Abstract
Background/Aim: Lung cancer is one of the most common malignant neoplastic diseases and by far the leading cause of cancer death worldwide. Recently, immune checkpoint inhibitors (ICIs) have received increasing attention for playing a crucial role in non-small cell lung cancer (NSCLC). Biomarkers, such as programmed cell death-ligand 1 (PD-L1) and tumor mutational burden (TMB), seemed to be helpful in selecting patients who are more likely to benefit from ICI treatment: however, their role has not yet been fully clarified.
Patients and Methods: In this retrospective study, we evaluated the relationship between pre-treatment peripheral blood neutrophil-to-lymphocyte ratio (NLR) and survival in 252 patients suffering from advanced NSCLC who had received pembrolizumab as their first-line immunotherapy.
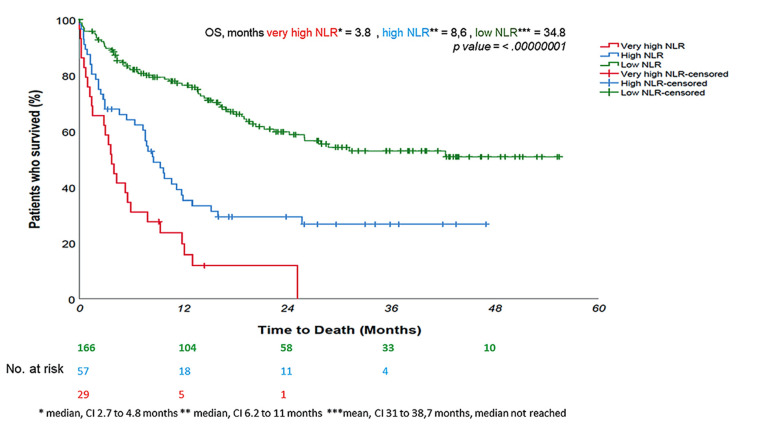
Results: Compared to their NLR low counterparts who had a median overall survival (OS) of 34.8 months, patients with NLRs above 4.8 had a median OS of 7.6 months (HR=3.26, 95%Cl=2.3-4.6, p-value<0.0000001). In multivariate Cox regression analysis, alongside other variables, such as metastatic sites, age, and sex, NLR and PD-L1 predicted progression-free survival and OS; furthermore, a very high NLR – over 10 – seemed to forecast a very dismal prognosis in patients undergoing immunotherapy, with sudden deaths in the days immediately following therapy (median OS=3.8 months).
Conclusion: NLR acts as a valuable and reliable prognostic factor in non-small cell lung carcinoma patients undergoing first line immunotherapy with pembrolizumab. Additional investigation is necessary to fully elucidate the underlying biological rationale, which can be found in myeloid derived suppressor cells, a heterogeneous population of cells with neutrophil-like immunophenotypic features.
Keywords: Non-small cell lung cancer, immunotherapy, pembrolizumab, neutrophils-to-lymphocytes ratio, myeloid derived suppressor cells, PD-L1, leukocytosis
Lung cancer is one of the most common malignant neoplastic diseases (1), with still poor prognosis despite the advances in the knowledge of the biology of this neoplasm. By far the most common cancer-related cause of death, lung cancer claims more lives each year than colon, breast, and prostate cancers combined (2). Small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) are the two main types according to histopathological diagnosis, with the former having a noticeably dismal prognosis compared to the latter. NSCLC represents about 85% of all lung carcinomas, with two predominant histological subtypes: adenocarcinoma and squamous cell carcinoma (3,4).
Recently, the immune system in cancer development and therapeutic response has taken on a pivotal role, and immune checkpoint inhibitors (ICIs) have received increasing attention due to their growing use in lung, skin, genitourinary, and other cancers treatment (5,6).
Biomarkers, such as programmed cell death-ligand 1 (PD-L1), tumor mutational burden (TMB) and neoantigen load, which may reflect the state of the tumor immune microenvironment, seemed to be helpful in selecting patients who are more likely to benefit from ICI treatment. However, the detection of these biomarkers greatly depends on the adequacy of tumor tissue. Therefore, there is an unmet need for biomarkers that can be easily assessed without requiring invasive procedures.
In NSCLC, ICIs, by targeting inhibitory receptors on T cells, have achieved tumor responses in a subset of patients across a wide range of cancer types; however, a not so small subset of patients undergoing ICI treatment do not experience clinical benefit. In less than half of patients with PD-L1 expression ≥50%, pembrolizumab yields an objective decrement in neoplastic mass, while 45% of those with negative PD-L1 respond to therapy with nivolumab and ipilimumab, suggesting that PD-L1 is far from an ideal biomarker predicting response to immunotherapy (7,8). Among patients with newly diagnosed lung cancer, almost 15% have leukocytosis (9). Granulocyte colony stimulating factor (G-CSF), macrophage colony-stimulating factor (M-CSF), and interleukin 6 (IL-6) levels have been shown to be significantly higher in patients with lung malignancies, associated to a worse prognosis (10). Leukocytosis is likely an upshot defined by the extent of cytokine production and is thus referred to as tumor-related leukocytosis.
Furthermore, even thrombocytosis has shown a correlation to many malignancies, and has been suggested to be an independent prognostic factor in lung cancer (11-13).
Tumor-related leukocytosis predicts poor survival in lung cancer patients (14,15). The pro-oncogenic role of G-CSF has been evaluated in preclinical models; tumor-secreted G-CSF has been shown to stimulate proliferation of myeloid-derived suppressor cells (MDSC). MDSCs are a heterogeneous population of cells with neutrophil-like immunophenotypic features. Polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs) have been found to hinder immune response activation in cancer and many other pathological conditions. Whereas in healthy individuals PMN-MDSCs are barely detectable, they accumulate in the blood, lymph nodes, and bone marrow of patients and cancer laboratory animals and inhibit T-cell-mediated antitumor activity (16). G-CSF mobilizes MDSCs from the bone marrow and makes them nest into distant organs, developing a pro-tumor microenvironment that fosters tumor cell diapedesis and metastasis (17,18). G-CSF is secreted by cancer cells and the tumor microenvironment, leading to the proliferation of neutrophils as well as MDSCs. MDSCs inhibit lymphocytes proliferation, increasing the number of neutrophils. Therefore, the neutrophil-to-lymphocyte ratio (NLR) represents only the epiphenomenon of a deeper immunoregulatory mechanism, which is indirectly related to disease progression and patients’ survival, while MDSCs directly contribute to disease progression and clinical outcome (19).
NLR represents a valuable and reliable candidate biomarker, because it is cost-effective and easily accessible (20,21). Biologically, NLR reflects systemic inflammation and could represent a sort of balance of the immune system in a malignant biological setting (22-24). Specifically, neutrophils can secrete immunosuppressive mediators and angiogenic factors, and can contribute to a pro-tumor microenvironment (25). In addition, low levels of circulating lymphocytes may correlate with lower levels of tumor-infiltrating lymphocytes (TILs) and a reduced anti-tumor T-cell response (26).
In NSCLC, higher neutrophil counts are correlated with reduced CD8+ T-lymphocytes counts (27). A high NLR in the pre-treatment setting seems to be independently and significantly associated with a short progression free-survival (PFS) and overall survival (OS) of patients with previously untreated NSCLC undergoing pembrolizumab monotherapy, even if PD-L1 expression on tumor cells is ≥50% (28). On the contrary, low NLR and high PD-L1 seem to be significantly associated with favorable outcomes (29), underlying the importance of this potential biomarkers for the choice of therapy, probably regardless of age, sex, tobacco use, performance status, histology, serum albumin level, and oncogenic drivers (30).
The aim of this retrospective study was to assess the prognostic role of NLR and platelet-to-lymphocyte ratio (PLR), alongside other variables, such as age, sex, metastatic sites, and PD-L1 tumoral proportion score (TPS) level in patients with advanced NSCLC treated with pembrolizumab.
Patients and Methods
Patients suffering from metastatic NSCLC, with TPS PD-L1 ≥50%, Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) ≤2, and who had undergone pembrolizumab as first-line monotherapy were eligible for this analysis. Patients were ineligible if they had oncogene-addicted malignancies (EGFR, ALK mutations/translocations), autoimmune disease requiring systemic steroid treatment, or active interstitial lung disease. Pembrolizumab was initially administered at 2 mg/kg intravenously over 60 min and later at 200 mg every 3 weeks, or at 400 mg every 6 weeks. Patients were not required to provide any written informed consent, and the study was conducted in accordance with the Declaration of Helsinki and Italian Privacy Protection Commissioner.
In each center, tumor PD-L1 expression was determined by immunoassay using the Ventana PD-L1 rabbit monoclonal antibody SP263 (Roche, Basel, Switzerland) and the BenchMark Ultra platform system (Roche). Representative tumor sections (with more than 100 neoplastic cells) of formalin-fixed and paraffin-embedded tissues were used: sections of 4 μM were prepared, heated, and mounted on polarized slides for immunohistochemistry staining. Every test was carried out with a session control (a positive staining in human tonsilla epithelial cells). Microscopical assessment of TPS was evaluated by an experienced pathologist and given as a percentage of positive cells on total tumoral cells.
Collection of peripheral venous blood was routinely analyzed by local laboratories for complete blood count (CBC). White blood cell count (WBC), absolute neutrophil count (NEU), absolute lymphocyte count (LYM), absolute platelets count (PLT) at baseline were analyzed: NLR and PLR were calculated from pre-treatment complete blood counts. Clinicopathological features and treatment history data were extracted from the medical records: tumor response was assessed using computed tomography and/or positron emitting tomography every 8-12 weeks. Progression free survival (PFS) was measured from pembrolizumab treatment start to clinical, metabolic, or radiographic progression or death from any cause. OS was measured from pembrolizumab treatment beginning to death from any cause.
Statistical analysis. Clinically relevant features, such as age (continuous), sex, histological type (adenocarcinoma, squamous, NSCLC NOS), PD-L1 TPS (continuous), metastatic sites, and baseline peripheral blood counts were used as variables. Kaplan–Meier analysis of PFS and OS was performed. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated using the univariate and multivariate Cox proportional hazard model. Both log-rank test and Cox model were analyzed using SPSS (IBM, Chicago, IL, USA). The cut-offs for NLR and PLR were identified by Survival Analysis for Continuous Explanatory Variable maximally selected rank statistics (Jamovi Software) (31,32).
Results
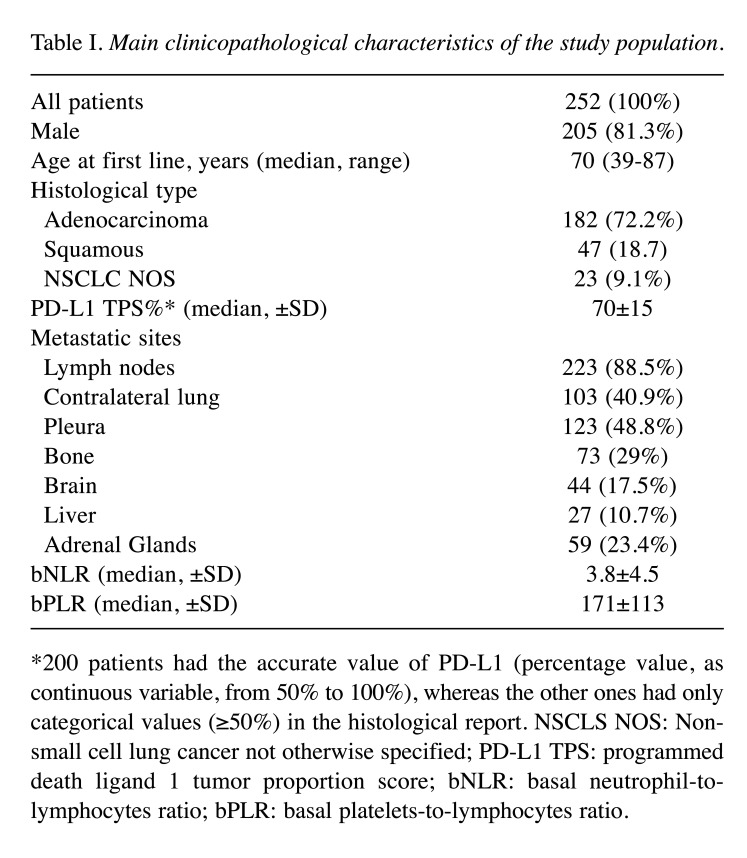
The analysis involved a series of 252 patients consecutively treated in four South Italy oncological centers according to clinical practice, between September 2017 and May 2022. Baseline characteristics and peripheral blood counts of these patients are shown in Table I. Of all patients, 205 (81.3%) were male. The median age was 70 years (range=39-87 years). One hundred eighty-two (72.2%) patients had adenocarcinoma histology. Twenty-seven (10.7%) patients had liver metastases (Table I).
Table I. Main clinicopathological characteristics of the study population.
*200 patients had the accurate value of PD-L1 (percentage value, as continuous variable, from 50% to 100%), whereas the other ones had only categorical values (≥50%) in the histological report. NSCLS NOS: Nonsmall cell lung cancer not otherwise specified; PD-L1 TPS: programmed death ligand 1 tumor proportion score; bNLR: basal neutrophil-tolymphocytes ratio; bPLR: basal platelets-to-lymphocytes ratio.
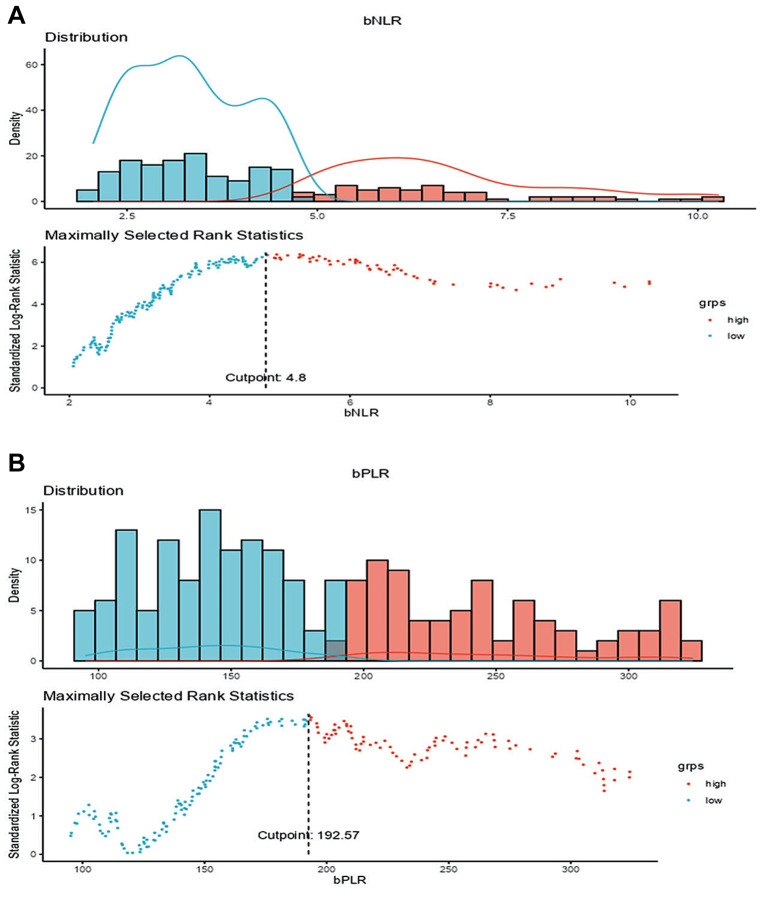
The median±SD NLR was 3.8±4.5 and PLR was 171±113. To evaluate OS, the cut-off values for NLR and PLR used were 4.8 (p-value<0.001) and 193 (p-value=0.003), respectively (Figure 1A and B); for PFS they were 5.29 (p-value<0.001) and 182 (p-value=0.013), respectively. For simplicity, it was decided to use the cut-off calculated on the OS also in the analysis of PFS.
Figure 1. Cut-offs for basal neutrophil-to-lymphocyte ratio (NLR) (A) and platelet-to-lymphocyte ratio (PLR) (B) were identified by survival analysis for continuous explanatory variable - maximally selected rank statistics (Jamovi Software), using overall survival as the outcome variable; the cut-off values for NLR and PLR were 4.8 (p-value<0.001) and 193 (p-value=0.003), respectively. Cut-offs were also calculated using progression-free survival as the outcome variable and were 5.29 (p-value<0.001) for NLR and 182 (p-value=0.013) for PLR, respectively (data not shown).
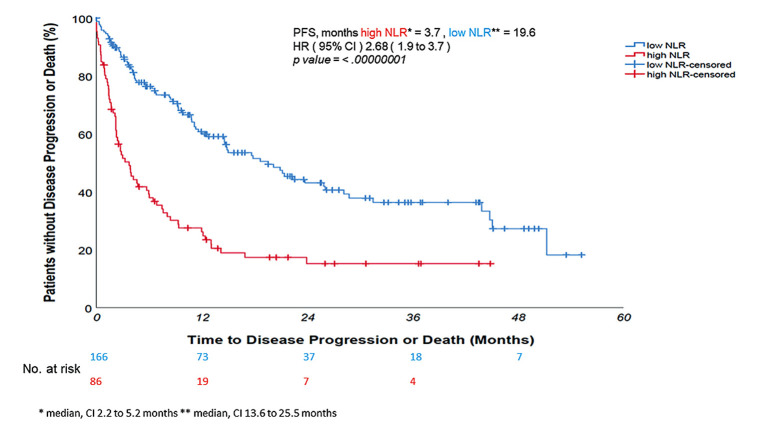
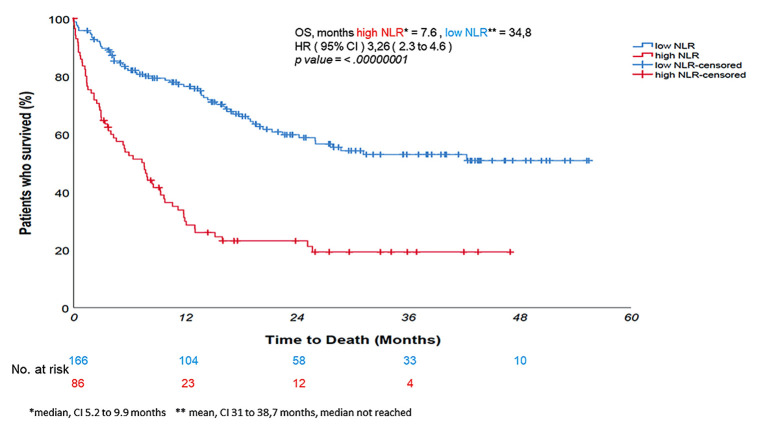
Univariate log rank test revealed that high NLR was associated with a significantly shorter PFS and OS - median 3.7 and 7.6 months vs. 19.6 and 34.8 months (mean value, median not reached) for the low NLR counterparts, respectively; p-value<0.0000001 (Figure 2 and Figure 3). At Cox proportional hazard univariate analysis, NLR ≥4.8 increased the risk of death by more than three-fold compared with the low NLR (HR=3.26, 95%CI=2.3-4.6, p-value<0.0000001) and the risk of disease progression by more than two-fold (HR=2.68, 95%CI=1.9-3.7, p-value<0.0000001). Multivariate analysis was performed using as risk factors all baseline characteristics. In both multivariate analysis for PFS and OS, NLR and PLR were analyzed as continuous variables, also because, despite the dichotomization easing the decision-making process in clinical practice, it may create rather than avoid problems by reducing the statistical power of the analysis (33).
Figure 2. Neutrophil-to-lymphocyte ratio (NLR) higher than 4.8 was associated with a significantly shorter progression-free survival – 3.7 months vs. 19.6 months of the NLR-low counterpart – p-value<0.0000001. Disease progression risk increased more than two times (hazard ratio=2.68).
Figure 3. Univariate log rank test revealed that neutrophil-to-lymphocyte ratio (NLR) equal or higher than 4.8 was associated with a significantly shorter overall survival - 7.6 months vs. 34.8 months of the NLR-low counterpart – p-value<0.0000001. Death risk increased more than threefold (hazard ratio=3.26).
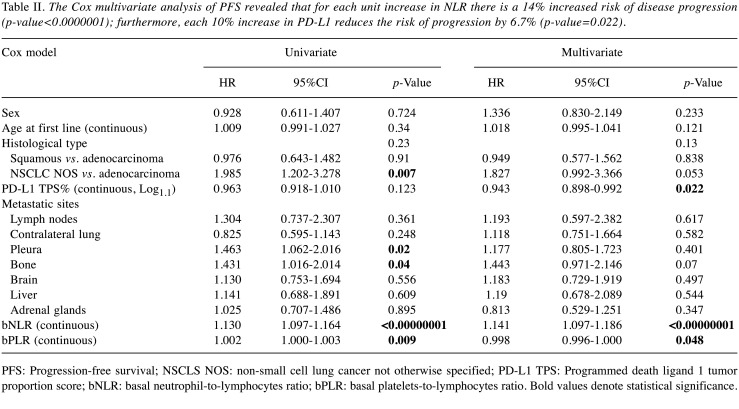
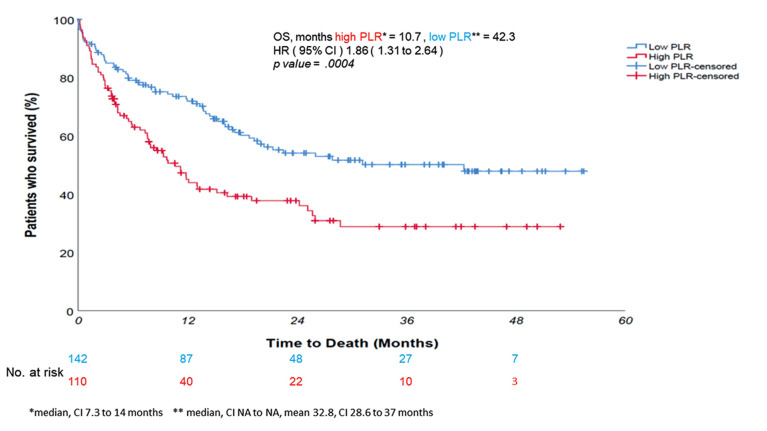
The multivariate analysis of PFS revealed that for each unit increase in NLR there was a 14% increased risk of disease progression (p-value<0.00000001); furthermore, each 10% increase in PD-L1 reduced the risk of progression by 6.7% (p-value=0.022) (Table II). Similarly, the multivariate analysis of OS found a 13.7% increased risk of death for each unit increase in NLR (p-value<0.00000001), with each 10% increase in PD-L1 reducing death risk by 7% (p-value=0.005) (Table III). Furthermore, a very high NLR – over 10 – seemed to forecast an even worse prognosis in patients undergoing immunotherapy, with sudden deaths in the weeks immediately following therapy (Figure 4). Although in univariate log-rank analysis PLR seemed to discriminate patients’ survival (Figure 5), in multivariate Cox regression, only NLR and PD-L1 retained statistical significance.
Table II. The Cox multivariate analysis of PFS revealed that for each unit increase in NLR there is a 14% increased risk of disease progression (p-value<0.0000001); furthermore, each 10% increase in PD-L1 reduces the risk of progression by 6.7% (p-value=0.022).
PFS: Progression-free survival; NSCLS NOS: non-small cell lung cancer not otherwise specified; PD-L1 TPS: Programmed death ligand 1 tumor proportion score; bNLR: basal neutrophil-to-lymphocytes ratio; bPLR: basal platelets-to-lymphocytes ratio. Bold values denote statistical significance.
Table III. The Cox multivariate analysis of OS found a 13.7% increased risk of death for each unit increase in NLR (p-value<0.00000001), with each 10% increase in PD-L1 reducing the death risk by 7% (p-value=0.005).
OS: Overall survival; NSCLS NOS: non-small cell lung cancer not otherwise specified; PD-L1 TPS: programmed death ligand 1 tumor proportion score; bNLR: basal neutrophil-to-lymphocytes ratio; bPLR: basal platelets-to-lymphocytes ratio. Bold values denote statistical significance.
Figure 4. A very high neutrophil-to-lymphocyte (NLR) – over 10 – seems to forecast an even worse prognosis in patients undergoing immunotherapy, with sudden deaths in the weeks immediately following therapy. Indeed, patients with NLR higher than 10 had a median overall survival of 3.8 months. In these patients, we postulated a detrimental effect of immunotherapy. The prediction of prognosis could help identify patients who are likely to benefit more from chemo-immunotherapy rather than immunotherapy only: indeed, chemotherapy could be effective in killing myeloidderived suppressor cells and therefore, hinder their immunosuppressive role.
Figure 5. In univariate log-rank analysis, platelet-to-limphocyte (PLR) discriminated patients’ survival, with a shorter survival of patients with PLR higher than 193 – overall survival (OS) 10.7 months vs. 42.4 – p-value=0.0004, hazard ratio for death risk=1.86. This finding was not retained at multivariate analysis for OS and was only slightly confirmed at multivariate analysis for progression-free survival (see Table II).
Discussion
Today, NLR is widely used across many medical disciplines, as a reliable and low-cost marker of cancer-related inflammation (34). Petrova et al. evaluated NLR and PLR in patients with NSCLC treated with pembrolizumab as a second line treatment, after they had undergone platinum-containing chemotherapy as a first line treatment. They assessed optimal cut-offs by receiver operating characteristics (ROC), with matching NLR and PLR with clinical benefit, defined as radiological response or stabilization for more than six months (35). Similarly, Hasegawa et al. found an NLR of 4.56 as the optimal cut-off by ROC, indicating as outcome variable disease progression, regardless of when it occurred (28). As also shown by Ksienski et al., we identified optimal cut-off points of slightly lower values for baseline NLR and PLR by using maximally selected rank statistics (36). Nevertheless, we suggest that both NLR and PD-L1 must be considered as continuous variables rather than dichotomous variables, and both should be included in the multivariate analysis: thus, their role can be fully understood, with an exponential trend in the risk as the NLR increases and PD-L1 decreases.
NLR and PD-L1 probably represent two sides of the same coin (or maybe 2 faces of an icosahedron), namely two mechanisms by which the tumor succeeds to evade the host's immune response: therefore, we believe it is restricting and inaccurate to evaluate them with a simple "high and low" score. MDSCs are an entity hiding behind neutrophilic leukocytosis, acting in synergy with PD-L1-expressing tumor cells to inactivate T cell immunosurveillance. Finally, alongside other common variables, such as metastatic sites, age, and sex, NLR and PD-L1 predict risk of disease progression and death; furthermore, a very high NLR seems to forecast an even worse prognosis in patients undergoing immunotherapy, with sudden deaths in the days immediately following therapy, strengthening the evidence that the prognosis worsens as NLR increases.
It could be interesting to evaluate immunotherapy treatment alone in patients with low NLR and PD-L1 values, and also explore the efficacy of the chemo-immunotherapy combination in patients with a high NLR. The prediction of prognosis could help identify patients who are likely to benefit more from chemo-immunotherapy rather than immunotherapy only: indeed, chemotherapy could be effective in killing MDSCs and therefore, hinder their immunosuppressive role.
Conflicts of Interest
The Authors have no conflicts of interest to declare in relation to this study.
Authors’ Contributions
Francesco Jacopo Romano conceived the study, performed statistical analysis, drafted and revised the manuscript. Ferdinando Riccardi contributed to the study concept, manuscript drafting and critical revision. All Authors contributed to data collection and manuscript revision.
References
- 1.Nixon NA, Blais N, Ernst S, Kollmannsberger C, Bebb G, Butler M, Smylie M, Verma S. Current landscape of immunotherapy in the treatment of solid tumours, with future opportunities and challenges. Curr Oncol. 2018;25(5):e373–e384. doi: 10.3747/co.25.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 3.Lim W, Ridge CA, Nicholson AG, Mirsadraee S. The 8th lung cancer TNM classification and clinical staging system: review of the changes and clinical implications. Quant Imaging Med Surg. 2018;8(7):709–718. doi: 10.21037/qims.2018.08.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osmani L, Askin F, Gabrielson E, Li QK. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): Moving from targeted therapy to immunotherapy. Semin Cancer Biol. 2018;52(Pt 1):103–109. doi: 10.1016/j.semcancer.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang T, Zhou C. The past, present and future of immunotherapy against tumor. Transl Lung Cancer Res. 2015;4(3):253–264. doi: 10.3978/j.issn.2218-6751.2015.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: the beginning of the end of cancer. BMC Med. 2016;14:73. doi: 10.1186/s12916-016-0623-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR, KEYNOTE-024 Investigators Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 8.Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, Minenza E, Linardou H, Burgers S, Salman P, Borghaei H, Ramalingam SS, Brahmer J, Reck M, O’Byrne KJ, Geese WJ, Green G, Chang H, Szustakowski J, Bhagavatheeswaran P, Healey D, Fu Y, Nathan F, Paz-Ares L. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093–2104. doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Granger JM, Kontoyiannis DP. Etiology and outcome of extreme leukocytosis in 758 nonhematologic cancer patients: a retrospective, single-institution study. Cancer. 2009;115(17):3919–3923. doi: 10.1002/cncr.24480. [DOI] [PubMed] [Google Scholar]
- 10.Katsumata N, Eguchi K, Fukuda M, Yamamoto N, Ohe Y, Oshita F, Tamura T, Shinkai T, Saijo N. Serum levels of cytokines in patients with untreated primary lung cancer. Clin Cancer Res. 1996;2(3):553–559. [PubMed] [Google Scholar]
- 11.Pedersen LM, Milman N. Prognostic significance of thrombocytosis in patients with primary lung cancer. Eur Respir J. 1996;9(9):1826–1830. doi: 10.1183/09031936.96.09091826. [DOI] [PubMed] [Google Scholar]
- 12.Maráz A, Furák J, Varga Z, Kahán Z, Tiszlavicz L, Hideghéty K. Thrombocytosis has a negative prognostic value in lung cancer. Anticancer Res. 2013;33(4):1725–1729. [PubMed] [Google Scholar]
- 13.Aoe K, Hiraki A, Ueoka H, Kiura K, Tabata M, Tanaka M, Tanimoto M. Thrombocytosis as a useful prognostic indicator in patients with lung cancer. Respiration. 2004;71(2):170–173. doi: 10.1159/000076679. [DOI] [PubMed] [Google Scholar]
- 14.Kasuga I, Makino S, Kiyokawa H, Katoh H, Ebihara Y, Ohyashiki K. Tumor-related leukocytosis is linked with poor prognosis in patients with lung carcinoma. Cancer. 2001;92(9):2399–2405. doi: 10.1002/1097-0142(20011101)92:9<2399::aid-cncr1588>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 15.Valero C, Lee M, Hoen D, Weiss K, Kelly DW, Adusumilli PS, Paik PK, Plitas G, Ladanyi M, Postow MA, Ariyan CE, Shoushtari AN, Balachandran VP, Hakimi AA, Crago AM, Long Roche KC, Smith JJ, Ganly I, Wong RJ, Patel SG, Shah JP, Lee NY, Riaz N, Wang J, Zehir A, Berger MF, Chan TA, Seshan VE, Morris LGT. Pretreatment neutrophil-to-lymphocyte ratio and mutational burden as biomarkers of tumor response to immune checkpoint inhibitors. Nat Commun. 2021;12(1):729. doi: 10.1038/s41467-021-20935-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou J, Nefedova Y, Lei A, Gabrilovich D. Neutrophils and PMN-MDSC: Their biological role and interaction with stromal cells. Semin Immunol. 2018;35:19–28. doi: 10.1016/j.smim.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bahar B, Acedil Ayc Iota B, Çoşkun U, Büyükberber S, Benekli M, Yildiz R. Granulocyte colony stimulating factor (G-CSF) and macrophage colony stimulating factor (M-CSF) as potential tumor markers in non small cell lung cancer diagnosis. Asian Pac J Cancer Prev. 2010;11(3):709–712. [PubMed] [Google Scholar]
- 18.Kowanetz M, Wu X, Lee J, Tan M, Hagenbeek T, Qu X, Yu L, Ross J, Korsisaari N, Cao T, Bou-Reslan H, Kallop D, Weimer R, Ludlam MJ, Kaminker JS, Modrusan Z, van Bruggen N, Peale FV, Carano R, Meng YG, Ferrara N. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc Natl Acad Sci USA. 2010;107(50):21248–21255. doi: 10.1073/pnas.1015855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tavakkoli M, Wilkins CR, Mones JV, Mauro MJ. A novel paradigm between leukocytosis, G-CSF secretion, neutrophil-to-lymphocyte ratio, myeloid-derived suppressor cells, and prognosis in non-small cell lung cancer. Front Oncol. 2019;9:295. doi: 10.3389/fonc.2019.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Zhang Z, Hu Y, Yan X, Song Q, Wang G, Chen R, Jiao S, Wang J. Pretreatment neutrophil-to-lymphocyte ratio (NLR) may predict the outcomes of advanced non-small-cell lung cancer (NSCLC) patients treated with immune checkpoint inhibitors (ICIs) Front Oncol. 2020;10:654. doi: 10.3389/fonc.2020.00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartlett EK, Flynn JR, Panageas KS, Ferraro RA, Sta Cruz JM, Postow MA, Coit DG, Ariyan CE. High neutrophil-to-lymphocyte ratio (NLR) is associated with treatment failure and death in patients who have melanoma treated with PD-1 inhibitor monotherapy. Cancer. 2020;126(1):76–85. doi: 10.1002/cncr.32506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bilen MA, Martini DJ, Liu Y, Lewis C, Collins HH, Shabto JM, Akce M, Kissick HT, Carthon BC, Shaib WL, Alese OB, Pillai RN, Steuer CE, Wu CS, Lawson DH, Kudchadkar RR, El-Rayes BF, Master VA, Ramalingam SS, Owonikoko TK, Harvey RD. The prognostic and predictive impact of inflammatory biomarkers in patients who have advanced-stage cancer treated with immunotherapy. Cancer. 2019;125(1):127–134. doi: 10.1002/cncr.31778. [DOI] [PubMed] [Google Scholar]
- 23.Xie X, Liu J, Yang H, Chen H, Zhou S, Lin H, Liao Z, Ding Y, Ling L, Wang X. Prognostic value of baseline neutrophil-to-lymphocyte ratio in outcome of immune checkpoint inhibitors. Cancer Invest. 2019;37(6):265–274. doi: 10.1080/07357907.2019.1639057. [DOI] [PubMed] [Google Scholar]
- 24.Tan Q, Liu S, Liang C, Han X, Shi Y. Pretreatment hematological markers predict clinical outcome in cancer patients receiving immune checkpoint inhibitors: A meta-analysis. Thorac Cancer. 2018;9(10):1220–1230. doi: 10.1111/1759-7714.12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nassar AH, Mouw KW, Jegede O, Shinagare AB, Kim J, Liu CJ, Pomerantz M, Harshman LC, Van Allen EM, Wei XX, McGregor B, Choudhury AD, Preston MA, Dong F, Signoretti S, Lindeman NI, Bellmunt J, Choueiri TK, Sonpavde G, Kwiatkowski DJ. A model combining clinical and genomic factors to predict response to PD-1/PD-L1 blockade in advanced urothelial carcinoma. Br J Cancer. 2020;122(4):555–563. doi: 10.1038/s41416-019-0686-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. 2019;19(3):133–150. doi: 10.1038/s41568-019-0116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kargl J, Busch SE, Yang GH, Kim KH, Hanke ML, Metz HE, Hubbard JJ, Lee SM, Madtes DK, McIntosh MW, Houghton AM. Neutrophils dominate the immune cell composition in non-small cell lung cancer. Nat Commun. 2017;8:14381. doi: 10.1038/ncomms14381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasegawa T, Yanagitani N, Utsumi H, Wakui H, Sakamoto H, Tozuka T, Yoshida H, Amino Y, Uematsu S, Yoshizawa T, Uchibori K, Kitazono S, Horiike A, Horai T, Kuwano K, Nishio M. Association of high neutrophil-to-lymphocyte ratio with poor outcomes of pembrolizumab therapy in high-PD-L1-expressing non-small cell lung cancer. Anticancer Res. 2019;39(12):6851–6857. doi: 10.21873/anticanres.13902. [DOI] [PubMed] [Google Scholar]
- 29.Banna GL, Signorelli D, Metro G, Galetta D, De Toma A, Cantale O, Banini M, Friedlaender A, Pizzutillo P, Garassino MC, Addeo A. Neutrophil-to-lymphocyte ratio in combination with PD-L1 or lactate dehydrogenase as biomarkers for high PD-L1 non-small cell lung cancer treated with first-line pembrolizumab. Transl Lung Cancer Res. 2020;9(4):1533–1542. doi: 10.21037/tlcr-19-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alessi JV, Ricciuti B, Alden SL, Bertram AA, Lin JJ, Sakhi M, Nishino M, Vaz VR, Lindsay J, Turner MM, Pfaff K, Sharma B, Felt KD, Rodig SJ, Gainor JF, Awad MM. Low peripheral blood derived neutrophil-to-lymphocyte ratio (dNLR) is associated with increased tumor T-cell infiltration and favorable outcomes to first-line pembrolizumab in non-small cell lung cancer. J Immunother Cancer. 2021;9(11):34824161. doi: 10.1136/jitc-2021-003536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The Jamovi project (Version 1.8) (Computer Software) Available at: https://www.jamovi.org. [Last accessed on October 17, 2022]
- 32.R Core Team. A Language and environment for statistical computing. (Version 4.0) (Computer software) Available at: https://cran.r-project.org. [Last accessed April 1, 2021]
- 33.Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25(1):127–141. doi: 10.1002/sim.2331. [DOI] [PubMed] [Google Scholar]
- 34.Zahorec R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl Lek Listy. 2021;122(7):474–488. doi: 10.4149/BLL_2021_078. [DOI] [PubMed] [Google Scholar]
- 35.Petrova MP, Eneva MI, Arabadjiev JI, Conev NV, Dimitrova EG, Koynov KD, Karanikolova TS, Valev SS, Gencheva RB, Zhbantov GA, Ivanova AI, Sarbianova II, Timcheva CV, Donev IS. Neutrophil to lymphocyte ratio as a potential predictive marker for treatment with pembrolizumab as a second line treatment in patients with non-small cell lung cancer. Biosci Trends. 2020;14(1):48–55. doi: 10.5582/bst.2019.01279. [DOI] [PubMed] [Google Scholar]
- 36.Ksienski D, Wai ES, Alex D, Croteau NS, Freeman AT, Chan A, Patterson T, Clarkson M, Fiorino L, Poonja Z, Fenton D, Irons S, Lesperance M. Prognostic significance of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio for advanced non-small cell lung cancer patients with high PD-L1 tumor expression receiving pembrolizumab. Transl Lung Cancer Res. 2021;10(1):355–367. doi: 10.21037/tlcr-20-541. [DOI] [PMC free article] [PubMed] [Google Scholar]