].
2. Materials
2.1. Heart Perfusion System
The Langendorff perfusion system (Radnoti). (Fig. 1a) includes heating coil (Fig. 1b), mouse heart chamber (Fig. 1c), MINI-PUMP variable flow (Fig. 1d), and heated circulating bath (Fig. 1e).
Fig. 1.
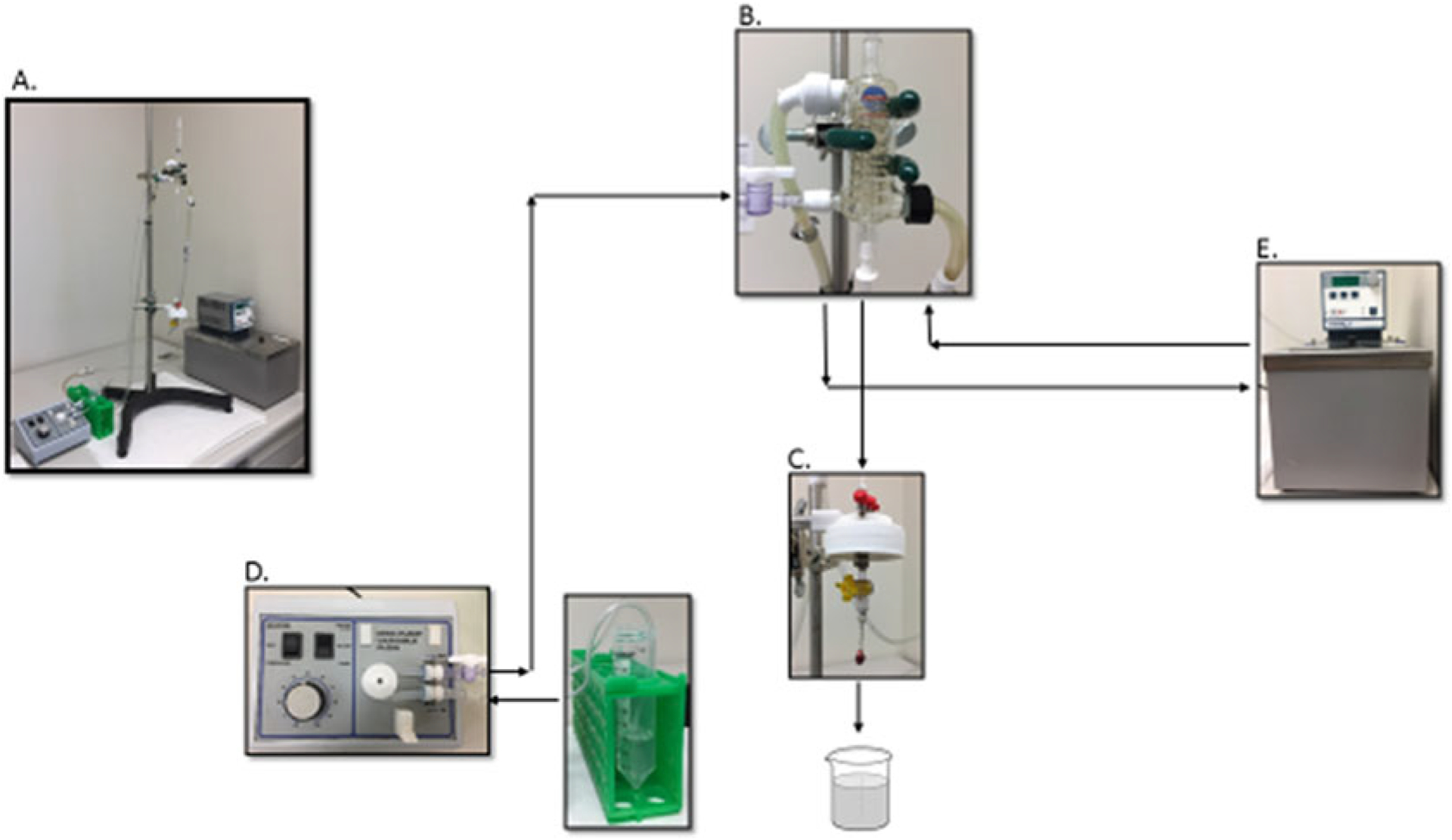
Langendorff perfusion system
2.2. Heart Cannulation and Cardiomyocytes Isolation System
Surgical Tools (Fig. 2): One big forceps, one small forceps, one big scissor, one small scissor, two fine-tipped forceps, and one fine-tipped surgical scissor.
1 ml syringe (Fig. 3b).
6–0 black-braided silk nonabsorbable, nonsterile surgical suture spool (SP102) (Fig. 3c).
60 × 15 mm petri dish.
50 ml conical Falcon tubes with Sefar Nylon Mesh filter top (Fig. 4).
Sefar Nylon Mesh Lab Pak, 250 Microns Square Opening, 12″ × 12″.
Bottle top filtration unit.
30 ml Luer lock syringe.
Syringe top filter, 0.2 μm.
Glassware: 10 ml beaker.
Hot plate stirrer.
Fig. 2.
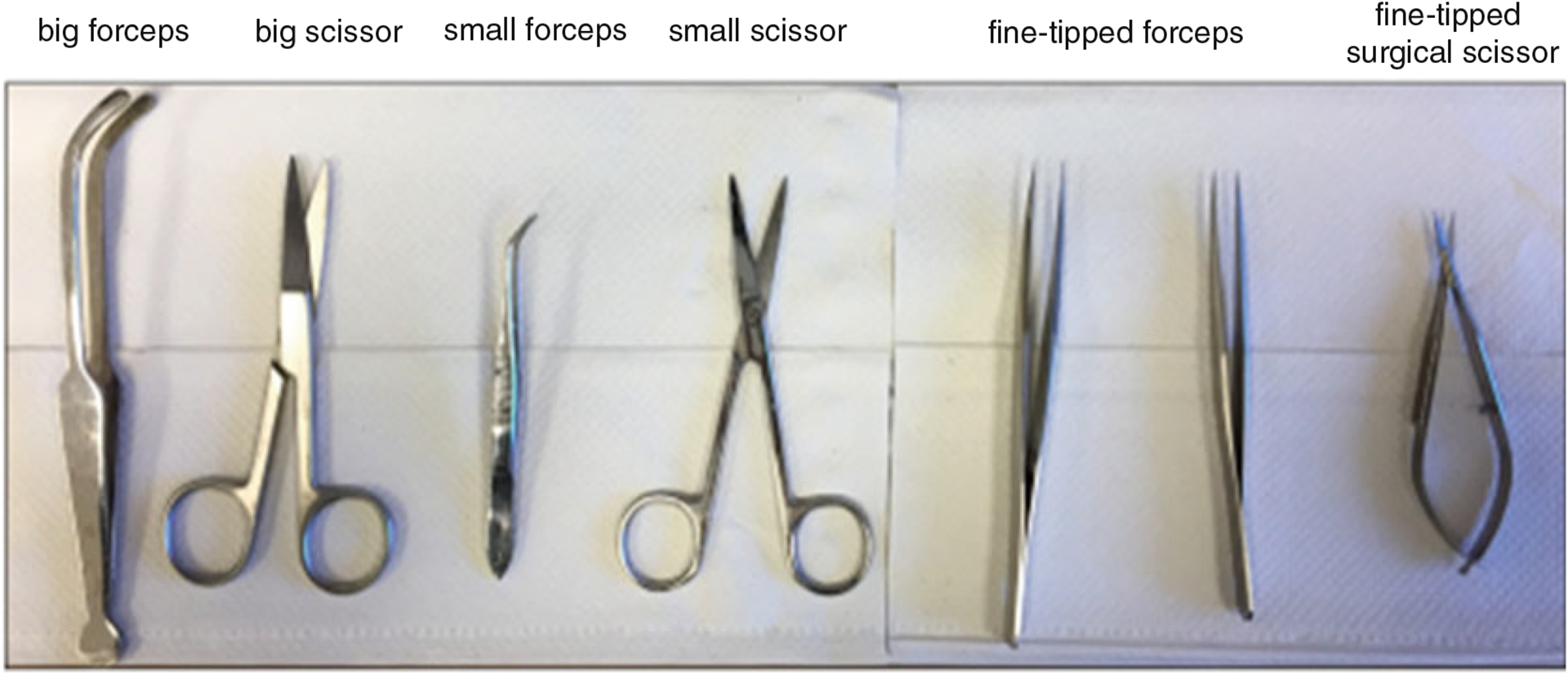
Surgical tools for isolating adult heart and loading heart into Langendorff apparatus
Fig. 3.
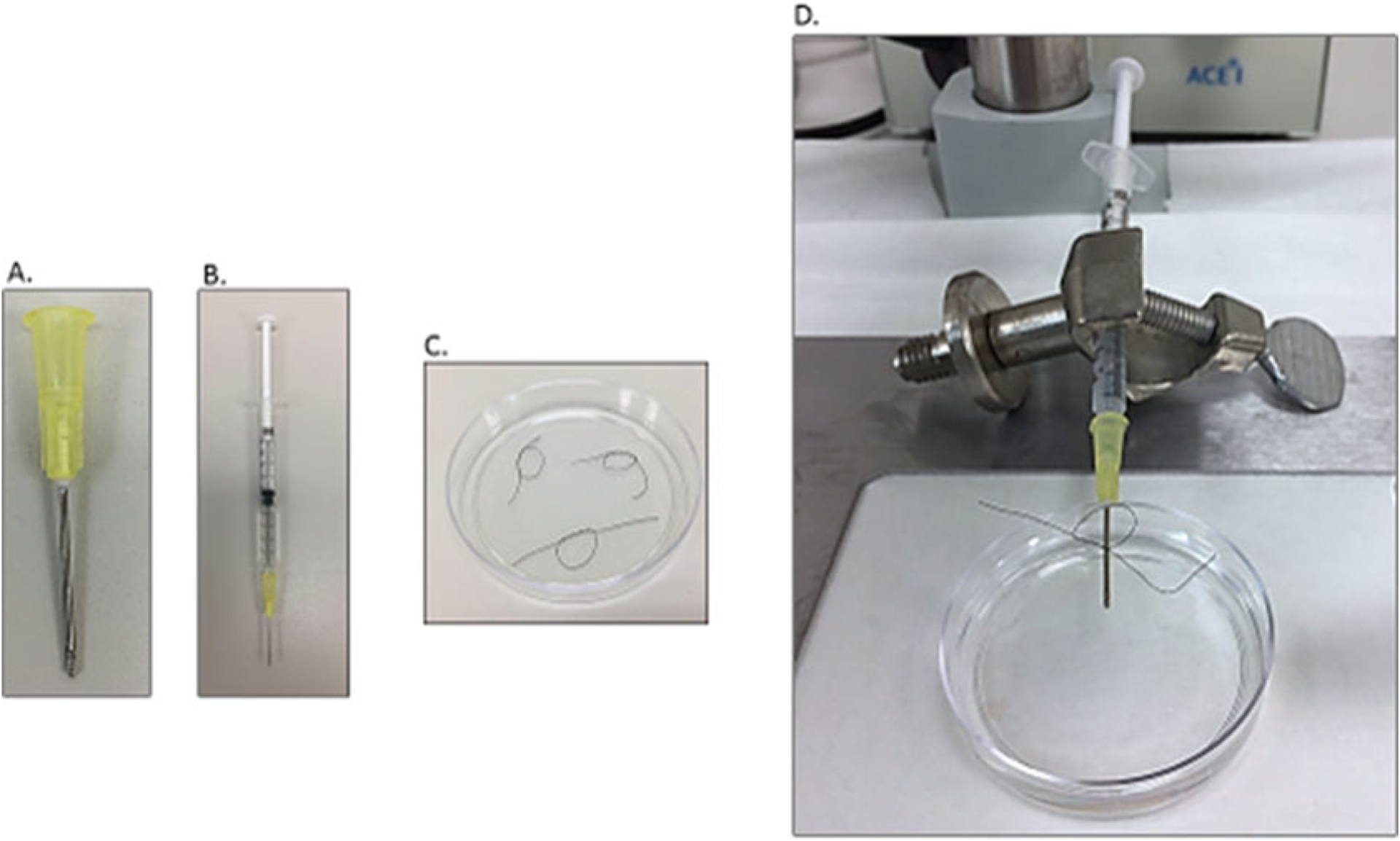
Heart cannulation system
Fig. 4.
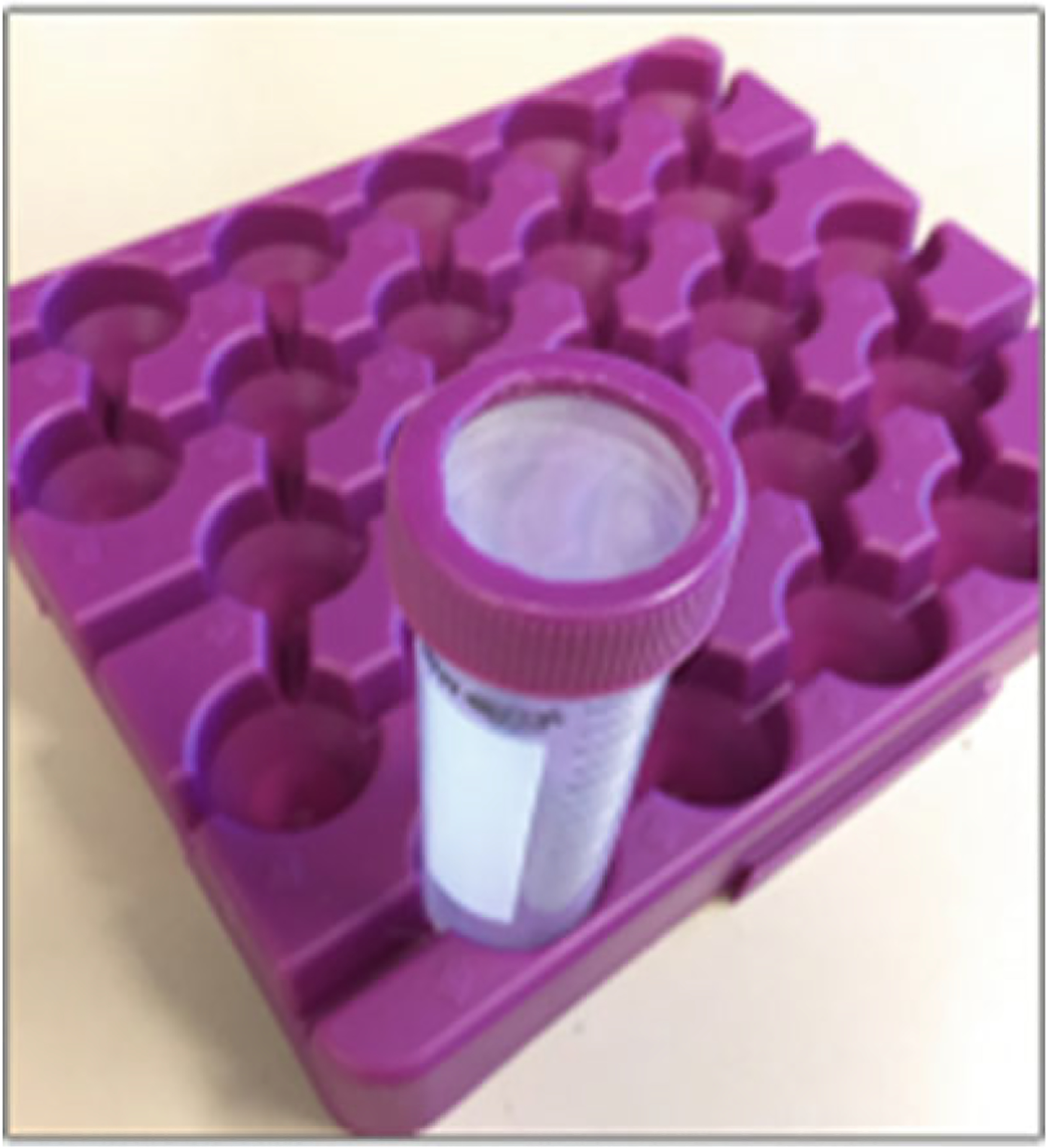
50 ml Falcon tube with Sefar Nylon Mesh filter top for accumulating filtered cardiomyocytes
2.3. Buffers
Basal Solution: 135 mM NaCl, 4 mM KCl, 1 mM MgCl2, 10 mM HEPES, 0.33 mM NaH2PO4 (see Note 3).
Perfusion Buffer: 1× basal solution with 10 mM d-(+)-Glucose, 10 mM BDM, and 5 mM taurine. Adjust the pH to 7.4 at 37 °C and filter with bottle top filtration unit (see Note 4).
Digestion Buffer: Add collagenase type 2 to perfusion buffer (pH has been adjusted to 7.4 at 37 °C) to a final concentration of 525 U/ml. Filter digestion buffer with a 30 ml Luer lock syringe and 0.2 μm syringe top filter (see Note 5).
Transfer Buffer A: 1× basal solution with 5.5 mM d-(+)-Glucose, 10 mM BDM, and 5 mg/ml BSA (see Note 6). Adjust pH to 7.4 at 37 °C. Filter with a 30 ml Luer lock syringe and 0.2 μm syringe top filter.
Transfer Buffer B: 137 mM NaCl, 5.4 mM KCl, 0.5 mM MgCl2, 10 mM HEPES, 5.5 mM d-(+)-Glucose, and 1.8 mM CaCl2. Adjust pH to 7.4 at 37 °C. Filter with a 30 ml Luer lock syringe and 0.2 μm syringe top filter (see Note 7).
2.3.1. Transfer Solution (See Note 8)
Transfer Solution 1: Add 0.2 ml transfer buffer B into 5.8 ml transfer buffer A, and the final [Ca2+] is 0.06 mM.
Transfer Solution 2: Add 0.8 ml transfer buffer B into 5.2 ml transfer buffer A, and the final [Ca2+] is 0.24 mM.
Transfer Solution 3: Add 2 ml transfer buffer B into 4 ml transfer buffer A, and the final [Ca2+] is 0.6 mM.
Transfer Solution 4: Add 4 ml transfer buffer B into 2 ml transfer buffer A, and the final [Ca2+] is 1.2 mM.
3. Methods
3.1. System Setup
The Langendorff Perfusion System (9): Assemble the instruments as indicated in Fig. 1. Make sure the whole system has no bubbles. Set the flow rate at 3 ml/min at the cannulation end (see Note 9).
Turn on the circulating water bath (Fig. 1e). Set the temperature of the water bath such that the temperature of the perfusion buffer at the cannulation end is at 37 °C.
Cannulation Apparatus (Fig. 3d): Make knots with two twist using 6–0 black-braided silk nonabsorbable suture (Fig. 3c). Connect the 20 G blunt-ended needle (Fig. 3a) onto a 1 ml syringe (Fig. 3b) and load about 500 μl of perfusion buffer to the syringe (Fig. 3b). Eliminate any bubbles from the syringe and then put the syringe onto the syringe holder (Fig. 3d). Add about 20 ml perfusion buffer to a 60 × 15 mm petri dish. Place the syringe on top of the petri dish such that the 20 G blunt-ended needle is immersed in the perfusion solution (Fig. 3d).
Keep perfusion buffer and digestion buffer in 100% O2 bubbling for the whole isolation process.
3.2. Heart Cannulation and Cardiomyocytes Isolation
Intraperitoneal inject 200 IU heparin sodium per mouse (about 6 weeks old) to prevent blood clotting before anesthetization. Allow about 10 min for the heparin to distribute through the bloodstream (see Note 10).
Anesthetize mouse with 2.5% isoflurane and 100% O2 by inhalation until reaching deep anesthetization with no response to a toe pinch.
Wipe mouse chest area with 75% ethanol and cut the skin open to expose the rib cage. Use the big forceps to grab the inferior end of the sternum and cut along the diaphragm’s anterior edge to open the thoracic cavity using the big scissors. Use the small scissors to cut the rib cage open to expose the thoracic cavity and the heart (see Note 11).
Remove heart by cutting off lungs and surrounding vessels carefully. Immediately immerse the heart in a 10 ml beaker, which contains 3–5 ml of perfusion buffer (see Note 12).
Transfer heart to the cannulation plate containing perfusion buffer (Fig. 3d). Make sure the entire heart and main vessels are submerged by the perfusion buffer to avoid air entering the heart. Using the fine-tipped forceps, cannulate the heart onto the 20 G blunt-ended needle through aorta vessel under a dissecting microscope. Leave the needle tip right above the aortic valves to allow for coronary perfusion. Proper perfusion through the coronary arteries will help digestion of the right ventricle. Firmly tie the aorta vessel onto the needle using the suture (Fig. 5a). Once the heart is secured, slowly inject the 500 μl perfusion buffer into the heart through the syringe attached to wash the remaining blood from the heart and vessels. Avoid introducing air bubbles into the heart and vessels (Fig. 5a).
Quickly trim off extra tissue if necessary. Remove the needle with the cannulated heart from the syringe and immediately fasten to the Langendorff perfusion system. Avoid introducing air bubbles (Fig. 5b).
Perfuse the heart with perfusion buffer for 3 min (see Note 13).
After 3 min of perfusion, switch to digestion buffer. Carefully monitor the digestion process. The heart should gradually become pale from the root to the tip. Gently squeeze the heart once every minute and collect a few drops of perfusate into a clean 60 × 15 mm petri dish and observe under an inverted microscope. Stop digestion when a single cardiomyocyte is present in the drop. The heart tissue should feel soft with no ventricular resistance (see Note 14).
Quickly remove the heart from the Langendroff perfusion system and place it into a 60 × 15 mm dish containing 5 ml transfer buffer A. Trim off extra tissue and vessels. Starting from the aortic opening of the left ventricle, gently tear the pericardium with fine-tipped forceps and tease the heart into 4–6 pieces (see Note 15).
Further dissociate the heart tissue using a plastic Pasteur pipette, whose tip had been cut off at an ~45° angle. Triturate up and down several times until the tissue pieces can easily enter the pipette (see Note 16).
Switch to a normal plastic Pasteur pipette. Pipette several times until the large pieces become smaller tissue strips.
Transfer the cell suspension into a 50 ml Falcon tube through a 250 μm filter top (Fig. 4). Rinse the petri dish with 3 ml more transfer buffer A and transfer to the same Falcon tube.
Place the Falcon tube upright and allow the cells to sediment by gravity for 15 min.
After 15 min, carefully remove the supernatant without irritating the cells.
Add 5 ml of transfer solution 1(0.06 mM [Ca2+]) to suspend the cells and mix by gently shaking the tube.
Repeat steps 14 and 15 with transfer solution 2 (0.24 mM [Ca2+]), 3 (0.6 mM [Ca2+]), and 4 (1.2 mM [Ca2+]).
After adding transfer solution 4, the isolated cardiomyocytes are ready for further culture or other experiments (see Note 17).
Fig. 5.
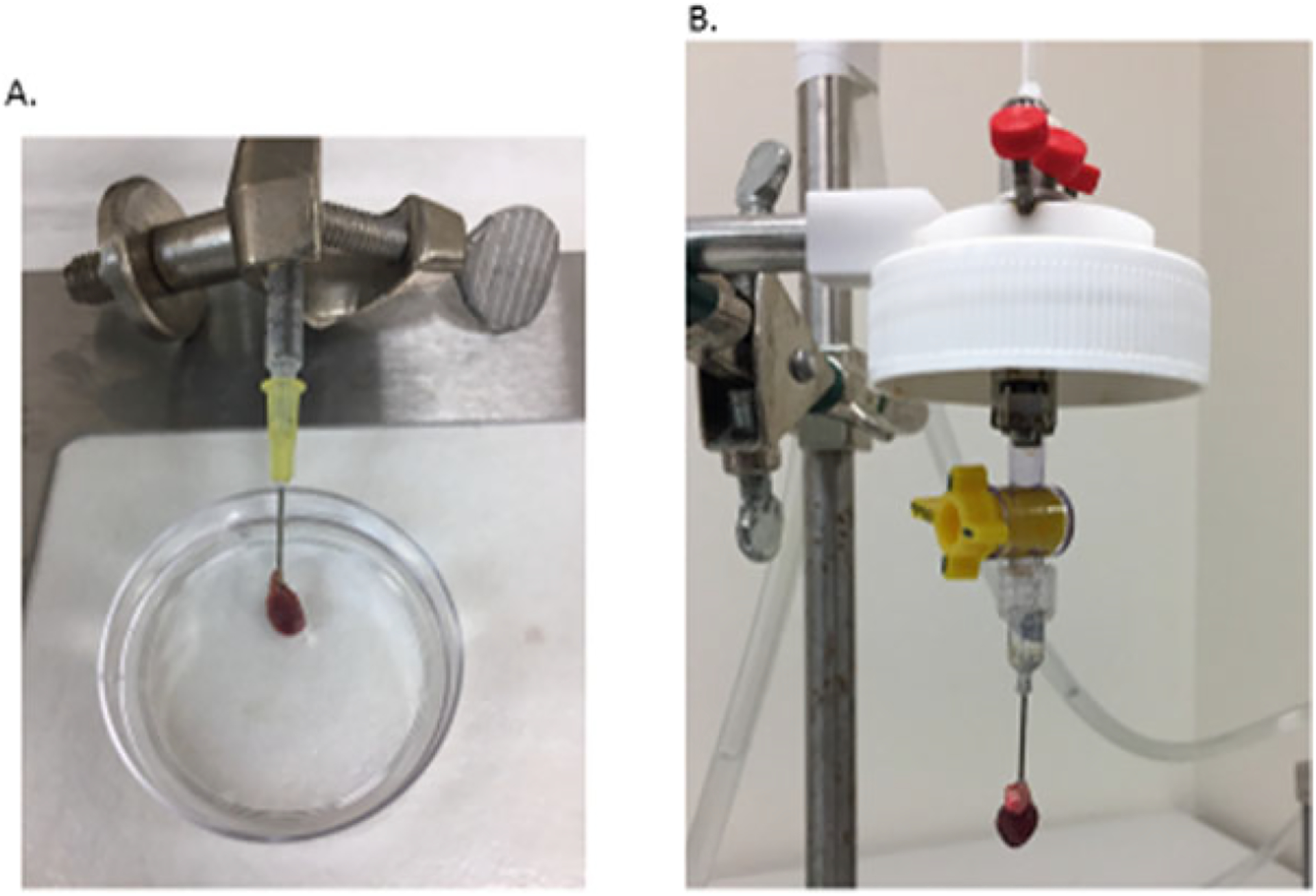
Heart cannulation
4. Notes
Can be made by removing the sharp tip of the regular 20 G needle and then smoothing the cut end using a sandstone.
Anything that can hold the 1 ml syringe to free your hands for the heart cannulation.
Basal solution can be made the day before the cardiomyocyte isolation experiment in a 1 L bottle and leave in 37 °C water bath over night with the cap tightened. If make the day of experiment, leave the basal solution in 37 °C water bath for at least 30 min to bring the temperature up to 37 °C.
It is recommended to use a hot plate stirrer while making the perfusion solution and adjusting the pH. Set the stirrer at a desired speed and the hot plate at 37 °C. Move basal solution from the 37 °C water bath to the hot plate stirrer. Add d-(+)-Glucose, BDM, and taurine to basal solution to make perfusion buffer as indicated in “Subheading 2.3, item 2” After adjusting the pH, bubble the perfusion buffer with 100% oxygen throughout the entire isolation procedure. One 3-month-old WT mouse heart needs about 155 ml of perfusion buffer to make digestion buffer, transfer buffer A, transfer buffer B, and to perfuse the heart. Thus, if starting with three mice hearts, making 500 ml perfusion buffer is suggested. Perfusion buffer of 1 l is good for six mice hearts.
Bubble the digestion buffer with 100% O2 throughout the duration of the isolation procedure. One 3 months old WT mouse heart needs about 25 ml of digestion buffer when the perfusion rate is set at 3 ml/min.
One mouse heart needs about 8 ml of transfer buffer A to neutralize the digestion buffer and 17 ml to make the transfer solution with increasing calcium concentration.
One mouse heart needs 7 ml of transfer buffer B to make the transfer solution with increasing calcium concentration.
This recipe is for one mouse heart, which is 6 ml of each transfer solution.
Before getting started, run the perfusion system with 75% ethanol for 5 min with the setting of the MINI-PUMP variable flow instrument at fast mode, then switch to ddH2O for 5 min. After ddH2O wash, switch to perfusion buffer and set the flow mode to slow. Adjust the flow speed to get the flow rate at 3 ml/min.
Make 1 IU/μl heparin in PBS. Inject about 200 μl per mouse.
Be careful not to accidentally cut the heart at this step.
Leave as much of the ascending aorta as possible to facilitate cannulation. Keeping the thymus gland with heat helps to identify the aorta vessel when cannulating. The aorta arch is right under the thymus when separating the two lobes of the thymus gland.
One can gently squeeze the heart to help remove the extra blood.
If the right ventricle area remains pink while the rest of the heart appears pale, it indicates that the needle tip was below the coronary opening such that the coronary was not properly perfused and the right ventricle was not well-digested.
A well-digested heart tissue will have cotton-like morphology, and cells start to dissociate when the pericardium is lightly torn.
Avoid generating bubbles. The transfer solution A starts to turn cloudy as more cells are dissociated. Modification of the pipette tip will decrease the shear stress on the cells during the dissociation process.
One can estimate the yield of the cardiomyocytes at this point by adding a few microliters of the cell suspension to a petri dish and observing under an inverted light microscope. A live cardiomyocyte should have a rod shape, and an injured or dead myocyte will become round (Fig. 6). This protocol generally yields about 80% of rod-shaped cardiomyocytes.
Fig. 6.
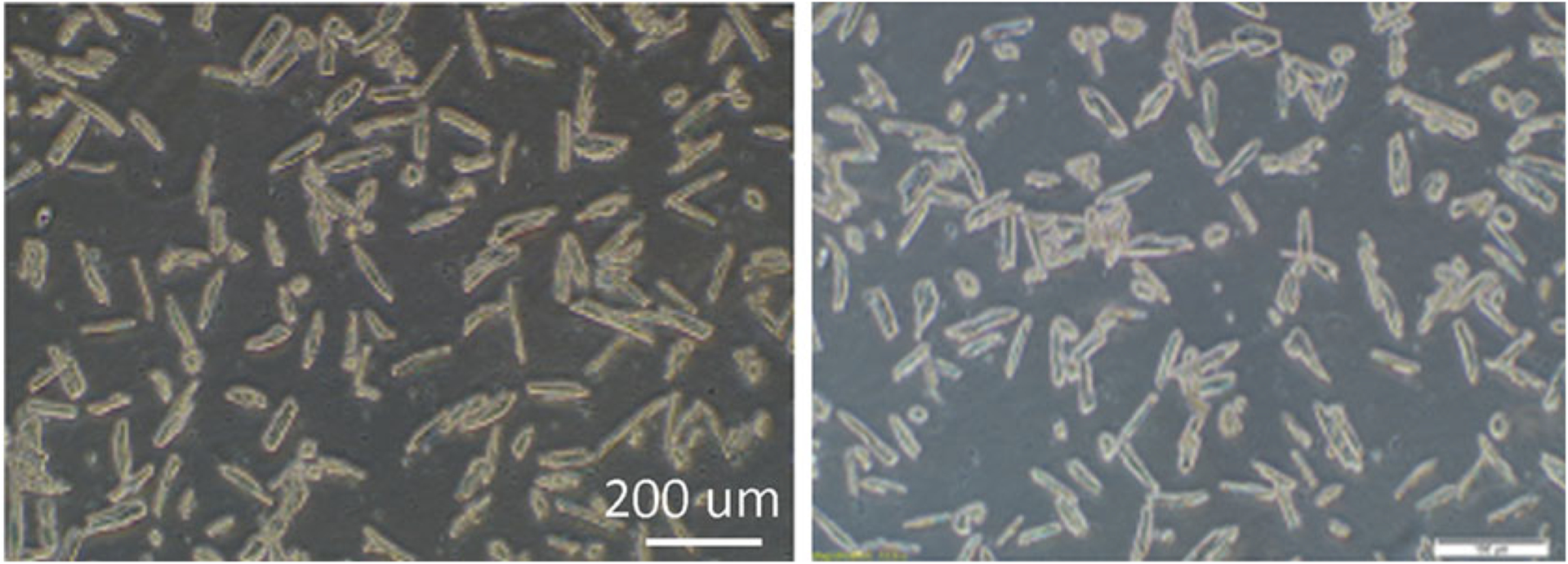
Isolated adult cardiomyocytes
References
- 1.Woodcock EA, Matkovich SJ (2005) Cardiomyocytes structure, function and associated pathologies. Int J Biochem Cell Biol 37:1746–1751 [DOI] [PubMed] [Google Scholar]
- 2.Ibrahim M, Gorelik J, Yacoub MH, Terracciano CM (2011) The structure and function of cardiac t-tubules in health and disease. Proc Biol Sci 278:2714–2723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piquereau J, Caffin F, Novotova M et al. (2013) Mitochondrial dynamics in the adult cardiomyocytes: which roles for a highly specialized cell? Front Physiol 4:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Louch WE, Sheehan KA, Wolska BM (2011) Methods in cardiomyocyte isolation, culture, and gene transfer. J Mol Cell Cardiol 51:288–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powell T, Twist VW (1976) A rapid technique for the isolation and purification of adult cardiac muscle cells having respiratory control and a tolerance to calcium. Biochem Biophys Res Commun 72:327–333 [DOI] [PubMed] [Google Scholar]
- 6.Liao R, Jain M (2007) Isolation, culture, and functional analysis of adult mouse cardiomyocytes. Methods Mol Med 139:251–262 [DOI] [PubMed] [Google Scholar]


