Abstract
Malocclusions negatively influence dental function, physical well-being, and quality of life. According to data provided by the World Health Organization (WHO), malocclusions represent the third most widespread oral health problem, after dental caries and periodontal diseases. In recent years, more and more people call for orthodontic treatment with fixed braces that aim at establishing a dental, aesthetic, and functional balance, compatible with a normal life. Our study assessed the histopathological and immunohistochemical changes that occurred in superficial marginal periodontal components, in a number of 23 patients diagnosed with malocclusions, as a result of orthodontic treatment with fixed metal braces. The purpose of the study was to evaluate the remodeling of the tissues in the superficial periodontium during orthodontic migration, microscopic aspects that are essential to evaluate the effectiveness of the orthodontic treatment.
Keywords: fixed orthodontic appliances, dental shifting, histopathological changes, superficial periodontium
Introduction
Malocclusions are caused by a complex of factors (hereditary and environmental) that can damage the occlusal harmony and negatively influence the dental function, physical well-being, and quality of life [1, 2, 3, 4]. World Health Organization (WHO) estimates malocclusions as the third most widespread oral health problem, after dental caries and periodontal diseases [5, 6].
Hereditary factors are considered causing factors, because they exert their influence during growth and, therefore, can lead to the development of a malocclusion, in the absence of associated factors. Genetic family inheritance has a strong influence on skeletal craniofacial dimensions, most commonly contributing to the occurrence of malocclusions [7, 8].
Environmental factors are more numerous, being represented by some bad habits, such as the finger sucking that may occur in infants and small children, or the obstruction of the nasal airways associated with oral breathing (caused by adenoid hypertrophy or palatal tonsils, rhinitis, nasal corneal hypertrophy, etc.), hormonal imbalances and disorders, such as gigantism induced by pituitary adenomas, craniofacial trauma, premature loss of primary teeth, congenital anatomical defects (e.g., lip split, palatal split), etc. [9, 10, 11, 12, 13].
The prevalence of malocclusion differs between different age groups, countries, and ethnic groups. Studies in recent years showed that only in the USA it affects 45% of children aged 8–11 years old and about 65% of adults [14]. It is also estimated that about 57–59% of people affected by malocclusion require orthodontic treatment.
The orthodontic treatment with fixed appliances aims at dental and facial balancing from an esthetic and functional point of view in patients with various degrees of impairment of structures that compose the dental–maxillary apparatus. Malocclusions require a detailed analysis of the data obtained by clinical and paraclinical examinations, to obtain a correct diagnosis and to develop a treatment plan in accordance with the therapeutic possibilities, the wishes, and possibilities of the patient. The treatment is performed with orthodontic appliances that, by exerting forces on the periodontal tissues, produce dental shifts followed by their alignment.
Aim
The purpose of this study was to evaluate the histopathological (HP) changes that occur in the marginal periodontium, as part of a complex process, triggered with the initiation of fixed orthodontic treatment.
Patients, Materials and Methods
This study was approved by the Ethics Committee of the University of Medicine and Pharmacy of Craiova, Romania (Approval No. 55/11.04.2022), in accordance with ethical guidelines and national and European legislation on human subjects. The clinical study included a number of 51 patients, aged between 14 and 30 years old, diagnosed with Angle Class I and Angle Class II malocclusions, examined within the Clinics Mandas Dental, Andent Smile and Petrident Orthodontics Medical Office between May 1, 2022 and May 31, 2022, in whom there was applied an orthodontic treatment with fixed braces, the straight wire technique.
To highlight the microscopic changes caused by orthodontic appliances in some patients, we proposed a HP and immunohistochemical (IHC) study to explain the clinical aspects.
The HP study included a number of 23 fragments of gingival tissue harvested from patients under treatment with fixed metallic orthodontic appliances, for about one year and six months, who presented at periodic clinical control with phenomena of hypertrophy, hyperplasia and gingival erosions. The fragments of gingival mucosa harvested after local anesthesia with 3% Mepivacaine hydrochloride were placed immediately in a 10% neutral buffered formalin fixing solution, after which they were included in histological paraffin, using the standard HP protocol. The collected samples of gingival mucosa were sent for HP and IHC examination in the Research Center for Microscopic Morphology and Immunology within the University of Medicine and Pharmacy of Craiova. Using the Microm HM 350 rotary microtome (equipped with a section transferring on water bath and Peltier cooling system), 4 μm thick sections were obtained, which were stained with Hematoxylin–Eosin (HE) and Goldner–Szekely (GS) trichrome, for an overall analysis of tissue and cellular changes present at the level of the marginal periodontium, in some orthodontic patients with fixed metallic appliances.
To highlight some particular aspects of the marginal periodontium, we propose to perform an IHC study, for completing the HP study.
For the IHC study, the histological sections were placed immediately after sectioning on poly-L-lysine covered slides to increase the histological adherence. After removing excess water, the samples were left in the thermostat, at 37ºC, for 24 hours to increase the material adhesion on the histological slides.
For our study, we used the following antibodies: anti-cluster of differentiation (CD)45RO (monoclonal mouse anti-human CD45RO, clone UCHL1, 1/100 dilution, Dako); anti-CD3 (monoclonal mouse anti-human CD3, clone F7.2.38, 1/50 dilution, Dako); anti-CD20 (monoclonal mouse anti-human CD20cy, clone L26, 1/50 dilution, Dako); anti-CD68 (monoclonal anti-human CD68, clone KP1, 1/100 dilution, Dako); anti-tryptase (monoclonal mouse anti-human mast cell tryptase, clone AA1, 1/500 dilution, Dako); anti-matrix metalloproteinase (MMP)-1 (monoclonal mouse anti-human tissue inhibitor of MMP-1, clone VT7, 1/100 dilution, Dako); anti-MMP-9 (polyclonal rabbit anti-human MMP-9, 1/100 dilution, Dako); anti-MMP-13 (monoclonal mouse anti-human recombinant collagenase-3 protein, clone NB110-5919, 1/100 dilution, Novus).
We mention that all the participants or parents/legal guardians of the patients included in the study group expressed their informed consent for collecting biological samples and the HP and IHC study.
Results
The orthodontic treatment performed by us was addressed to young people, aged between 14 and 30 years old, with Angle Class I malocclusions caused by carious processes and early tooth extractions from the Korkhaus area, with Angle Class II Subdivision 1 malocclusions caused by the predominantly oral breathing and vicious habits of finger sucking during childhood and Angle Class II Subdivision 2 malocclusions caused by genetic factors, patients also having other family members with the same malocclusion.
Clinical examination was performed periodically in all patients to perform the necessary corrections of orthodontic appliances and to prevent or treat any complications induced by the orthodontic treatment. Of the 51 patients included in our study, 19 presented a good oral hygiene, the remaining 32 patients having deficient oral hygiene with abundant deposits of mineralized bacterial plaque (tartar), but also new deposits. These were found especially around the brackets and other elements that make up the orthodontic system.
A number of 14 patients reported that they had intermittent gingival bleeding during the gingival–dental brushing, but also during the mastication of more consistent foods. In them, we identified bleeding at examining time of the dental ditch with the dental probe and while applying the air spray from the dental unit.
In patients with deficient oral hygiene, the clinical examination found areas with congested gums, sensitive to pressure and even gingival hyperplasia characterized by abnormal gum increases (Figures 1, 2, 3, 4), especially in the interdental spaces, most likely due to local inflammatory processes induced by changes in the bacterial plaque, because of the application of orthodontic appliances.
Figure 1.
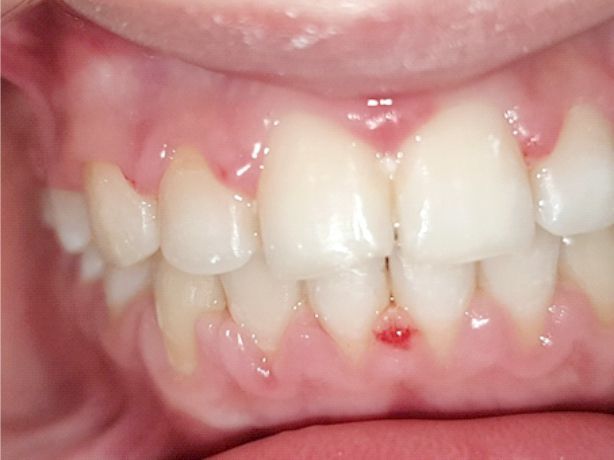
Inflammatory gingivitis immediately after the removal of a fixed metallic orthodontic appliance in a 16-year-old patient.
Figure 2.
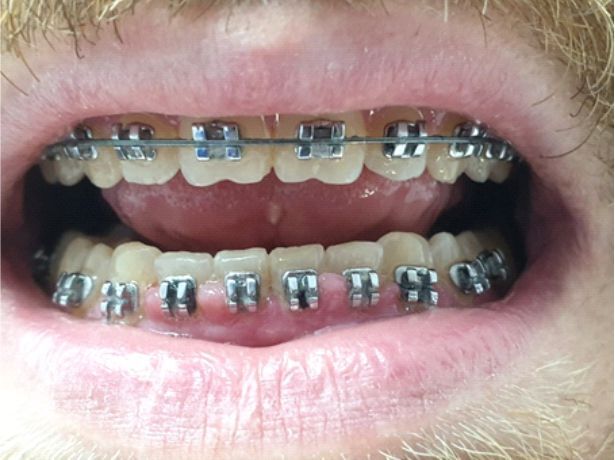
Gingival hyperplasia at the lower arch during treatment with a fixed metallic orthodontic appliance in a 26-year-old patient.
Figure 3.
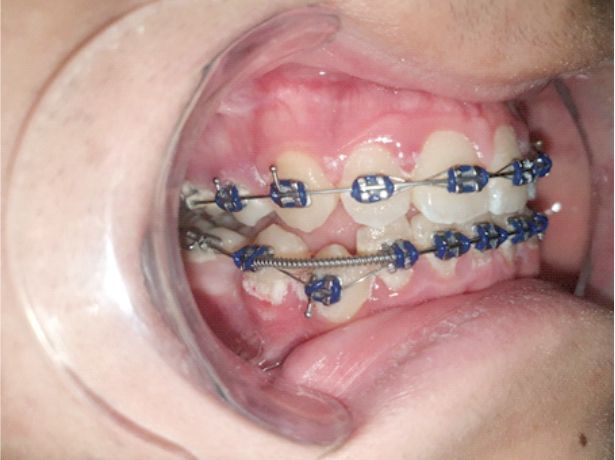
Inflammatory gingivitis and bacterial plaque at the upper and lower arches during treatment with a fixed metallic orthodontic appliance in a 15-year-old patient.
Figure 4.
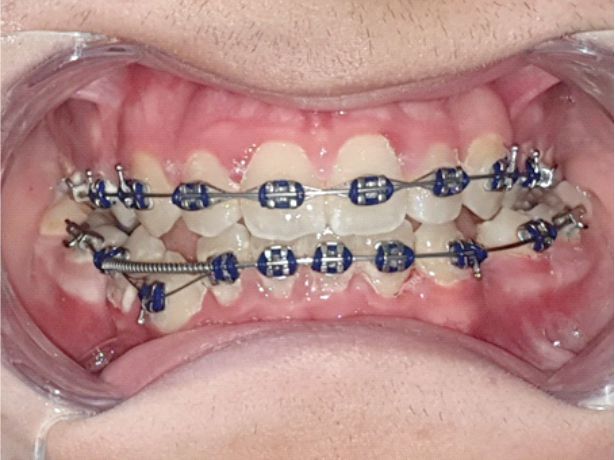
Inflammatory gingivitis and bacterial plaque at the upper and lower arches during treatment with a fixed metallic orthodontic appliance in a 15-year-old patient.
The microscopic analysis of HP samples showed the presence of important morphological changes of the gingival mucosa structure in some patients with fixed orthodontic appliances. Gingivitis caused by the orthodontic appliances had different aspects and intensities from one patient to another, which shows that in the etiopathogenesis of gingivitis there are factors related to the host reactivity. Morphological changes in the superficial (covering) periodontium were identified both in the gingival epithelium and in the underlying connective tissue. Most often, in the gingival epithelium there were highlighted areas of hypertrophy and hyperplasia, characterized by increase of the gingival epithelium thickness, on account of the spiny layer (Figure 5). In other patients, the microscopic examination showed a reduction in the thickness of the gingival epithelium, sometimes associated with intraepithelial edema, the widening of the intercellular spaces, the vacuolization of the cytoplasm of the epithelial cells and even cell necrosis (Figure 6). Other times there were highlighted areas of gingival epithelium with extended cell necrosis (Figure 7).
Figure 5.
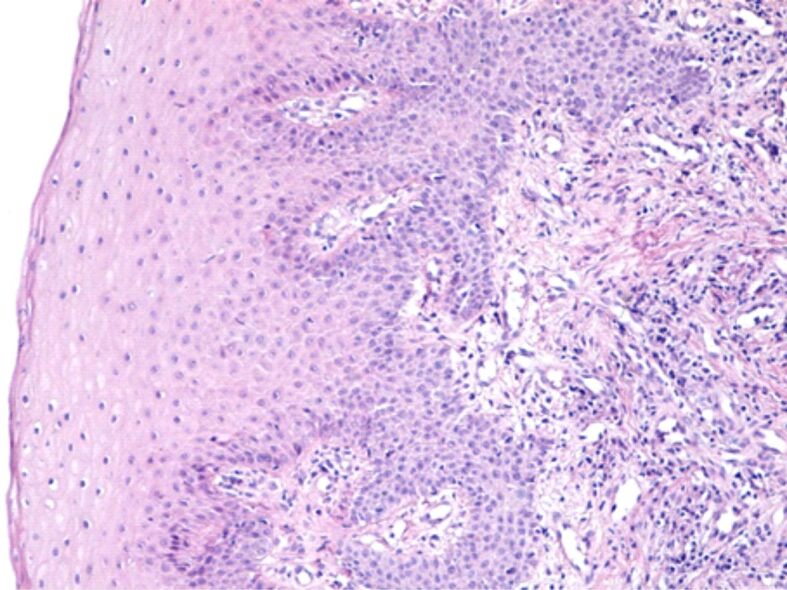
Overall image of the gingival mucosa where we observe the increase of the thickness of the cover epithelium. Hematoxylin–Eosin (HE) staining, ×100
Figure 6.
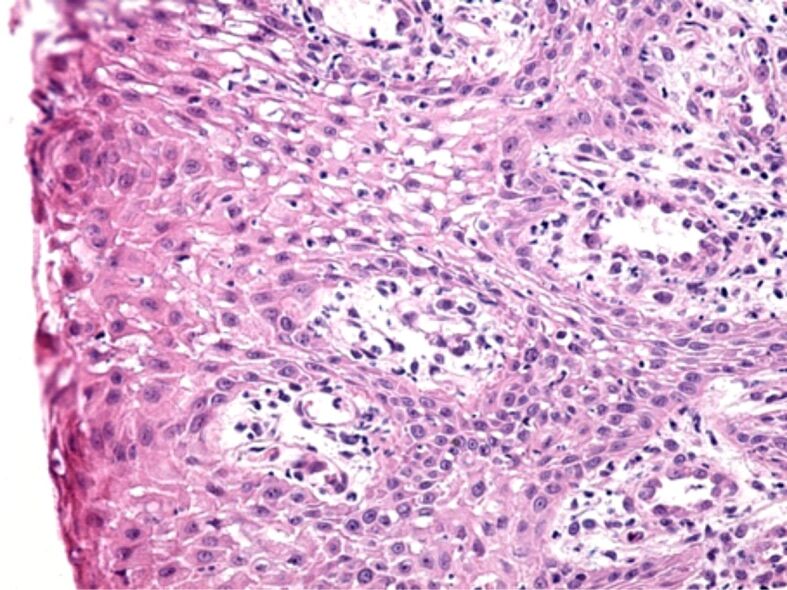
Swollen gingival epithelium, with the broadening of the intracellular spaces, cytoplasmic vacuoles, and cell necrosis. HE staining, ×200.
Figure 7.
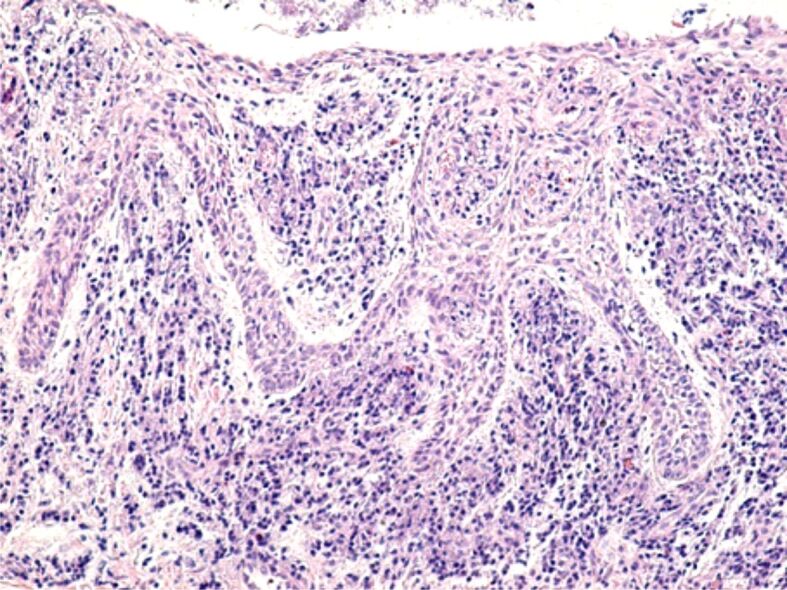
Image of gingival epithelium with extended areas of epithelial necrosis. HE staining, ×100
The gingival chorion was frequently the headquarters of an acute inflammatory process, characterized by accumulation of increased amounts of inflammatory cells, especially granulocytes (Figures 8 and 9) or chronic inflammation, characterized by the accumulation of round and macrophage mononuclear cells (Figure 10). The arrangement of the inflammatory infiltrate was totally heterogeneous, being identified on the same HP sample strongly infiltrated chorion areas with inflammatory cells, but also areas with moderate or even poor content of inflammatory cells. Most often, chronic infiltrates were associated with the development of an angiogenesis network, necessary to develop tissue regeneration processes. Although there was not identified any a cause to explain the heterogenous distribution of inflammatory cells, we believe that this would be due to the presence of antigens represented by bacteria in the subgingival plaque or other antigens synthesized by them.
Figure 8.
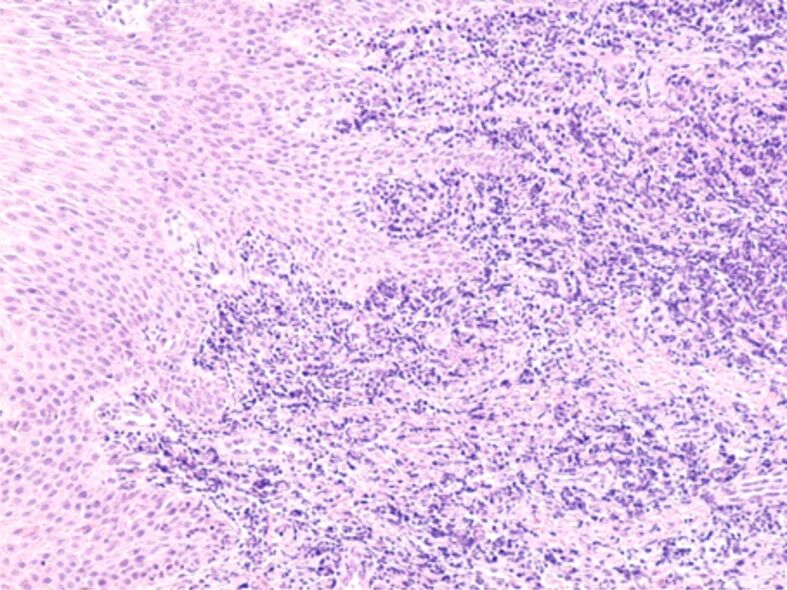
Gingival chorion strongly infiltrated with inflammatory cells of lymphocyte and granulocyte type. HE staining, ×100.
Figure 9.
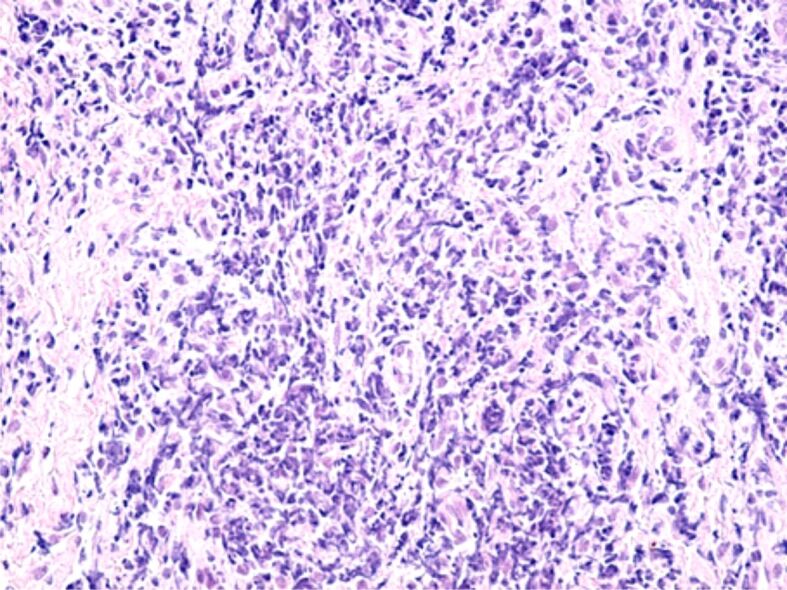
Image of acute, abundant inflammatory infiltrate mainly made up of granulocytes, present in the gingival chorion. HE staining, ×200.
Figure 10.
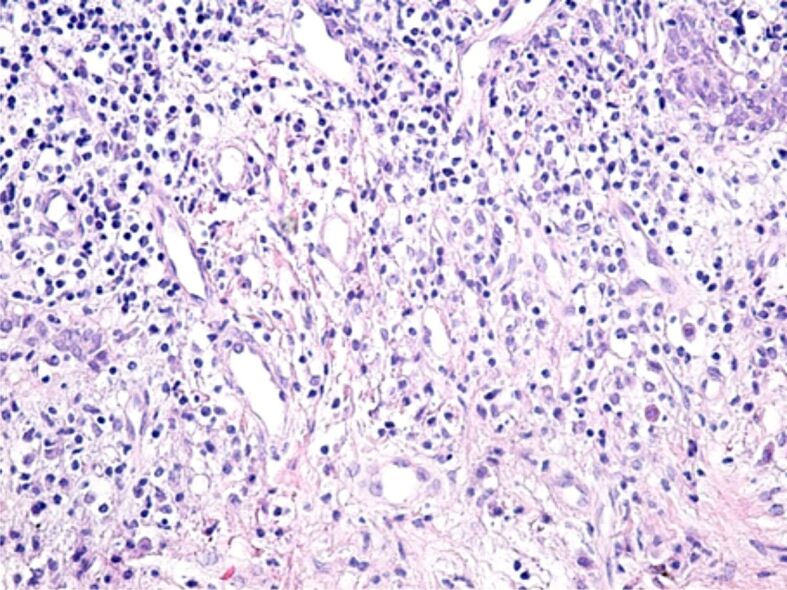
Chronic, moderate inflammatory infiltrate, made up of lymphocytes, plasmocytes and macrophages, associated with the development of a dense network of angiogenesis vessels. HE staining, ×200.
Other microscopic changes highlighted on some HP samples were the tissue edema present more frequently in the papillary chorion (Figure 11) or the intensification of collagen fibrosis processes, in the deep chorion (Figure 12).
Figure 11.
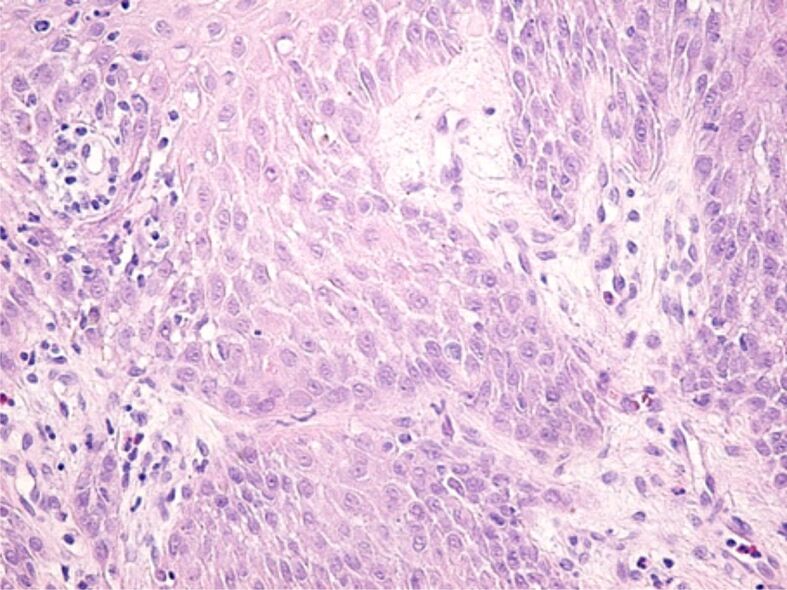
Moderate tissue edema in the papillary chorion and gingival epithelium. HE staining, ×200.
Figure 12.
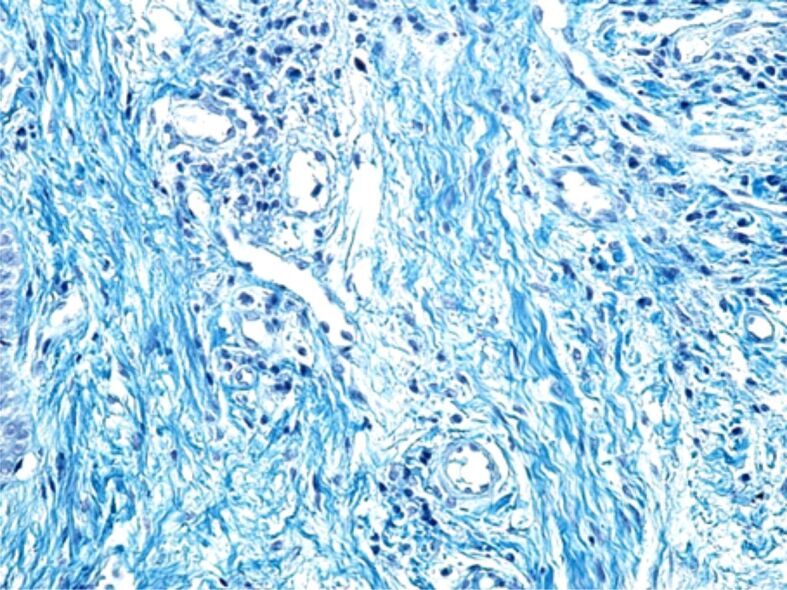
Collagen fibrosis occurring in the deep chorion. Goldner–Szekely (GS) trichrome staining, ×200.
IHC study allowed us to highlight the types of inflammatory cells present in the superficial periodontium, as well as the activity of some MMPs, in individuals wearing orthodontic braces.
Specific marking of inflammatory cells once again showed that their distribution in the connective tissue of the superficial periodontium was totally heterogenous. Of all the inflammatory cells, the most numerous were CD3+ lymphocytes and T-lymphocytes with CD45RO+ memory (Figures 13 and 14), which shows that the gingival inflammatory reaction caused by orthodontic braces is dominated by cellular immunity. Compared to T-lymphocytes, B-lymphocytes and macrophages were less numerous in the connective tissue of the superficial periodontium (Figure 15), showing that humoral immunity is less involved in the immune defense mechanisms. Also, the presence of macrophages (Figure 16) indicates the existence of antigens in the periodontal structure, even of bacteria or cellular or tissue residues subject to the local phagocytosis process. Mastocytes (Figure 17), specifically highlighted by the anti-tryptase antibody, were detected in a higher number around the blood vessels and at the limit between the epithelium and the gingival chorion.
Figure 13.
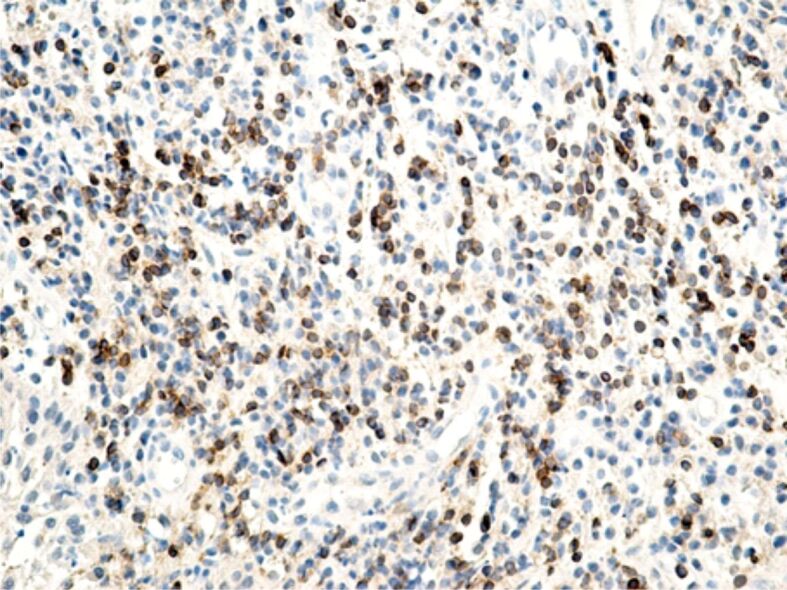
Chronic, abundant inflammatory infiltrate with a relatively high content of CD3+ T-lymphocytes. Anti-CD3 antibody immunomarking, ×200. CD3: Cluster of differentiation 3.
Figure 14.
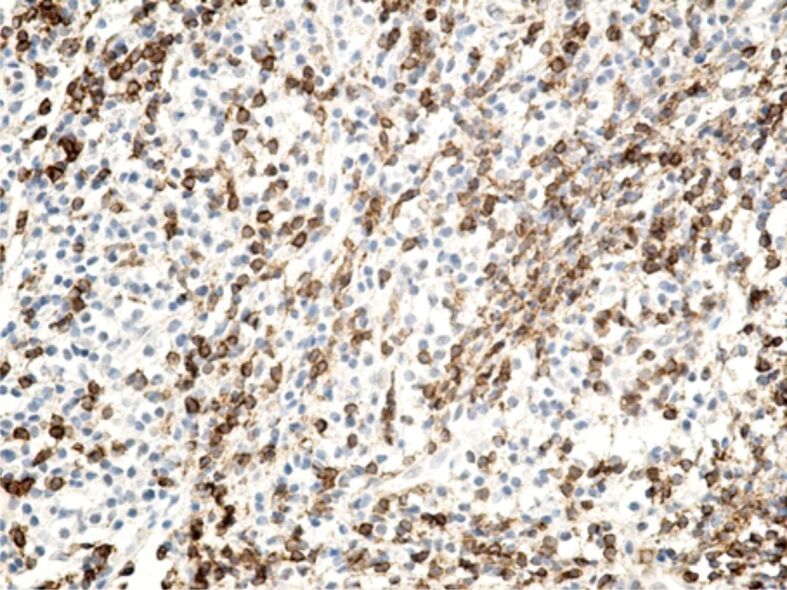
Image of highly infiltrated superficial periodontium with lymphocyte cells, where a large part of the cells are T-lymphocytes with CD45RO+ memory. Anti-CD45RO antibody immunomarking, ×200. CD45RO: Cluster of differentiation 45RO.
Figure 15.
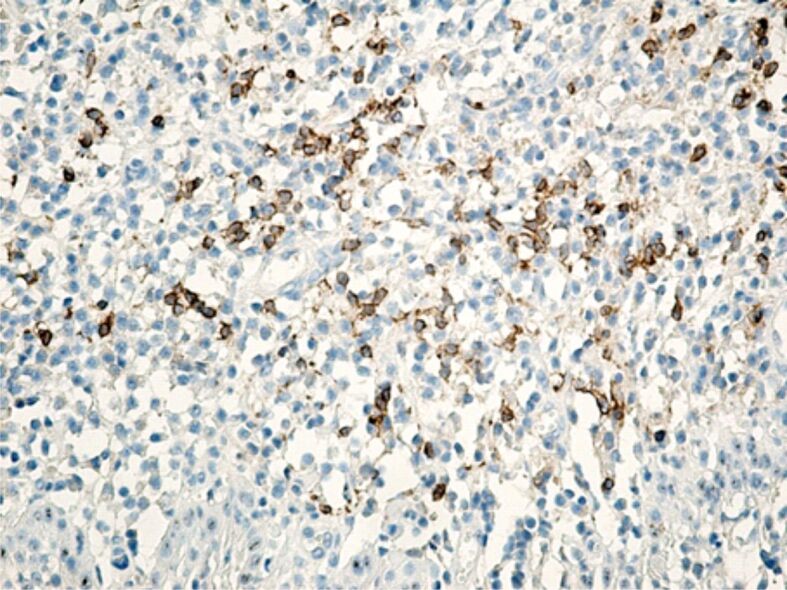
Strongly infiltrated gingival chorion with mononuclear round cells, in which CD20+ B-lymphocytes have a moderate representation. Anti-CD20 antibody immunomarking, ×200. CD20: Cluster of differentiation 20.
Figure 16.
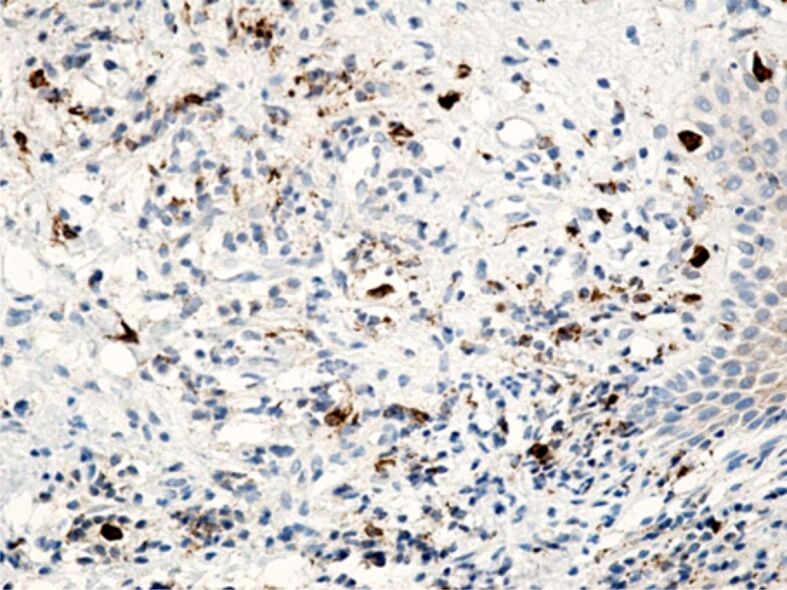
Gingival chorion infiltrated with inflammatory cells, where macrophages are in a relatively small number. Anti-CD68 antibody immunomarking, ×200. CD68: Cluster of differentiation 68.
Figure 17.
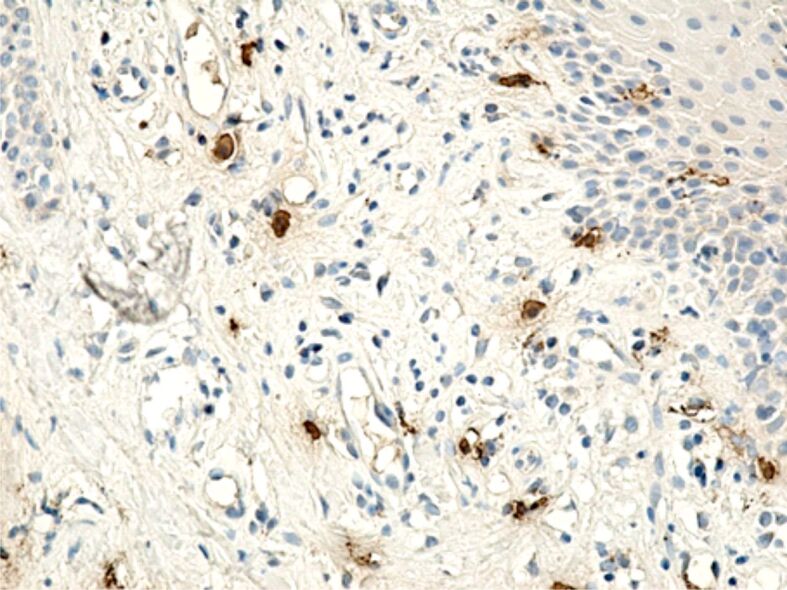
Image of gingival chorion, where the presence of mastocytes is observed in an increased number around blood vessels and the junction between the epithelium and the gingival chorion. Anti-tryptase antibody immunomarking, ×200.
Regarding the MMPs reaction, it correlated with the intensity of the inflammatory process, especially with the number of lymphocytes and macrophages present in the inflammatory foci. Of the three MMPs investigated by us (MMP-2, MMP-9, MMP-13), the most intense reaction was for MMP-1 (interstitial collagenase) that was highlighted in both inflammatory infiltrate cells, as well as in the fibroblasts (Figure 18). MMP-9 or collagenase IV was intensely positive in some inflammatory cells (lymphocytes, neutrophils), moderately positive in cells of vascular endothelium and poorly positive in the fibroblasts (Figure 19). MMP-13 (collagenase III) generally had a less intense expression, being detected in both inflammatory cells (lymphocytes, macrophages, granulocytes) and also free in the extracellular environment (Figure 20). The positive reaction of these MMPs highlights the restructuring processes of the intracellular conjunctive matrix.
Figure 18.
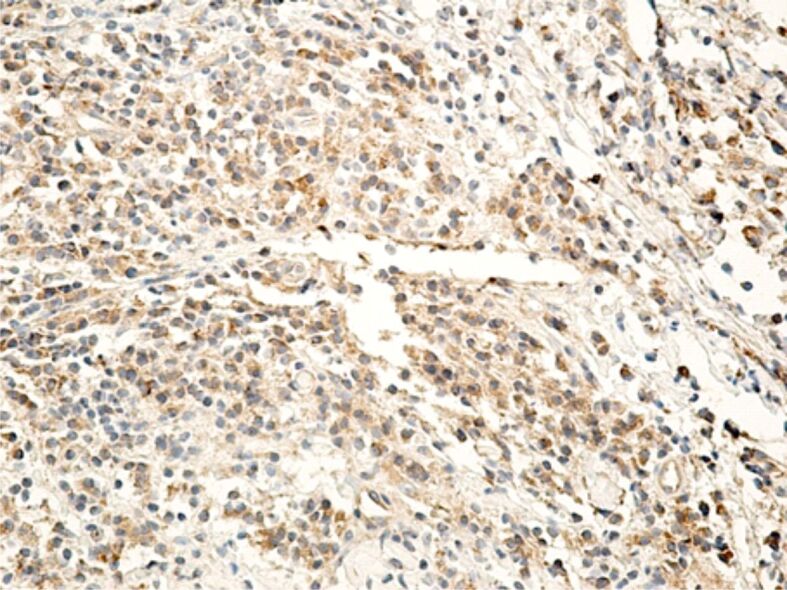
Gingival chorion area, strongly infiltrated with inflammatory cells, with an intense reaction to MMP-1. Anti-MMP-1 antibody immunomarking, ×200. MMP-1: Matrix metalloproteinase-1.
Figure 19.
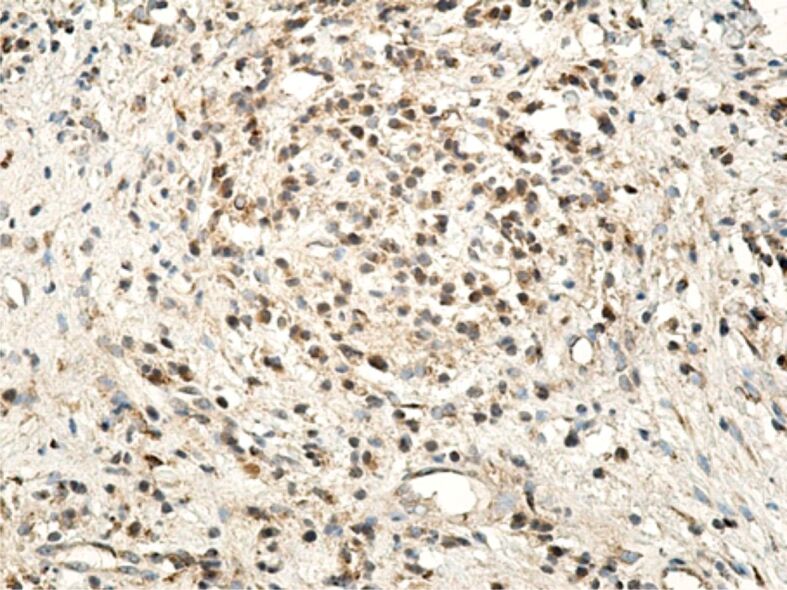
Gingival chorion with a positive reaction of the lymphocytes and endothelial cells for MMP-9. Anti-MMP-9 antibody immunomarking, ×200. MMP-9: Matrix metalloproteinase-9.
Figure 20.
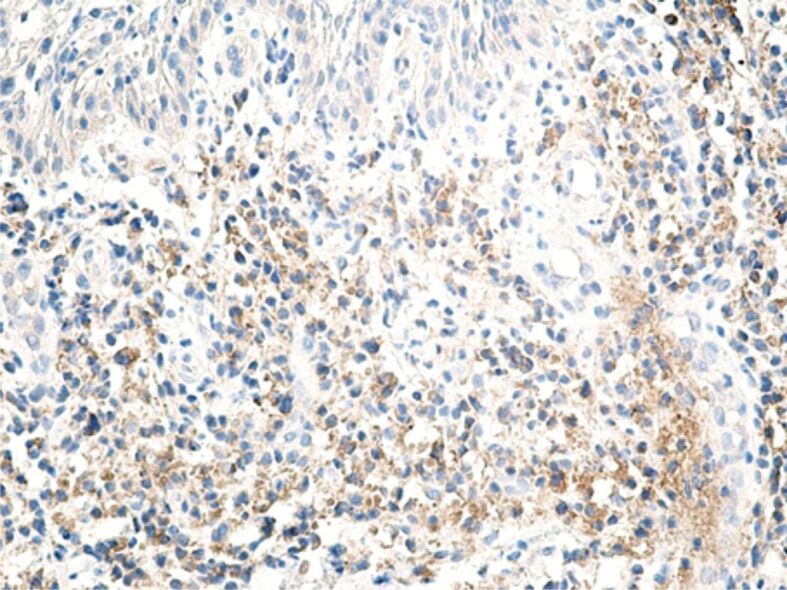
Microscopic image of a superficial periodontal area, where inflammatory cells have a moderate or weak reaction for MMP-13. Anti-MMP-13 antibody immunomarking, ×200. MMP-13: Matrix metalloproteinase-13.
Discussions
Currently, due to the increase of facial and oral aesthetic standards, fixed orthodontic appliances became highly used in the treatment of malocclusions. Through the orthodontic treatment with fixed appliances, the bone and dental changes that occur at the level of the maxillary dental system are corrected. Simultaneously with solving malocclusions, there also improves the gingival health, resulting in obtaining an aesthetic–periodontal harmony [15, 16].
Remodeling of tissues surrounding teeth are essential for the efficiency of the orthodontic treatment. This remodeling is due to the exertion of orthodontic forces. Thus, changes in the bone, dental and periodontal tissues are produced, particularly including the periodontal ligaments, the alveolar bone, and the gum. By exposure to forces with a different amplitude, frequency and mechanical force, these tissues respond through clinical and HP changes [17, 18]. It is shown that the orthodontic movement of teeth depends on the exerted force and the biological response from the surrounding tissues [17, 19]. The loading and compression forces, caused by the orthodontic braces, change the periodontal blood flow, resulting in a local inflammatory process that ensures a favorable microenvironment for the deposit or resorption of the alveolar bone, eventually leading to the movement of the teeth [20].
The exertion of mechanical forces on the teeth will lead to the adaptation of the cells in the area, to maintain and repair both the bone structure and the odontoideum and the periodontium. Thus, dental shifts are achieved through dynamic resorption and bone apposition processes [21].
Bone remodeling involves a balance between the activity of osteoblasts and osteoclasts. This process is regulated by a series of proteins interacting through complex mechanisms. Dental shifts for orthodontic purposes are dependent on the response of bone tissue to the forces exerted by the orthodontic appliances. During orthodontic treatment, the connection between bone remodeling and exerted orthodontic forces is extremely important [21, 22].
Dental position changes for orthodontic purposes can be made rapidly or slowly. The dental shifting speed varies according to the characteristics of the force, the response of the periodontal ligament, but also the biological response of the patient [17, 23].
Numerous studies showed that in the use of fixed orthodontic appliances there always appears an associated degree of trauma, including the use of low intensity orthodontic forces, which affect both the hard and soft tissues. Research performed on laboratory animals showed that the periodontal ligament subject to orthodontic stress had an intense cellular activity, involving fibroblasts, endothelial cells, osteoblasts, osteocytes and cementoblasts [17].
The exertion of orthodontic forces causes vascularization and blood flow changes, as well as the synthesis and release of key molecules for remodeling phenomena, such as neurotransmitters, cytokines, growth factors and metabolites of arachidonic acid in the periodontal ligament [17].
A microscopic study on the marginal periodontium in cases of dental trauma unrelated to orthodontic treatment showed the presence of necrosis areas associated with vascular congestion and hemorrhage. Also, many macrophages, T-lymphocytes, angiogenesis vessels, as well as a moderate number of B-lymphocytes were observed during the IHC study [24].
Cellular and tissue reactions begin within the first 24–48 hours after applying the orthodontic appliances, when the dental shifting in the alveoli occur rapidly. As a result of the compression and elongation of the periodontal ligament fibers, in the pressure and tension areas, respectively, the process of recruiting osteoclasts and osteoblasts begins, as well as the extravasation and chemotactic activity of the inflammatory cells. The presence of hyalinization portions presented right from this initial stage [17, 25, 26].
During the next stage (stage 2), which lasts between four and 20 days, the dental shifting stops, due to the disturbances in the bloodstream and the presence of the hyalinization areas. Only the removal of necrotic tissue and bone resorption from adjacent trabecular spaces (indirect resorption) and from the direction of viable periodontal ligament (undermining resorption) allow the retake of dental shifting. This complex process requires the presence of macrophages and osteoclasts. These cells act in tandem to remove the necrosis tissues from the compressed areas of the periodontal ligament and the adjacent alveolar bone. In areas subject to tension, osteoblasts increase and begin the production of bone matrix (osteoid). New progeny cells from the fibroblast-like population are recruited (pericytes). These cells proliferate and migrate to the alveolar bone surface, along the Sharpey elongated fibers. Simultaneously, fibroblasts in the areas of the periodontal ligament in tension begin the multiplication process and reshape the surrounding matrix [17, 27].
Stages 3 and 4 of the orthodontic dental shifting, also known as the acceleration stage and the linear stage, respectively, begin about 40 days after the initial exertion of forces. In the pressure areas, there are observed collagen fibers without orientation and irregular bone areas indicating a direct bone resorption. Tension areas have a bone apposition, and bone remodeling in these stages are performed at a certain rate, regardless of the intensity of the force [17, 25].
The initial stage of the orthodontic dental shifting is characterized by predominantly exudative acute inflammatory phenomena, with periodontal vasodilation and with the migration of capillary leukocytes. A few days later, the acute phase is remitted, being progressively replaced by a chronic predominantly proliferative inflammation, which involves fibroblasts, endothelial cells, osteoblasts, and cells originating in the red bone marrow. Also, leukocytes continue to migrate from the capillaries and modulate the remodeling process [17, 26].
Adverse reactions that may occur at the periodontal level during orthodontic shifting include hypertrophy and gingival hyperplasia, retractions, invaginations, and gingival erosions [16].
Normally, the increase of the gingival volume was considered an inflammatory reaction following the accumulation of bacterial plaque [16, 28].
Our study analyzed the microscopic and IHC changes that occurred in patients wearing orthodontic appliances for a longer time. We believe that microscopic changes in our group are caused by both the mechanical force caused by the orthodontic appliances, but also by the bacterial plaque. It is known that the use of orthodontic brackets leads to changes in the composition of the bacterial plaque, both quantitatively and qualitatively, also increasing the risk of periodontal infection [29, 30]. By preventing access to good oral hygiene and creating microbial niches, orthodontic brackets prevent good oral hygiene, resulting in a serious threat to periodontal health [31]. The accumulation of gingival plaque then induces inflammatory changes in the gingival tissues.
We mentioned that the inflammatory responses to this aggression represented by the orthodontic appliances vary greatly from one individual to another. We consider, like other authors, that these answers may depend on the quality and/or quantity of the bacterial biofilm, but also on the host’s immune response [32, 33].
Conclusions
Through the remodeling initiated at the level of the superficial periodontal components, orthodontic treatment with fixed appliances can induce major HP changes in these tissues, showing different aspects depending on the patient’s reactivity, oral hygiene, as well as the accuracy of the orthodontic treatment. The superficial periodontium is the core of a chronic inflammatory process and tissue remodeling that can persist throughout the entire period of orthodontic treatment. The cellular and molecular response is extremely complex and explains the clinical changes of the gingival mucosa and periodontium as a whole.
Conflict of interests
None to declare.
Authors’ contribution
Mahmoud Katta and Adela Cristina Lazăr equally contributed to the manuscript.
References
- 1.Liu Z, McGrath C, Hägg U. The impact of malocclusion/orthodontic treatment need on the quality of life. A systematic review. Angle Orthod. 2009;79(3):585–591. doi: 10.2319/042108-224.1. [DOI] [PubMed] [Google Scholar]
- 2.Anthony SN, Zimba K, Subramanian B. Impact of malocclusions on the oral health-related quality of life of early adolescents in Ndola, Zambia. Int J Dent. 2018;2018:7920973–7920973. doi: 10.1155/2018/7920973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naseri N, Baherimoghadam T, Kavianirad F, Haem M, Nikmehr S. Associations between malocclusion and self-esteem among Persian adolescent population. J Orthod Sci. 2020;9:6–6. doi: 10.4103/jos.JOS_23_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almotairy N, Almutairi F. A nation-wide prevalence of malocclusion traits in Saudi Arabia: a systematic review. J Int Soc Prev Community Dent. 2022;12(1):1–11. doi: 10.4103/jispcd.JISPCD_251_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo L, Feng Y, Guo HG, Liu BW, Zhang Y. Consequences of orthodontic treatment in malocclusion patients: clinical and microbial effects in adults and children. BMC Oral Health. 2016;16(1):112–112. doi: 10.1186/s12903-016-0308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alhammadi MS, Halboub E, Fayed MS, Labib A, El-Saaidi C. Global distribution of malocclusion traits: a systematic review. Dental Press J Orthod. 2018;23(6):40_e1–40_e10. doi: 10.1590/2177-6709.23.6.40.e1-10.onl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zere E, Chaudhari PK, Sharan J, Dhingra K, Tiwari N. Developing Class III malocclusions: challenges and solutions. Clin Cosmet Investig Dent. 2018;10:99–116. doi: 10.2147/CCIDE.S134303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dascălu IT, Ţuculină MJ, Răescu M, Popescu SM, Corega C, Vaida L, Bold A. Modifications of the marginal paradentium to a case of Angle Class III/1 malocclusion. Rom J Morphol Embryol. 2013;54(3 Suppl):857–862. [PubMed] [Google Scholar]
- 9.Zou J, Meng M, Law CS, Rao Y, Zhou X. Common dental diseases in children and malocclusion. Int J Oral Sci. 2018;10(1):7–7. doi: 10.1038/s41368-018-0012-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abate A, Cavagnetto D, Fama A, Maspero C, Farronato G. Relationship between breastfeeding and malocclusion: a systematic review of the literature. Nutrients. 2020;12(12):3688–3688. doi: 10.3390/nu12123688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung JC. Redirecting the growth pattern with rapid maxillary expander and chin cup treatment: changing breathing pattern from oral to nasal. World J Orthod. 2006;7(3):236–253. [PubMed] [Google Scholar]
- 12.Sugawara Y, Ishihara Y, Takano-Yamamoto T, Yamashiro T, Kamioka H. Orthodontic treatment of a patient with unilateral orofacial muscle dysfunction: the efficacy of myofunctional therapy on the treatment outcome. Am J Orthod Dentofacial Orthop. 2016;150(1):167–180. doi: 10.1016/j.ajodo.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 13.Freitas HV, Alves CMC, Silva LFGE, Pereira ALP, Hugo FN, Thomaz EBAF. Alterations of oral functions and dental malocclusions in adolescents: a cross-sectional population-based study. Cien Saude Colet. 2021;26(Suppl 3):5261–5272. doi: 10.1590/1413-812320212611.3.07992020. [DOI] [PubMed] [Google Scholar]
- 14.Proffit WR, Fields HW, Moray LJ. Prevalence of malocclusion and orthodontic treatment need in the United States: estimates from the NHANES III survey. Int J Adult Orthodon Orthognath Surg. 1998;13(2):97–106. [PubMed] [Google Scholar]
- 15.Del Santo. Periodontium and orthodontic implications: biological basics. Int J Stomatol Res. 2012;1(2):6–16. [Google Scholar]
- 16.Gorbunkova A, Pagni G, Brizhak A, Farronato G, Rasperini G. Impact of orthodontic treatment on periodontal tissues: a narrative review of multidisciplinary literature. Int J Dent. 2016;2016:4723589–4723589. doi: 10.1155/2016/4723589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krishnan V, Davidovitch Z. Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofacial Orthop. 2006;129(4):469_e1–469_e32. doi: 10.1016/j.ajodo.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Reitan K. Tissue behavior during orthodontic tooth movement. Am J Orthod. 1960;46(12):881–900. [Google Scholar]
- 19.Gameiro GH, Pereira-Neto JS, Magnani MBB, Nouer DF. The influence of drugs and systemic factors on orthodontic tooth movement. J Clin Orthod. 2007;41(2):73–78. [PubMed] [Google Scholar]
- 20.Duarte L, Bezerra AP, Flores-Mir C, De Luca, Pereira LJ, Vega Gonçalves. Activation and installation of orthodontic appliances temporarily impairs mastication. Angle Orthod. 2022;92(2):275–286. doi: 10.2319/061221-469.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Jia L, Zheng Y, Li W. Bone remodeling induced by mechanical forces is regulated by miRNAs. Biosci Rep. 2018;38(4):BSR20180448–BSR20180448. doi: 10.1042/BSR20180448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kular J, Tickner J, Chim SM, Xu J. An overview of the regulation of bone remodelling at the cellular level. Clin Biochem. 2012;45(12):863–873. doi: 10.1016/j.clinbiochem.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 23. Rygh P , Brudvik P . In: The periodontal ligament in health and disease . 2nd . Berkovitz BKB , Moxham BJ , Newman HN , editors. London-Baltimore : Mosby-Wolfe ; 1995 . The histological responses of the periodontal ligament to horizontal orthodontic forces . [Google Scholar]
- 24.Dascălu IT, Manolea HO, Coleş E, Dăguci C, Bătăiosu M, Andrei CM, Ţuculină MJ, Diaconu OA, Dăguci L. The prevalence of crown injuries to frontal teeth at schoolchildren aged 6 to 14 and their effects on the periodontal tissue. Rom J Morphol Embryol. 2016;57(2 Suppl):729–735. [PubMed] [Google Scholar]
- 25.von Böhl, Maltha JC, Von Den, Kuijpers-Jagtman AM. Focal hyalinization during experimental tooth movement in beagle dogs. Am J Orthod Dentofacial Orthop. 2004;125(5):615–623. doi: 10.1016/j.ajodo.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 26.von Böhl, Maltha JC, Von den, Kuijpers-Jagtman AM. Changes in the periodontal ligament after experimental tooth movement using high and low continuous forces in beagle dogs. Angle Orthod. 2004;74(1):16–25. doi: 10.1043/0003-3219(2004)074<0016:CITPLA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 27.Bozal CB, Fiol JA, Ubios AM. Early osteocyte response to bone resorption stimuli. Acta Odontol Latinoam. 2001;14(1-2):24–29. [PubMed] [Google Scholar]
- 28.Eid HA, Assiri HAM, Kandyala R, Togoo RA, Turakhia VS. Gingival enlargement in different age groups during fixed orthodontic treatment. J Int Oral Health. 2014;6(1):1–4. [PMC free article] [PubMed] [Google Scholar]
- 29.Pinto AS, Alves LS, Zenkner JEDA, Zanatta FB, Maltz M. Gingival enlargement in orthodontic patients: effect of treatment duration. Am J Orthod Dentofacial Orthop. 2017;152(4):477–482. doi: 10.1016/j.ajodo.2016.10.042. [DOI] [PubMed] [Google Scholar]
- 30.Vincent-Bugnas S, Borsa L, Gruss A, Lupi L. Prioritization of predisposing factors of gingival hyperplasia during orthodontic treatment: the role of amount of biofilm. BMC Oral Health. 2021;21(1):84–84. doi: 10.1186/s12903-021-01433-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pellegrini P, Sauerwein R, Finlayson T, McLeod J, Covell DA Jr, Maier T, Machida CA. Plaque retention by self-ligating vs elastomeric orthodontic brackets: quantitative comparison of oral bacteria and detection with adenosine triphosphate-driven bioluminescence. Am J Orthod Dentofacial Orthop. 2009;135(4):426–427. doi: 10.1016/j.ajodo.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 32.Trombelli L, Farina R. A review of factors influencing the incidence and severity of plaque-induced gingivitis. Minerva Stomatol. 2013;62(6):207–234. [PubMed] [Google Scholar]
- 33.Zanatta FB, Ardenghi TM, Antoniazzi RP, Pinto TMP, Rösing CK. Association between gingivitis and anterior gingival enlargement in subjects undergoing fixed orthodontic treatment. Dental Press J Orthod. 2014;19(3):59–66. doi: 10.1590/2176-9451.19.3.059-066.oar. [DOI] [PMC free article] [PubMed] [Google Scholar]


