Abstract

Anthriporphyrinoid and its dimeric homologues were synthesized by Suzuki–Miyaura coupling and subsequent oxidation. Both porphyrinoids were smoothly converted to their PdII complexes and were further decorated by Suzuki–Miyaura coupling with thiophene derivatives and subsequent oxidative fusion reaction to provide multiply fused compounds. Most PdII anthriporphyrinoids have been structurally well characterized to be planar for monomeric and helically twisted for dimeric species. The dimeric anthriporphyrinoids show paratropic ring currents due to their global antiaromatic networks, the extent of which increases with an increase of conjugated network. Multiply fused dimeric anthriporphyrinoids show helical structures, fully reversible six redox potentials, small HOMO–LUMO gaps, and absorption tails reaching in the near-infrared region, suggesting the high potential of this approach to explore molecular graphene. Optical separations of the dimeric helical species were accomplished, and racemization barrier heights were determined.
Short abstract
Multiply thiophene- and benzothiophene-fused PdII anthriporphyrinoids were synthesized. While the monomeric complexes are nonaromatic, the dimeric complexes show paratropic ring currents owing to the global 32π- and 36π-electronic circuits. Multiple fusions of the dimeric complexes allowed six fully reversible redox potentials, small HOMO−LUMO gaps, and broad absorption tails reaching at NIR, and further, caused helical conformations. The optical separations were accomplished and their racemization barrier heights were determined.
1. Introduction
“Molecular-graphenes” represent a class of discrete 2-dimensional and highly conjugated molecular sheets, which is widely regarded as the molecular model of graphene.1,2 These species are useful in understanding the structure–property relationships of graphenes and stimulating the organic synthesis of graphene materials with a discrete structure. Porphyrinoids can be an important structural motif of molecular graphenes. Representative examples include porphyrin tapes A that exhibit the remarkably strong absorption reaching in the infrared region,3 [26]hexaphyrin doubly fused with porphyrins B,4 a porphyrin sheet, C, that has a strong paratropic ring current just at the central planar cyclooctatetraene,5 and a porphyrin fused with four anthracene units, D.6−9 These compounds constitute a subgroup of “porphyrinoid molecular graphenes”, which are promising in light of the remarkable optical and electronic properties of porphyrins (Scheme 1).
Scheme 1. Multiply Fused Porphyrinoids (A, B, C, and D) and Earring Porphyrin E.

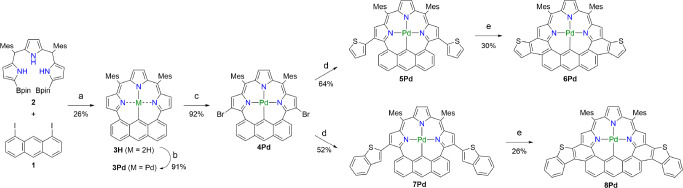
In 2016, we reported “earring porphyrin” E, in which the tripyrrin moiety is directly linked at the 3,7-positions of a NiII porphyrin, forming a cavity that can serve as a coordination ligand toward transition metal.10 Further fusion of heterocycles on the periphery of earring porphyrins led to multiply fused porphyrinoids with absorption bands reaching at ca. 2200 nm.11 These compounds can be regarded as “porphyrinoid molecular graphenes”. As an extension of these studies, we designed and synthesized anthracene-based earring porphyrinoids, anthriporphyrinids such as 3H and 10H, as new porphyrinic molecular graphenes, where a tripyrrin segment is directly linked at 1,8-positions or 1,4,5,8-positions of anthracene to form a cavity serving as an effective ligand toward PdII ion (Schemes 2 and 3).
Scheme 2. Synthesis of Anthriporphyrinoids 6Pd and 18Pd.
Conditions and reagents: (a) Pd2(dba)3, Sphos, Cs2CO3, CsF, Toluene, DMF, 115 °C; (b) Pd(OAc)2, NaOAc, CH2Cl2, MeOH; (c) NBS, CH2Cl2, 25 °C; (d) 2-thienylboronic acid, Pd2(dba)3, PPh3, Cs2CO3, CsF, toluene, DMF, 115 °C (for 5Pd); 2-benzothienylboronic acid, Pd2(dba)3, PPh3, Cs2CO3, CsF, toluene, DMF, 115 °C (for 7Pd); (e) DDQ, TfOH, CH2Cl2, 0 °C. Mes = 2,4,6-trimethylphenyl, Bpin = pinacolatoboryl.
Scheme 3. Synthesis of Anthracene-Sharing Dimeric Porphyrinoids 13Pd and 15Pd.
Conditions and reagents: (a) Pd2(dba)3, Sphos, Cs2CO3, CsF, toluene, DMF, 115 °C; (b) Pd(OAc)2, NaOAc, CH2Cl2, MeOH; (c) NBS, CH2Cl2, 25 °C; (d) 2-thienylboronic acid, Pd2(dba)3, PPh3, Cs2CO3, CsF, toluene, DMF, 115 °C (for 12Pd); 2-benzothienylboronic acid, Pd2(dba)3, PPh3, Cs2CO3, CsF, toluene, DMF, 115 °C (for 14Pd); (e) DDQ, TfOH, CH2Cl2, 0 °C. Mes = 2,4,6-trimethylphenyl, Bpin = pinacolatoboryl.
2. Results and Discussion
2.1. Synthesis
Synthesis of anthriporphyrinoids 3H is shown in Scheme 2. Suzuki–Miyaura cross-coupling reaction of 1,8-diiodoanthracene121 with α,α′-diboryltripyrrane 2(10) and subsequent oxidation with DDQ gave 1,8-anthriporphyrin 3H in 26% yield. In line with the structure, high-resolution matrix-assisted laser desorption ionization time-of-flight (HR-MALDI-TOF) mass measurements showed the parent ion peak of 3H at m/z = 631.2870, calcd for (C46H37N3)+ = 631.2982 ([M]+). The structure of 3H was confirmed by X-ray diffraction analysis, which showed that there were two different conformations (Figure 1a,b and Supporting Information).
Figure 1.

X-ray structures: (a) top view and (b) side view of 3H (structure of one conformers); (c) top view and (d) side view of 3Pd; (e) top view and (f) side view of 6Pd. The thermal ellipsoids were scaled to 50% probability for 3H, 3Pd, and 6Pd (solvent molecules and hydrogen atoms on carbon were omitted for clarity).
Treatment of 3H with Pd(OAc)2 in the presence of NaOAc in CH2Cl2/MeOH afforded 3Pd in 91%. The structure of 3Pd has been unambiguously determined by X-ray diffraction analysis. 3Pd exhibits a roughly planar structure with a larger mean-plane-deviation (MPD) of 0.605(4) Å. PdII ion resides at the center of the macrocycle with C–Pd bond of 2.047(3) Å and three N–Pd bonds of 2.013(3), 2.089(3), and 2.015(3) Å. The diagonal C–N and N–N distances are 4.136(4) Å and 4.025(4) Å, respectively. The 1H NMR spectrum of 3Pd displays four signals at 9.10, 9.01, 8.64, and 7.89 ppm due to the anthracene protons and three signals at 7.79, 7.20, and 6.78 ppm due to the pyrrolic β-protons, suggesting nonaromatic character for the anthriporphyrin part. The nonaromatic nature was also indicated by small negative values (−0.97 to −0.58 ppm) of nucleus-independent chemical shift NICS(0) values (Figure S73).
Gratifyingly, dibrominated anthriporphyrinoid 4Pd was obtained in a high yield of 92% by bromination with NBS in CH2Cl2, similarly to the earring porphyrin E.11 Cross-coupling of 4Pd with 2-thiopheneboronic acid afforded doubly thiophene-appended 5Pd and subsequent Scholl reaction with DDQ and TfOH in CH2Cl2 (1:100) at 0 °C for 5 min furnished doubly thiophene-fused complex 6Pd in a two-step yield of 19% yield (Scheme 2). X-ray diffraction analysis revealed a C2v-symmetric structure with a MPD value of 0.498(4) Å for 6Pd (Figure 3). The 1H NMR spectrum exhibits a singlet at 7.82 ppm and two doublets at 7.67 and 7.53 ppm due to the anthracene protons, two doublets at 7.24 and 7.03 ppm due to the thiophene protons, and two singlets at 6.18 and 5.93 ppm for the pyrrolic β-protons. The NICS(0) values of 6Pd at the center of the macrocycle were calculated be to +3.78 to +7.56 ppm (Figure S74).
Figure 3.

UV/vis/NIR absorption spectra of 3Pd, 6Pd, and 8Pd in CH2Cl2.
Similarly, 4Pd was coupled with 2-benzothienylboronic acid to give 7Pd in 52%, which was oxidized with DDQ/TfOH in CH2Cl2 to afford doubly benzothiophene-fused complexes 8Pd in 26%. The 1H NMR spectrum of 8Pd displays a singlet at 7.77 ppm and two doublets at 8.26 and 8.10 ppm due to the anthracene protons, three signals at 7.68, 7.38, and 7.28 ppm due to the benzothiophene protons and two singlets at 6.21 and 5.93 ppm for the pyrrolic β-protons. The NICS(0) values of 8Pd at the center of the macrocycle were calculated be to +3.44 to +7.56 ppm (Figure S74).
Synthesis of anthracene-sharing dimeric porphyrinoid 10H is shown in Scheme 3. Reduction of 1,4,5,8-tetrachloroanthraquinone with NaBH4 in isopropanol gave 1,4,5,8-tetrachloroanthracene,13 which was converted to 1,4,5,8-tetraiodoanthracene (9) via borylation14,15 and subsequent iodination.10,16−22 Under similar conditions, reaction of 9 with 2 gave dimer 10H in 10% yield. 10Pd was readily obtained in 76% yield upon treatment of 10H with Pd(OAc)2 under similar conditions. The structure of 10Pd was unambiguously determined by X-ray diffraction analysis. Two different conformations were found in the crystal of 10Pd. Both conformations are helically twisted with large twist angles of 67.54(6)° and 67.54(5)° for the two tripyrrin units. The anthracene unit of 10Pd is also highly distorted with twist angles of 47.8(2)° and 46.9(3)° (Figure 2).
Figure 2.

X-ray structures: (a) top view and (b) side view of 10Pd (structure of one conformer); (c) top view and (d) side view of 13Pd; (e) top view and (f) side view of 15Pd. The thermal ellipsoids were scaled to 50% probability for 10Pd, 13Pd, and 15Pd (solvent molecules and hydrogen atoms on carbon were omitted for clarity).
The 1H NMR spectrum of 10Pd is symmetric, showing a singlet at 6.95 ppm due to the anthracene protons and three signals at 6.21, 6.07, and 5.58 ppm due to the pyrrolic β-protons, which are distinctly high-field shifted as compared with those of 3Pd, suggesting a weak paratropic ring current for 10Pd. Actually, the NICS(0) values of 10Pd were calculated to be +8.51 to +8.81 ppm (Figure S73), and anisotropy of induced current density (AICD) plots of 10Pd showed a counterclockwise circuit. This may be ascribed to the 32π-electronic global network as indicated by the bold black line in Scheme 3.
Bromination of 10Pd with NBS in CH2Cl2 gave tetrabrominated PdII complexes 11Pd in 42%. Under the same reaction conditions, 11Pd was converted to quadruply thiophene-appended dimer 12Pd in 36% yield, which was oxidized with DDQ and TfOH to give 13Pd in 31% yield. The structure of 13Pd was also confirmed by X-ray analysis. Two similar but different conformations were found in the crystal of 13Pd. Both structures display a twisted conformation consisting of two PdII coordinated porphyrinoids with MPDs of 0.643(13) and 0.610(14) Å and of 0.632(13) and 0.624(13) Å. The dihedral angles of the two porphyrinoids were 43.69(6)° and 43.21(6)°, and the anthracene unit of 13Pd is also highly distorted with twist angles of 53.4(5)° and 51.6(4)° (Figure 2). Intriguingly, 13Pd contains two dithia[5]helicene segments. The 1H NMR spectrum of 13Pd displays two doublets at 6.54 and 6.18 ppm due to the thiophene protons and two singlets at 4.47 and 4.45 ppm due to the pyrrolic β-protons. It is apparent that the chemical shifts of the pyrrolic β-protons of 13Pd are high-field shifted as compared with 10Pd, indicating a larger paratropic ring current. In line with this, the NICS(0) values in the center of macrocycle were calculated to be +13.75 to +16.58 ppm (Figure S74). Further, the AICD plot of 13Pd displayed a moderate global paratropic ring current. This may be ascribed to the presence of an additional global 36π-electronic network in 13Pd, as indicated in blue in Scheme 3, besides the 32π-electronic network.
Finally, 11Pd were coupled with 2-benzothienylboronic acid to give benzothiophene-appended PdII complexes 14Pd in 31% yield, which was oxidized with DDQ and TfOH in CH2Cl2 to afford quadruply benzothiophene-fused complexes 15Pd in 66% yield.
The structure of 15Pd has been revealed by X-ray diffraction analysis to be considerably twisted owing to the multiply fused structures. Actually, 15Pd possesses two dithia[7]helicene segments at the periphery. The two roughly planar PdII porphyrinoids segments show MPD of 0.578(4) and 0.545(4) Å, and the dihedral angle of these units is 40.84(2)°, and the anthracene unit of 15Pd is also highly distorted with twist angles of 52.93(14)° (Figure 2). The 1H NMR spectrum of 15Pd shows signals at 6.78–6.74 and 6.51–6.47 ppm due to the benzothiophene protons and two singlets at 4.43 and 4.42 ppm for the pyrrolic β-protons. These data suggest an enhanced paratropic ring current of 15Pd as compared with 13Pd. The NICS(0) values were calculated to be +14.11 to +17.09 ppm (Figure S74), and the AICD plot showed a clear global paratropic ring current.
2.2. UV/vis/NIR Absorption Spectra
The UV/vis/NIR absorption spectra of 3Pd, 6Pd, and 8Pd are shown in Figure 3. The absorption spectrum of 3Pd shows structured bands in the range of 600–880 nm, which are distinctly red-shifted as compared with that of 3H (SI). Intriguingly, the absorption spectra of fused anthriporphyrinoids 6Pd and 8Pd display broad and red-shifted absorption bands up to 1350 nm as a rare case of monomeric porphyrinoid, indicating that the peripheral fusion is really effective to reduce the HOMO–LUMO gaps.
The absorption spectra of the dimeric PdII complexes 10Pd, 13Pd, and 15Pd are shown in Figure 4. It is notable that these complexes show strong absorption bands at 400, 450, and 800 nm, along with a weak absorption tail reaching at 1300, 1700, and 1900 nm for 10Pd, 13Pd, and 15Pd, respectively. These spectral features are consistent with their antiaromatic character. On the basis of these results, their optical HOMO–LUMO gaps have been estimated to be 0.95, 0.73, and 0.65 eV, respectively, indicating that the peripheral fusion is effective to lower the HOMO–LUMO gap.
Figure 4.

UV/vis/NIR absorption spectra of 10Pd, 13Pd, and 15Pd in CH2Cl2.
2.3. Electrochemical Properties
The electrochemical properties were investigated by cyclic voltammetry and differential pulse voltammetry. On the basis of the data in Table 1, the HOMO–LUMO gaps of 3Pd and 10Pd were estimated to be respectively smaller than those of 3H and 10H, and the HOMO–LUMO gaps of the dimeric complexes (10Pd, 13Pd, and 15Pd) are smaller than those of the monomeric complexes (3Pd, 6Pd, and 8Pd). The HOMO–LUMO gaps decreased in the order of 3Pd (1.60 eV) > 6Pd (1.23 eV) > 8Pd (1.20 eV) > 10Pd (0.97 eV) > 13Pd (0.81 eV) > 15Pd (0.78 eV). Remarkably, dimeric complex 10Pd showed two oxidation waves and four reduction waves. While the fourth reduction wave was quasi reversible, the other waves were reversible. Fused complexes 13Pd and 15Pd showed two reversible oxidation waves and four reversible reduction waves, all being at considerably higher potentials as compared with 10Pd. Naturally, the HOMO–LUMO gaps of 13Pd and 15Pd are quite small, being 0.81 and 0.78 eV, respectively. These properties encourage the potential use of these fused anthriporphyrinoids as an electron reservoir. These electrochemical data are roughly in accord with the absorption spectra and TD-DFT calculation (Figures S75–82).
Table 1. Electrochemical Potentials in CH2Cl2a.
| Ered.4 | Ered.3 | Ered.2 | Ered.1 | Eox.1 | Eox.2 | ΔEHLb | |
|---|---|---|---|---|---|---|---|
| 3H | — | — | –1.68 | –1.33 | 0.56c | — | 1.89 |
| 3Pd | — | — | –1.76 | –1.15 | 0.45 | — | 1.60 |
| 6Pdd | — | — | –1.34 | –0.72 | 0.51c | — | 1.23 |
| 8Pdd | — | — | –1.22 | –0.65 | 0.55c | — | 1.20 |
| 10H | — | — | –1.24 | –1.11 | 0.42 | 0.57 | 1.53 |
| 10Pd | –2.48e | –2.19 | –1.03 | –0.75 | 0.22 | 0.52 | 0.97 |
| 13Pd | –1.77 | –1.55 | –0.79 | –0.44 | 0.37 | 0.70 | 0.81 |
| 15Pd | –1.81 | –1.56 | –0.74 | –0.36 | 0.42 | 0.74 | 0.78 |
Potentials [V] vs ferrocene/ferrocenium ion. Scan rate 0.05 V s–1; glassy carbon working electrode, Pt wire counter electrode, Ag/AgNO3 reference electrode, supporting electrolyte 0.1 M nBu4NPF6 in CH2Cl2.
Electrochemical HOMO–LUMO gaps (ΔEHL = Eox.1 – Ered.1 [eV]).
Irreversible peaks.
Solubilities are very poor.
Quasi reversible.
2.4. Circular Dichroism and Racemization Barrier
Subsequently, the largely twisted 13Pd and 15Pd were subjected to enantiomeric resolution using chiral column chromatography (Figure S91). The chromatograms of 13Pd and 15Pd racemates in hexane/CH2Cl2 (1:1) did show two well-separated peaks. The isolated first and second peaks clearly provided mirror-signed circular dichroism (CD) spectral profiles, indicating that enantiomeric resolution was achieved (Figure 5). Both CD spectra (i.e., for 13Pd and 15Pd) were simulated using the SpecDis software package23−25 with high similarity factors (0.91 for 13Pd and 0.86 for 15Pd, respectively) (Figures S92 and S93), and thus, the absolute structure of the second eluent 13Pd2 was determined with high accuracy as (M,M), and the first eluent 13Pd1 was determined as (P,P). The absolute structure of the second eluent 15Pd2 was determined as (M,M) as well, while the first eluent 15Pd1 was determined to be (P,P).
Figure 5.

CD spectra of (a) 13Pd and (b) 15Pd in the range from 300 to 900 nm in hexane/CH2Cl2 (1:1).
The activation barrier of the twist inversion processes (ΔG‡298) of 13Pd in toluene was experimentally determined as 113 kJ mol–1 at 298 K (Figure S94). The Eyring plot provides the enthalpy (ΔH‡ = 53.1 kJ mol–1) and the entropy (ΔS‡ = −0.20 kJ mol–1 K–1) for the racemization process. No racemization of 15Pd between the (P,P)- and (M,M)-isomers was detected in toluene at 100 °C for 15 h, suggesting the high configurational stability of 15Pd.
3. Conclusions
In summary, PdII complexes of anthriporphyrinoid and its dimeric homologues were synthesized and further decorated to multiply thiophene- and benzothiophene-fused compounds. While the monomeric PdII complexes are nonaromatic, the dimeric complexes show paratropic ring currents owing to the global 32π- and 36π-electronic circuits. Multiple fusions of the dimeric complexes allowed fully reversible six redox potentials, small HOMO–LUMO gaps, and broad absorption tails reaching at NIR, and further, caused helical conformations. Finally, their optical separations were accomplished and their racemization barrier heights were determined.
Acknowledgments
The work is supported by the National Natural Science Foun-dation of China (Grant Nos. 22201072, 22071052, 21772036, 21602058, 21705055), Scientific Research Fund of Hunan Provincial Science and Technology Department (Grant No. 2021JJ40333), and Science and Technology Innovation Program of Hunan Province (2021RC4059)
Supporting Information Available
This material is available free of charge via the Internet at http://pubs.acs. The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscentsci.2c01218.
Experimental procedures and characterization data for all products, including 1H NMR, UV–vis, X-ray single crystal data and computational work (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Cai J. M.; Ruffieux P.; Jaafar R.; Bieri M.; Braun T.; Blankenburg S.; Muoth M.; Seitsonen A. P.; Saleh M.; Feng X.; Müllen K.; Fasel R. Atomically precise bottom-up fabrication of graphene nanoribbons. Nature. 2010, 466, 470–473. 10.1038/nature09211. [DOI] [PubMed] [Google Scholar]
- Talirz L.; Söde H.; Dumslaff T.; Wang S.; Sanchez-Valecia J.; Liu J.; Shinde P.; Pignedoli C. A.; Liang L.; Meunier V.; Plumb N. C.; Shi M.; Feng X.; Narita A.; Müllen K.; Fasel R.; Ruffieux P. On-Surface synthesis and characterization of 9-atom wide armchair graphene nanoribbons. ACS Nano 2017, 11, 1380–1388. 10.1021/acsnano.6b06405. [DOI] [PubMed] [Google Scholar]
- Tsuda A.; Osuka A. Fully conjugated porphyrin tapes with electronic absorption bands that reach into infrared. Science 2001, 293, 79–82. 10.1126/science.1059552. [DOI] [PubMed] [Google Scholar]
- Mori H.; Tanaka T.; Lee S.; Lim J. M.; Kim D.; Osuka A. meso-meso Linked porphyrin-[26]hexaphyrin-porphyrin hybrid arrays and their triply linked tapes exhibiting strong absorption bands in the NIR region. J. Am. Chem. Soc. 2015, 137, 2097–2106. 10.1021/ja5130034. [DOI] [PubMed] [Google Scholar]
- Nakamura Y.; Aratani N.; Shinokubo H.; Takagi A.; Kawai T.; Matsumoto T.; Yoon Z. S.; Kim D. Y.; Ahn T. K.; Kim D.; Muranaka A.; Kobayashi N.; Osuka A. A directly fused tetrameric porphyrin sheet and its anomalous electronic properties that arise from the planar cyclooctatetraene core. J. Am. Chem. Soc. 2006, 128, 4119–4127. 10.1021/ja057812l. [DOI] [PubMed] [Google Scholar]
- Davis N. K. S.; Thompson A. L.; Anderson H. L. A porphyrin fused to four anthracenes. J. Am. Chem. Soc. 2011, 133, 30–31. 10.1021/ja109671f. [DOI] [PubMed] [Google Scholar]
- Mori H.; Tanaka T.; Osuka A. Fused porphyrinoids as promising near-infrared absorbing dyes. J. Mater. Chem. C 2013, 1, 2500–2519. 10.1039/c3tc00932g. [DOI] [Google Scholar]
- Tanaka T.; Osuka A. Conjugated porphyrin arrays: synthesis, properties and applications for functional materials. Chem. Soc. Rev. 2015, 44, 943–969. 10.1039/C3CS60443H. [DOI] [PubMed] [Google Scholar]
- Stȩpień M.; Gońta E.; Żyła M.; Sprutta N. Heterocyclic nanographenes and other polycyclic heteroaromatic compounds: synthetic routes, properties, and applications. Chem. Rev. 2017, 117, 3479–3716. 10.1021/acs.chemrev.6b00076. [DOI] [PubMed] [Google Scholar]
- Rao Y.; Kim T.; Park K. H.; Peng F.; Liu L.; Liu Y.; Wen B.; Liu S.; Kirk S. R.; Wu L.; Chen B.; Ma M.; Zhou M.; Yin B.; Zhang Y.; Kim D.; Song J. π-Extended “earring” porphyrins with multiple cavities and near-infrared absorption. Angew. Chem., Int. Ed. 2016, 55, 6438–6442. 10.1002/anie.201600955. [DOI] [PubMed] [Google Scholar]
- Wu L.; Li F.; Rao Y.; Wen B.; Xu L.; Zhou M.; Tanaka T.; Osuka A.; Song J. Synthesis, structures, and near-IR absorption of heterole-fused earring porphyrins. Angew. Chem., Int. Ed. 2019, 58, 8124–8128. 10.1002/anie.201903446. [DOI] [PubMed] [Google Scholar]
- Gu X.; Lai Y.-H. Triptycene: no homoconjugation effect for extending optical properties of π-conjugated oligomers. Org. Lett. 2010, 12, 5200–5203. 10.1021/ol102147f. [DOI] [PubMed] [Google Scholar]
- Niermeier P.; Teichmann L.; Neumann B.; Stammler H.-G.; Mitzel N. W. 1,4,5,8-Tetraethynylanthracene - synthesis, UV/VIS absorption spectroscopy and its application as building block for tetradentate acceptor molecules. Eur. J. Org. Chem. 2018, 2018, 6780–6786. 10.1002/ejoc.201801317. [DOI] [Google Scholar]
- Yang W.; Roy S. S.; Pitts W. C.; Nelson R. L.; Fronczek F. R.; Jurss J. W. Electrocatalytic CO2 reduction with cis and trans conformers of a rigid dinuclear rhenium complex: comparing the monometallic and cooperative bimetallic pathways. Inorg. Chem. 2018, 57, 9564–9575. 10.1021/acs.inorgchem.8b01775. [DOI] [PubMed] [Google Scholar]
- Wada T.; Muckerman J. T.; Fujita E.; Tanaka K. Substituents dependent capability of bis(ruthenium-dioxolene-terpyridine) complexes toward water oxidation. Dalton Trans. 2011, 40, 2225–2233. 10.1039/C0DT00977F. [DOI] [PubMed] [Google Scholar]
- Thompson A. L. S.; Kabalka G. W.; Akula M. R.; Huffman J. W. The conversion of phenols to the corresponding aryl halides under mild conditions. Synthesis 2005, 2005, 547–550. 10.1055/s-2005-861791. [DOI] [Google Scholar]
- Wu H.; Hynes J. Copper-catalyzed chlorination of functionalized arylboronic acids. Org. Lett. 2010, 12, 1192–1195. 10.1021/ol9029337. [DOI] [PubMed] [Google Scholar]
- Zhang G.; Lv G.; Li L.; Chen F.; Cheng J. Copper-catalyzed halogenation of arylboronic acids. Tetrahedron Lett. 2011, 52, 1993–1995. 10.1016/j.tetlet.2011.02.075. [DOI] [Google Scholar]
- Yang H.; Li Y.; Jiang M.; Wang J.; Fu H. General copper-catalyzed transformations of functional groups from arylboronic acids in water. Chem.—Eur. J. 2011, 17, 5652–5660. 10.1002/chem.201003711. [DOI] [PubMed] [Google Scholar]
- Niu L.; Yang H.; Yang D.; Fu H. functionalizations of aryl C-H bonds in 2-arylpyridines via sequential borylation and copper catalysis. Adv. Synth. Catal. 2012, 354, 2211–2217. 10.1002/adsc.201100930. [DOI] [Google Scholar]
- Ren Y.-L.; Tian X.-Z.; Dong C.; Zhao S.; Wang J.; Yan M.; Qi X.; Liu G. A simple and effective copper catalyst for the conversion of arylboronic acids to aryl iodides at room temperature. Catal. Commun. 2013, 32, 15–17. 10.1016/j.catcom.2012.11.022. [DOI] [Google Scholar]
- Fujimoto K.; Yorimitsu H.; Osuka A. Facile preparation of β-haloporphyrins as useful precursors of β-substituted porphyrins. Org. Lett. 2014, 16, 972–975. 10.1021/ol4037049. [DOI] [PubMed] [Google Scholar]
- Bruhn T.; Schaumlöffel A.; Hemberger Y.; Pescitelli G.. SpecDis, Version 1.71; Berlin, Germany, 2017; https://specdis-software.jimdo.com.
- Bruhn T.; Schaumlöffel A.; Hemberger Y.; Bringmann G. SpecDis: quantifying the comparison of calculated and experimental electronic circular dichroism spectra. Chirality 2013, 25, 243–249. 10.1002/chir.22138. [DOI] [PubMed] [Google Scholar]
- Pescitelli G.; Bruhn T. Good computational practice in the assignment of absolute configurations by TDDFT calculations of ECD spectra. Chirality 2016, 28, 466–474. 10.1002/chir.22600. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.