Abstract
The superantigenic function of toxic shock syndrome toxin 1 (TSST-1) is generally regarded as an important determinant of its lethal effects in humans or experimental animals. This study examined the role of superantigenicity in a BALB/c mouse model of lethal TSST-1-induced hypersensitivity to lipopolysaccharide (LPS). In this model, TSST-1 greatly potentiated both LPS-induced lethality, as well as LPS-induced serum tumor necrosis factor alpha (TNF-α) activity. Although BALB/c-SCID mice were resistant to these LPS enhancement effects of TSST-1, BALB/c-SCID mice reconstituted with T cells were completely susceptible to the enhancement effect of TSST-1 on LPS-induced serum TNF-α. Mice pretreated with cyclosporine (Cs) or neutralizing antibodies against gamma interferon (IFN-γ) did not develop lethal LPS hypersensitivity when injected with TSST-1, and these agents reduced the enhancement effect of TSST-1 on LPS-induced serum TNF-α by 99 and 85%, respectively. Cs pretreatment also completely inhibited the known capacity of TSST-1 to amplify LPS-induced levels of IFN-γ in serum. In contrast, mice given Cs after a priming injection of TSST-1, but before LPS, still exhibited lethal hypersensitivity to LPS. Cs given after TSST-1 also did not inhibit enhancement of LPS-induced serum TNF-α by TSST-1 but inhibited the enhancement effect of TSST-1 on LPS-induced serum IFN-γ by 50%. These experiments support the theory that TSST-1-induced hypersensitivity to LPS is mediated primarily by IFN-γ derived from superantigen-activated T cells.
Staphylococcus aureus causes approximately 6,000 cases of toxic shock syndrome each year in the United States (61). Most of these cases are caused by strains of S. aureus elaborating toxic shock syndrome toxin 1 (TSST-1) (8), a pyrogenic toxin superantigen (PTSAg) (14). Although TSST-1 is unique among PTSAgs in its capacity to cross mucosal surfaces (7, 45), it shares considerable structural and functional homology with other PTSAgs. The PTSAg family of bacterial exotoxins presently includes the staphylococcal enterotoxins (SEA to SEI, excluding SEF) and the streptococcal pyrogenic exotoxins (SPE A, B, C, F, G, H, and J, and streptococcal superantigen [14]). Each of these proteins has or is predicted to have the ability to cause fever and stimulate T-cell proliferation as a superantigen (20, 42, 58). Most PTSAgs also exhibit a third property: the capacity to enhance the susceptibility of rabbits to the lethal effects of gram-negative lipopolysaccharide (LPS). TSST-1, which has been shown to enhance the lethality of LPS by as much as 50,000-fold (60), is among the most potent of agents known to sensitize animals to the lethal effects of LPS (22). The LPS enhancement effects of PTSAgs are typically measured by testing the lethality of LPS in animals that have been primed with an injection of a PTSAg several hours prior to injection of LPS. However, rabbits injected with TSST-1 were shown to develop a dose-dependent state of LPS hypersensitivity that was detectable for up to 48 h (60). It was further shown that a log increase in the priming dose of TSST-1 resulted in a log decrease in the 50% lethal dose (LD50) of LPS (60). Administration of cyclosporine (Cs) or nonsteroidal anti-inflammatory drugs failed to prevent the development of LPS hypersensitivity in rabbits injected with TSST-1 (31, 54). In contrast, prior administration of methylprednisolone prevented TSST-1-induced hypersensitivity to LPS (54). Although the relevance of pathogenic interactions between PTSAgs and LPS is difficult to estimate, the highly lethal effects of these interactions in animal models predict that PTSAgs could substantially amplify the toxicity of LPS in humans.
In vivo neutralization of tumor necrosis factor alpha (TNF-α) has been shown to block the lethal effects of LPS in animal models (3, 43, 71), demonstrating that TNF-α is a critical mediator of lethal shock caused by LPS. In addition, many of the pathologic manifestations that develop in animals injected with LPS have also appeared in response to injection of purified TNF-α (43, 70, 72). Mice injected with a staphylococcal PTSAg (SEA, SEB, or TSST-1) in combination with LPS develop significantly higher serum TNF-α levels compared to mice treated with each toxin alone (5, 28, 65, 67). This finding has suggested that the lethal LPS enhancement effects of PTSAgs could be mediated primarily by increases in plasma TNF-α levels. In support of this hypothesis, SEB or SEA failed to induce lethal hypersensitivity in mice deficient of the p55 receptor for TNF-α (5, 67). Moreover, SEA failed to enhance the lethality of LPS in mice lacking major histocompatibility complex (MHC) class II molecules (65), and administration of Cs or neutralization of gamma interferon (IFN-γ) protected mice against the lethal LPS enhancement effects of SEB (5, 38). These latter observations indicated that the superantigenic function of SEA or SEB may be required for their ability to enhance the lethal effects of LPS in mice. Nevertheless, the role of T cells in animal models of PTSAg-induced hypersensitivity to LPS remains controversial. Several in vitro studies have suggested that PTSAgs such as SEA and TSST-1 can potentially sensitize monocyte/macrophage cells to LPS in the absence of T cells (17, 25, 26, 32, 44, 53, 73). Moreover, experiments in rabbits suggest that TSST-1-induced hypersensitivity to LPS can occur independently of T-cell activation (31, 49). Finally, it is not known whether the protective effects of Cs during SEB-induced hypersensitivity to LPS were due to this drug's inhibitory effects on T-cell-independent cytokine production (10, 19, 59).
PTSAgs may enhance LPS-induced TNF-α synthesis in vivo by stimulating the release of macrophage-activating factors such as IFN-γ, granulocyte-macrophage colony-stimulating factor (GM-CSF), or CD40 ligand from T cells. IFN-γ in particular has been shown to function as a critical mediator of lethality in states of LPS hypersensitivity evoked by LPS itself or by gram-positive bacteria. Neutralization of IFN-γ prevented the development of generalized Shwartzman-like reactions in mice (4), and neutralizing antibodies to IFN-γ also blocked the development of LPS hypersensitivity in mice injected with killed Propionibacterium acnes (34). Furthermore, it is well known that purified IFN-γ augments TNF-α synthesis by LPS-stimulated mononuclear phagocytes in vitro (23, 46, 51, 52, 62), and mice or rabbits pretreated with purified IFN-γ developed hypersensitivity to the TNF-α-inducing (27) or lethal (33) effects of LPS, respectively. Although mice pretreated with Cs or anti-IFN-γ antibodies were protected from SEB-induced hypersensitivity to LPS (5, 38), it is not known whether this result was associated with reductions in circulating TNF-α or IFN-γ.
In this report we investigated the relationship between superantigenicity and LPS hypersensitivity in mice treated with TSST-1. Despite the natural resistance of mice to the lethal effects of PTSAgs (13, 41), PTSAgs have been shown to enhance the lethality of LPS in mice by as much as 20-fold (35, 65, 68). TSST-1 is also known to have significant mitogenic potential toward T lymphocytes isolated from appropriate strains of inbred mice (55). In a strain of BALB/c mice susceptible to the lethal LPS enhancement effects of TSST-1, we discovered that the capacity of TSST-1 to enhance LPS-induced lethality or LPS-induced serum TNF-α was dependent on the presence of T cells. In addition, mice pretreated with CsA or neutralizing antibodies against IFN-γ were resistant to the LPS enhancement effects of TSST-1, as measured by lethality or TNF-α levels in serum. The potent capacity of TSST-1 to upregulate LPS-induced IFN-γ levels in serum (67) was also suppressed in mice pretreated with Cs. In contrast, mice treated with Cs after injection of TSST-1 but before injection of LPS still developed a lethal illness associated with elevated levels of TNF-α and IFN-γ in serum. These findings are consistent with a model in which the lethal LPS enhancement effects of TSST-1 are mediated primarily by IFN-γ generated by superantigen-activated T cells.
MATERIALS AND METHODS
Animals.
BALB/cJ and C57BL/6J mice were purchased from Jackson Laboratory (Bar Harbor, Maine). BALB/c-AnNCr, SCIDNCr, and C57BL/6NCr mice were purchased from the National Cancer Institute (Frederick, Md.). All mice were 6- to 9-week-old females weighing 20 to 25 g. SCIDNCr mice were housed in a specific-pathogen-free facility and handled according to specific-pathogen-free guidelines. Blood was drawn by means of retro-orbital puncture from mice anesthetized with metaphane, and euthanasia was done by means of cervical dislocation. Lethality was monitored for 72 h after injection of LPS in each experiment.
Reagents.
TSST-1 was purified from strain MN8 of S. aureus as previously described (6). The concentration of purified TSST-1 was determined by use of a quantitative double-immunodiffusion assay with rabbit antiserum raised against TSST-1 (6). LPS was purified from Salmonella enterica serovar Typhimurium by use of the hot-phenol extraction method (74). LPS preparations were quantified by use of a Limulus gel aggregation assay and stored at −20°C in phosphate-buffered saline (PBS) until use. Cs concentrate (Sandimmune injection; 50 mg/ml) for intravenous injection was purchased from Fairview Homecare Supply (St. Paul, Minn.). Cs vehicle contained 650 mg of Cremophor El (Sigma Chemical Co, St. Louis, Mo.) per ml and 32.9% by volume of absolute ethanol. Neutralizing rat monoclonal antibody (MAb) to mouse IFN-γ, rat immunoglobulin G2a (IgG2a) isotype control MAb, and murine recombinant TNF-α were obtained from R&D Systems (Minneapolis, Minn.). Actinomycin D-mannitol and polymyxin B sulfate were obtained from Sigma.
Drug and toxin administration.
Lyophilized exotoxins or LPS were diluted in sterile, pyrogen-free PBS and filter sterilized (0.2-μm pore size) prior to injection. Mice were injected intraperitoneally (i.p.) with TSST-1 or LPS in 100-μl volumes of PBS. Cs or its vehicle were diluted 1:10 into PBS immediately prior to use and injected i.p. in a volume of 200 μl. Antibody solutions were injected as indicated in the results section. Cs or antibody solutions given at least 12 h prior to the LPS were treated with polymyxin B (2 μg/ml) to neutralize low-level LPS contamination (16).
Measurement of IFN-γ and TNF-α in serum.
The IFN-γ level in serum was measured by use of an enzyme-linked immunosorbent assay purchased from R&D Systems. TNF-α levels in serum were determined by using a bioassay for serum cytotoxicity, performed as follows. Blood samples were allowed to clot for 2 h at room temperature and then stored overnight at 4°C. Separated serum samples were prediluted 1:5 into RPMI cell culture medium (Gibco-BRL, Grand Island, N.Y.) containing 10% fetal calf serum (Sigma), filter sterilized (0.2-μm pore size), and frozen to −70°C until used. Murine WEHI 164 clone 13 target cells (18) were obtained from the laboratory of David Dunn (University of Minnesota, Minneapolis). Subconfluent cells were seeded into 96-well plates at 1.5 × 105 cells/well in 100 μl of cell culture medium and incubated for 2 to 5 h at 37°C in 7% CO2 to allow for adherence. Then, 50 μl of cell culture medium containing actinomycin D-mannitol (8 μg/ml) was added to each culture. One hour later, 50 μl of serially diluted serum samples was added to each well. After 18 h of incubation, cell viability was measured by the reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma). MTT (5 mg/ml in PBS) was diluted 1:10 into each well. Four hours later, culture supernates were aspirated, and insoluble MTT product was resolubilized in 100 μl of 0.1 N HCl in isopropanol. The concentration of reduced MTT in each well was determined spectrophotometrically by subtraction of the absorbance reading at 630 nm from that measured at 570 nm. One unit of TNF-α activity per milliliter was defined as the quantity of TNF-α in serum required to produce 50% cytolysis of WEHI cells. The concentration of murine recombinant TNF-α that typically resulted in 50% cytolysis was 1.0 pg/ml. TNF-α levels in serum were reported as the mean ± the standard error of the mean.
Although this bioassay is also sensitive to TNF-β activity, we found that approximately 90% of LPS-induced serum cytotoxicity in rabbits was neutralized in blocking experiments with antibodies to rabbit TNF-α (Pharmingen, San Diego, Calif.; M. M. Dinges and P. M. Schlievert, unpublished data). This result held true regardless of whether rabbits were unprimed or primed with TSST-1.
Repopulation of SCIDNCr mice with splenic CD3+ T cells from BALB/c-AnNCr mice.
Splenocytes were obtained from donor BALB/c-AnNCr mice by gently teasing apart spleens while irrigating the splenic capsule with medium. Red blood cells were removed by the use of ACK lysing buffer (36), and remaining leukocytes were loaded onto CD3+ T-cell enrichment columns (R&D Systems). The yield of purified T cells typically represented 10% of the total splenocyte number applied to the columns, and 71 to 85% of purified T cells expressed surface CD3 by fluorescence-activated cell sorter analyses, with the degree of purity dependent on the column age. Recipient SCIDNCr mice were injected with 2.0 × 107 T cells in 0.5 ml of PBS via the lateral tail vein. T-cell-reconstituted mice were injected with toxins 3 days after injection of T cells.
Statistical analyses.
Statistical analysis was performed on log10 transformed scores of measured TNF-α values. Serum samples containing less than 20 U of TNF-α activity per ml were assigned a value of 10 U/ml prior to log transformation. A two-tailed Student t test between independent means for samples with unequal variances was used to determine the significance of differences between group means. LD50 statistics were calculated as previously described (57).
RESULTS
Lethality of LPS in four strains of mice primed with an injection with TSST-1.
Four strains of mice (BALB/cJ, BALB/c-AnNCr, C57Bl/6J, and C57Bl/6NCr) were tested for LPS hypersensitivity after a priming injection of TSST-1. Groups of three mice were injected with LPS (400 μg/kg) at 4 or 12 h after a priming injection of TSST-1 (200 μg/mouse). Control mice received injections of PBS instead of TSST-1 or LPS. Lethality was observed only in BALB/c-AnNCr mice primed for 12 h with TSST-1. The lethality of LPS in unprimed or TSST-1-primed BALB/c-AnNCr mice is shown in Table 1. The LD50 of LPS alone in BALB/c-AnNCr mice was approximately 2,000 μg/kg. A priming dose of 200 μg of TSST-1 per kg reduced the LD50 of LPS in these mice to less than 200 μg/kg. Lethality was consistently observed within 48 h of LPS injection. Control mice injected with the same doses of TSST-1 or LPS alone did not develop lethal illness.
TABLE 1.
Lethality of TSST-1 and LPS in BALB/c-AnNCr mice
| Dose (μg/kg) of toxin injected (i.p.)
|
Lethalityb (no. of animals alive/total no.) | |
|---|---|---|
| TSST-1 | LPSa | |
| 4,000 | 0/6 | |
| 2,000 | 2/6 | |
| 1,000 | 1/3 | |
| 400 | 3/3 | |
| 200 | 400 | 0/6 |
| 200 | 200 | 0/3 |
| 200 | 40 | 3/3 |
Mice were injected with LPS 12 h after injection of TSST-1 or PBS.
Lethality occurred within 48 h.
LPS-induced serum TNF-α levels in mice primed with TSST-1.
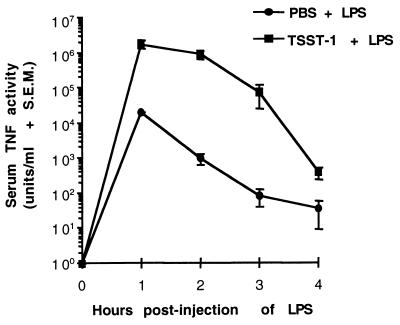
The development of LPS-induced serum TNF-α activity was first measured in groups of three BALB/c-AnNCr mice primed with either TSST-1 (200 μg/kg) or PBS (unprimed) for 12 h (Fig. 1). TNF-α activity was measured in serum samples collected every hour for 4 h following the injection of LPS (400 μg/kg). Increases in TNF-α activity in serum were detectable in both primed and unprimed mice, but levels of TNF-α in serum in mice primed with TSST-1 were significantly greater than those measured in unprimed mice at each time point tested (P ≤ 0.05). Levels of TNF-α activity in serum were measured at 1 h postinjection of LPS in both primed and unprimed mice, but the magnitude of the priming effect of TSST-1 on TNF-α levels was greatest at 2 h postinjection of LPS. At this time point, the mean level of TNF-α in the serum of primed animals was approximately 1,000-fold greater than that of unprimed mice. Control mice primed with 200 μg of TSST-1 per kg, followed by an injection of PBS 12 h later, did not exhibit detectable levels of serum TNF-α activity at the time of PBS injection or at 2 h postinjection of PBS (data not shown).
FIG. 1.
Time course of LPS-induced serum TNF-α activity in BALB/c-AnNCr primed with TSST-1. Groups of three mice were injected i.p. with 200 μg of TSST-1 per kg or an equal volume of PBS. All mice were injected i.p. with 400 μg of LPS per kg 12 h later. Levels of TNF-α were plotted against a log scale because of the large magnitude by which LPS-induced TNF-α levels were enhanced by TSST-1. Mice injected with 200 μg of TSST-1 per kg and then PBS 12 h later did not develop detectable levels of TNF-α in serum.
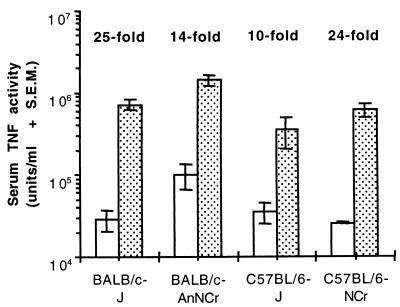
To determine if the greater susceptibility of BALB/c-AnNCr mice was correlated with LPS-induced serum TNF-α levels, baseline and TSST-1-primed serum TNF-α responses to LPS were measured in BALB/c-AnNCr, BALB/cJ, C57BL/6J, and C57BL/6NCr mice at 1 h postinjection of LPS (Fig. 2). Compared to the other strains, BALB/c-AnNCr mice developed the highest baseline levels of circulating TNF-α activity in response to LPS injected alone. This strain also developed the highest peak levels of LPS-induced serum TNF-α when primed with TSST-1. However, the magnitude by which TSST-1 enhanced LPS-induced serum TNF-α levels in BALB/c-AnNCr mice was lower than that measured in BALB/cJ or C57BL/6NCr mice. Therefore, only total circulating TNF-α levels were predictive of lethality among these strains, not the factor by which TSST-1 increased LPS-induced TNF-α levels.
FIG. 2.
Magnitude of baseline and TSST-1-primed serum TNF-α responses to LPS in four strains of inbred mice. Groups of three to four mice were injected i.p. with 200 μg of TSST-1 (dotted bars) per kg or an equal volume of PBS (clear bars). All mice were injected i.p. with 400 μg of LPS per kg 12 h later, and the levels of TNF-α in serum were measured 1 h following the injection of LPS. The magnitude by which TSST-1 enhanced LPS-induced TNF-α levels in serum is shown for each strain.
LPS-induced levels of TNF-α in serum in SCIDNCr mice primed with TSST-1.
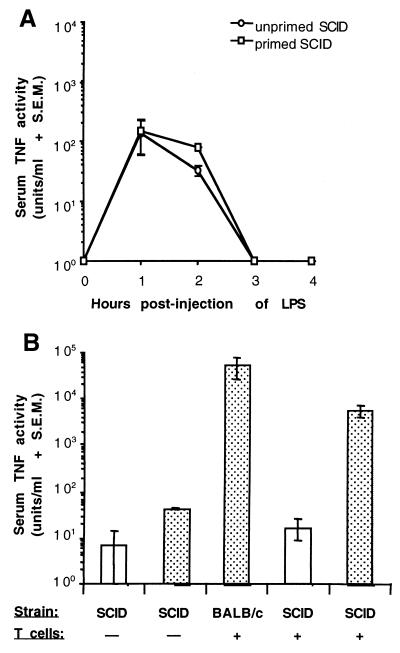
The capacity of TSST-1 to enhance LPS-induced levels of TNF-α in serum was next examined in BALB/c-SCIDNCr mice (Fig. 3A). Two groups of three mice were injected with either 200 μg of TSST-1 per kg or an equivalent volume of PBS, followed 12 h later by the injection of LPS (400 μg/kg), and the levels of TNF-α in serum were measured every hour for 4 h postinjection of LPS. Although a 2.4-fold difference between mean levels of LPS-induced TNF-α in unprimed and primed SCIDNCr mice approached significance at 2 h postinjection of LPS (P = 0.057), no significant differences were detected between primed and unprimed SCIDNCr mice at each time point tested. In addition, injection of TSST-1 (200 μg/kg), followed 12 h later by an injection of LPS (400 μg/kg), failed to induce mortality in SCIDNCr mice.
FIG. 3.
(A) Time course of LPS-induced serum TNF-α activity in BALB/c-SCIDNCr mice primed with TSST-1. Groups of three mice were injected i.p. with 200 μg of TSST-1 (primed) per kg or PBS (unprimed). All mice were injected with LPS (400 μg/kg, i.p.) 12 h later. (B) Effects of TSST-1 on LPS-induced serum TNF-α activity in BALB/c-SCIDNCr mice repopulated with T cells. Recipient SCIDNCr mice received 2.0 × 107 splenic T cells from donor BALB/c-AnNCr mice 3 days prior to injection of TSST-1. Groups of three or four mice (BALB/c-AnNCr or BALB/c-SCIDNCr) were injected i.p. with 200 μg of TSST-1 (dotted bars) per kg or PBS (clear bars). All mice were injected with LPS (400 μg/kg, i.p.) 12 h later. Serum TNF-α activity was measured 2 h postinjection of LPS.
The ability of TSST-1 to enhance LPS-induced serum TNF-α activity was also examined in SCIDNCr mice repopulated with splenic T cells from BALB/c-AnNCr mice (Fig. 3B). Groups of three or four mice were injected with 200 μg of TSST-1 (primed) per kg or an equivalent volume of PBS (unprimed), followed 12 h later by the injection of LPS (400 μg/kg). Levels of LPS-induced TNF-α were measured in serum samples collected at 2 h postinjection of LPS, the time point at which the TNF-α enhancement effect of TSST-1 was greatest in Fig. 1. In SCIDNCr mice reconstituted with T cells, the mean level of LPS-induced TNF-α in serum was significantly greater in TSST-1-primed mice compared to unprimed mice (P ≤ 0.01). Moreover, after a priming with TSST-1, the LPS-induced serum TNF-α responses of T-cell-reconstituted SCIDNCr mice and BALB/c-AnNCr mice were statistically equivalent. In this experiment, an approximately fivefold difference was detected between the mean levels of LPS-induced TNF-α in unprimed and primed SCIDNCr mice. This finding stood in contrast to our previous comparison of these two groups at 2 h postinjection of LPS (Fig. 3A), because this difference reached statistical significance (P ≤ 0.05). Adoptive transfer of T cells into SCIDNCr mice did not significantly enhance LPS-induced TNF-α levels in serum in the absence of priming with TSST-1 (Fig. 3B).
Effect of Cs or anti-IFN-γ on TSST-1-induced hypersensitivity to LPS in mice.
Groups of four BALB/c-AnNCr mice were preinjected with diluted Cs (40 mg/kg), an equivalent volume (200 μl) of PBS, or an equivalent volume of diluted Cs vehicle 4 h prior to an injection of TSST-1. In a separate experiment, groups of four mice were injected i.p. with 640 μg of IFN-γ MAb (1.0 ml) or an equivalent volume of PBS 2 h prior to injection of TSST-1. Pretreated mice were then primed with TSST-1 (200 μg/kg) and challenged 12 h later with LPS (400 μg/kg). As shown in Table 2, pretreatment with either Cs or anti-IFN-γ MAb completely prevented the mortality induced by LPS in mice primed with TSST-1. In contrast, diluted Cs vehicle did not prevent LPS-induced mortality in mice primed with TSST-1.
TABLE 2.
Effects of Cs or anti-IFN-γ MAbs on TSST-1 induced hypersensitivity to LPS in mice
| Treatment
|
Lethality (no. of animals alive/total no.) | |||
|---|---|---|---|---|
| Csa | Anti-IFN-γb | TSST-1c | LPSd | |
| − | − | + | + | 0/4 |
| + | − | + | + | 4/4 |
| − | + | + | + | 5/5 |
CsA (40 mg/kg, i.p.) was injected in 200 μl of polymyxin B-treated PBS 4 h prior to injection of TSST-1. Control mice received an equivalent volume of PBS or Cs vehicle diluted in PBS.
Anti-IFN-γ MAb (640 μg/mouse, i.p.) was injected in 1.0 ml of polymyxin B-treated PBS 2 h prior to injection of TSST-1. Control mice received an equivalent volume of PBS.
Mice were injected with TSST-1 (200 μg/kg, i.p.).
Mice were injected with LPS (400 μg/kg, i.p.) 12 h after injection of TSST-1.
Effect of Cs on LPS-induced TNF-α in serum in mice primed for 12 h with TSST-1.
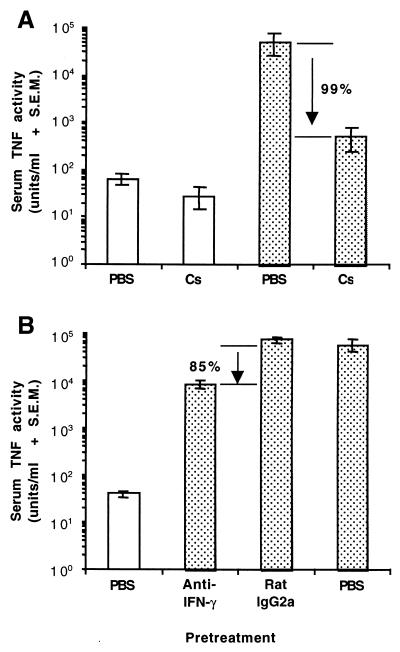
Groups of four BALB/c-AnNCr mice were pretreated with Cs (40 mg/kg) and then primed with 200 μg of TSST-1 per kg 4 h later. Control mice were treated with equivalent volumes of PBS. All mice were challenged with LPS 12 h after injection of TSST-1, and the levels of TNF-α in serum were measured 2 h postinjection of LPS. Cs pretreatment significantly reduced levels of LPS-induced TNF-α in mice primed with TSST-1 (P ≤ 0.01) but did not significantly modify the baseline serum TNF-α response of mice to LPS alone (Fig. 4A). Pretreatment with Cs reduced circulating TNF-α levels by approximately 99% in TSST-1-primed mice injected with LPS.
FIG. 4.
Effects of Cs (A) or anti-IFN-γ MAb (B) on LPS-induced serum TNF-α activity in BALB/c-AnNCr mice primed with TSST-1. Groups of four mice were pretreated with Cs (40 mg/kg, i.p.) or anti-IFN-γ MAb (750 mg/mouse, i.p.) at 4 or 2 h, respectively, prior to injection of TSST-1. Mice were then injected i.p. with 200 μg of TSST-1 (dotted bars) per kg or PBS (clear bars). Control mice received equal volumes of PBS instead of Cs, rat IgG2a instead of anti-IFN-γ MAb, or PBS instead of TSST-1. All mice were injected with LPS (400 μg/kg, i.p.) 12 h later. TNF-α activity in serum was measured 2 h postinjection of LPS. Solutions of Cs or MAb were treated with 2 μg of polymyxin B per ml to remove contaminating LPS. The percent reductions in circulating TNF-α activity caused by Cs or anti-IFN-γ MAb are shown.
Effect of anti-IFN-γ MAbs on LPS-induced TNF-α levels in serum in mice primed for 4 h with TSST-1.
Groups of four BALB/c-AnNCr mice were pretreated i.p. with 750 μg of anti-IFN-γ MAb or isotype control MAb (rat IgG2a) per mouse in 1.0 ml of PBS. Mice were then primed with 200 μg of TSST-1 per kg 2 h after the MAb administration. A third group of mice was injected with equivalent volumes of PBS instead of MAb or TSST-1. It was assumed that the dose of anti-IFN-γ MAb given would be inadequate to neutralize all TSST-1-induced IFN-γ activity for a 12-h priming period. In this experiment, mice were therefore challenged with 400 μg of LPS 4 h after the priming injection of TSST-1. Serum samples were again collected for measurement of the TNF-α levels at 2 h postinjection of LPS. The levels of LPS-induced serum TNF-α in TSST-1-primed mice were significantly lower (P ≤ 0.01) in mice pretreated with anti-IFN-γ MAb compared to mice pretreated with an isotype control antibody (Fig. 4B). The mean LPS-induced serum TNF-α level in TSST-1-primed mice pretreated with anti-IFN-γ was approximately 8,000 U/ml. The mean LPS-induced TNF-α level in the sera of TSST-1-primed mice pretreated with an equal volume of PBS was approximately 56,000 U/ml. Anti-IFN-γ MAb therefore reduced the amount of circulating TNF-α activity by approximately 85% in TSST-1-primed mice challenged with LPS. Levels of LPS-induced serum TNF-α activity in TSST-1-primed mice were not significantly influenced by administration of the isotype control MAb (Fig 4B).
Effect of Cs on the challenge phase of LPS-induced lethality in mice primed for 12 h with TSST-1.
TSST-1, SEA, and SEB have previously been shown to augment LPS-induced the levels of IFN-γ, as well as LPS-induced TNF-α, in serum (5, 65, 66). The protective effects of Cs or anti-IFN-γ measured above could therefore have been due to the suppression of IFN-γ synthesized after administration of TSST-1 during the priming phase or after the administration of LPS in the challenge phase. To reveal the impact of LPS-induced IFN-γ on LPS hypersensitivity in mice primed with TSST-1, we tested the effects of Cs given 10 h after the administration of TSST-1 but 2 h before LPS. Since LPS-induced IFN-γ expression in unprimed mice was found to be T-cell-independent and yet still sensitive to inhibition by Cs (10), we hypothesized that Cs would effectively inhibit LPS-induced IFN-γ synthesis by both T-cell and non-T-cell populations in mice that had previously been injected with TSST-1.
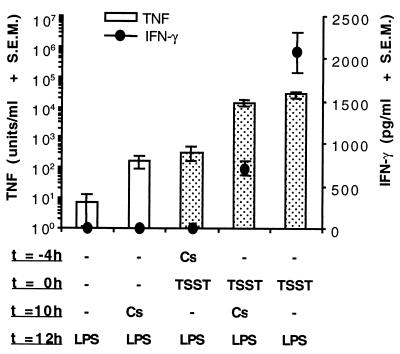
Groups of four mice were given Cs (40 mg/kg) either 4 h before or 10 h after the priming dose of TSST-1 (200 μg/kg). The challenge dose of LPS (400 μg/kg) was given 12 h after the TSST-1. The levels of TNF-α and IFN-γ in serum measured 12 h after LPS injection are shown in Fig. 5. Although Cs given 2 h before LPS appeared to enhance slightly the baseline levels of LPS-induced serum TNF-α in unprimed mice, this effect was not significant (P = 0.137). In addition, Cs given after TSST-1 in primed mice did not significantly suppress the enhancement effect of TSST-1 on LPS-induced TNF-α. With regards to the effects of Cs and TSST-1 on LPS-induced IFN-γ levels in serum, we found that Cs given before TSST-1 reduced LPS-induced IFN-γ to undetectable levels, while Cs given after TSST-1 reduced LPS-induced IFN-γ levels by Ca. 65%. These effects of Cs on IFN-γ levels in serum were both significant (P ≤ 0.01).
FIG. 5.
Effects of Cs given before or after injection of TSST-1 on LPS-induced serum TNF-α and IFN-γ. Groups of three BALB/c-AnNCr mice were injected i.p. with 200 μg of TSST-1 per kg (dotted bars) or PBS (clear bars), followed 12 h later by LPS (400 μg/kg, i.p.). Mice were given Cs (40 mg/kg, i.p.) either 4 h prior to injection of TSST-1 or 2 h prior to injection of LPS. Control mice received equal volumes of PBS instead of TSST-1 or Cs. Cytokine activity in serum was measured 2 h postinjection of LPS. The solutions injected were not treated with polymyxin B.
In a parallel experiment, we tested the capacity of Cs given after TSST-1 to prevent LPS-induced lethality in mice sensitized to LPS with TSST-1. In contrast to Cs given prior to TSST-1, Cs given after TSST-1 failed to prevent LPS-induced lethality in TSST-1-sensitized mice (data not shown). Surprisingly, the onset of LPS-induced mortality was uniformly more rapid in mice given Cs (or its vehicle) after TSST-1 than in control mice given PBS (or nothing) after the injection of TSST-1 (data not shown).
DISCUSSION
SEA, SEB, and TSST-1 greatly potentiate LPS-induced production cytokines such as TNF-α, IFN-γ, interleukin-1 (IL-1), IL-2, and IL-6 (5, 28, 48, 65, 66). The capacity of these staphylococcal PTSAgs to upregulate the host TNF-α response is of considerable interest because the toxicity of TNF-α has been well established in animal models of gram-negative septic shock (3, 43, 70–72). PTSAg effects on IFN-γ production are also important because this cytokine not only enhances host sensitivity to LPS (27, 33) but synergistically augments the lethal effects of circulating TNF-α on host tissues (69). Lethal interactions between IFN-γ and TNF-α have been characterized in other models of LPS hypersensitivity, such as LPS hypersensitivity induced by d-galactosamine (47) or by a priming injection of LPS itself in the lethal Shwartzman reaction (4, 30). However, these models differed from PTSAg-induced LPS hypersensitivity in two important ways. First, in contrast to PTSAg-sensitized mice, mice sensitized to LPS with d-galactoasmine or with LPS itself did not exhibit increased levels of LPS-induced serum TNF-α (30, 47). Second, conventional α/β T cells were not required for the induction of LPS hypersensitivity by d-galactosamine or by LPS itself in the lethal Shwartzman reaction (29, 47). Studies of staphylococcal enterotoxin-induced hypersensitivity to LPS in MHC class II-deficient mice suggested that superantigenic activation of α/β T cells had a major impact on sensitivity to LPS (65). The present study therefore examined the effects of PTSAg-induced IFN-γ on LPS-induced TNF-α and further defined the cellular requirements for PTSAg-induced hypersensitivity to LPS.
We first determined whether the LPS enhancing effects of TSST-1 were in fact dependent on the presence of T cells. A murine model was adopted in which a sublethal priming injection of TSST-1 reduced the lethal dose of LPS in mice by as much as 20-fold (Table 1). This is the greatest increase in LPS sensitivity that has been attributed to a PTSAg in mice (35, 65, 68), and lethality was observed in response to doses of TSST-1 and LPS that were lower than those required to cause lethality in previously characterized murine models (5, 65–67). In addition, the injected doses of TSST-1 and LPS were separated by 12 h, a time period known to lead to maximal priming of LPS-induced serum TNF-α in mice treated with TSST-1 (28). As predicted, T-cell-deficient SCIDNCr mice failed to develop lethal hypersensitivity to LPS after injection of TSST-1, and these mice were almost completely resistant to the priming effect of TSST-1 on LPS-induced serum TNF-α (Fig. 3A). Although TSST-1 enhanced LPS-induced TNF-α by as much as fivefold in SCIDCr mice, any T-cell-independent effects of TSST-1 on LPS-induced TNF-α may not be relevant in comparison to the 1,000-fold TNF-α enhancement effect of TSST-1 measured in BALB/c-ANnCr mice (Fig. 1). Adoptive T-cell transfer from BALB/c-AnNCr mice into SCIDNCr mice completely reconstituted the LPS enhancement activity of TSST-1 in these mice, as measured by LPS-induced serum TNF-α levels (Fig. 3B). The resistance of SCIDNCr mice to the LPS sensitizing effects of TSST-1 was therefore due specifically to T-lymphocyte deficiency rather than to changes in other factors involved in the host's response to LPS. These results also addressed the in vivo relevance of T-cell-independent stimulation of antigen-presenting cells by PTSAgs such as TSST-1 and SEA (17, 25, 26, 32, 44, 53, 73). In the absence of T cells, TSST-1–MHC class II interactions were not sufficient to induce hypersensitive TNF-α responses to LPS. In addition, interactions between TSST-1 and natural killer (NK) cells were not sufficient to cause hypersensitivity to LPS, because SCID mice are known to retain normal or exaggerated NK cell responses (15).
The relationship between T-cell activation, the levels of TNF-α in serum, and lethality was next examined by testing the effects of Cs on the LPS enhancement activity of TSST-1. As was observed in a murine model of SEB-induced hypersensitivity to LPS (5), Cs completely prevented LPS-induced lethality in mice primed with TSST-1 (Table 2). However, it was further shown in this study that Cs potently inhibited the synergistic effects of TSST-1 and LPS on the levels of TNF-α in serum (Fig. 4A). Since Cs did not significantly modify the baseline serum TNF-α responses of unprimed mice to LPS, the immunosuppressive effects of Cs were most likely restricted to the T-cell-dependent component of the LPS-induced serum TNF-α response in mice primed with TSST-1. The protective effects of Cs in this model can be attributed to the capacity of Cs to prevent induction of T-cell effector molecules such as IL-2, IFN-γ, GM-CSF, and CD40 ligand (1, 12, 21, 64). Analysis of SEB-induced cytokine gene expression in vivo has shown that substantial amounts of IL-2, IFN-γ, TNF-α, and TNF-β are expressed in lymphoid tissues after the injection of SEB into mice (2, 24, 40). Each of these cytokines may function as macrophage-activating factors serving to upregulate macrophage responsiveness to LPS (50). Prior administration of Cs almost completely inhibited SEB-induced expression of these cytokines in vivo (2, 24), indicating that T-cell activation was required for their secretion.
Of particular relevance to this study are prior studies in which the in vivo neutralization of IFN-γ activity prevented lethal hypersensitivity reactions to LPS in mice primed with SEA or SEB (5, 38). In view of these findings, it was not surprising that TSST-1 also induced a state of hypersensitivity to LPS that was preventable by means of passive immunization against IFN-γ. The mechanism underlying the protective effects of anti-IFN-γ in our model was further investigated, and it was shown that neutralizing MAb against IFN-γ, administered prior to injection of TSST-1, potently inhibited the synergistic induction of serum TNF-α activity by TSST-1 and LPS (Fig. 4B). In mice primed with TSST-1, the dose of anti-IFN-γ MAb administered caused an 85% reduction in LPS-induced serum TNF-α activity. Although TSST-1 still had significant priming effects on LPS-induced serum TNF-α in mice pretreated with either anti-IFN-γ or Cs, the large reductions in the serum load of TNF-α caused by Cs and anti-IFN-γ were associated with survival. The residual increases in the LPS-induced serum TNF-α responses may not be dependent on IFN-γ or Cs-sensitive cytokines. Alternatively, the doses of anti-IFN-γ or Cs given may have been insufficient to neutralize all relevant cytokine activity during the 4- or 12-h priming phases, respectively. Cs in particular has a serum half-life of 8 h in humans, and it is possible that Cs given 16 h prior to LPS allows breakthrough of TSST-induced priming effects.
In addition to their capacity to enhance LPS-induced serum TNF-α, SEA, SEB, and TSST-1 also greatly enhance the levels of LPS-induced IFN-γ in serum (5, 38, 48, 65, 66), a result that has been attributed to the action of superantigen-induced IL-12 on host IFN-γ production (48). It was previously shown that blockade of IFN-γ activity imposed at the time of LPS injection prevented lethality in mice challenged with LPS within 2 h of SEB (5, 38). Neutralization of IFN-γ imposed after combined injection of a PTSAg and LPS may prevent high cocirculating levels of IFN-γ and TNF-α from having toxic synergistic effects on host tissues (69). Since we did not assess the effects of anti-IFN-γ given after TSST-1, but before LPS, it remained possible that the protection conferred by anti-IFN-γ was due at least in part to neutralization of IFN-γ produced after administration of LPS. However, Cs given after TSST-1 but before LPS inhibited LPS-induced IFN-γ by 65% (Fig. 5), and this effect did not delay LPS-induced mortality or reduce LPS-induced TNF-α levels in mice primed with TSST-1. It therefore seems likely that the protective effects of anti-IFN-γ were mainly due to neutralization of IFN-γ triggered by TSST-1 during the 12 h preceding the injection of LPS. In addition, the failure of Cs to prevent lethal LPS hypersensitivity when given after TSST-1 indicates that the protective effects of CsA given before TSST-1 were due to the suppression of events triggered by TSST-1 rather than by LPS. The inhibition of TSST-1-induced IFN-γ by Cs may underlie not only the capacity of Cs to block LPS-induced TNF-α and lethality in mice primed with TSST-1, but also the capacity of Cs to prevent the burst of serum IFN-γ induced by LPS in mice primed with TSST-1 (Fig. 5). IFN-γ has been shown to upregulate its own expression in vivo (11), and TSST-1-induced IFN-γ could be required for the upregulation of IFN-γ production in response to the LPS. The Cs-resistant IFN-γ response remains to be elucidated in mice treated with CsA after injection of TSST-1, but this finding suggests that Cs is only partially effective in blocking LPS-induced IFN-γ production from cells that have been preactivated by TSST-1. Interestingly, Cs or its vehicle given 2 h before LPS actually enhanced the onset of LPS-induced lethality in mice primed with TSST-1. The Cs vehicle contains both ethanol and castor oil, and it is possible that these agents enhanced the toxicity of LPS by causing chemical peritonitis immediately prior to injection of LPS.
In conclusion, our experiments lend strong support to a model of PTSAg-induced LPS hypersensitivity in which host macrophages are sensitized to LPS by intercellular signaling molecules derived from superantigen-activated T cells. A T-cell-driven source of IFN-γ is a major determinant of LPS-induced serum TNF-α activity in mice sensitized to LPS with TSST-1. Although we have emphasized the role of TNF-α in LPS hypersensitivity induced by TSST-1, our data do not exclude the possibility that TSST-1 has important effects on other host factors that interact with LPS. For example, the sensitivity of macrophage or endothelial cells to LPS is influenced by acute-phase reactants, such as LPS binding protein (63), and also by soluble or cell-associated CD14 (75). Modulation of either of these proteins could underlie hypersensitive responses to LPS in vivo. Furthermore, PTSAgs such as SEB and TSST-1 are known to cause activation of endothelial cells by binding directly to poorly characterized receptors on these cells (9, 37, 39). Interactions between PTSAgs and endothelial cells may result in endothelial cell hypersensitivity to circulating LPS-CD14 complexes (56) or to vasoactive mediators induced by LPS. Extant research has not addressed these mechanisms in the context of PTSAg-induced hypersensitivity to LPS.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grant AI22159 from the National Institute of Allergy and Infectious Diseases. Martin Dinges was supported by USPHS training grant AI07421.
Melodie Bahan and John McCormick are gratefully acknowledged for help during the preparation of the manuscript.
REFERENCES
- 1.Batiuk T D, Kung L, Halloran P F. Evidence that calcineurin is rate-limiting for primary human lymphocyte activation. J Clin Investig. 1997;100:1894–1901. doi: 10.1172/JCI119719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bette M, Schafer M K, van Rooijen N, Weihe E, Fleischer B. Distribution and kinetics of superantigen-induced cytokine gene expression in mouse spleen. J Exp Med. 1993;178:1531–1539. doi: 10.1084/jem.178.5.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beutler B, Milsark I W, Cerami A C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985;229:869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- 4.Billiau A, Heremans H, Vandekerckhove F, Dillen C. Anti-interferon-γ antibody protects mice against the generalized Shwartzman reaction. Eur J Immunol. 1987;17:1851–1854. doi: 10.1002/eji.1830171228. [DOI] [PubMed] [Google Scholar]
- 5.Blank C, Luz A, Bendigs S, Erdmann A, Wagner H, Heeg K. Superantigen and endotoxin synergize in the induction of lethal shock. Eur J Immunol. 1997;27:825–833. doi: 10.1002/eji.1830270405. [DOI] [PubMed] [Google Scholar]
- 6.Blomster-Hautamaa D A, Schlievert P M. Purification of toxic-shock syndrome toxin-I. Methods Enzymol. 1988;165:37–43. doi: 10.1016/s0076-6879(88)65009-9. [DOI] [PubMed] [Google Scholar]
- 7.Bohach G A, Jablonski L, Roggiani M, Sadler I, Schlievert P M, Mitchell D, Ohlendorf D. Biological activity of pyrogenic exotoxins delivered at the mucousal surface. In: Arbuthnott J, Furman B, editors. European Conference on Toxic Shock Syndrome, International Congress and Symposium Series. Vol. 229. New York, N.Y: Royal Society of Medicine Press, Ltd.; 1998. p. 170. [Google Scholar]
- 8.Bohach G A, Fast D J, Nelson R D, Schlievert P M. Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and related illnesses. Crit Rev Microbiol. 1990;17:251–272. doi: 10.3109/10408419009105728. [DOI] [PubMed] [Google Scholar]
- 9.Campbell W N, Fitzpatrick M, Ding X, Jett M, Gemski P, Goldblum S E. SEB is cytotoxic and alters EC barrier function through protein tyrosine phosphorylation in vitro. Am J Physiol. 1997;273:L31–L39. doi: 10.1152/ajplung.1997.273.1.L31. [DOI] [PubMed] [Google Scholar]
- 10.Cockfield S M, Ramassar V, Halloran P F. Regulation of IFN-γ and TNF-α expression in vivo. Effects of cycloheximide and cyclosporine in normal and lipopolysaccharide-treated mice. J Immunol. 1993;150:342–352. [PubMed] [Google Scholar]
- 11.Cockfield S M, Ramassar V, Noujaim J, van der Meide P H, Halloran P F. Regulation of IFN-γ expression in vivo. IFN-γ up-regulates expression of its mRNA in normal and lipopolysaccharide-stimulated mice. J Immunol. 1993;150:717–725. [PubMed] [Google Scholar]
- 12.Denecker G, Vandenabeele P, Grooten J, Penning L C, Declercq W, Beyaert R, Buurman W A, Fiers W. Differential role of calcium in tumor necrosis factor-mediated apoptosis and secretion of granulocyte-macrophage-stimulating factor in a T cell hybridoma. Cytokine. 1997;9:631–638. doi: 10.1006/cyto.1997.0218. [DOI] [PubMed] [Google Scholar]
- 13.Dinges M M, Jessurun J, Schlievert P M. Comparisons of mouse and rabbit models of toxic shock syndrome. In: Arbuthnott J, Furman B, editors. European Conference on Toxic Shock Syndrome, International Congress and Symposium Series. Vol. 229. New York, N.Y: Royal Society of Medicine Press, Ltd.; 1998. pp. 167–168. [Google Scholar]
- 14.Dinges M M, Orwin P M, Schlievert P M. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev. 2000;13:16–34. doi: 10.1128/cmr.13.1.16-34.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dorshkind K, Pollack S B, Bosma M J, Phillips R A. Natural killer (NK) cells are present in mice with severe combined immunodeficiency (scid) J Immunol. 1985;134:3798–3801. [PubMed] [Google Scholar]
- 16.Duff G W, Atkins E. The inhibitory effect of polymyxin B on endotoxin-induced endogenous pyrogen production. J Immunol Methods. 1982;52:333–340. doi: 10.1016/0022-1759(82)90005-9. [DOI] [PubMed] [Google Scholar]
- 17.Espel E, Garcia-Sanz J A, Aubert V, Menoud V, Sperisen P, Fernandez N, Spertini F. Transcriptional and translational control of TNF-α gene expression in human monocytes by major histocompatability complex class II ligands. Eur J Immunol. 1996;26:2417–2424. doi: 10.1002/eji.1830261023. [DOI] [PubMed] [Google Scholar]
- 18.Espevik T, Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986;95:99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- 19.Espevik T, Figari I S, Shalaby M R, Lackides G A, Lewis G D, Shepard H M, Palladino M A., Jr Inhibition of cytokine production by cyclosporin A and transforming growth factor β. J Exp Med. 1987;166:571–576. doi: 10.1084/jem.166.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleischer B, Schrezenmeier H. T cell stimulation by staphylococcal enterotoxins. Clonally variable response and requirement for major histocompatibility complex class II molecules on accessory or target cells. J Exp Med. 1988;167:1697–1707. doi: 10.1084/jem.167.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuleihan R, Ramesh N, Horner A, Ahern D, Belshaw P J, Alberg D G, Stamenkovic I, Harmon W, Geha R S. Cyclosporin A inhibits CD40 ligand expression in T lymphocytes. J Clin Investig. 1994;93:1315–1320. doi: 10.1172/JCI117089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galanos C, Freudenberg M A. Mechanisms of endotoxin shock and endotoxin hypersensitivity. Immunobiology. 1993;187:346–356. doi: 10.1016/S0171-2985(11)80349-9. [DOI] [PubMed] [Google Scholar]
- 23.Gifford G E, Lohmann-Matthes M L. Gamma-interferon priming of mouse and human macrophages for induction of tumor necrosis factor production by bacterial lipopolysaccharide. J Natl Cancer Inst. 1987;78:121–124. doi: 10.1093/jnci/78.1.121. [DOI] [PubMed] [Google Scholar]
- 24.Gimeno R, Codony-Servat J, Montserrat P, Rodriguez-Sanchez J L, Juarez C. Stat1 implication in the immune response to superantigens in vivo. J Immunol. 1996;156:1378–1386. [PubMed] [Google Scholar]
- 25.Grossman D, Cook R G, Sparrow J T, Mollick J A, Rich R R. Dissociation of the stimulatory activities of staphylococcal enterotoxins for T cells and monocytes. J Exp Med. 1990;172:1831–1841. doi: 10.1084/jem.172.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grossman D, Lamphear J G, Mollick J A, Betley M J, Rich R R. Dual roles for class II major histocompatibility complex molecules in staphylococcal enterotoxin-induced cytokine production and in vivo toxicity. Infect Immun. 1992;60:5190–5196. doi: 10.1128/iai.60.12.5190-5196.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heinzel F P. The role of IFN-γ in the pathology of experimental endotoxemia. J Immunol. 1990;145:2920–2924. [PubMed] [Google Scholar]
- 28.Henne E, Campbell W H, Carlson E. Toxic shock syndrome toxin 1 enhances synthesis of endotoxin-induced tumor necrosis factor in mice. Infect Immun. 1991;59:2929–2933. doi: 10.1128/iai.59.9.2929-2933.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heremans H, van Damme J, Dillen C, Dijkmans R, Billiau A. Interferon-γ, a mediator of lethal lipopolysaccharide-induced Shwartzman-like shock reactions in mice. J Exp Med. 1990;171:1853–1869. doi: 10.1084/jem.171.6.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heremans H, Dillen C, van Damme J, Billiau A. Essential role for natural killer cells in the lethal lipopolysaccharide-induced Shwartzman-like reaction in mice. Eur J Immunol. 1994;24:1155–1160. doi: 10.1002/eji.1830240522. [DOI] [PubMed] [Google Scholar]
- 31.Igarashi H, Fujikawa H, Usami H. Effects of drugs on the pyrogenicity of toxic shock syndrome toxin 1 and its capacity to enhance susceptibility to the lethal effects of endotoxic shock in rabbits. Rev Infect Dis. 1989;11(Suppl. 1):S210–S213. doi: 10.1093/clinids/11.supplement_1.s210. [DOI] [PubMed] [Google Scholar]
- 32.Ikejima T, Dinarello C A, Gill D M, Wolff S M. Induction of human interleukin-1 by a product of Staphylococcus aureus associated with toxic shock syndrome. J Clin Investig. 1984;73:1312–1320. doi: 10.1172/JCI111334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jurkovich G J, Mileski W J, Maier R V, Winn R K, Rice C L. Interferon-γ increases sensitivity to endotoxin. J Surg Res. 1991;51:197–203. doi: 10.1016/0022-4804(91)90094-3. [DOI] [PubMed] [Google Scholar]
- 34.Katschinski T, Galanos C, Coumbos A, Freudenberg M A. Gamma-interferon mediates Propionibacterium acnes-induced hypersensitivity to lipopolysaccharide in mice. Infect Immun. 1992;60:1994–2001. doi: 10.1128/iai.60.5.1994-2001.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim Y B, Watson D W. A purified group A streptococcal pyrogenic exotoxin. Physiochemical and biological properties including the enhancement of susceptibility to endotoxin lethal shock. J Exp Med. 1970;131:611–622. doi: 10.1084/jem.131.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kruisbeek A M. Isolation and fractionation of mononuclear cell populations. In: Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. Vol. 1. New York, N.Y: Greene Publishing Associates/Wiley Interscience; 1992. p. 3.1.1. [Google Scholar]
- 37.Kushnaryov V M, MacDonald H S, Reiser R F, Bergdoll M S. Reaction of toxic shock syndrome toxin 1 with endothelium of human umbilical cord vein. Rev Infect Dis. 1989;11(Suppl. 1):S282–S287. doi: 10.1093/clinids/11.supplement_1.s282. [DOI] [PubMed] [Google Scholar]
- 38.LeClaire R D, Kell W, Bavari S, Smith T J, Hunt R E. Protective effects of niacinamide in staphylococcal enterotoxin-B-induced toxicity. Toxicology. 1996;107:69–81. doi: 10.1016/0300-483x(95)03202-q. [DOI] [PubMed] [Google Scholar]
- 39.Lee P K, Vercellotti G M, Deringer J R, Schlievert P M. Effects of staphylococcal toxic shock syndrome toxin 1 on aortic endothelial cells. J Infect Dis. 1991;164:711–719. doi: 10.1093/infdis/164.4.711. [DOI] [PubMed] [Google Scholar]
- 40.Litton M J, Sander B, Murphy E, O'Garra A, Abrams J S. Early expression of cytokines in lymph nodes after treatment in vivo with Staphylococcus enterotoxin B. J Immunol Methods. 1994;175:47–58. doi: 10.1016/0022-1759(94)90330-1. [DOI] [PubMed] [Google Scholar]
- 41.Marrack P, Blackman M, Kushnir E, Kappler J. The toxicity of staphylococcal enterotoxin B in mice is mediated by T cells. J Exp Med. 1990;171:455–464. doi: 10.1084/jem.171.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:1066. [PubMed] [Google Scholar]
- 43.Mathison J C, Wolfson E, Ulevitch R J. Participation of tumor necrosis factor in the mediation of gram-negative bacterial lipopolysaccharide-induced injury in rabbits. J Clin Investig. 1988;81:1925–1937. doi: 10.1172/JCI113540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mehindate K, al-Daccak R, Damdoumi F, Mourad W. Synergistic effect between CD40 and class II signals overcome the requirement for class II dimerization in superantigen-induced cytokine gene expression. Eur J Immunol. 1996;26:2075–2080. doi: 10.1002/eji.1830260917. [DOI] [PubMed] [Google Scholar]
- 45.Melish M, Fukunaga C, Murata S. TSST-1 dissemination in a vaginal model for TSS. In: Arbuthnott J, Furman B, editors. European Conference on Toxic Shock Syndrome, International Congress and Symposium Series. Vol. 229. New York, N.Y: Royal Society of Medicine Press, Ltd.; 1998. p. 169. [Google Scholar]
- 46.Meltzer M S. Macrophage activation for tumor cytotoxicity: characterization of priming and triggering signals during lymphokine activation. J Immunol. 1981;127:179–183. [PubMed] [Google Scholar]
- 47.Miethke T, Duschek K, Wahl C, Heeg K, Wagner H. Pathogenesis of toxic shock syndrome: T cell mediated lethal shock caused by the superantigen TSST-1. Eur J Immunol. 1993;23:1494–1500. doi: 10.1002/eji.1830230715. [DOI] [PubMed] [Google Scholar]
- 48.Muraille E, Pajak B, Urbain J, Moser M, Leo O. Role and regulation of IL-12 in the in vivo response to staphylococcal enterotoxin B. Intern Immunol. 1999;11:1403–1410. doi: 10.1093/intimm/11.9.1403. [DOI] [PubMed] [Google Scholar]
- 49.Murray D L, Earhart C A, Mitchell D T, Ohlendorf D H, Novick R P, Schlievert P M. Localization of biologically important regions on toxic shock syndrome toxin 1. Infect Immun. 1996;64:371–374. doi: 10.1128/iai.64.1.371-374.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nacy C A, Meltzer M S. T-cell-mediated activation of macrophages. Curr Opin Immunol. 1991;3:330–335. doi: 10.1016/0952-7915(91)90033-w. [DOI] [PubMed] [Google Scholar]
- 51.Pace J L, Russell S W. Activation of mouse macrophages for tumor cell killing. I. Quantitative analysis of interactions between lymphokine and lipopolysaccharide. J Immunol. 1981;126:1863–1867. [PubMed] [Google Scholar]
- 52.Pace J L, Russell S W, Torres B A, Johnson H M, Gray P W. Recombinant mouse gamma interferon induces the priming step in macrophage activation for tumor cell killing. J Immunol. 1983;130:2011–2013. [PubMed] [Google Scholar]
- 53.Parsonnet J, Gillis Z A. Production of tumor necrosis factor by human monocytes in response to toxic-shock-syndrome toxin-1. J Infect Dis. 1988;158:1026–1033. doi: 10.1093/infdis/158.5.1026. [DOI] [PubMed] [Google Scholar]
- 54.Peterson P K, Schlievert P M, Conroy W, Kelly J A, Spika J, Quie P G. Protection against staphylococcal pyrogenic exotoxin type C-enhanced endotoxin lethality with methylprednisolone and IgG. J Infect Dis. 1983;147:358. doi: 10.1093/infdis/147.2.358. [DOI] [PubMed] [Google Scholar]
- 55.Poindexter N J, Schlievert P M. Toxic-shock-syndrome toxin 1-induced proliferation of lymphocytes: comparison of the mitogenic response of human, murine, and rabbit lymphocytes. J Infect Dis. 1985;151:65–72. doi: 10.1093/infdis/151.1.65. [DOI] [PubMed] [Google Scholar]
- 56.Pugin J, Ulevitch R J, Tobias P S. A critical role for monocytes and CD14 in endotoxin-induced endothelial cell activation. J Exp Med. 1993;178:2193–2200. doi: 10.1084/jem.178.6.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reed L J, Muench H. A simple method for estimating fifty per cent endpoints. Am J Hyg. 1938;27:493. [Google Scholar]
- 58.Regelmann W E, Gray E D, Wannamaker L W. Characterization of the human cellular immune response to purified group A streptococcal blastogen A1. J Immunol. 1982;128:1631–1636. [PubMed] [Google Scholar]
- 59.Remick D G, Nguyen D T, Eskandari M K, Strieter R M, Kunkel S L. Cyclosporine A inhibits TNF production without decreasing TNF mRNA levels. Biochem Biophys Res Commun. 1989;161:551–555. doi: 10.1016/0006-291x(89)92634-x. [DOI] [PubMed] [Google Scholar]
- 60.Schlievert P M. Enhancement of host susceptibility to lethal endotoxin shock by staphylococcal pyrogenic exotoxin type C. Infect Immun. 1982;36:123–128. doi: 10.1128/iai.36.1.123-128.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schlievert P M. Incidence studies of toxic shock syndrome. In: Arbuthnott J, Furman B, editors. European Conference on Toxic Shock Syndrome, International Congress and Symposium Series. Vol. 229. New York, N.Y: Royal Society of Medicine Press, Ltd.; 1998. p. 167. [Google Scholar]
- 62.Schreiber R D, Ziegler H K, Calamai E G, Unanue E R. Two signal requirement for macrophage tumoricidal activity. Fed Proc. 1981;40:1002. [Google Scholar]
- 63.Schumann R R, Leong S R, Flaggs G W, Gray P W, Wright S D, Mathison J C, Tobias P S, Ulevitch R J. Structure and function of lipopolysaccharide binding protein. Science. 1990;249:1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- 64.Sigal N H, Dumont F H. Cyclosporin A, FK-506, and rapamycin: pharmacologic probes of lymphocyte signal transduction. Annu Rev Immunol. 1992;10:519–560. doi: 10.1146/annurev.iy.10.040192.002511. [DOI] [PubMed] [Google Scholar]
- 65.Stiles B G, Bavari S, Krakauer T, Ulrich R G. Toxicity of staphylococcal enterotoxins potentiated by lipopolysaccharide: major histocompatibility complex class II molecule dependency and cytokine release. Infect Immun. 1993;61:5333–5338. doi: 10.1128/iai.61.12.5333-5338.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stiles B G, Krakauer T, Bonventre P F. Biological activity of toxic shock syndrome toxin 1 and a site-directed mutant, H135A, in a lipopolysaccharide-potentiated mouse lethality model. Infect Immun. 1995;63:1229–1234. doi: 10.1128/iai.63.4.1229-1234.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stiles B G, Campbell Y G, Castle R M, Grove S A. Correlation of temperature and toxicity in murine studies of staphylococcal enterotoxins and toxic shock syndrome toxin 1. Infect Immun. 1999;67:1521–1525. doi: 10.1128/iai.67.3.1521-1525.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sugiyama H, McKissic E M, Bergdoll M S, Heller B. Enhancement of bacterial endotoxin lethality by staphylococcal enterotoxin. J Infect Dis. 1964;114:111. doi: 10.1093/infdis/114.2.111. [DOI] [PubMed] [Google Scholar]
- 69.Talmadge J E, Bowersox O, Tribble H, Lee S H, Shepard H M, Liggitt D. Toxicity of tumor necrosis factor is synergistic with γ-interferon and can be reduced with cyclooxygenase inhibitors. Am J Pathol. 1987;128:410–425. [PMC free article] [PubMed] [Google Scholar]
- 70.Tracey K J, Beutler B, Lowry S F, Merryweather J, Wolpe S, Milsark I W, Hariri R J, Fahey T J D, Zentella A, Albert J D, et al. Shock and tissue injury induced by recombinant human cachectin. Science. 1986;234:470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- 71.Tracey K J, Fong Y, Hesse D G, Manogue K R, Lee A T, Kuo G C, Lowry S F, Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 72.Tracey K J, Lowry S F, Fahey T J D, Albert J D, Fong Y, Hesse D, Beutler B, Manogue K R, Calvano S, Wei H, et al. Cachectin/tumor necrosis factor induces lethal shock and stress hormone responses in the dog. Surg Gynecol Obstet. 1987;164:415–422. [PubMed] [Google Scholar]
- 73.Trede N S, Geha R S, Chatila T. Transcriptional activation of IL-1 beta and tumor necrosis factor-alpha genes by MHC class II ligands. J Immunol. 1991;146:2310–2315. [PubMed] [Google Scholar]
- 74.Westphal O, Luderitz O, Blister F. Uber die extraktion von bacterien mit phenol/wasser. Z Naturforsch. 1952;7b:148. [Google Scholar]
- 75.Ziegler-Heitbrock H W, Ulevitch R J. CD14: cell surface receptor and differentiation marker. Immunol Today. 1993;14:121–125. doi: 10.1016/0167-5699(93)90212-4. [DOI] [PubMed] [Google Scholar]