Abstract
Background
Breast cancer is traditionally classified into three clinical subtypes based on hormone receptor and HER2 status (i.e., luminal-like, HER2-positive, and triple negative). Each subtype has distinct clinical-pathological and molecular characteristics and requires tailored treatments. Recent research efforts have been focusing on a new classification, identifying the so-called “HER2-low” category, including tumors characterized by a low level of HER2 expression (immunohistochemistry score 1+ or 2+ without in situ hybridization amplification). Emerging evidence shows that patients with HER2-low tumors can derive benefit from selected anti-HER2 therapies. This represents a major advancement in the field of breast oncology, where a broader proportion of patients with breast cancer can ultimately benefit from new effective targeted treatment strategies.
Summary
The antibody-drug conjugate trastuzumab deruxtecan has proven impressive efficacy in patients with HER2-low breast cancer, and several other drugs are currently under investigation in this subset of patients. Additional investigation is needed to address open issues that exist in this field, including appropriate pathological assessment of HER2-low status, clarification of its prognostic implications, and global access to newly approved drugs.
Key Message
Our review aims to summarize the available evidence regarding HER2-low breast cancer, illustrating the current challenges that are being addressed and the future perspectives in this exciting new field.
Keywords: Breast cancer, HER2-low, Trastuzumab deruxtecan
Introduction
Breast cancer is traditionally classified into three clinical subtypes according to estrogen receptors (ER), progesterone receptors (PgR), and HER2 status. Tumors expressing ER and PgR but not HER2 (i.e., HER2-negative) are defined as luminal-like cancers, further classified into luminal A-like and luminal B-like carcinomas depending on the level of tumor cell proliferation. Luminal A-like tumors, which express high levels of ER and PgR as well as relatively low levels of tumor cell proliferation, are usually associated with a relatively good prognosis. Triple negative (TN) cancers do not express ER/PgR or HER2, and are characterized by a relatively poor prognosis [1, 2]. Luminal B-like carcinomas constitute a spectrum of tumors with variable proliferation levels, and some also show HER2 overexpression and amplification. The HER2 status is defined in clinical practice by a combination of immunochemistry (IHC) and/or in situ hybridization (ISH) aimed at identifying HER2 protein overexpression and HER2 gene amplification [3]. According to the 2018 American Society of Clinical Oncology/College of American Pathologists guidelines, tumors with an IHC score 3+ or 2+ harboring HER2 amplification by ISH are defined as HER2-positive [4]. These tumors were historically associated with a poor prognosis before the introduction of anti-HER2 treatments [5]. Tumors characterized by IHC 2+ score without ISH amplification or IHC 1+ score have historically been labeled as “HER2-negative” because of the lack of oncogene addiction stemming from HER2 gene amplification. Nevertheless, as shown by the scores 1+ and 2+ in IHC they display a non-negligible level of HER2 receptors on the cell membrane and constitute the so-called “HER2-low” breast cancer category. By considering this definition, about 40–55% of all breast cancers are ultimately HER2-low [6, 7]. As mentioned above, these tumors are classified as HER2-negative, with treatment recommendations based on hormone receptor status and no differences between breast cancers with IHC score 0 and 1+ or 2+ with ISH negative. Nevertheless, nowadays, emerging evidence is showing that patients with HER2-low breast cancers also can benefit from selected anti-HER2 therapies [6]. The aim of this review is to summarize the evidence available regarding HER2-low breast cancer, illustrating the current challenges that are being addressed and the future perspectives in this exciting new field.
HER2-Low Status: An Easy Assessment?
HER2 overexpression is scored as 2+ by immunohistochemistry when weak to moderate complete membrane staining is observed in >10% of neoplastic cells [4]. An IHC score 1+ is assigned when an incomplete membrane staining with a barely perception in >10% of tumor cells is identified, and its interpretations may lead to discrepancies and misinterpretations [4]. Another feature complicating this scenario is the heterogeneity of HER2 IHC staining [8]. The antibodies used for IHC staining can impact on the level of HER2 expression, particularly with regard to the HER2-low subgroup. The most used assays are HercepTest and VENTANA 4B5. The agreement rate between these techniques is 73.2%, with a higher detection of HER2-low cancers in favor of VENTANA 4B5 [9]. Among 500 samples, the 4B5 assay identified 28.0% as IHC 1+/2+ compared with 11.6% identified by HercepTest. A total of 21.6% (95% CI, 15.1–29.4) of tumors identified as IHC 1+/2+ by 4B5 was classified concordantly by HercepTest. A high rate of concordance (98.3%; 95% CI, 96.2–99.5) was found in defining the IHC 0 samples [9]. Moreover, 200 stained slides from 100 independent cases were analyzed by 5 different pathologists to define IHC status. Overall kappa concordance was 0.79 (p < 0.001), but it was 0.67 for 1+ and 0.74 for 2+ (p < 0.001), confirming the difficulties in finding a real concordance of 1+ [7]. In another study, 170 biopsy samples were analyzed for IHC score by 18 pathologists, and the concordance rate between 0 and 1+ was attested at only 26%, while it was at 58% regarding 2+ and 3+ [10].
Clinicopathologic and Genomic Features
Hormone Receptor Status
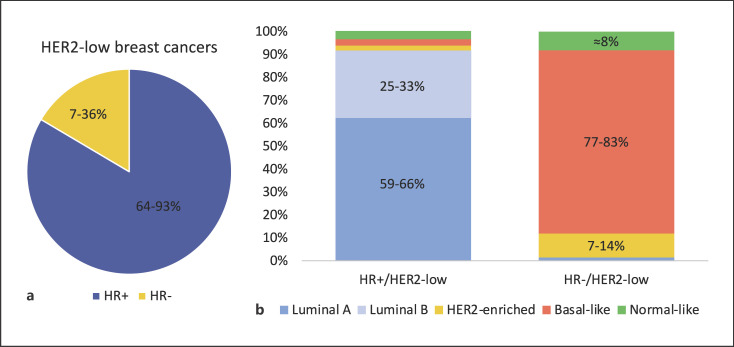
Different studies have evaluated the clinicopathological characteristics of HER2-low breast cancer. Most of them are consistent with the observation that the majority of HER2-low tumors express hormone receptors (Fig. 1a). For example, in the study by Schettini et al. [7], 88.2% of cases had positive hormone receptor status among 2,195 patients with HER2-low tumors. Consistently, in a pooled analysis among 1,098 patients with HER2-low breast cancer included in 4 neoadjuvant trials, 64% of them had hormone receptor-positive disease [11]. This trend was also confirmed in a retrospective analysis of 804 primary breast cancers: among them, 51% were HER2-low. The vast majority of them (336, 82%) was hormone receptor-positive and 74 (18%) were hormone receptor-negative [12]. Moreover, another study conducted at the Dana-Farber Cancer Center enrolled 5,235 patients with HER2-negative early breast cancer of whom 55.7% had HER2-low disease. The rate of hormone receptor-positive breast cancers was higher in the HER2-low cohort (90.6% vs. 81.8%, p < 0.001) as compared to HER2 0 subgroup, and HER2-low breast cancer rate increased progressively with the increase of ER expression [13]. Regarding nodal involvement and tumor size, data are not completely consistent. In the study published by Schettini et al., the HER2-low subtype was characterized by a more frequent nodal involvement (p = 0.010) and larger tumor size (p = 0.007) when compared to HER2 0 breast cancers [7]. On the contrary, in a large Korean study of 30,491 cases, among patients affected by hormone receptor-positive early breast cancer, the HER2-low subgroup was characterized by fewer T4 tumors, higher histological grade, and negative lymphatic invasion. In this analysis, clinicopathologic features of HER2-low breast cancers differed slightly according to hormone receptor status: hormone receptor-positive tumors were more common in premenopausal patients and had a higher histological grade. In contrast, among TN breast cancers, HER2-low tumors were more prevalent in older patients, with a higher neutrophil-to-lymphocyte ratio [14]. HER2-low breast cancers seem to be characterized by a lower rate of pathological complete response after neoadjuvant therapy when compared with HER2 0 tumors (29.2% vs. 39.0%, p = 0.0002). These data were confirmed within the hormone receptor-positive subgroup (17.5% vs. 23.6%, p = 0.024) but not in the hormone receptor-negative subgroup (50.1% vs. 48%, p = 0.21) [11].
Fig. 1.
a Hormone receptor status distribution within HER2-low breast cancer. Original figure based on data available in the literature [7, 8, 11, 12, 13, 14]. b PAM50 intrinsic subtype distribution in HER2-low tumors. The figure illustrates the percentages of PAM50 intrinsic subtype within HR+/HER2-low and HR−/HER2-low subgroups (i.e., for HR+/HER2-low: luminal A 58.9–65.9%, luminal B 25.4–33.4%, HER2-enriched 1.2–3.0%, basal-like 1.9–3.6%, normal-like 2.8–3.9%. For HR−/HER2-low: luminal A 1.4–1.6%, luminal B 0%, HER2-enriched 7.1–13.7%, basal-like 76.7–83.3%, normal-like 7.9–8.2%). Original figure based on data available in the literature [7, 12]. HR, hormone receptor.
HER2 Status Evolution
As HER2 expression can change from primary tumor to disease relapse, the prevalence of HER2-low status can also differ between the two settings. In a study published by Miglietta et al. [15], samples from primary tumors and recurrences were analyzed for 547 patients. HER2-low status was found in 34.2% of the primary tumor samples and in 37.3% of the recurrence samples. The HER2 status discordance rate between primary and relapse was 38.0%. In 15.2% of cases, the HER2 status changed from HER2 0 to HER2-low and the opposite happened in 14.1% of cases. HER2-low status turned out to be more unstable in the hormone receptor-positive subgroup as compared to that in TN group: in the hormone receptor-positive subgroup, 47.3% was HER2-low on the primary tumor and 53.8% was HER-low at relapse, and 5.1% turned into HER2-positive. As regards to TN breast cancers, 35.4% of cases were HER2-low on the primary and 36.2% at recurrence [15]. Another study investigating samples for primary tumor and relapse included 232 patients with metastatic HER2-negative breast cancer. Consistently, a significant discordance in the HER2 status was observed: an increase in the HER2 score was found in 44% of HER2 0 breast cancers, while 22% of HER2-low primary breast cancers were HER2 0 at relapse. This study also showed that HER2-low status is more unstable within the hormone receptor-positive subgroup (57% of HER2-low on the primary vs. 64% on the metastatic site) as compared with TN breast cancer (36% vs. 38%, respectively) [16]. Moreover, anti-HER2 treatments can impact the expression of both HER2 and hormone receptors due to the connection existing between the ER and HER2 signaling pathway. Breast cancers developing an acquired resistance to anti-HER2 and/or endocrine treatments can either lose or gain HER2 and ER expressions [17]. This could be relevant also in HER2-low tumors, where hormone receptor status seems to play a potential prognostic and predictive value. These data further highlight the importance of performing a biopsy at relapse for treatment decision-making.
Transcriptomic/Genomic Landscape of HER2-Low Disease
The genomic landscape of HER2-low disease seems to have some peculiar features and to differ as compared to non-HER2-low tumors. Moreover, the distribution of molecular intrinsic subtypes exhibits an interesting heterogeneity [18] also within the HER2-low category (for instance, according to the expression of hormone receptors). In the analysis by Schettini et al., among 3,689 patients with HER2-negative early breast cancer, 1,576 cases were evaluated for the PAM50 intrinsic subtype. Intrinsic subtype distribution was significantly different between HER2-low cases (IHC 1+ and 2+ with ISH not amplified) and tumors with IHC 0. A higher rate of luminal A and luminal B tumors was found in HER2-low tumors compared to tumors with IHC 0. Conversely, the proportion of HER2-enriched and basal-like tumors was lower. As shown in Figure 1b, among HER2 low-breast cancers, PAM50 intrinsic subtype distribution varies considerably according to hormone receptor status [7]. Consistently, in another retrospective study including 404 HER2-low breast cancers tested with PAM50, HER2-enriched tumors were more frequent among the hormone receptor-negative subgroup (13.7% vs. 1.2%) as well as the basal-like subtype (76.7% vs. 3.6%) [12]. Similar results were found by Zhang et al. [8]. These authors retrospectively reviewed Mammaprint and Blueprint results from 281 breast cancer cases. Their analysis showed that within the HER2-low subgroup, 65.5% of cases were luminal A (low-risk), 28.8% luminal B, 1.1% HER2-enriched, and 4.6% basal-like. In the HER2-low/hormone receptor positive cohort, 70.4% of tumors were classified as luminal A, 28.4% as luminal B, and 1.2% as HER2-enriched. No basal-like tumors were seen in this subgroup. Among the HER2-low/TN subgroup, 66.7% were basal-like and 33.3% were luminal B [8].
In the study by Schettini et al. among the hormone receptor-positive cohort, the HER2-low group showed higher ERBB2 levels as compared with the HER2 0 tumors (1.4-fold mean difference, p < 0.001). ERBB2 levels were higher as the HER2 IHC score increased, with a fold mean difference of 1.7 between HER2 2+ and HER2 0. Within the hormone receptor-negative cohort, HER2-low breast cancers had significantly higher ERBB2 levels in comparison with HER2 0 (p = 0.027), but no significant difference was found within the HER2-low subgroups (IHC 0, 1+, 2+). TN breast cancers expressed a higher rate of proliferation-related genes in comparison with the hormone receptor-positive population, irrespectively of HER2 status [7]. In a study published by Van den Ende et al. [19], HER2-low breast cancers seem to express genes linked to poorer outcome and lower immune response when compared to HER2 0 tumors. According to the data published by Agostinetto et al. [12], a positive correlation between ERBB2 and ESR1 levels was found in the HER2-low cohort (Spearman 0.38, p < 0.001).
Recently, an integrative genomic and transcriptomic analysis has provided further insights into the biology of this wide spectrum of breast carcinomas. By analyzing a cohort of treatment-naive 99 HER2-low carcinomas, Berrino et al. [20] observed that these tumors harbor a constellation of somatic mutations that significantly differs from HER2-positive and HER2-negative tumors. Significant differences were also observed across score 1+ and score 2+ within the HER2-low category. In a comparative gene expression analysis, score 1+ cancers showed overlapping gene expression features with score 0 tumors, whereas score 2+ tumors with equivocal HER2 gene copy numbers harbor the most distinct transcriptional profile with the highest ERBB2 mRNA levels overall. The integrated DNA-based comprehensive genomic profiling revealed that potentially targetable alterations could be identified in 52% of patients, including mainly PIK3CA and ERBB2 mutations. These data provide an overview of the potential biological features of HER2-low breast cancer beyond low levels of HER2 expression in tumor cells [20].
Prognostic Implications of HER2-Low Status
Several studies have evaluated the prognostic implications of HER2-low status, both in the metastatic and early settings, with conflicting results so far (Table 1). In the study by Schettini et al. [7] in the metastatic setting, no differences were detected in terms of overall survival (OS) between HER2-low and HER2 0 subgroups, independently of hormone receptor status at a median follow-up of 90.3 months. In the study conducted by Won et al. [14], the HER2-low group showed better breast cancer-specific survival than the HER2 0 subgroup, while no difference was reported in terms of OS. Agostinetto et al. [12] did not observe any significant difference in disease-free interval, progression-free interval, and OS between HER2-low and non-HER2-low breast cancers paired by hormone receptor status. However, HER2-low/hormone receptor-positive tumors had a better progression-free interval and disease-free interval as compared to HER2-low hormone receptor-negative tumors. In another retrospective cohort of 675 patients receiving neoadjuvant chemotherapy, pathological complete response was more frequent in patients with HER2 0 tumors than in those with HER2-low breast cancer. However, in an exploratory analysis of survival data with a median follow-up of approximately 10 months, no differences were found in terms of disease-free survival (DFS), distant DFS, or OS between HER2-low and HER2 0 cancers when separately analyzing hormone receptor-positive and negative tumors [13]. In the analysis performed among early breast cancers by Denkert et al. [11], HER2-low status was significantly related to features usually associated with favorable prognosis as fewer G3 tumors, a lower ki67 index, and a lower number of TP53 mutations compared with HER2 0. HER2-low breast cancer patients had significantly longer OS than patients with HER2 0.
Table 1.
Characteristics and main results of the studies evaluating the prognostic implications of HER2−low status
| Study | Setting | Patients, n | HER2−low (%) | HER2−low in HR + subgroup | HER2−low in HR− subgroup | Main outcomes |
|---|---|---|---|---|---|---|
| Schettini et al. [7] | Metastatic and early | 3,689 | 2,203 (59.7%) | 65.4% | 36.5% | OS: no significant difference between HR+/HER2−low and HR+/HER2 0 (p = 0.234) and between HR−/HER2−low and HR−/HER2 0 (p = 0.533) |
|
| ||||||
| Won et al. [14] | Metastatic and early | 30,491 | 9,506 (31.2%) | 33.6% | 23.0% | OS: no difference between HR+/HER2−low and HR+/HER2 0 (p = 0.086) and between HR−/HER2−low and HR−/HER2 0 (p = 0.170) BCSS: higher for HR+/HER2−low than HR+/HER2 0 (99.4% and 99.1 %, p = 0.003) and for HR−/HER2−low than HR−/HER2 0 (97.2% vs. 95.9%, p = 0.023) |
|
| ||||||
| Agostinetto et al. [12] | Metastatic and early | 804 | 410 (51%) | 53% | 43.5% | OS: no significant difference between HER2−low/HR+ and HER2 0/HR+ (p = 0.47) and between HER2−Low/HR− and HER2 0/HR−(p = 0.52) DFI and PFI: higher for HER2−low/HR+ than HER2−low/HR− BC (p = 0.01 and p = 0.0066) |
|
| ||||||
| Gampenrieder et al. [43] Metastatic and early | 1,729 | 608 (35.2%) | 39.8% | 22.5% | OS: no significant difference between HR+/HER2−low and HR+/HER2 0 (38.9 vs. 38.9 mp = 0.171) and between HR−/HER2−low and HR−/HER2 0 (16.6 vs.12.7 m, p = 0.585) | |
|
| ||||||
| Tarantino et al. [13] | Early | 5,235 | 2,917 (55.7%) | 58.2% | 39.3% | OS: no significant difference between HR+/HER2−low and HR+/HER2 0 (p = 0.26) and between HR−/HER2−low and HR−/HER2 0 (p = 0.65) DFS and DDFS: no significant difference between HR+/HER2−low and HR+/HER2 0 (p = 0.11 and p = 0.13) and between HR−/HER2−low and HR−/HER2 0 (p = 0.38 and p = 0.43) pCR: lower rate in HER2−low than HER2 0 (16.6% vs. 26.8%, p = 0.002). No significant difference when restricting analysis to HR + tumors (p = 0.08) |
|
| ||||||
| Denkert et al. [11] | Early | 2,310 | 1,098 (47.5%) | 61.2% | 34% | 3y DFS: no significant difference between HR+/HER2−low and HR+/HER2 0 (82.8% vs. 79.3%, p = 0.39), higher for HR−/HER2−low than HR−/HER2 0 (84.5% vs. 74.4%, p = 0.0076) 3y OS: no significant difference between HR+/HER2−low and HR+/HER2 0 (92.3% vs. 88.4%, p = 0.13) and between HR−/HER2−low and HR−/HER2 0 (90.2% vs. 84.3%, p = 0.016) pCR: lower in HER2−low than HER2 0 (29.2% vs. 39.9%, p = 0.0002). Lower in HR+/HER2−low than HR+/HER2 0 (17.5% vs. 23.6%, p = 0.024). Higher in HR−/HER2−low than HR−/HER2 0 (50.1% vs. 48.9%, p = 0.21) |
|
| ||||||
| Mutai et al. [44] | Early | 3,608 | 304 (50%) | NA | NA | OS: higher in HER2−low than HER2 0 among patient with high genomic risk (89% vs. 68%, p = 0.01) DFS: higher in HER2−low than HER2 0 among patient with high genomic risk (80% vs. 59%, p = 0.01) DDFS: higher in HER2−low than HER2 0 among patient with high genomic risk (89% vs. 59%, p = 0.002) |
|
| ||||||
| Tan et al. [45] | Early | 28,280 | 12,260 (43.4%) | 45.9% | 29.4% | DFS: higher in HER2−low than HER2 0 (p < 0.001) OS: higher in HER2−low than HER2 0 (p < 0.001) |
BCSS, breast cancer-specific survival; DDFS, distant disease-free survival; DFI, disease-free interval; DFS, disease-free survival; HR+, hormone receptor positive; HR−, hormone receptor negative; OS, overall survival; PFI, progression-free interval.
The evidence to date is insufficient to draw solid conclusions on the prognostic role of HER2-low with controversial findings across different studies, and with most evidence suggesting no significant impact on survival outcomes, in absence of specific targeted treatments. Interestingly, HER2-low expression seems to be continuously associated with the level of ER expression [21].
Therapeutic Implications of HER2-Low Status
Monoclonal Antibodies
The first anti-HER2 agents investigated in HER2-low breast cancer were monoclonal antibodies (mAbs). In the phase III trial NSABP-B47, no benefit in invasive DFS was found for HER2-low high-risk patients who received trastuzumab in addition to standard adjuvant chemotherapy [22]. Pertuzumab showed limited activity in patients with HER2-low tumors: when administered as monotherapy in pretreated HER2-low metastatic breast cancer only 2 patients out of 78 (2.5%) achieved partial response [23]. Disappointing results were also obtained with margetuximab: no responses were obtained in 25 patients with HER2-low breast cancer included in a phase II trial [24]. These discouraging results emphasized the assumption that mAbs are highly dependent on the HER2 signaling pathway [25] and led to investigate alternative anti-HER2 agents with different mechanisms of action.
Antibody-Drug Conjugates
An exploratory analysis performed on 21 HER2 non-amplified tumors included in a clinical trial testing ado-trastuzumab emtansine for HER2-positive disease showed no significant activity of this antibody-drug conjugate (ADC) in this setting [26]. Nevertheless, new-generation ADCs have shown promising results. The poor results obtained with mAbs seem to be due to the heterogeneous expression of HER2 and their dependence on the HER2 signaling pathway. In contrast, ADCs represent an innovative class of drugs, combining the selectivity of targeted therapy with the cytotoxicity of chemotherapy. They consist of the mAb (the vehicle) and the cytotoxic agent (payload) connected by a synthetic linker. Through the diffusion of hydrophobic payloads from antigen-expressing cells to neighboring antigen-negative cells (the so-called bystander effect), they may overcome the heterogeneity of HER2 expression [27].
Trastuzumab deruxtecan (T-DXd) is an ADC, consisting of trastuzumab conjugated to deruxtecan, a topoisomerase I inhibitor, via a cleavable but stable linker. Its potent cytotoxic effect is due to its high drug-to-antibody ratio (DAR) of 8:1, which allows a large amount of the cytotoxic payload to reach the targeted cells [28]. Beyond its potent activity in patients with HER2-positive metastatic breast cancer as shown in the phase II DESTINY-Breast 01 [29] and phase III DESTINY-Breast 03 [30] trials, T-DXd has also been evaluated in HER2-low metastatic breast cancer. A phase Ib study included 54 patients that were heavily pretreated for metastatic HER2-low breast cancer. The overall response rate (ORR) was 37.0% and the median duration of response was 10.4 months. In the phase III DESTINY-Breast04 trial, at a median follow-up of 18.4 months, T-DXd successfully prolonged both progression-free survival (PFS) and OS as compared to the treatment of the physician's choice in patients that had received one or 2 previous lines of chemotherapy, and in case of hormone receptor positive-cancer, at least one line of endocrine therapy. Of the 557 patients enrolled in the study, the vast majority (494, 89%) had hormone receptor-positive disease, 58% had IHC 1+ score and 42% were IHC 2+/ISH not amplified. The study showed a nearly doubled PFS in hormone receptor-positive patients (10.1 vs. 5.4 months, hazard ratio 0.51, p < 0.001) and an almost 50% reduction in risk of disease progression in the overall study population (PFS 9.9 vs. 5.1 months, hazard ratio 0.50, p < 0.001). A 36% reduction in the risk of death was found in the hormone receptor-positive population (23.9 vs. 17.5 months, hazard ratio 0.64, p < 0.003) and in the overall population (23.4 vs. 16.8 months, hazard ratio 0.64, p = 0.0010). An exploratory analysis restricted to patients with TN breast cancer showed consistent results with improved PFS (8.5 vs. 2.9 months, hazard ratio 0.46) and OS (18.2 vs. 8.3 months, hazard ratio 0.48) in the experimental arm [31]. No new side effects were observed with T-DXd, with gastrointestinal toxicity, myelotoxicity, and alopecia being the most common. Interstitial lung disease occurred in 12.1% of patients treated with T-DXd, remaining one of the most relevant side effects and requiring careful and proactive monitoring. In August 2022, the US Food and Drug Administration (FDA) approved T-DXd for the treatment of HER2-low metastatic breast cancer, making it the first approved therapy for this patients' subgroup [32].
The relevant results of the DAISY trial, analyzing the association between T-DXd efficacy and HER2 expression, have been recently presented. In this study, pretreated metastatic breast cancer patients were divided into three different cohorts (HER2-positive, HER2-low, HER2 0). All the cohorts received T-DXd until progression or unacceptable toxicity. The ORR was 71% in the HER2-positive subgroup, 37.5% in HER2-low subgroup, and 30% in patients with HER2 0 disease, with a median PFS of 11.1, 6.7, and 4.2 months, respectively. These data show without any doubt that T-DXd antitumor activity is strongly associated with HER2 expression, but that some activity exists also in patients with tumors showing no HER2 expression or at least not efficiently detectable with current methodologies [33]. A subset of HER2 0 tumors shows some HER2 staining at IHC, though incomplete and faint, in ≤10% of tumor cells. This recently defined HER2 “ultra-low” subset might be responsible for the positive results observed in anti-HER2 trials in HER2-negative tumors [25]. In this perspective, more research is required to shed light on the predictive value of the HER2 “ultra-low” status, along with more effective methodologies to correctly identify tumors responsive to new potent anti-HER2 ADCs.
Ongoing clinical trials are investigating whether T-DXd in combination with endocrine therapy (NCT04553770) is also an effective treatment for early stage hormone receptor-positive/HER2-low breast cancer patients. T-DXd is currently being tested in combination with anastrozole and fulvestrant in the advanced setting (NCT04556773). In the advanced stage, the combination of T-DXd with pembrolizumab (NCT04042701) and nivolumab (NCT03523572) is being studied in phase Ib trials. Finally, the DESTINY-Breast06 (NCT04494425) will provide information on the role of T-DXd in chemo-naive breast cancer patients with hormone receptor-positive/HER2-low breast cancer.
Another promising ADC is trastuzumab-duocarmazine (SYD985) [34]. In the phase I study, 47 HER2-low breast cancer patients were enrolled in the dose-expansion cohort. Despite a significant rate of any grade toxicities (71%), the trial showed promising results in terms of ORR (28% in hormone receptor-positive and 40% in hormone receptor-negative/HER2-low breast cancer) [34]. However, the subsequent phase III study was restricted only to HER2-positive metastatic breast cancer [35].
Vaccines
Several combinations of drugs with different mechanisms of action are currently being tested in patients with HER2-low disease (Tables 2, 3), including vaccines. The nelipepimut-S vaccine consists of a HER2-derived peptide, in combination with the immunoadjuvant granulocyte-macrophage colony-stimulating factor [36]. A phase IIb trial tested adjuvant treatment with trastuzumab and granulocyte-macrophage colony-stimulating factor with or without vaccine in high-risk HER2-low early breast cancer. The combination showed a significantly better DFS in the subgroup of hormone receptor-negative patients [37], but a subsequent phase III study showed no difference in DFS for HER2-low patients receiving nelipepimut-S as adjuvant treatment (24-month DFS rate 89.9% in the vaccine group vs. 83.8% in the control group, hazard ratio 0.62); however, nelipepimut-S significantly improved DFS in the subgroup of patients with hormone receptor-negative breast cancer [38]. Interestingly, the new generation of trials testing therapeutic vaccines tends to focus on the early setting, where patients are still immunocompetent, and an immune reaction is more likely to occur, compared to the advanced setting. Indeed, some initial discouraging results observed in clinical trials testing therapeutic vaccines in breast cancer could be, at least in part, explained by the characteristics of the patients included in these studies, with most of them having heavily pretreated metastatic disease and thus less likely to develop a strong immune reaction [39].
Table 2.
Available results from the main trials in early and metastatic HER2−low breast cancer
| Drug | NCT | Ref | Phase | Overall BC pts (HER2−low pts) | Setting | Treatments | Main results |
|---|---|---|---|---|---|---|---|
| mAbs | |||||||
| Trastuzumab | NCT01275677 | Fehrenbacher L. et al. [22] | III | 3,270 (3,270) | Early, adjuvant | Adjuvant CT with or without trastuzumab | 5y DFS: 89.8% versus 89.2% HR 0.98; p = 0.85 |
| Pertuzumab | NCT02491892 | Gianni L. et al. [23] | II | 78 (74) | Advanced | Pertuzumab monotherapy | ORR: PR 2.5% (2 pt s) |
|
| |||||||
| ADCs | |||||||
| T_DXd | NCT02564900 | Modi S. etal. [46] | 54 (54) | Advanced | T-DXd monotherapy | ORR: 37% mDOR: 10.4 months mPFS: 11.1 months |
|
| NCT03734029 | Modi S. et al DESTINY-Breast04 [31] | III | 557 (557) | Advanced | T-DXd versus TPC | PFS in HR+: 10.1 versus 5.4 months HR0.51; p < 0.001 PFS in all: 9.9 versus 5.1 months HR0.50; p < 0.001 OS in HR+: 23.9 versus 17.5 months HR 0.64; p = 0.003 OS in all: 23.4 versus 16.8 months HR 0.64; p = 0.001 |
|
| NCT04420598 | Pérez-García et al DEBBRAH [47] | II | 21 (8) | Advanced, CNS | T-DXd monotherapy | ORR CNS: 46.2% | |
| NCT04132960 | Mosele et al. [33] | II | 179 (73) | Advanced | T-DXd monotherapy | ORR: 71 % in HER2−positive, 37.5% in HER2−low, and 30% in HER2 0 patients PFS: 11.1 in HER2−positive, 6.7 in HER2−low, and 4.2 months in HER2 0 | |
| Trastuzumab | NCT02277717 | Banerji U. et al. [34] | 99 (49) | Advanced | SYD985 monotherapy | ORR in HR+: 28% | |
| Duocarmazina | ORR in TNBC: 40% PFS in HR+: 4.1 month PFS in TNBC: 4.9 month | ||||||
| XMT-1522 | NCT02952729 | Hamilton et al. [48] | 19 (not available) Advanced | XMT-1522 in monotherapy | DCR: 83% | ||
|
| |||||||
| Combinations therapies | |||||||
| Nelipepimut-S | NCT01570036 | Hickerson et al. [37] | IIb | 275 (275) | Early, adjuvant | Nelipepimut-S + GM-CSF + trastuzumab versus trastuzumab + GM-CSF | 24-months DFS ITT population: 89.9% versus 83.8%, Hazard Ratio 0.62, CI 0.31–1.25 24-months DFS rate TNBC: 92.6% versus 70.2%, Hazard Ratio 0.26, CI: 0.08–0.81 |
| NCT01479244 | Mittendorf et al. PRESENT [38] | III | 758 (758) | Early, adjuvant | Nelipepimut-S + GM-CSF versus placebo + GM-CSF | 3-year DFS: 77.1 % versus 77.5% | |
| GP2 | NCT00524277 | Mittendorf et al. [49] | II | 180 (79) | Early, adjuvant | GP2 + GM-CSF versus GM-CSF alone | 5-year DFS: 88% versus 81% |
| AE37 | NCT00524277 | Mittendorf et al. [49] | II | 301 (154) | Early, adjuvant | AE37 + GM-CSF versus GM-CSF alone | DFS: 77.2% versus 65.7% |
| Nivolumab Zenocutuzumab |
NCT03523572 NCT03321981 |
Hamilton et al. [50] Pistilli et al. [42] |
Ib II | 54 (16) 50 (50) |
Advanced Advanced |
Nivolumab + T-DXd Zenocutuzumab (MCLA-128) + ET |
ORR37.5%in HER2 low DCR 75.0% in HER2 low mPFS 6.3 months in HER2 low CBR (CR + PR + SD at 24 weeks): 16.7% |
BC, breast cancer; BOR, best overall response; CBR, clinical benefit rate; CDK4/6i, cyclin-dependent kinase 4/6 inhibitors; CNS, central nervous system; DCR, disease control rate; DFS, disease free survival; ET, endocrine therapy; GM-CSF, granulocyte-macrophage colony-stimulating facto; HR, hazard ratio; ITT, intention to treat; ORR, objective response rate; OS, overall survival; PFS, progression-free survival.
Table 3.
Main trials ongoing in HER2−low breast cancer
| Type of drug | Drugs tested | NCT | Phase | Status | Population | Primary endpoints |
|---|---|---|---|---|---|---|
| Advanced breast cancer | ||||||
| ADC | T-DXd + pembrolizumab | NCT04042701 | lb | Recruiting | HER2−positive and HER2−low BC | MTD, ORR |
| T-DXd + durvalumab | NCT03742102 | lb/11 | Recruiting | HER2−low TNBC | AEs | |
| T-DXd + durvalumab + paclitaxel + ET | NCT04556773; DESTINY-Breast08 | lb (module 2) | Recruiting | HER2−low BC | AEs | |
| T-DXd versus TPC | NCT04494425; DESTINY-Breast06 | III | Recruiting | HER2−low and HR + BC | PFS | |
| MRG002 | NCT04742153 | II | Recruiting | HER2−low BC | ORR | |
| RC48-ADC | NCT04400695 | III | Recruiting | HER2−low bc | PFS | |
| RC48-ADC | NCT05331326 | II | Recruiting | HER2−expressng BC | ORR | |
| SYD985 + paclitaxel | NCT04602117;ISPY-P1 | I/IIb | Recruiting | HER2−positive and HER2−low BC | AEs, CBR, ORR | |
| ARX788 | NCT02512237; ACE-Breast03 | II | Recruiting | HER2−positive and HER2−low BC | ORR | |
| ARX788 | NCT03255070 | Recruiting | HER2−positive tumors | AEs, ORR | ||
| ARX788 | NCT05018676 | II | Recruiting | HER2−low BC | ORR | |
| RC48-ADC | NCT03052634 | lb | Active, not recruiting | HER2−positive and HER2−low BC | RP2D | |
| RC48-ADC versus TPC | NCT04400695 | III | Recruiting | HER2−low BC | PFS | |
| A166 | NCT03602079 | I/II | Active, not recruiting | HER2−positive tumors | DLT | |
| BAY2701439 | NCT04147819 | Recruiting | HER2−positive tumors | AEs | ||
| FS-1502 | NCT03944499 | Recruiting | HER2−positive tumors | AEs | ||
| Bi/trispecific antibody | MCLA-128 | NCT03321981 | II | Active, not recruiting | HER2−positive and HR+/HER2−low tumors | CBR at 24 weeks |
| Zanidatamab | NCT02892123 | Active, not recruiting | HER2−expressing cancer | rate of DLT | ||
| Runimotamab | NCT03448042 | Recruiting | HER2−expressing cancer | rate of AEs | ||
| IBI315 | NCT04162327 | Recruiting | HER2−expressing cancer | AUC,t1/2,Vd,Cmax | ||
| SAR443216 | NCT05013554 | Recruiting | HER2−expressing cancer | MTD, rate of AEs | ||
| mAB | IMM2902 | NCT05076591 | Recruiting | HER2−expressing cancer | DLT, MTD, AEs | |
|
| ||||||
| Early breast cancer | ||||||
| Vaccine | AST-301, pNGVL3-hlCD | NCT05163223 | II | Recruiting | HER2−low BC | 2 yr iDFS |
| TKI inhibitor | Pyrotinib | NCT05165225 | II | Recruiting | HR+/HER2−low BC | pCR |
| ADCs | T-Dxd + Anastrozole | NCT04553770 | II | Recruiting | HR+/HER2−low BC | pCR |
| T-Dxd | NCT04132960 (DAISY) | II | Active, not recruiting | HER2+, HER2−low, and HER2 0 B C | Antitumor activity | |
ADC, antibody drug conjugates; AEs, adverse events; AUC, area under the plasma concentration-time curve; bc, breast cancer; BOR, best overall response; CBR, clinical benefit rate; Cmax, Maximum plasma concentration; DLT, dose limiting toxicity; iDFS, invasive disease free survival; mAB, monoclonal antibody; MTD, maximum tolerated dose; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; RP2D, recommended phase II dose; TKI, tyrosine kinase inhibitors; T1/2, Terminal elimination half-life; Vd, apparent volume of distribution; pCR, pathological complete response.
Other Treatment Strategies
In preclinical studies, combination therapies with T-DXd and an anti-PD-1 antibody were more effective than monotherapy, possibly due to increased numbers of tumor-infiltrating CD8+ T cells and upregulated PD-L1 and major histocompatibility complex class I expression on tumor cells [40]. Preliminary data from phase Ib/II trials of T-DXd in combination with pembrolizumab, nivolumab, and durvalumab support the rationale for combining anti-HER2 therapies with immunotherapy in HER2-low breast cancer (NCT04042701, NCT03523572, NCT03742102). Another promising drug is zenocutuzumab (MCLA128), a bi-specific humanized IgG1 antibody characterized by a specific crosstalk between ER and HER2 [41]. In a phase II study, the drug was tested in hormone receptor-positive/HER2-low metastatic breast cancer refractory to endocrine therapy and CDK4-6 inhibitors. Eight patients out of 50 (16%) in the experimental arm achieved clinical benefit at week 24, and 1 patient showed partial response followed by long-lasting disease stabilization. Moreover, the addition of zenocutuzumab to endocrine therapy led to restoration of endocrine sensitivity in 17% of them [42].
Conclusions
While traditionally only patients with HER2-positive breast cancer are expected to derive benefit from anti-HER2 therapies, emerging evidence is showing an impressive activity of selected anti-HER2 therapies in patients with HER2-low tumors. In August 2022, the ADC T-DXd was approved by the FDA for the treatment of patients with advanced HER2-low breast cancer, representing the first approved treatment indication in this setting and thus marking an important milestone. Despite the impressive advances in this field, several open challenges still exist. First, after FDA approval, there is an urgent need to revise the treatment algorithm for these patients, and T-DXd should be quickly and broadly introduced in clinical practice, facilitating drug access at a global level. Second, a high heterogeneity seems to exist also within the HER2-low category, starting from the distinction between hormone receptor-positive and hormone receptor-negative disease. The predictive role of hormone receptor status has shown conflicting results across different clinical trials in HER2-low breast cancer, and further investigation is needed to clarify its role. Finally, a consensus on the appropriate pathological assessment of HER2-low status should be sought in order to provide a consistent assessment and reduced discrepancy rate in pathological evaluation. Furthermore, following the DAISY trial results, it will be of outmost importance to define novel methodological strategies to correctly identify within the current HER2 0 category, the cases that might also benefit from T-DXd and other anti-HER2 ADCs.
Conflict of Interest Statement
Dr. Molinelli received honoraria from Novartis and Lilly outside the submitted work. Dr. Jacobs declares no conflicts of interest. Dr. Agostinetto received consultancy fees or honoraria from Eli Lilly and Sandoz and support to attend medical conferences from Roche, Novartis, Eli Lilly, and Genetic, Istituto Gentili (all disclosures are outside the submitted work). Dr. Marchiò received honoraria from Bayer, Roche, AstraZeneca, and Daiichi Sankyo. Dr. De Azambuja received honoraria and/or participated to advisory board from Roche/GNE, Novartis, Seattle Genetics, Zodiac, Libbs, and Pierre Fabre; received travel grants from Roche/GNE and GSK/Novartis; and received research grant to his institution from Roche/GNE, AstraZeneca, GSK/Novartis, and Servier. Dr. Schettini received personal fees from Novartis for educational material. Dr. Lambertini played an advisory role for Roche, Lilly, Novartis, Astrazeneca, Pfizer, Seagen, Gilead, MSD, and Exact Sciences and received speaker honoraria from Roche, Daiichi Sankyo, Lilly, Novartis, Pfizer, Sandoz, Libbs, and Takeda and travel grants from Gilead outside the submitted work.
Funding Sources
Matteo Lambertini acknowledges the support by the Italian Association for Cancer Research (“Associazione Italiana per la Ricerca sul Cancro”, AIRC; MFAG 2020 ID 24698), the Italian Ministry of Health (5×1000 funds 2017), and the IRCCS Ospedale Policlinico San Martino (“Ricerca Corrente” funds). Francesco Schettini is the recipient of a 2020 European Society for Medical Oncology (ESMO) Fellowship − Translational and of the 2021 BBVA Foundation/Hospital Clinic of Barcelona Joan Rodés − Jose Baselga Advanced Research Contract in Oncology. Any views, opinions, findings, conclusions, or recommendations expressed in this material are those solely of the author(s) and do not necessarily reflect those of funding entities.
Author Contributions
All the authors contributed equally.
Funding Statement
Matteo Lambertini acknowledges the support by the Italian Association for Cancer Research (“Associazione Italiana per la Ricerca sul Cancro”, AIRC; MFAG 2020 ID 24698), the Italian Ministry of Health (5×1000 funds 2017), and the IRCCS Ospedale Policlinico San Martino (“Ricerca Corrente” funds). Francesco Schettini is the recipient of a 2020 European Society for Medical Oncology (ESMO) Fellowship − Translational and of the 2021 BBVA Foundation/Hospital Clinic of Barcelona Joan Rodés − Jose Baselga Advanced Research Contract in Oncology. Any views, opinions, findings, conclusions, or recommendations expressed in this material are those solely of the author(s) and do not necessarily reflect those of funding entities.
References
- 1.Harbeck N, Gnant M. Breast cancer. Lancet. 2017;389:1134–50. doi: 10.1016/S0140-6736(16)31891-8. [DOI] [PubMed] [Google Scholar]
- 2.Waks AG, Winer EP. Breast cancer treatment a review. JAMA. 2019;321:288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 3.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ. Strategies for subtypes dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the primary therapy of early breast cancer 2011. Ann Oncol. 2011;22:1736–47. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, et al. Human epidermal growth factor receptor 2 testing in breast cancer American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline focused update. Arch Pathol Lab Med. 2018;142:1364–82. doi: 10.5858/arpa.2018-0902-SA. [DOI] [PubMed] [Google Scholar]
- 5.Cossetti RJD, Tyldesley SK, Speers CH, Zheng Y, Gelmon KA. Comparison of breast cancer recurrence and outcome patterns between patients treated from 1986 to 1992 and from 2004 to 2008. J Clin Oncol. 2015;33:65–73. doi: 10.1200/JCO.2014.57.2461. [DOI] [PubMed] [Google Scholar]
- 6.Tarantino P, Hamilton E, Tolaney SM, Cortes J, Morganti S, Ferraro E, et al. HER2-low breast cancer pathological and clinical landscape. J Clin Oncol. 2020;38:1951–62. doi: 10.1200/JCO.19.02488. [DOI] [PubMed] [Google Scholar]
- 7.Schettini F, Chic N, Brasó-Maristany F, Paré L, Pascual T, Conte B, et al. Clinical and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer. 2021;7((1)):1. doi: 10.1038/s41523-020-00208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H, Katerji H, Turner BM, Audeh W, Hicks DG. HER2-low breast cancers incidence, HER2 staining patterns, clinicopathologic features, MammaPrint and BluePrint genomic profiles. Mod Pathol. 2022;35((8)):1075–82. doi: 10.1038/s41379-022-01019-5. [DOI] [PubMed] [Google Scholar]
- 9.Scott M, Vandenberghe ME, Scorer P, Boothman A-M, Barker C. Prevalence of HER2 low in breast cancer subtypes using the VENTANA anti-HER2/neu (4B5) assay. Jco. 2021;39((15_Suppl)):1021. [Google Scholar]
- 10.Fernandez AI, Liu M, Bellizzi A, Brock J, Fadare O, Hanley K, et al. Examination of low ERBB2 protein expression in breast cancer tissue. JAMA Oncol. 2022;8((4)):1–4. doi: 10.1001/jamaoncol.2021.7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denkert C, Seither F, Schneeweiss A, Link T, Blohmer JU, Just M, et al. Clinical and molecular characteristics of HER2-low-positive breast cancer pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 2021;22(((8)):1151–61. doi: 10.1016/S1470-2045(21)00301-6. [DOI] [PubMed] [Google Scholar]
- 12.Agostinetto E, Rediti M, Fimereli D, Debien V, Piccart M, Aftimos P, et al. HER2-low breast cancer molecular characteristics and prognosis. Cancers. 2021;13((11)):2824. doi: 10.3390/cancers13112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tarantino P, Jin Q, Tayob N, et al. Prognostic and biologic significance of ERBB2-low expression in early-stage breast cancer. JAMA Oncol. 2022;8((8)):1177–83. doi: 10.1001/jamaoncol.2022.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Won HS, Ahn J, Kim Y, Kim JS, Song JY, Kim HK, et al. Clinical significance of HER2-low expression in early breast cancer a nationwide study from the Korean Breast Cancer Society. Breast Cancer Res. 2022;24((1)):22. doi: 10.1186/s13058-022-01519-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miglietta F, Griguolo G, Bottosso M, Giarratano T, Lo Mele M, Fassan M, et al. Evolution of HER2-low expression from primary to recurrent breast cancer. NPJ Breast Cancer. 2021;7((1)):137. doi: 10.1038/s41523-021-00343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tarantino P, Gandini S, Nicolò E, Trillo P, Giugliano F, Zagami P, et al. Evolution of low HER2 expression between early and advanced-stage breast cancer. Eur J Cancer Oxf Engl. 2022;163:35–43. doi: 10.1016/j.ejca.2021.12.022. [DOI] [PubMed] [Google Scholar]
- 17.Agostinetto E, Debien V, Marta GN, Lambertini M, Piccart-Gebhart M, de Azambuja E. CDK4/6 and PI3K inhibitors a new promise for patients with HER2-positive breast cancer. Eur J Clin Invest. 2021;51((7)) doi: 10.1111/eci.13535. https://onlinelibrary.wiley.com/doi/10.1111/eci.13535 . [DOI] [PubMed] [Google Scholar]
- 18.Schettini F, Brasó-Maristany F, Kuderer NM, Prat A. A perspective on the development and lack of interchangeability of the breast cancer intrinsic subtypes. NPJ Breast Cancer. 2022;8((1)):85. doi: 10.1038/s41523-022-00451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Ende NS, Smid M, Timmermans A, van Brakel JB, Hansum T, Foekens R, et al. HER2-low breast cancer shows a lower immune response compared to HER2-negative cases. Sci Rep. 2022;12((1)):12974. doi: 10.1038/s41598-022-16898-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berrino E, Annaratone L, Bellomo SE, Ferrero G, Gagliardi A, Bragoni A, et al. Integrative genomic and transcriptomic analyses illuminate the ontology of HER2-low breast carcinomas. Genome Med. 2022;14((1)):98. doi: 10.1186/s13073-022-01104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tarantino P, Jin Q, Tayob N, Jeselsohn R, Schnitt SJ, Vincuilla J, et al. 1MO Prognostic and biologic significance of HER2-low expression in early breast cancer. Ann Oncol. 2022;33:S124. doi: 10.1001/jamaoncol.2022.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fehrenbacher L, Cecchini RS, Geyer CE, Rastogi P, Costantino JP, Atkins JN, et al. NSABP B-47/NRG oncology phase III randomized trial comparing adjuvant chemotherapy with or without trastuzumab in high-risk invasive breast cancer negative for HER2 by FISH and with IHC 1+ or 2. J Clin Oncol. 2020;38:444–53. doi: 10.1200/JCO.19.01455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gianni L, Lladó A, Bianchi G, Cortes J, Kellokumpu-Lehtinen PL, Cameron DA, et al. Open-label, phase II multicenter, randomized study of the efficacy and safety of two dose levels of Pertuzumab, a human epidermal growth factor receptor 2 dimerization inhibitor, in patients with human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28:1131–7. doi: 10.1200/JCO.2009.24.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.U.S. National Library of Medicine Available from https://clinicaltrials.gov/ct2/show/results/NCT01828021. [DOI] [PubMed]
- 25.Venetis K, Crimini E, Sajjadi E, Corti C, Guerini-Rocco E, Viale G, et al. HER2 low and novel complementary biomarkers expanding the spectrum of HER2 positivity in breast cancer. Front Mol Biosci. 2022;9:834651–12. doi: 10.3389/fmolb.2022.834651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burris HA, Rugo HS, Vukelja SJ, Vogel CL, Borson RA, Limentani S, et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J Clin Oncol. 2011;29((4)):398–405. doi: 10.1200/JCO.2010.29.5865. [DOI] [PubMed] [Google Scholar]
- 27.Nicolò E, Zagami P, Curigliano G. Antibody-drug conjugates in breast cancer the chemotherapy of the future? Curr Opin Oncol. 2020;32(((5)):494–502. doi: 10.1097/CCO.0000000000000656. [DOI] [PubMed] [Google Scholar]
- 28.Doi T, Shitara K, Naito Y, Shimomura A, Fujiwara Y, Yonemori K, et al. Safety and antitumour activity of trastuzumab deruxtecan (DS-8201) a HER2-targeting antibody-drug conjugate in patients with advanced breast and gastric or gastro-oesophageal tumours a phase 1 dose-escalation study. Lancet Oncol. 2017;18:1512–22. doi: 10.1016/S1470-2045(17)30604-6. [DOI] [PubMed] [Google Scholar]
- 29.Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382:610–21. doi: 10.1056/NEJMoa1914510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cortés J, Kim S-B, Chung W-P, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med. 2022;386:1143–54. doi: 10.1056/NEJMoa2115022. [DOI] [PubMed] [Google Scholar]
- 31.Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387((1)):9–20. doi: 10.1056/NEJMoa2203690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.U.S. Food and Drug Administration Approves first targeted therapy for HER2-low breast cancer [Internet] 2022. Available from https://www.fda.gov/news-events/press-announcements/fda-approves-first-targeted-therapy-her2-low-breast-cancer.
- 33.Mosele MF, Lusque A, Dieras V, Deluche E, Ducoulombier A, Pistilli B, et al. LBA1 Unraveling the mechanism of action and resistance to trastuzumab deruxtecan (T-DXd) biomarker analyses from patients from DAISY trial. Ann Oncol. 2022;33:S123. [Google Scholar]
- 34.Banerji U, van Herpen CML, Saura C, Thistlethwaite F, Lord S, Moreno V, et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer a phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019;20:1124–35. doi: 10.1016/S1470-2045(19)30328-6. [DOI] [PubMed] [Google Scholar]
- 35.Saura Manich C, O'Shaughnessy J, Aftimos PG, van den Tweel E, Oesterholt M, Escrivá-de-Romaní SI. Primary outcome of the phase III SYD985.002/TULIP trial comparing [vic-]trastuzumab duocarmazine to physician's choice treatment in patients with pre-treated HER2-positive locally advanced or metastatic breast cancer. Ann Oncol. 2021;2((suppl_5)):S1283–S1346. [Google Scholar]
- 36.Eiger D, Agostinetto E, Saúde-Conde R, de Azambuja E. The exciting new field of her2-low breast cancer treatment. Cancers. 2021;13((5)):1–18. doi: 10.3390/cancers13051015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hickerson A, Clifton GT, Hale DF, Peace KM, Holmes JP, Vreeland TJ, et al. Final analysis of nelipepimut-S plus GM-CSF with trastuzumab versus trastuzumab alone to prevent recurrences in high-risk HER2 low-expressing breast cancer a prospective, randomized, blinded, multicenter phase IIb trial. Jco. 2019;37((8_Suppl)):1. [Google Scholar]
- 38.Mittendorf EA, Lu B, Melisko M, Price Hiller J, Bondarenko I, Brunt AM, et al. Efficacy and safety analysis of Nelipepimut-S vaccine to prevent breast cancer recurrence a randomized, multicenter, phase III clinical trial. Clin Cancer Res. 2019;25:4248–54. doi: 10.1158/1078-0432.CCR-18-2867. [DOI] [PubMed] [Google Scholar]
- 39.Agostinetto E, Montemurro F, Puglisi F, Criscitiello C, Bianchini G, Del Mastro L, et al. Immunotherapy for HER2-positive breast cancer clinical evidence and future perspectives. Cancers. 2022;14((9)):2136. doi: 10.3390/cancers14092136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iwata TN, Ishii C, Ishida S, Ogitani Y, Wada T, Agatsuma T. A HER2-targeting antibody-drug conjugate, trastuzumab deruxtecan (DS-8201a), enhances antitumor immunity in a mouse model. Mol Cancer Ther. 2018;17:1494–503. doi: 10.1158/1535-7163.MCT-17-0749. [DOI] [PubMed] [Google Scholar]
- 41.You G, Won J, Lee Y, et al. Bispecific antibodies a smart arsenal for cancer immunotherapies. Vaccines. 2021;9((7)):724. doi: 10.3390/vaccines9070724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pistilli B, Wildiers H, Hamilton EP, Ferreira AA, Dalenc F, Vidal M, et al. Clinical activity of MCLA-128 (zenocutuzumab) in combination with endocrine therapy (ET) in ER+/HER2-low non-amplified metastatic breast cancer (MBC) patients (pts) with ET-resistant disease who had progressed on a CDK4/6 inhibitor (CDK4/6i) Jco. 2020;38((15_Suppl)):1037. [Google Scholar]
- 43.Gampenrieder SP, Rinnerthaler G, Tinchon C, Petzer A, Balic M, Heibl S, et al. Landscape of HER2-low metastatic breast cancer (MBC) results from the Austrian AGMT_MBC-Registry. Breast Cancer Res. 2021 Dec 14;23((1)):112. doi: 10.1186/s13058-021-01492-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mutai R, Barkan T, Moore A, Sarfaty M, Shochat T, Yerushalmi R, et al. Prognostic impact of HER2-low expression in hormone receptor positive early breast cancer. Breast. 2021 Dec;60:62–9. doi: 10.1016/j.breast.2021.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tan RSYC, Ong WS, Lee KH, Lim AH, Park S, Park YH, et al. HER2 expression copy number variation and survival outcomes in HER2-low non-metastatic breast cancer an international multicentre cohort study and TCGA-METABRIC analysis. BMC Med. 2022 Mar 17;20((1)):105. doi: 10.1186/s12916-022-02284-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Modi S, Park H, Murthy RK, Iwata H, Tamura K, Tsurutani J, et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer results from a Phase Ib Study. J Clin Oncol. 2020 Jun 10;38(((17)):1887–96. doi: 10.1200/JCO.19.02318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pérez-García JM, Batista MV, Cortez P, Ruiz-Borrego M, Cejalvo JM, de la Haba-Rodriguez J, et al. Trastuzumab deruxtecan in patients with central nervous system involvement from HER2-positive breast cancer the DEBBRAH trial. Neuro Oncol. 2022 May 26;:noac144. doi: 10.1093/neuonc/noac144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hamilton EP, Barve MA, Bardia A, Beeram M, Bendell JC, Mosher R, et al. Phase 1 dose escalation of XMT-1522 a novel HER2-targeting antibody-drug conjugate (ADC) in patients (pts) with HER2-expressing breast lung and gastric tumors. J Clin Oncol. 2018;36((15_suppl)):2546. [Google Scholar]
- 49.Mittendorf EA, Ardavanis A, Litton JK, Shumway NM, Hale DF, Murray JL, et al. Primary analysis of a prospective single-blinded phase II trial evaluating the HER2 peptide GP2 vaccine in breast cancer patients to prevent recurrence. Oncotarget. 2016 Oct 4;7((40)):66192–201. doi: 10.18632/oncotarget.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hamilton E, Shapiro CL, Petrylak D, Boni V, Martin M, Conte GD, et al. Abstract PD3-07 trastuzumab deruxtecan (T-DXd; DS-8201) with nivolumab in patients with HER2-expressing, advanced breast cancer: a 2-part, phase 1b, multicenter, open-label study. Cancer Res. 2021;81((4_Suppl)):PD3–PD07. [Google Scholar]