Abstract
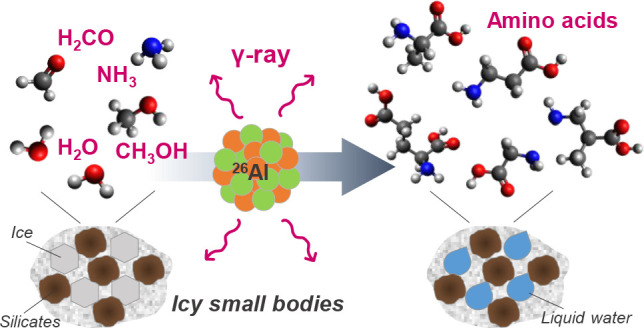
Carbonaceous chondrites contain life’s essential building blocks, including amino acids, and their delivery of organic compounds would have played a key role in life’s emergence on Earth. Aqueous alteration of carbonaceous chondrites is a widespread process induced by the heat produced by radioactive decay of nuclides like 26Al. Simple ubiquitous molecules like formaldehyde and ammonia could produce various organic compounds, including amino acids and complex organic macromolecules. However, the effects of radiation on such organic chemistry are unknown. Hence, the effects of gamma rays from radioactive decays on the formation of amino acids in meteorite parent bodies are demonstrated here. We discovered that gamma-ray irradiation of aqueous formaldehyde and ammonia solutions afforded a variety of amino acids. The amino acid yields had a linear relationship with the total gamma-ray dose but were unaffected by the irradiation dose rates. Given the gamma-ray production rates in the meteorite parent bodies, we estimated that the production rates were reasonable compared to amino acid abundances in carbonaceous chondrites. Our findings indicate that gamma rays may contribute to amino acid formation in parent bodies during aqueous alteration. In this paper, we propose a new prebiotic amino acid formation pathway that contributes to life’s origin.
Short abstract
We experimentally demonstrate that various amino acids were produced by gamma-ray irradiation to aqueous solutions of NH3, H2CO, and CH3OH simulating aqueous alteration in small bodies in the early solar system.
Introduction
Amino acids found in carbonaceous chondrites are of interest for cosmochemistry and in the study of the origin of life; however, the origins of these amino acids are not well-known. Icy grains in the interstellar and outer solar nebulae could provide reactive environments for prebiotic chemistry made possible by the energy provided by ultraviolet (UV) light and cosmic rays. The results of laboratory experiments, for example, indicated that interstellar ice analogues irradiated with protons or UV light at ∼10 K and subsequently warmed up to room temperature produced amino acid precursors that were then acid-hydrolyzed to produce amino acids.1−3 The parent bodies of chondritic meteorites were formed by the accretion of icy dusts ∼4.5 billion years ago; they then underwent aqueous alteration, as made evident by the presence of hydrated minerals. Furthermore, as a result of aqueous alteration, the complexity and diversity of organic compounds are expected to have increased. Indeed, the results of hydrothermal experiments indicate that amino acids and macromolecular organic matter were produced during aqueous alteration.4−9 Notably, ammonia and aldehydes6,7,9−11 or hexamethylenetetramine8 have been used to successfully synthesize various amino acids. Ammonia and aldehydes produced macromolecular organic compounds similar to those found in carbonaceous chondrites, as well as N-containing cyclic compounds and various other organic compounds.4,5,12,13 Radioactive nuclides like 26Al have been regarded as the most effective heat sources that drive the aqueous alteration process.14−16 The 26Al nuclide undergoes conversion to 26Mg with β+ decay with a half-life of 7.17 × 105 y and a decay constant of 3.01 × 10–14 s–1. The decay process produces 3.12 MeV per atom mostly as gamma rays.17 In the case of the parent body of the most common carbonaceous chondrites—CM (Mighei-group) chondrites, the total gamma-ray radiation produced in the parent body should be 6.3 MGy based on the canonical values for the 26Al/27Al ratio (∼5 × 10–5)16 and the abundance of Al in the Murchison meteorite (1.14 wt %).18 It should be noted that the canonical 26Al/27Al ratio highly depends on the timing of asteroid formation—earlier formation leads to higher values. The effects of such radiations on organic matter formation, on the other hand, have not been studied. In the present study, we used gamma-ray irradiation to simulate the aqueous alteration of the meteorite parent body.
Results and Discussion
Amino Acid Formation by Gamma-Ray Irradiations
We prepared aqueous solutions of ammonia, formaldehyde, and methanol in sealed glass tubes with the following molar ratios: H2O:NH3:HCHO:CH3OH = 100:6:8:1 (2.8 M ammonia, 3.7 M formaldehyde, and 0.47 M methanol). Although the initial compositions of chondrite parent bodies are unknown, in similar—but not aqueously altered—bodies like comets, simple molecules such as NH3, HCHO, and CH3OH are expected to be found in the following mutual ratios: H2O:NH3:HCHO:CH3OH = 100:≤1.5:≤4:≤4.19 The aforementioned sealed glass tubes containing the described solutions were then irradiated with gamma rays emitted from 60Co gamma-ray sources at various dose rates and durations—from 0.5 to 20 kGy h–1 and from 3 to 20 h, respectively.
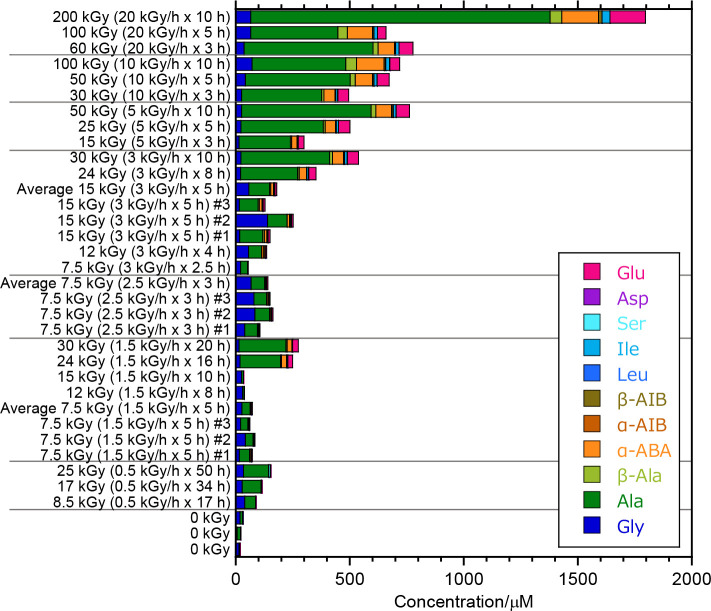
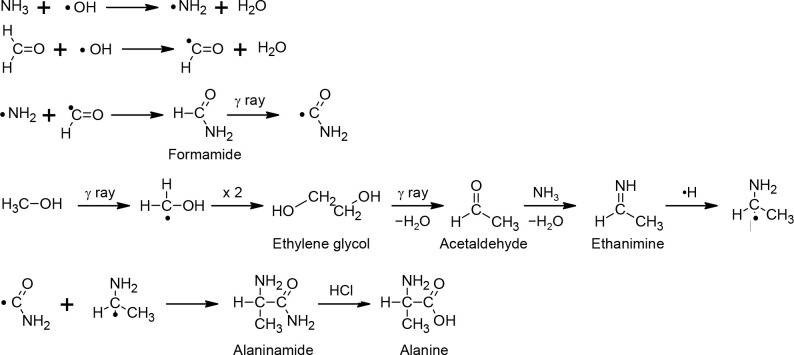
Gamma-ray irradiation of formaldehyde and ammonia aqueous solutions (Figures 1 and S1; Table S1) resulted in amino acid production in amounts that were generally superior to those produced in the control samples, which consisted of nonirradiated formaldehyde and ammonia solutions. After acid hydrolysis of the irradiated samples, α-amino acids, mostly alanine with some glycine, as well as α-aminobutyric acid (ABA) and glutamic acid, were found to have formed. β-Amino acids (i.e., β-alanine and β-aminoisobutyric acid (AIB)) were also found to have been produced. Note that these amino acids were attempted to be identified using standards, and thus one cannot exclude the possibilities of formations of other amino acids. These results were somewhat similar to those obtained in heating experiments previously conducted by our group on ammonia, formaldehyde, and methanol aqueous solutions with similar—but slightly different—concentrations,9 but alanine was significantly dominant in our gamma-ray irradiation products. High-energy gamma rays are likely to trigger the decomposition of HCHO, NH3, CH3OH, and H2O into radicals. One of the possible reaction pathways could be illustrated as Figure 2, based on the radical formations induced by gamma rays20−26 in addition to the formation of alanine from ammonia and aldehyde.7 OH radicals produced by gamma-rays efficiently remove hydrogen from molecules and trigger further radical reactions. NH2 radical and HCO radical are produced by ammonia20 and formaldehyde,21 and then formamide is produced.22 Ethylene glycol is produced from the CH2OH radial which is formed from methanol by gamma rays.23,24 Ethylene glycol then produces acetaldehyde25 which reacts with ammonia and produces ethanimine.26 Ethanimine could react with a hydrogen radical and produce CH3CHNH2 radical and then react with NH2CO radical and produce alaninamide. Finally, acid hydrolysis of alaninamide produces alanine.7
Figure 1.
Amino acids produced as a result of gamma-ray irradiation experiments conducted on ammonia, formaldehyde, and methanol aqueous solutions. Notably, “0 kGy” indicates nonirradiated solutions, which were used as controls. The experiments at values for the gamma-ray dose rate and test duration of 3 kG h–1 × 5 h, 2.5 kG h–1 × 3 h, and 1.5 kG h–1 × 5 h were conducted in triplicates; the average and raw results are reported.
Figure 2.
Possible pathways for alanine formation from ammonia, formaldehyde, and methanol aqueous solutions with gamma-ray irradiation based on the radical formations induced by gamma rays20−26 in addition to the formation of alanine from ammonia and aldehyde.7
We prepared three separate samples for each gamma-ray irradiation experiment conducted at values for the gamma-ray dose rate and test duration of 3 kG h–1 × 5 h, 2.5 kG h–1 × 3 h, and 1.5 kG h–1 × 5 h, and satisfactory reproducibility was observed, except for the case of the amount of glycine detected in one sample (3 kG h–1 × 5 h sample #2) (Figure 1 and Table S1). Glycine production was inconsistent in general—likely due to relatively high concentration of glycine in the control samples, so we did not use this amino acid in our quantitative analysis. Furthermore, we confirmed that the d/l ratio of the alanine produced by gamma-ray irradiation was approximately 1, indicating that environmental contamination was minimal (see Supporting Information).
Correlation between Amino Acid Yields and Gamma-Ray Dose
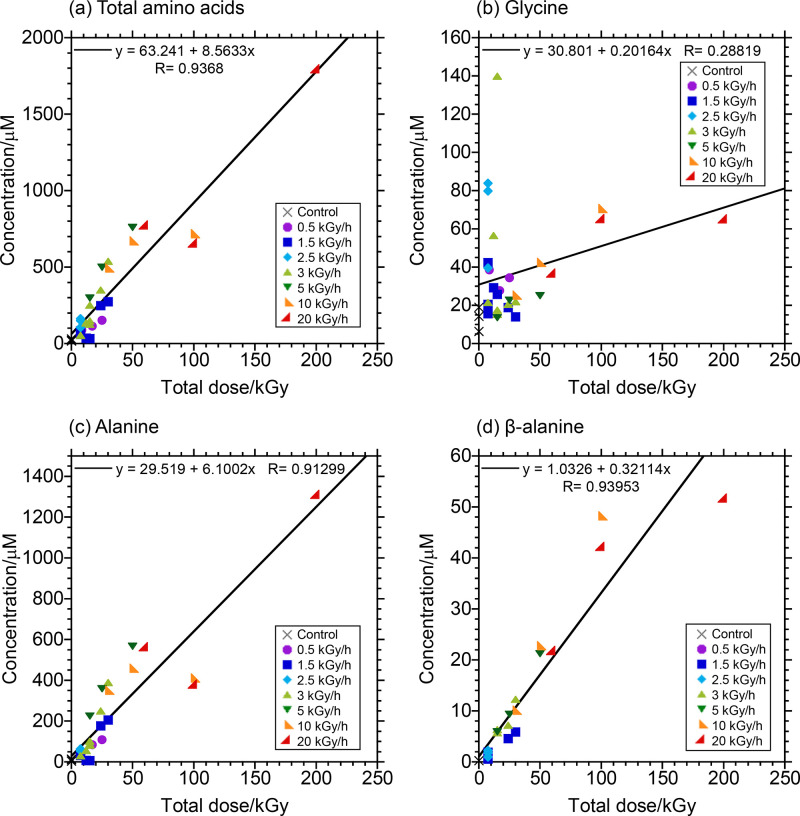
The amino acid yields increased with the total gamma-ray dose irradiated on the samples (Figure 3a–d). The 200 kGy (20 kGy h–1 × 10 h) of irradiation resulted in the highest yield of amino acids—1.797 mM in total which is equivalent to 5.918 mM of carbon. Considering that the starting solution contains 4.22 M of carbon, 0.14% of carbon was converted to amino acids. In fact, the amounts of alanine and β-alanine produced were highly correlated with the total gamma-ray dose (R = 0.91 and R = 0.94, respectively). No correlation was instead observed between glycine yield and gamma-ray dose. Possibly, as a result of the high proportion of alanine in the total amino acids produced, the total amino acid yield correlated well with the total gamma-ray dose (R = 0.94). Indeed, the relationships just described were unaffected by changes in the gamma-ray dose rate (Gy h–1). To infer the effects on the amino acid yields of the gamma-ray dose rates, the amino acid yields were plotted against the irradiation time for each value for the gamma-ray dose rate (see Figure S2a–d). In the case of alanine and β-alanine, the amino acid yield and irradiation time correlated fairly well with each other for each dose rate, and the slopes of the plots, which are equivalent to the amino acid yield rates, correlated well with the gamma-ray dose rate (Figure S2e). This confirms the idea that a linear relationship exists between alanine yield and total gamma-ray dose. Specifically, the linear relationship between total gamma-ray dose (D) and alanine concentration (CAla) could be expressed as follows:
| 1 |
Figure 3.
Amino acid yields as a function of the total gamma-ray dose. (a) Total amino acid, (b) glycine, (c) alanine, and (d) β-alanine concentrations versus the total gamma-ray dose.
The linear relationship between total gamma-ray dose (D) and β-alanine concentration (Cβala) could be expressed as follows:
| 2 |
These amino acid yields are based on the amino acid concentrations in water. Thus, we estimated the amino acid concentrations in the parent bodies using a water/rock ratio of 0.3–0.6 (v/v) for CM2 chondrites.27,28 Notably, the water weight fraction can be calculated as (0.45/2.31)/(1 + 0.45/2.31) = 0.163 using a value for the rock density of 2.31 g cm–3 (in the case of the Murchison meteorite29) and a value for the water density of approximately 1 g cm–3. The amino acid yield per g of meteorite parent body (m) can thus be expressed as follows with the molar mass of alanine (89.09 g mol–1):
| 3 |
| 4 |
Implications for Amino Acid Formation in Parent Bodies
One can estimate the expected amino acid yields in the parent bodies using the dynamic correlations represented by eqs 1–4. The number of 26Al atoms (N26Al) can be calculated as follows:
| 5 |
where λ denotes the decay constant of 26Al (λ = 3.01 × 10–14 s–1). The gamma-ray dose is linearly correlated to the number of decayed 26Al [= N26Al(0) (1– e–λt)]. Therefore, the total gamma-ray dose D and the gamma-ray dose rate dD/dt can be calculated based on eq 5, as well as on the value of the total gamma-ray dose (6.3 MGy), as follows:
| 6 |
| 7 |
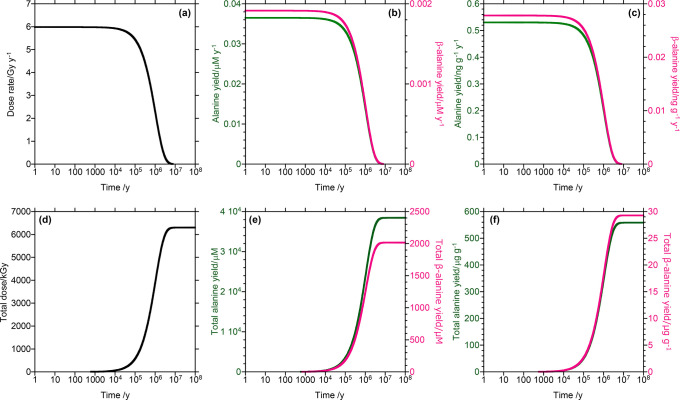
The alanine and β-alanine yields were calculated using eqs 1–4; the values thus obtained are presented in Figure 4 and Table 1. Based on the calculated data, a time duration between 103 and 105 y is required for amino acids alanine and β-alanine to be produced in the 1.3–1.4-μg g–1 concentration ranges observed in the Murchison meteorite.30 These are just rough estimates, however, and not all amino acids found in meteorites can be attributed to gamma-ray-induced reactivity. Nevertheless, evidence from the present study indicates that gamma rays may contribute to the formation of amino acids in meteorites.
Figure 4.
Calculated yields of alanine and β-alanine. (a) Gamma-ray dose rate expected in the parent bodies of CM chondrites, and calculated yields of alanine and β-alanine per year (b) in the liquid phase and (c) in the whole rock. (d) Total gamma-ray dose expected in the parent bodies of CM chondrites and calculated total yields of alanine and β-alanine (e) in the liquid phase and (f) in the whole rock.
Table 1. Calculated Yields of Alanine and β-Alanine in the CM Parent Bodya.
| yield
rate/μM y–1 |
yield rate/ng g–1 y–1 |
total yield/μM |
total yield/μg g–1 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| time/y | dose rate/Gy y–1 | alanine | β-alanine | alanine | β-alanine | total dose/kGy | alanine | β-alanine | alanine | β-alanine |
| 1 | 6.0 | 0.037 | 0.0019 | 0.53 | 0.028 | 0.0060 | 0.037 | 0.0019 | 0.00053 | 2.8 × 10–5 |
| 10 | 6.0 | 0.037 | 0.0019 | 0.53 | 0.028 | 0.060 | 0.37 | 0.019 | 0.0053 | 0.00028 |
| 100 | 6.0 | 0.037 | 0.0019 | 0.53 | 0.028 | 0.60 | 3.7 | 0.19 | 0.053 | 0.0028 |
| 1,000 | 6.0 | 0.036 | 0.0019 | 0.53 | 0.028 | 6.0 | 36 | 1.9 | 0.53 | 0.028 |
| 10,000 | 5.9 | 0.036 | 0.0019 | 0.53 | 0.028 | 60 | 3.6 × 102 | 19 | 5.3 | 0.28 |
| 100,000 | 5.4 | 0.033 | 0.0017 | 0.48 | 0.025 | 5.7 × 102 | 3.5 × 103 | 1.8 × 102 | 51 | 2.7 |
| 1,000,000 | 2.3 | 0.014 | 0.00074 | 0.21 | 0.011 | 3.9 × 103 | 2.4 × 104 | 1.2 × 103 | 3.4 × 102 | 18 |
| 10,000,000 | 0.00045 | 2.7 × 10–6 | 1.4 × 10–7 | 4.0 × 10–5 | 2.1 × 10–6 | 6.3 × 103 | 3.8 × 104 | 2.0 × 103 | 5.6 × 102 | 29 |
Amounts of alanine and β-alanine expected to be produced in the parent body of CM chondrites reported in yields per year (yield rates) and total yields in the liquid phase (μM) as well as in the whole rock (ng g–1 or μg g–1).
Note that the actual gamma-ray source in the parent bodies was mainly 26Al, but its half-life is 7.17 × 105 y, which is unrealistic to use for laboratory experiments. Thus, we use 60Co (half-life of 5.27 y). In addition to the half-life (expected dose rate), the decay energies of 60Co (1.173 MeV and 1.332 MeV) are different from those of 26Al, but the difference in decay energies should not significantly affect the number of molecules formed per energy depositions.
Although the apparent amino acid production rate (k) obtained here includes the effects of decomposition of the amino acids during the gamma-ray irradiation, the long-term stability of amino acids is worth discussing in the present context. According to the decomposition kinetics data collected in a previously published study, at room temperature, 50% of alanine is expected to decompose in aqueous solution over a period of 1.5 × 107 y; moreover, 99.9% of alanine is expected to decompose over ∼1.5 × 108 y under the same conditions.31 The said time frames are long enough for the specified amino acids to have the opportunity to form (<107 y of alteration). However, the half-life of alanine was determined to decrease to ∼105 y at 50 °C and ∼103 y at 80 °C.31 As a result, the final alanine concentration in the parent bodies should have been affected by the thermal decomposition of alanine if the alteration temperature was over several tens of degrees Celsius. In general, β-alanine is thermodynamically more stable than alanine; however, both have been found to exhibit similar stabilities under alkaline conditions.32 Based on our findings, the very similar abundances of alanine and β-alanine observed in the Murchison meteorite (∼1.3 and ∼1.4 μg g–1, respectively)30 despite the preferential formation of alanine over β-alanine could be attributed to the higher stability of β-alanine. Notably, the above discussion is relevant to free amino acids; however, amino acids bound to macromolecular species, which would yield amino acids as a result of acid hydrolysis, would exhibit higher stability than free amino acids. For example, amino acids present in the complex amino acid precursor synthesized by proton irradiation of gas mixtures, in a process intended to simulate the interstellar medium, exhibited higher stability than free amino acids when subjected to heating33 or gamma-ray irradiation.34,35 Solid-state amino acids are known to be gamma-ray-resistant; for example, in the solid state, ∼70% of alanine was observed to survive irradiation with a 3.2-MGy dose of gamma rays,36 although decomposition of amino acids was accelerated at least a factor of 10 with the presence of dry silica powder.37 Thus, following the aqueous alteration, amino acids should be stable over long periods of time at low temperatures (<0 °C) in the interior of asteroids.
Conclusions
A Strecker-like reaction involving HCN, ammonia, and aldehydes (or ketones) in the presence of water was the classic scenario for α-amino acid formation in meteorite parent bodies, and a Michael addition of ammonia to unsaturated nitriles was the classic scenario for the formation of β-amino acids in the same context.38,39 Moreover, aldehydes and ammonia can produce a variety of amino acids, including α-, β-, and γ-amino acids,6,7,9,10 as well as hexamethylenetetramine, which easily decomposes into formaldehyde and ammonia.8 In addition to these possibilities, gamma rays could assist the formation of amino acids in the parent bodies. Our findings point to the possibility of gamma-ray-induced amino acid formation from ubiquitous simple molecules such as formaldehyde and ammonia in the presence of water inside small bodies during the early stages of the formation of the Solar System. The gamma-ray-induced production of amino acids could be a novel prebiotic amino acid formation pathway that could have contributed to the origins of life on early Earth, as building blocks of life were delivered through the fall of meteorites.
Methods
Gamma-Ray-Irradiation Experiments
The sample solutions were prepared according to the method described by Elmasry et al.9 In detail, 200 μL of starting solutions containing 2.8 M ammonia, 3.7 M formaldehyde, and 0.47 M methanol at molar ratios of H2O:NH3:HCHO:CH3OH = 100:6:8:1 was prepared from 55.5 μL of 37% (mass/mass) formaldehyde aqueous solution (containing 5% methanol) and 41.5 μL of 25% ammonia aqueous solution purchased from Wako Pure Chemical Corporation, in addition to 103 μL of pure water. After O2 was removed from the system by keeping it under a vacuum while cooling it with liquid nitrogen, each solution was flame-sealed into glass tubes (6 mm in diameter). Even though we attempted to remove O2, some O2 could be left in the sample tubes, but O2 in general tends to inhibit the formation of amino acids by oxidation, and thus at least, we did not overestimate the yields of amino acids. These solution-containing tubes were exposed to gamma-ray irradiation at ambient temperature using 60Co sources at the Laboratory for Nuclear Reactors, Tokyo Institute of Technology and the Research Laboratory for Quantum Beam Science, Institute of Scientific and Industrial Research, Osaka University (Rabbit11) (Figure S3). The same protocols were employed to prepare the control samples, which were not irradiated with gamma rays. All the utilized glassware was baked at 500 °C for 4 h prior to use in all experiments, and the water used in the experiments and analytical procedures was prepared using a Millipore Milli-Q system.
Note that the temperatures during gamma-ray irradiation experiments should not exceed ∼70 °C, even if all the energy was used to increase temperature for the 200 kGy (maximum dose) experiment. Comparing to the heating experiment without gamma rays at 80 °C for 24 h (Table S1), the total amino acid yield was ∼15 μM, which is much less compared to those of ∼1800 μM produced by 200 kGy of gamma-ray irradiation. This fact excludes the possibility of the effects of heating by gamma-ray irradiation in our experiments.
Amino Acid Analyses
The amino acid analyses were conducted according to the method described by Kebukawa et al.6 The gamma-ray-irradiated and control samples were acid-hydrolyzed with 6 M HCl for 24 h at 110 °C to release the bound amino acids, which is the standard technique for amino acid analysis.40,41 Following acid hydrolysis, the samples were dried by vacuum centrifugation conducted at 60 °C. Each dried sample was then dissolved in 0.4 mL up to 1.5 mL of water (depending on the amino acid concentrations). A 10-μL aliquot of each of the obtained aqueous sample solutions was filtered through a 0.45-μm membrane filter before being analyzed using a high-performance liquid chromatography (HPLC) system. Notably, the HPLC system included a system controller (Shimadzu CBM-20A), HPLC pumps (Shimadzu LC-20AD), a polystyrene-type ion exchange column (Shimadzu Shimpack ISC-07/S1504) heated at 55 °C in a column heater (CTO-20AC), and a fluorescence detector (Shimadzu RF-20Axs) with a 358 nm excitation wavelength and a 450 nm emission wavelength. A postcolumn derivatization process was conducted using a solution comprising o-phthalaldehyde 0.104 g L–1, N-acetyl-l-cysteine 0.65 g L–1, Na2CO3 40.7 g L–1, H3BO3 13.5 g L–1, K2SO4 18.8 g L–1, and polyoxyethylene lauryl ether 0.2 g L–1. Gradient elution was performed using a Shimadzu amino acid mobile phase kit (Na type) with the following eluents: (A) pH 3.23 sodium citrate buffer (containing 0.2 M Na+ and 7% (v/v) ethanol); (B) pH 10.00 sodium citrate buffer (containing ca. 0.73 M Na+ and 0.2 M boric acid); and (C) 0.2 M sodium hydroxide aqueous solution (for conditioning purposes). The flow rate of the carrier solution was 0.300 mL min–1 with (A) and (B) gradients; 100% (A) at first 0–15 min, the ratio of (B) was increased from 0% to 16% at 15–35 min, 16% (B) was kept at 35–40 min, the ratio of (B) was increased from 60% to 100% at 40–50 min, 100% (B) at 50–60 min, and finally 100% (A) at 60–65 min. Aspartic acid, serine, glutamic acid, glycine, alanine, α-aminoisobutyric acid, α-aminobutyric acid, isoleucine, leucine, β-alanine, and β-aminoisobutyric acid were quantified using commercial amino acid standard solutions (Wako Amino Acids Mixture Standard Solution, Type B and Type AN-2), in addition to an α-aminoisobutyric acid standard.
Gas Chromatography/Mass Spectrometry Analysis of Alanine
We also conducted gas chromatography/mass spectrometry (GC/MS) measurements to determine the d/l ratio of alanine to ensure that the obtained amino acids were not contaminated by environmental sources, based on the fact that environmental alanine is characterized by a low d/l ratio, whereas the gamma-ray-triggered alanine synthesis would produce a racemic mixture for the said amino acid. Prior to GC/MS analysis, the acid-hydrolyzed samples were derivatized using the method described by Ubukata et al. (2007).42 Subsequently, 45 μL of 2,2,3,3,4,4,4-heptafluoro-1-butanol and 15 μL of pyridine were combined in a small vial and vortexed for ∼10 s. The sample solution (50–100 μL) was transferred into the vial, which was then vortexed. Afterward, 30 μL of chloroform and 10 mg of NaCl were added to the vial, before the said vial was vortexed once again. A syringe was used to inject 1 μL of the organic phase present in the vial into a GC/MS system (Shimadzu QP-2020) with an Agilent J&W CP-Chirasil-l-Val capillary column (25 m × 0.25 mm i.d. and 0.12-μm film thickness). A splitless injection mode was used; an injector temperature of 200 °C, an inlet pressure of 40.6 kPa, and a He flow of 1 mL/min were applied. The column temperature was maintained at 50 °C for 1 min, before it was initially made to increase to 150 °C at a rate of 5 °C min–1 and then to 200 °C at a rate of 7 °C min–1. Electron ionization was conducted at 70 eV, the ion source temperature was set to 200 °C, and scanning was conducted in selected ion monitoring (SIM) mode at m/z = 116 (the most intense fragmentation peak due to derivatized alanine).
The area ratios of the peaks due to d-alanine and l-alanine in the chromatograms obtained for the gamma-ray-irradiated sample (5 kGy h–1 × 3 h) and for the dl-alanine standard had values of 0.840 and 0.877, respectively (Figure S4). Therefore, the value of the d/l-alanine ratio in the gamma-ray-irradiated sample was determined to be ∼0.96, pointing to the racemic nature of the amino acid produced as a result of the gamma-ray-irradiation experiment. Evidence thus indicates that only minor environmental alanine contamination of the samples subjected to irradiation with gamma rays had occurred.
Acknowledgments
We thank Shusuke Misawa for the earlier sets of experiments. We are grateful to Dr. Takashi Ubukata for discussion on possible reaction mechanisms. Two anonymous reviewers are greatly appreciated for improvements to our manuscript. A part of gamma-ray irradiation with 60Co was performed in the Research Laboratory for Quantum Beam Science, Institute of Scientific and Industrial Research, Osaka University. We thank the Japan Society for the Promotion of Science KAKENHI (Grant Numbers JP17H06458, JP17H06456, JP17H02991, JP19K21895, JP21K18648, and JP21H00036).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscentsci.2c00588.
Supplementary Figure 1, typical HPLC chromatograms of gamma-ray irradiated samples; Figure 2 amino acid yields as a function of irradiation time for each gamma-ray dose rate; Figure 3, photos of the 60Co facility and samples before and after the irradiation; Figure 4, GC/MS chromatograms of gamma-ray irradiated sample. Supplementary Table 1, yields of amino acids by gamma-ray irradiations (PDF)
Author Contributions
Y.K. designed the experiments, analyzed the data, and wrote the manuscript. S.A. performed gamma-ray-irradiation experiments with A.T., I.Y., and Y.K. Additionally, S.A. performed the amino acid analysis under the supervision of K.K. and Y.K. All authors contributed to the discussion and the final text.
The authors declare no competing financial interest.
Supplementary Material
References
- Kobayashi K.; Kasamatsu T.; Kaneko T.; Koike J.; Oshima T.; Saito T.; Yamamoto T.; Yanagawa H. Formation of amino acid precursors in cometary ice environments by cosmic radiation. Adv. Space Res. 1995, 16 (2), 21–26. 10.1016/0273-1177(95)00188-K. [DOI] [PubMed] [Google Scholar]
- Bernstein M. P.; Dworkin J. P.; Sandford S. A.; Cooper G. W.; Allamandola L. J. Racemic amino acids from the ultraviolet photolysis of interstellar ice analogues. Nature 2002, 416 (6879), 401–403. 10.1038/416401a. [DOI] [PubMed] [Google Scholar]
- Muñoz Caro G.; Meierhenrich U.; Schutte W.; Barbier B.; Arcones Segovia A.; Rosenbauer H.; Thiemann W.-P.; Brack A.; Greenberg J. Amino acids from ultraviolet irradiation of interstellar ice analogues. Nature 2002, 416 (6879), 403–406. 10.1038/416403a. [DOI] [PubMed] [Google Scholar]
- Cody G. D.; Heying E.; Alexander C. M. O. D.; Nittler L. R.; Kilcoyne A. L. D.; Sandford S. A.; Stroud R. M. Establishing a molecular relationship between chondritic and cometary organic solids. Proc. Natl. Acad. Sci. U. S. A. 2011, 108 (48), 19171–19176. 10.1073/pnas.1015913108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebukawa Y.; Kilcoyne A. L. D.; Cody G. D. Exploring the potential formation of organic solids in chondrites and comets through polymerization of interstellar formaldehyde. Astrophys. J. 2013, 771 (1), 19. 10.1088/0004-637X/771/1/19. [DOI] [Google Scholar]
- Kebukawa Y.; Chan Q. H. S.; Tachibana S.; Kobayashi K.; Zolensky M. E. One-pot synthesis of amino acid precursors with insoluble organic matter in planetesimals with aqueous activity. Sci. Adv. 2017, 3 (3), e1602093 10.1126/sciadv.1602093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga T.; Naraoka H. A new family of extraterrestrial amino acids in the Murchison meteorite. Sci. Rep. 2017, 7 (1), 636. 10.1038/s41598-017-00693-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradoff V.; Remusat L.; McLain H. L.; Aponte J. C.; Bernard S.; Danger G.; Dworkin J. P.; Elsila J. E.; Jaber M. Impact of phyllosilicates on amino acid formation under asteroidal conditions. ACS Earth Space Chem. 2020, 4 (8), 1398–1407. 10.1021/acsearthspacechem.0c00137. [DOI] [Google Scholar]
- Elmasry W.; Kebukawa Y.; Kobayashi K. Synthesis of organic matter in aqueous environments simulating small bodies in the Solar System and the effects of minerals on amino acid formation. Life 2021, 11 (1), 32. 10.3390/life11010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox S.; Windsor C. R. Formaldehyde and ammonia as precursors to prebiotic amino acids. Science 1971, 174 (4013), 1040–1041. 10.1126/science.174.4013.1040. [DOI] [PubMed] [Google Scholar]
- Marshall W. L. Hydrothermal synthesis of amino acids. Geochim. Cosmochim. Acta 1994, 58 (9), 2099–2106. 10.1016/0016-7037(94)90288-7. [DOI] [Google Scholar]
- Naraoka H.; Yamashita Y.; Yamaguchi M.; Orthous-Daunay F.-R. Molecular evolution of N-containing cyclic compounds in the parent body of the Murchison meteorite. ACS Earth Space Chem. 2017, 1 (9), 540–550. 10.1021/acsearthspacechem.7b00058. [DOI] [Google Scholar]
- Isono Y.; Tachibana S.; Naraoka H.; Orthous-Daunay F.-R.; Piani L.; Kebukawa Y. Bulk chemical characteristics of soluble polar organic molecules formed through condensation of formaldehyde: Comparison with soluble organic molecules in Murchison meteorite. Geochem. J. 2019, 53 (1), 41–51. 10.2343/geochemj.2.0551. [DOI] [Google Scholar]
- Urey H. C. The cosmic abundances of potassium, uranium, and thorium and the heat balances of the Earth, the Moon, and Mars. Proc. Natl. Acad. Sci. U. S. A. 1955, 41 (3), 127–144. 10.1073/pnas.41.3.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray C. M.; Compston W. Excess 26Mg in the Allende Meteorite. Nature 1974, 251 (5475), 495–497. 10.1038/251495a0. [DOI] [Google Scholar]
- MacPherson G. J.; Davis A. M.; Zinner E. K. The distribution of aluminum-26 in the early Solar System - A reappraisal. Meteoritics 1995, 30 (4), 365–386. 10.1111/j.1945-5100.1995.tb01141.x. [DOI] [Google Scholar]
- Castillo-Rogez J.; Johnson T. V.; Lee M. H.; Turner N. J.; Matson D. L.; Lunine J. 26Al decay: Heat production and a revised age for Iapetus. Icarus 2009, 204 (2), 658–662. 10.1016/j.icarus.2009.07.025. [DOI] [Google Scholar]
- Jarosewich E. Chemical analyses of meteorites: A compilation of stony and iron meteorite analyses. Meteoritics 1990, 25 (4), 323–337. 10.1111/j.1945-5100.1990.tb00717.x. [DOI] [Google Scholar]
- Mumma M. J.; Charnley S. B. The chemical composition of comets-emerging taxonomies and natal heritage. Annu. Rev. Astron. Astrophys. 2011, 49, 471–524. 10.1146/annurev-astro-081309-130811. [DOI] [Google Scholar]
- Grachev V. A.; Sazonov A. B. Radiolysis of aqueous ammonia solutions: Mathematical modeling. High Energy Chem. 2021, 55 (6), 472–481. 10.1134/S0018143921060072. [DOI] [Google Scholar]
- López-Islas A.; Colín-García M.; Negrón-Mendoza A. Stability of aqueous formaldehyde under γ irradiation: prebiotic relevance. Int. J. Astrobiol. 2019, 18 (5), 420–425. 10.1017/S1473550418000368. [DOI] [Google Scholar]
- Rawlings J. M. C.; Williams D. A.; Viti S.; Cecchi-Pestellini C.; Duley W. W. Episodic explosions in interstellar ices. Mon. Not. R. Astron. Soc. 2013, 430 (1), 264–273. 10.1093/mnras/sts601. [DOI] [Google Scholar]
- Seki H.; Nagai R.; Imamura M. γ-Radiolysis of a Binary Mixture of Methanol and Water. The Formation of Formaldehyde in the Radiolysis of Liquid Methanol. Bull. Chem. Soc. Jpn. 1968, 41 (12), 2877–2881. 10.1246/bcsj.41.2877. [DOI] [Google Scholar]
- Castillo-Rojas S.; Landeros J. C.; Negrón-Mendoza A.; Navarro-González R. Radiolysis of aqueous formaldehyde relevant to cometary environments. Adv. Space Res. 1992, 12 (4), 57–62. 10.1016/0273-1177(92)90154-P. [DOI] [PubMed] [Google Scholar]
- Burchill C. E.; Perron K. M. Radiation-induced Rearrangement of Ethylene Glycol in Aqueous Solution. Can. J. Chem. 1971, 49 (14), 2382–2389. 10.1139/v71-389. [DOI] [Google Scholar]
- Alouini Z.; Seux R. Cinetiques et mecanismes de l’action oxydative de l’hypochlorite sur les acides α-amines lors de la desinfection des eaux. Water Res. 1987, 21 (3), 335–343. 10.1016/0043-1354(87)90213-2. [DOI] [Google Scholar]
- Zolensky M. E.; Mittlefehldt D. W.; Lipschutz M. E.; Wang M.-S.; Clayton R. N.; Mayeda T. K.; Grady M. M.; Pillinger C.; B D. CM chondrites exhibit the complete petrologic range from type 2 to 1. Geochim. Cosmochim. Acta 1997, 61 (23), 5099–5115. 10.1016/S0016-7037(97)00357-8. [DOI] [Google Scholar]
- Clayton R. N.; Mayeda T. K. Oxygen isotope studies of carbonaceous chondrites. Geochim. Cosmochim. Acta 1999, 63 (13), 2089–2104. 10.1016/S0016-7037(99)00090-3. [DOI] [Google Scholar]
- Macke R. J.; Consolmagno G. J.; Britt D. T. Density, porosity, and magnetic susceptibility of carbonaceous chondrites. Meteorit. Planet. Sci. 2011, 46 (12), 1842–1862. 10.1111/j.1945-5100.2011.01298.x. [DOI] [Google Scholar]
- Glavin D. P.; Dworkin J. P.; Aubrey A.; Botta O.; Doty J. H.; Martins Z.; Bada J. L. Amino acid analyses of Antarctic CM2 meteorites using liquid chromatography-time of flight-mass spectrometry. Meteorit. Planet. Sci. 2006, 41, 889–902. 10.1111/j.1945-5100.2006.tb00493.x. [DOI] [Google Scholar]
- Truong N.; Monroe A. A.; Glein C. R.; Anbar A. D.; Lunine J. I. Decomposition of amino acids in water with application to in-situ measurements of Enceladus, Europa and other hydrothermally active icy ocean worlds. Icarus 2019, 329, 140–147. 10.1016/j.icarus.2019.04.009. [DOI] [Google Scholar]
- Kitadai N. Predicting thermodynamic behaviors of non-protein amino acids as a function of temperature and pH. Orig. Life Evol. Biosph. 2016, 46 (1), 3–18. 10.1007/s11084-015-9457-y. [DOI] [PubMed] [Google Scholar]
- Kurihara H.; Takano Y.; Kaneko T.; Obayashi Y.; Kobayashi K. Stability of Amino Acids and Related Compounds in Simulated Submarine Hydrothermal Systems. Bull. Chem. Soc. Jpn. 2012, 85 (5), 624–630. 10.1246/bcsj.20110349. [DOI] [Google Scholar]
- Takano Y.; Kaneko T.; Kobayashi K.; Hiroishi D.; Ikeda H.; Marumo K. Experimental verification of photostability for free- and bound-amino acids exposed to gamma-rays and UV irradiation. Earth Planets Space 2004, 56 (7), 669–674. 10.1186/BF03352529. [DOI] [Google Scholar]
- Kobayashi K.; Mita H.; Kebukawa Y.; Nakagawa K.; Kaneko T.; Obayashi Y.; Sato T.; Yokoo T.; Minematsu S.; Fukuda H.; et al. Space Exposure of Amino Acids and Their Precursors during the Tanpopo Mission. Astrobiology 2021, 21 (12), 1479–1493. 10.1089/ast.2021.0027. [DOI] [PubMed] [Google Scholar]
- Cataldo F.; Ragni P.; Iglesias-Groth S.; Manchado A. A detailed analysis of the properties of radiolyzed proteinaceous amino acids. J. Radioanal. Nucl. Chem. 2011, 287 (3), 903–911. 10.1007/s10967-010-0851-7. [DOI] [Google Scholar]
- Pavlov A. A.; McLain H. L.; Glavin D. P.; Roussel A.; Dworkin J. P.; Elsila J. E.; Yocum K. M. Rapid Radiolytic Degradation of Amino Acids in the Martian Shallow Subsurface: Implications for the Search for Extinct Life. Astrobiology 2022, 22, 1099. 10.1089/ast.2021.0166. [DOI] [PubMed] [Google Scholar]
- Pizzarello S.; Cooper G. W.; Flynn G. J.. The nature and distribution of the organic material in carbonaceous chondrites and interplanetary dust particles. In Meteorites and the Early Solar System II; Lauretta D. S., McSween J. H. Y., Eds.; University of Arizona Press: Tucson, 2006; pp 625–651. [Google Scholar]
- Elsila J. E.; Aponte J. C.; Blackmond D. G.; Burton A. S.; Dworkin J. P.; Glavin D. P. Meteoritic amino acids: Diversity in compositions reflects parent body histories. ACS Cent. Sci. 2016, 2, 370–379. 10.1021/acscentsci.6b00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherfurd S. M.; Gilani G. S. Amino acid analysis. Curr. Protoc. Protein Sci. 2009, 58 (1), 11.9.1–11.9.37. 10.1002/0471140864.ps1109s58. [DOI] [PubMed] [Google Scholar]
- Simkus D. N.; Aponte J. C.; Elsila J. E.; Parker E. T.; Glavin D. P.; Dworkin J. P. Methodologies for Analyzing Soluble Organic Compounds in Extraterrestrial Samples: Amino Acids, Amines, Monocarboxylic Acids, Aldehydes, and Ketones. Life 2019, 9 (2), 47. 10.3390/life9020047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubukata M.; Kusai A.; Taniuchi T.; Kaneko T.; Kobayashi K. Unimolecular Decomposition of N-Ethoxycarbonyl Heptafluorobutyl Ester Derivatives of Amino Acids upon Electron Ionization. J. Mass Spectrom. Soc. Jpn. 2007, 55 (4), 271–277. 10.5702/massspec.55.271. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.